Abstract
There is growing evidence that generation of adenosine from ATP, which is mediated by the CD39/CD73 enzyme pair, predetermines immunosuppressive and pro-angiogenic properties of myeloid cells. We have previously shown that the deletion of the TGFβ type II receptor gene (Tgfbr2) expression in myeloid cells is associated with decreased tumor growth suggesting pro-tumorigenic effect of TGFβ signaling. In this study, we tested the hypothesis that TGFβ drives differentiation of myeloid-derived suppressor cells (MDSCs) into pro-tumorigenic terminally differentiated myeloid mononuclear cells (TDMMCs) characterized by high levels of cell surface CD39/CD73 expression. We found that TDMMCs represent a major cell subpopulation expressing high levels of both CD39 and CD73 in the tumor microenvironment. In tumors isolated from MMTV-PyMT/TGFRIIKO mice, an increased level of TGFβ protein was associated with further increase in number of CD39+CD73+ TDMMCs compared to MMTV-PyMT/TGFRIIWT control tumors with intact TGFβ signaling. Using genetic and pharmacological approaches, we demonstrated that the TGFβ signaling mediates maturation of MDSCs into TDMMCs with high levels of cell surface CD39/CD73 expression and adenosine-generating capacity. Disruption of TGFβ signaling in myeloid cells resulted in decreased accumulation of TDMMCs, expressing CD39 and CD73, and was accompanied by increased infiltration of T lymphocytes, reduced density of blood vessels and diminished progression of both Lewis Lung carcinoma and spontaneous mammary carcinomas. We propose that TGFβ signaling can directly induce the generation of CD39+CD73+ TDMMCs, thus contributing to the immunosuppressive, pro-angiogenic, and tumor-promoting effects of this pleiotropic effector in the tumor microenvironment.
Introduction
CD39 (ectonucleoside triphosphate diphosphohydrolase-1) hydrolyzes extracellular ATP and ADP into AMP, which is then processed into adenosine by the CD73 (ecto-5′-nucleotidase). There is growing evidence that the cell surface CD39/CD73 enzyme pair plays an important role in regulating antitumor responses by catabolizing the tumor-suppressing ATP to the tumor-promoting adenosine. Antitumor effects of extracellular ATP are explained by its direct inhibition of tumor cell growth (1) and by acting as a “natural adjuvant” (2) or “danger signal” (3) that activates the immune system (4–6). Conversely, tumor-promoting effects of extracellular adenosine have been attributed to suppression of immune responses through A2A adenosine receptor-mediated inhibition of T cell proliferation (7) and A2A/2B receptor-mediated stimulation of tumor angiogenesiss (8–10). Therefore, cell populations present in tumors that express CD39 and CD73 including T regulatory cells (11, 12), MDSCs (13), endothelial cells (14), and some types of cancer cells (15, 16) or even exosomes (17), have attracted much attention lately due to their involvement in ATP-CD39–CD73–adenosine pathway, which can shift the balance from tumor-suppressive extracellular ATP toward tumor-promoting extracellular adenosine. However, studies on CD39 and CD73 expression so far have focused only on separate cell subpopulations without taking into account other cells present in the microenvironments of tumors.
The pleiotropic cytokine, transforming growth factor beta (TGFβ), is a part of the tumor microenvironment and plays a critical role in the regulation of tumor growth. High levels of TGFβ are present in many types of tumors, including melanomas and carcinomas of the breast, colon, esophagus, stomach, liver, lung, pancreas, and prostate, as well as hematologic malignancies (18, 19). Through its pleiotropic effects on immune cells, TGFβ maintains a delicate balance between immunosuppression and activation of the immune system. For example, genetic ablation of Smad4-dependent signaling in T lymphocytes resulted in spontaneous development of gastrointestinal tumors (20) suggesting tumor-suppressive role of TGFβ signaling in T cells. However, T cell specific TGFβ signaling can also contribute to suppression of anti-tumor immunity directly via inhibition of generation and activity of cytotoxic T lymphocytes, or indirectly through induction of regulatory T cells (21).
In myeloid cells, we have recently shown that genetic disruption of the TGFβ type II receptor gene, Tgfbr2, in LysM-Cre/Tgfbr2KO mice resulted in decreased implanted tumor growth suggesting pro-tumorigenic effects of TGFβ signaling (22). Indeed, TGFβ has been shown to promote recruitment of myeloid-derived suppressor cells (MDSCs) into tumors (23). MDSCs may contribute to immunosuppressive tumor networks through a variety of mechanisms including generation of reactive oxygen species, secretion of TGFβ, depletion of L-arginine, cystine and cysteine (24). However, in the tumor microenvironment, MDSCs rapidly mature into terminally differentiated myeloid cells which include neutrophils, macrophages and dendritic cells (25). Differentiated cells of the myeloid lineage represent a major component of the leukocyte infiltrate of many solid tumors. These cells are composed of multiple distinct subpopulations with pro- or anti-tumorigenic properties depending on stimuli that triggered their differentiation (26–29).
In the current study, we report for the first time that CD45+CD11b+CD11c+F4/80+MHCII+Gr-1− terminally differentiated myeloid mononuclear cells (TDMMCs) represent a major cell subpopulation in tumors expressing high levels of both CD39 and CD73, and that TGFβ acting on myeloid cells can directly regulate the generation of CD39/CD73 TDMMCs, thus contributing to the tumor-promoting effects of this pleiotropic effector of tumor microenvironment.
Materials and Methods
Mice and cell lines
TGFβRIIMyeKO and TGFβRIIMyeWT mice, on a C57BL6 background, and MMTV-PyMT/TGFβRIIfloxed and MMTV-PyMT/TGFβRIIKO, on a FVB background, were established and maintained as described (30). To generate MMTV-PyMT/TGFβRIIMeyKO mice we first crossed LysM-Cre mice (FVB background, kindly provided by Timothy Blackwell, Vanderbilt University, Nashville) with MMTV-PyMT mice and then MMTV-PyMT/TGFβRIIfloxed mice with MMTV-PyMT/LysM-Cre mice. The studies were approved by IACUC at Vanderbilt University Medical Center.
LLC cell line (CRL-1642) was obtained from American Type Culture Collection (Manassas, VA, USA) and maintained following the manufacturer’s protocols. LLC cells (5×105 cells) were injected s.c. into the right flank of mice.
Flow Cytometry Analysis
Single-cell suspension from explant of LLC tumor was prepared after collagenase I/ hyaluronidase digestion for 1 hr as describe (31). Collagenase I/Dispase II solution was used to obtain cell suspension from MMTV-PyMT tumors (32). After treatment with FcR Blocking Reagent, cells (106 cells/ml) were incubated with the relevant antibodies for 25 minutes at 4°C. If not stated otherwise, all antibodies were obtained from eBioscience, Inc. (San Diego, CA) and from Biolegend, Imc. (San Diego, CA). Data acquisition was performed on a LSRII and FACSCalibur flow cytometers (BD Biosciences, Franklin Lakes, NJ) and the data were analyzed with FlowJo software. Antigen negativity was defined as having the same fluorescent intensity as the isotype-matched control antibody.
Generation of cells from bone marrow hematopoietic progenitors
Bone marrow cells were harvested from the femurs and tibias of TGFβRIIMyeWT or TGFβRIIMyeKO mice. Hematopoietic progenitor cells (Lin−) were isolated using lineage cell depletion kit and LS columns from Miltenyi Biotec Inc. (Auburn, CA) according to the manufacturer’s instructions. Resulting cells were >50% CD117-positive as assayed by flow cytometry. Hematopoietic progenitor cells were cultured at initial concentration of 5 × 104 cells/mL concentration in RPMI medium containing 10% FBS, 20 mM Hepes, 50 μM 2-mercaptoethanol, 1X antibiotic-antimycotic solution (Sigma, St. Louis, MO) and supplemented with granulocyte-macrophage colony stimulating factor (GM-CSF; 20 ng/mL) and IL-6 (10 ng/ml; both from R&D Systems, Inc., Minneapolis, MN) (33) for 3–4 days under humidified atmosphere of air/CO2 (19:1) at 37°C.
Adenosine generation assay
The optimal number of myeloid cells (5 × 104) per assay was determined in ancillary studies (Supplementary Figure 1). Magnetically sorted CD11b+, Gr-1+ or Gr-1− myeloid cells were resuspended in 50 μl of modified Tyrode’s buffer (20 mM HEPES, 10 mM glucose, 5 mM KCI, 120 mM NaCI, 2 mM CaCI2, pH 7.5) containing 2 μM erythro-9-(2-hydroxy-3-nonyl) adenine (R&D Systems/Tocris Biosciences). The reaction was started with addition of 50 μl of the same buffer containing 20 μM of [8-14C] adenosine 5′-diphosphate (ADP; American Radiolabeled Chemicals, St. Louis, MO). After 10 min incubation period at 37°C, the reaction was stopped with addition of trichloroacetic acid (5% final concentration) and tubes were immediately placed on ice. Radioactive [8-14C] adenosine, generated by CD39+CD73+ myeloid cells, was separated from [8-14C] nucleotides on columns of acidic aluminum oxide (1.3g per column) by elution with 4 ml 0.005 N hydrochloric acid as described previously (34). 14C radioactivity in eluents was measured with a liquid scintillation counter (LS6000IC; Beckman, Fullerton, CA) and adenosine concentrations were calculated from calibration curves.
ATP breakdown assay
Magnetically sorted CD11b+ cells from tumors were resuspended in modified Tyrode’s buffer (20 mM HEPES, 10 mM glucose, 5 mM KCI, 120 mM NaCI, 2 mM CaCI2, pH 7.4) at a concentration of 105 cells/ml and incubated in the presence of 5 μM ATP for 10 min at 37°C. Concentrations of remaining unhydrolyzed ATP in supernatants were determined using ATP Determination Kit (A22066, Life Technologies/Molecular Probes, Eugene, OR) according to the manufacturer’s instructions.
Whole-Lung Mounting
Mice were sacrificed by anesthetic overdose. Lungs were processed as described (35). The tumor nodules in lung were then counted.
Measurements of secreted vascular endothelial growth factor (VEGF)
Magnetically sorted CD11b+, Gr-1+ or Gr-1− myeloid cells were resuspended in RPMI media at a concentration of 106 cells/ml and incubated in the absence or presence of 10 μM 5′-N-ethylcarboxamido adenosine (NECA; Sigma) for 6 hours at 37°C. Mouse VEGF concentrations in supernatants were quantified using the DuoSet ELISA Development Systems (R&D Systems) according to the manufacturer’s instructions.
Statistical Analysis
Data were analyzed using the GraphPad Prism 5.02 software (GraphPad Software Inc., San Diego, CA) and presented as mean ± SEM. Comparisons between two groups were performed using two-tailed unpaired t tests. Multiple comparisons were performed using one-way ANOVA with appropriate post-hoc tests. A p-value < 0.05 was considered significant.
Results
Terminally differentiated myeloid mononuclear cells (TDMMC) represent a major cell subpopulation characterized by high level of CD39 and CD73 expression in mouse mammary carcinomas
To identify tumor cell subpopulations contributing to the generation of adenosine, we compared the cell surface expression of CD39 (NTPDase-1) and CD73 (5′-nucleotidase) in cell subpopulations of tumors obtained from MMTV-PyMT mice on day 21 after tumor palpation with their expression on cells from normal mammary gland tissue of naïve FVB mice. Using simultaneous flow cytometric analysis of several surface markers, we identified a number of phenotypically distinct subpopulations within CD45-positive immune cells, that consisted mainly of T lymphocytes and myeloid cells, and within CD45-negative cells, which were represented by fibroblasts, epithelial, and endothelial cells. Gating strategy used to define cell subpopulations and their percentage in normal mammary glands or tumors are summarized in Table 1. Representative cytofluorimetric contour plots of CD39 and CD73 expression in different cell subpopulations from tumor and normal mammary gland tissues are shown in Figure 1A.
Table.
Cell subpopulations* in normal mammary glands and tumors extracted from MMTV-PyMT mice.
| % of CD45 positive cell | % of CD45 negative cell | |||
|---|---|---|---|---|
| Normal MG | MMTV- PyMT tumor | Normal MG | MMTV- PyMT tumor | |
| TDMMC (CD45+CD11b+CD11c+F4/80+MHCII+Gr1−) | 14.9±0.7 | 76.5±5.4 | - | - |
| MDSC (CD45+CD11b+CD11c−F4/80−MHCII−Gr1+) | 2.1±0.09 | 9.2±1.7 | - | - |
| T cells (CD45+CD3+) | 73.3±3.8 | 11.7±0.6 | - | - |
| Fibroblasts (CD45−CD326−CD140a+) | - | - | 81.7.0±6.8 | 11.4±1.3 |
| Epithelial/Cancer cells (CD45−CD326+CD140a−) | - | - | 8.9±1.5 | 75.9±6.9 |
| Endothelial (CD45−CD326−CD31+CD102+) | - | - | 7.3±0.8 | 1.8±0.4 |
Single cell suspensions obtained from normal mammary gland (MG) or MMTV-PyMT tumors were initially gated as DAPI-negative population to exclude dead cells. Then, viable cells were adjusted to singlets using FSC-A/ FSC-H dot plot. Viable singlet cell population was then used to identify different cell subpopulation based on cell surface markers indicated in the table. Data are presented as mean ± SEM of five animals in each group.
Mean values of percentage of CD45+ cells were 49.6±5.3% and 18.3±7.1% in normal MG and MMTV-PyMT tumor, respectively. Total cell numbers in cell suspensions from normal mammary glands and tumors were 1.1±0.3 × 107 cell and 9.8±2.6 × 107 cell per g of tissue, respectively.
Figure 1. Analysis of the cell-surface expression of CD39 and CD73 in different cell subpopulations in normal pre-tumor mammary glands and tumors from MMTV-PyMT mice.
(A) Representative flow cytometric contour plots showing percentage of CD39+CD73+ cells within different cell subpopulations in normal mammary gland (upper panel) and tumor (lower panel) from MMTV-PyMT mice. (B) Graphical representation of data from flow cytometric analysis of CD39/CD73 expressing cells. Numbers of cells expressing CD39 and CD73 per gram of tissue were calculated from percentages of each cell subpopulation and corresponding percentages of CD39+CD73+ cells. Isotype-matched antibodies were used to determine positivity or negativity for particular markers and set the location of gates. Variation in gates location is due to different levels of autofluorescence in each individual cell subpopulation. Data represent mean±SEM of five independent experiments with 2 mice/each. (C and D) Cell surface expression of CD39 (C) and CD73 (D) in subpopulations of epithelial and myeloid cells obtained from normal MG (open bars) or tumor (closed bars). Bars represent the average Δ of mean fluorescence intensity (ΔMFI, geometric mean fluorescence intensity corresponded to isotype-matched antibody subtracted from geometric MFI of specific antibody) from five independent experiments with 2 mice/each. Asterisks indicate the level of statistical significance: *- p<0.05; **- p< 0.01;***- p< 0.001; unpaired two-tailed t test.
Tumor growth resulted in an increase in the number of cells expressing the enzyme pair CD39 and CD73 known to be a major contributor into extracellular adenosine generation (36). Mean values of CD39+CD73+ cell numbers were 19.2±4.5 and 2.7±0.8 ×106 per gram of tissue in tumors and normal mammary glands, respectively (p<0.01, n=5, unpaired t test). An increase in CD39+CD73+ cell numbers was detected in every cell subpopulation examined, except for fibroblasts (Figure 1B). The largest numbers of cells expressing CD39 and CD73 were found within the cell population with epithelial characteristics most likely represented by tumor cells and within the myeloid cell population comprised of TDMMC and MDSC subpopulations. Although the numbers of tumor and myeloid cells expressing CD39 and CD73 were comparable, the expression levels of these ectonucleotidases on their surface, calculated as a difference in mean fluorescence intensity (δMFI), differed greatly (Figure 1C, D). CD11b+Gr1+ cells in normal mammary glands expressed high levels of both CD39 and CD73 on their surface, whereas the expression levels of these ectonucleotidases on epithelial cells and TDMMCs were significantly lower. In the tumor microenvironment, however, the expression of CD73 and CD39 on TDMMC dramatically increased by 2- and 30-fold, respectively. In contrast, the expression levels of CD73 and CD39 on epithelial cells were actually reduced by 20 and 80%, respectively. Although the expression of CD73 on MDSCs was also increased, the expression of CD39 was decreased by 50%. Therefore, we conclude that, in the tumor microenvironment, TDMMCs become a major cell subpopulation characterized by high levels of CD39 and CD73 expression.
Elevated levels of TGFβ protein in the tumor are associated with an increase in CD39+CD73+ terminally differentiated myeloid mononuclear cells
We have recently reported that conditional deletion of Tgfbr2 in mammary epithelium of MMTV-PyMT/TGFRIIKO mice results in shortened tumor latency and a dramatic increase in the number of metastases in the lungs (37). We have also reported that these aggressive tumors are characterized by more than 2-fold increase in TGFβ levels compared to MMTV-PyMT/TGFβRIIfloxed (38).
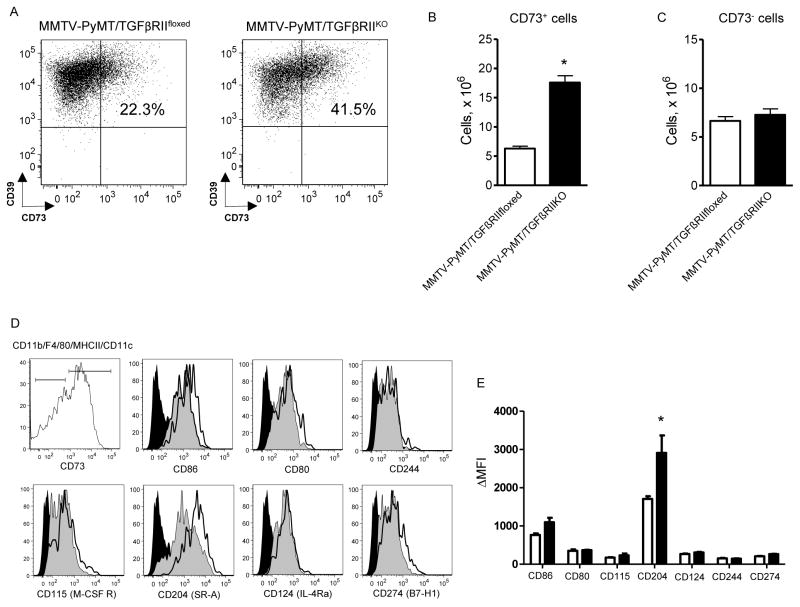
In this study, we used MMTV-PyMT/TGFβRIIKO and MMTV-PyMT/TGFβRIIfloxed mice to determine if changes in the tumor microenvironment, characterized by increased TGFβ levels, can affect tumor-associated populations of CD39+CD73+-expressing TDMMCs. Indeed, cytofluorimetric analysis revealed a 2.8-fold increase in the number of CD39+CD73+ TDMMCs in tumors extracted from MMTV-PyMT/TGFRIIKO mice, compared to MMTV-PyMT/TGFβRIIfloxed controls (Figure 2A and 2B). In contrast, we found no differences in the number of CD39+CD73− TDMMCs (Figure 2C), tumor cells, fibroblasts or endothelial cells (data not shown). Of interest, analysis of other cell surface markers on TDMMCs revealed higher expression levels of the class A scavenger receptor (SRA), also known as CD204, in CD39+CD73+ TDMMCs compared to CD39+CD73− TDMMCs (Figures 2D, E). Thus, our in vivo experiments provided indirect evidence of a potential role for TGFβ in regulation of CD39+CD73+ TDMMC accumulation in tumor tissue.
Figure 2. Analysis of CD39+CD73+ TDMMC populations in tumors from MMTV-PyMT/TGFRIIKO and MMTV-PyMT mice.
(A) Representative dot plots showing percentage of CD39+CD73+ cells within TDMMC subpopulation in tumors extracted from MMTV-PyMT mice lacking Tgfbr2 expression in mammary epithelial cells (TGFβRIIKO, right dot plot) or MMTV-PyMT/TGFβRIIfloxed control (left dot plot) animals on day 28 after tumor palpation. (B and C) Graphic representation of data from flow cytometric analysis of CD73+ (B) and CD73− (C) TDMMCs in tumors extracted from MMTV-PyMT/TGFβRIIKO (closed bars) mice or tumors from MMTV-PyMT/TGFβRIIfloxed (open bars) control animals. Data represent mean±SEM from three independent experiments with 2 mice/each. (D) Representative cytofluorimetric histograms demonstrating the expression of cell markers of mature myeloid cells in subsets of CD39+CD73− and CD39+CD73+ TDMMCs. Gray shaded histograms correspond to the expression of antigens on cell surface of CD39+CD73- TDMMCs, open histograms represent the expression on CD39+CD73+ TDMMCs and black histograms represent isotype-matched antibodies. (E) Graphical representation of mature myeloid cell markers expression on CD39+CD73− (open bars) and CD39+CD73+ (closed bars) TDMMCs. Data are expressed as δMFI and presented as mean±SEM from five independent experiments with 2 mice/each. * - p<0.05, TGFβRIIKO versus TGFβRIIfloxed; unpaired two-tailed t test.
TGFβ signaling promotes differentiation of MDSCs into CD39+CD73+ TDMMC
The tumor microenvironment directs MDSC differentiation towards terminally differentiated myeloid cells, characterized by pro-tumorigenic properties (25). To determine whether TGFβ can directly affect differentiation of myeloid cells into CD39+CD73+ -expressing TDMMCs, we performed in vitro experiments with TGFβ stimulation of MDSCs.
MDSCs were generated from Lin− hematopoietic progenitor cells (HPC) as described (33). After day 3 in culture, more than 95% of cells expressed CD11b and Gr-1, the cell surface markers characteristic of the MDSC phenotype (39). The percentage of Gr-1 negative cells, which also expressed markers of terminally differentiated myeloid cells, including F4/80, MHCII and CD11c, was less than 2% of CD11b+ cells (data not shown). Slow spontaneous differentiation of MDSCs and accumulation of F4/80+MHCII+CD11c+Gr1− TDMMC was observed within the next 24 hours (day 4) of incubation in the absence of TGFβ. The expression of CD39 was detected on cell surface of all TDMMCs. At least one-fourth of TDMMCs also expressed CD73. Incubation of cells in the presence of TGFβ resulted in enhanced MDSC differentiation into TDMMCs and increased expression of CD73. Numbers of CD39+CD73+ TDMMCs were increased 2.9-fold in the presence of TGFβ compared to vehicle (Figure 3A and 3C). The potent and selective inhibitor of type I receptor activin receptor-like kinase 5 (ALK5) SB431542, at a concentration of 2 μM, abrogated the effect of TGFβ in myeloid cells (Figure 3C). Accordingly, MDSC numbers were decreased in the presence of TGFβ compared to vehicle and the TGFβ inhibitor SB431542 abrogated these induced effects (data not shown). To determine whether the effect of TGFβ is mediated via activation of TGFβRII, we performed experiments using Lin− HPC isolated from mice lacking Tgfbr2 in myeloid cells (Tgfbr2MyeKO). Representative flow cytometric dot plots showing percentage of MDSCs and TDMMCs, including CD39+CD73+ TDMMCs, in the absence or presence of TGFβ are shown in Figure 3B. Conditional deletion of Tgfbr2 in myeloid cells isolated from TGFβRIIMyeKO mice resulted in a significant attenuation of CD39+CD73+ TDMMC accumulation (Figure 3C), suggesting a functional importance of intact TGFβ signaling. The effect of TGFβ was dose-dependent with an EC50 of 80 pg/ml (Figure 3D), close to the reported affinity of TGFβ 1 to TGFβ receptors (40). It should be noted that only CD73+, but not CD73- TDMMC, generated in vitro from MDSCs in the presence of TGFβ, also expressed a high level of CD204 (Figure 3E and 3F), highlighting the similarity between the in vivo and in vitro TDMMC phenotypes. Of interest, CD73high intermediate populations of cells with a Gr-1low/dim and (F4/80/MHCII/CD11c)low/dim phenotype had higher CD204 expression compared to corresponding CD73low intermediate populations (data not shown). Therefore, we conclude that TGFβ, acting via TGFβRII, guides MDSC differentiation into CD39+CD73+ terminally differentiated myeloid cells.
Figure 3. TGFβ/TGFβRII signaling promotes differentiation of MDSCs into CD39+CD73+TDMMCs.
(A and B) Lin- HPCs were isolated from bone marrow of TGFβRIIMyeWT (A) or TGFβRIIMyeKO (B) mice. Representative flow cytometric dot plots showing percentage of TDMMCs (CD11b+F4/80+MHCII+CD11c+Gr1−) and MDSC (CD11b+F4/80−MHCII−CD11c−Gr1+) and percentage of CD73+ cells within the population of TDMMCs in the absence or presence of 1 ng/ml of TGFβ. (C) Graphical representation of data from flow cytometric analysis of CD39+CD73+TDMMC accumulation expressed as cell numbers per well in the absence (open bars) or presence of 1 ng/ml TGFβ(black bars) alone, or in combination with 2 μM SB431542 (hatched bars). (D) Dose-dependent curve for TGFβ to induce an increase in numbers (per well) of CD39+CD73+TDMMCs generated from TGFβRIIMyeWT Lin- HPCs. (C–D) For details please see Materials and Methods. The data are expressed as means and SEM of three independent experiments. (E and F) Flow cytometric histograms (E) showing expression of CD204 on cell surface of CD39+CD73− (gray-shaded) and CD39+CD73+ (open) TDMMCs and graphical representation of flow cytometric data. (F) Data represent mean±SEM from three independent experiments. (G) Adenosine production by Gr-1− and Gr-1+ populations of myeloid cells derived from WT (Lin−) HPCs in vitro. Data are presented as mean±SEM of adenosine concentrations, n=6. (H) Adenosine production by myeloid (CD11b+) cells isolated from LLC tumors grown in TGFβRIIMyeWT (WT) or TGFβRIIMyeKo (KO) mice. Data are presented as mean±SEM of adenosine concentrations, n=4 animals in each group. (I) ATP breakdown by myeloid (CD11b+) cells isolated LLC tumors grown in TGFβRIIMyeWT (WT) or TGFβRIIMyeKO (KO). Data are presented as mean±SEM of ATP concentrations remaining in media, n=5. Statistical significance was calculated by unpaired two-tailed t test (F, G-I), or one-way ANOVA with Bonferroni’s (C) or Dunnett’s (D) post-hoc tests; *- p<0.05; **- p< 0.01;***- p< 0.001.
To compare adenosine-generating capacities of TDMMCs and MDSCs, we prepared myeloid cells from Lin− HPCs in the presence of TGFβ as described above and magnetically separated them into Gr-1− and Gr-1+ cell populations. Cells (5×104 in 0.1 ml) were incubated in the presence of 10 μM ADP for 10 minutes. Figure 3G shows that adenosine generation by Gr-1− cell population containing TDMMCs was more than 1.5 times higher compared to Gr-1+ cells, which are represented mostly by MDSCs. We then compared adenosine-generating capacities of myeloid (CD11b+) cells isolated from LLC tumors grown in mice lacking Tgfbr2 in myeloid cells (TGFβRIIMyeKO) or TGFβRIIMyeWT control animals. As seen in Figure 3H, adenosine generation by myeloid cells lacking TGFβ RII was more than 4 times lower compared to wild-type cells. Myeloid cells lacking TGFβ RII were also less efficient in ATP breakdown. As seen in Figure 3I, incubation of these cells (104 in 0.15 ml) in the presence of 5 μM ATP for 10 minutes decreased ATP concentration in supernatant to 1.5±0.04 μM, whereas the same number of wild-type cells decreased extracellular ATP concentration to 0.17±0.04 μM. Taken together, our results suggest that TGFβ signaling is important for differentiation of MDSCs into CD39+CD73+TDMMCs which are characterized by high adenosine-producing capacity.
Myeloid cell-specific deletion of Tgfbr2 is associated with decreased accumulation of CD39+CD73+ TDMMCs and reduced tumor growth and lung metastasis
To evaluate the effect of Tgfbr2 deletion on differentiation of MDSCs into CD39+CD73+ TDMMCs within the tumor microenvironment, we first analyzed the number of these cells in LLC tumors grown in mice lacking Tgfbr2 in myeloid cells (TGFβRIIMyeKO) or TGFβRIIMyeWT control animals. Flow cytometric examination of single cell suspensions revealed that there was no difference in the proportion of tumor-infiltrating CD45+ immune cells between TGFβ RIIMyeKO and control TGFβ RIIMyeWT mice (31.2±5.8% and 34.7±7.4%, respectively; p>0.05, n=5, unpaired t test). We next analyzed TDMMC and MDSC subpopulations within CD11b positive immune cells. Figure 4A depicts representative flow cytometric dot plots showing percentage of tumor myeloid cell subpopulations and the expression of CD39 and CD73. We found that the conditional deletion of Tgfbr2 in myeloid cells resulted in increased numbers of MDSCs and reduced numbers of TDMMCs (Figure 4B). The number of CD39+CD73+ TDMMCs was decreased approximately by one-third in TGFβ RIIMyeKO mice compared to TGFβ RIIMyeWT animals. Importantly, tumor growth was significantly reduced in mice lacking Tgfbr2 in myeloid cells compared to control animals (22) implicating the tumor-promoting role for CD39+CD73+ TDMMC in a Lewis lung carcinoma (LLC) isograft model.
Figure 4. Myeloid cell-specific deletion of Tgfbr2 is associated with decreased accumulation of CD39+CD73+ TDMMCs and reduced tumor growth and lung metastasis.
(A) Single cell suspensions were prepared from Lewis lung carcinoma tumors (3 weeks) extracted from TGFβRIIMyeWT (left) and TGFβRIIMyeKO (right) mice. Representative FACS dot plots showing percentage of TDMMCs (left upper quadrant) and MDSCs (right lower quadrant). Subpopulation of TDMMCs was gated and analyzed for expression of CD39 and CD73. (B) Graphical representation of data from flow cytometric analysis of MDSCs, TDMMCs and CD39+CD73+ TDMMCs in tumors extracted from TGFβRIIMyeWT (open bars) and TGFβRIIMyeKO (closed bars) mice. (C) Mammary tumor onset in MMTV-PyMT/TGFβRIIMyeWT and MMTV-PyMT/TGFβRIIMyeKO mice. Age of onset is the time that a palpable mammary tumor first appears. T50 denotes the age at which 50% of mice first possess a tumor, and n is the number of mice examined. (D) Weight of tumor tissue in MMTV-PyMT/TGFβRIIMyeWT and MMTV-PyMT/TGFβRIIMyeKO mice on day 28 after tumor palpation. The total weight of tumors from all 10 mammary glands is indicated. (E) Number of metastatic foci in lungs. (F and G) Representative cytofluorimetric dot plots demonstrating percentage of MDSCs, TDMMCs and CD39+CD73+TDMMCs in tumors extracted from (F) MMTV-PyMT/TGFβRIIMyeWT and (G) MMTV-PyMT/TGFβRIIMyeKO mice. (H) Graphical representation of data from FACS analysis of MDSCs and TDMMCs in MMTV-PyMT/TGFβRIIMyeWT and MMTV-PyMT/TGFβRIIMyeKO mice on day 28 after tumor palpation. Data represent mean±SEM three independent experiments with 2 mice/each. * - p<0.05; unpaired two-tailed t test.
To investigate effects of Tgfbr2 deletion in myeloid cells on different aspects of multistage breast carcinogenesis, we took an advantage of the mouse line established in our laboratory with spontaneous mammary gland tumorigenesis, in which Tgfbr2 gene was specifically deleted in myeloid cells (MMTV-PyMT/TGFβRIIMyeKO). The advantage of MMTV-PyMT/TGFβRIIMyeKO/WT model is its close similarity to human breast cancer characterized by the development of metastatic lesions in the lungs. No difference was observed in tumor latency between MMTV-PyMT/TGFβRIIMyeKO and MMTV-PyMT/TGFβRIIMyeWT mice (Figure 4C). However, tumor weight was significantly reduced and the average number of lung metastasis was decreased in mice with ablated Tgfbr2 in myeloid cells, compared to control (Figure 4D and 4E). Total cell numbers in single-cell suspensions obtained from MMTV-PyMT/TGFβRIIMyeKO tumors were lower than those in MMTV-PyMT/TGFβRIIMyeWT mice (43.6±5.5 and 97.7±11.3 ×106 cell/tumor, respectively, p<0.01; n=5, unpaired t test), reflecting attenuation of tumor growth in animals lacking Tgfbr2. However, the percentage of CD45+ cells was comparable between tumors obtained either from MMTV-PyMT/TGFβRIIMyeKO or MMTV-PyMT/TGFβRIIMyeWT mice with respective mean values of 25.3%±5.4% and 29.1%±7.1%, (p>0.05, n=5, unpaired t test). Representative cytofluorimetric dot plots showing the percentage of MDSCs and TDMMCs within CD45+ cell population are shown in Figures 4F and 4G. Flow cytometric analysis revealed that the number of MDSCs was significantly increased in MMTV-PyMT/TGFβRIIMyeKO compared to control (Figure 4H). The higher number of MDSCs were reciprocated by decreased numbers of TDMMCs including CD39+CD73+TDMMCs, indicating impaired differentiation of MDSCs into CD39+CD73+TDMMCs in animals lacking myeloid cell specific TGFβRII signaling.
Myeloid cell-specific deletion of Tgfbr2 is associated with decreased tumor angiogenesis and immunosuppression
Immune cells expressing high levels of CD39 and CD73 have been suggested to contribute to an increase of extracellular adenosine seen in tumor microenvironment (11, 12), which in turn can promote angiogenesis (41) and the tumor-associated immunosuppression (13, 42, 43). In agreement with this concept, we observed a lower density of CD31 positive blood vessels in tumors extracted from MMTV-PyMT/TGFβRIIMyeKO mice compared to tumors from MMTV-PyMT/TGFβRIIMyeWT (Figure 5A and 5B). To determine if myeloid cells could be a source of angiogenic factors, we magnetically separated WT bone marrow-derived myeloid cells into Gr-1− and Gr-1+ cell populations and measured VEGF secretion from these cells in response to their stimulation with the non-hydrolyzed adenosine analog NECA (10−5 M). Figure 5C shows that even in the absence of NECA, Gr-1− cells tended to produce higher levels of VEGF compared to Gr-1+ cells, though the difference between basal VEGF levels did not reach statistical significance. Stimulation of Gr-1− cells with NECA significantly increased their VEGF secretion by nearly 3-fold, whereas NECA had no significant effect on VEGF secretion from Gr-1+ cells. Therefore, our data suggest that in contrast to MDSCs (Gr-1+), TDMMCs are capable not only to generate high levels of adenosine but also respond to stimulation of their adenosine receptors with a significant increase in VEGF secretion.
Figure 5. Myeloid cell-specific deletion of Tgfbr2 leads to decreased tumor angiogenesis and immunosuppression.
(A) Double immunofluorescence microscopy of frozen tumor sections stained with antibodies against CD31 (green) and cytokeratin 8 (red). Bar, 100 μm. (B) Graphical representation of vessel density: CD31 positive vessels were quantified within the tumor epithelium and given as fraction of cytokeratin-8 positive area. Quantification of immunofluorescence was performed with ImageJ software (National Institutes of Health). Bars represent mean±SEM measurements in five fields per tumor of 5 animals. (C) VEGF secretion by Gr-1− and Gr-1+ populations of myeloid cells derived from WT (Lin−) HPCs in the absence (open bars) or presence (closed bars) of NECA. Data are presented as mean±SEM of VEGF concentrations, n=4. (D) VEGF secretion by myeloid (CD11b+) cells isolated from LLC tumors grown in TGFβRIIMyeWT (WT) or TGFβRIIMyeKO (KO) mice in the absence (open bars) or presence (closed bars) of NECA. Data are presented as mean±SEM of VEGF concentrations, n=4 animals in each group. (E) Percentage of CD3 positive T lymphocytes in tumors extracted from MMTV-PyMT/TGFβRIIMyeWT (open bars) and MMTV-PyMT/TGFβRIIMyeKO (closed bars) mice. Data represent mean±SEM from five tumors. (F) Total numbers of CD3+ T lymphocytes including numbers of CD3+CD4+ and CD3+CD8+ T lymphocytes in tumors extracted from MMTV-PyMT/TGFβRIIMyeWT (open bars) and MMTV-PyMT/TGFβRIIMyeKO (closed bars) mice. Data represent mean±SEM from three tumors. (G) Representative cytofluorographic outlier contour plots of CD154 and CD69 expression on CD3+CD4+ T lymphocytes (upper panels), CD137 and CD107a expression on CD3+CD8+ T lymphocytes (middle panels) and intracellular INFγ staining of CD3+CD8+ T lymphocytes (lower panels) in tumors extracted from MMTV-PyMT/TGFβRIIMyeWT (left panels) and MMTV-PyMT/TGFβRIIMyeKO (right panels) mice. Asterisks indicate the level of statistical significance calculated by unpaired two-tailed t test (B, E and F) or one-way ANOVA with Bonferroni’s (C and D) post-hoc test; * - p<0.05, ** - p<0.01, *** - p<0.001, and ns - not statistically significant difference.
Next, we compared VEGF secretion from myeloid (CD11b+) cells isolated from LLC tumors grown in mice lacking Tgfbr2 in myeloid cells TGFβRIIMyeKO or TGFβRIIMyeWT control animals. As seen in Figure 5D, tumor-derived WT myeloid cells tended to produce higher levels of VEGF compared to myeloid cells lacking TGFβ RII, though the difference between basal VEGF levels did not reach statistical significance. Stimulation of WT myeloid cells with NECA significantly increased their VEGF secretion by 2-fold, whereas NECA had no significant effect on VEGF secretion from TGFβRIIMyeKO myeloid cells.
We have also found that the percentage of CD3e+ T lymphocytes was significantly increased in mammary tumors obtained from mice lacking Tgfbr2 gene expression in myeloid cells compared to control animals (Figure 5E). Accordingly, the absolute number of CD3e+ T lymphocytes, and particularly CD3e+CD8+ T lymphocytes, was also significantly higher in tumors obtained from MMTV-PyMT/TGFβRIIMyeKO mice compared to MMTV-PyMT/TGFβRIIMyeWT control animals (Figure 5F). Although no difference in total numbers of CD3e+CD4+ T lymphocytes was seen between tumors obtained from TGFβRIIMyeKO and TGFβRIIMyeWT (Figure 5F), we found that the proportion of activated CD154+CD3e+CD4+ T lymphocytes (44) was 2.5-fold higher in tumors obtained from mice lacking TGFβRII in myeloid cells compared to control animals (Figure 5G). Similarly, the proportion of activated cytotoxic CD107a+CD3e+CD8+ T lymphocytes (45) was 2-fold higher in tumors obtained from TGFβRIIMyeKO mice compared to control animals. Remarkably, the proportion of INFγ-producing CD3e+CD8+ T lymphocytes was 3-fold higher in tumors obtained from MMTV-PyMT/TGFβRIIMyeKO mice (Figure 5G). Taken together, our results suggest that TGFβ signaling is important for generation of CD39+CD73+ TDMMCs capable to increase extracellular adenosine in tumor microenvironment, which in turn can promote angiogenesis and the tumor-associated immunosuppression.
Discussion
In the current study, we adopted a systematic approach to simultaneously analyze CD39 and CD73 expression on various cell populations present in tumors from MMTV-PyMT mice, a clinically-relevant spontaneous breast tumor model. We discovered that, whereas TDMMCs represent a minor cell subpopulation expressing relatively low levels of CD39 and CD73 in normal mammary gland tissue, they become a major cell subpopulation expressing high levels of both CD39 and CD73 in the tumor microenvironment. In contrast to normal mammary gland, where CD140a+ fibroblasts are the major cell population, characterized by CD39 and CD73 surface expression, the majority of cells expressing high level of both CD39 and CD73 in tumors were represented by myeloid cells. We and others have previously reported that the entire subset of Gr-1high granulocytic myeloid derived suppressor cells (G-MDSC) is characterized by high expression of CD73. In contrast, only minor fraction of Gr-1−/low myeloid cells expressed CD73 when generated in vitro from Lin− HPC (13) or in melanoma and pancreatic adenocarcinoma mouse tumor models (46). In agreement with these data, we have found that the majority of MDSCs and a lesser fraction of TDMMCs expressed CD39 and CD73 in tumors from MMTV-PyMT mice. However, due to significant accumulation of TDMMCs in breast tumors these cells represented a major subpopulation with high expression levels of both CD39 and CD73. Changes in the tumor microenvironment associated with elevated TGFβ resulted in a further increase in number of CD39+CD73+ TDMMCs. Therefore, we hypothesized that TGFβ acting on myeloid cells can directly regulate generation of CD39+CD73+ TDMMCs, thus contributing to the tumor-promoting effects of this pleiotropic effector of tumor microenvironment.
TGFβ has been characterized as both tumor-promoting and tumor-suppressing growth factor depending on the phase of tumor development, local microenvironmental milieu and cell type (47). We have previously shown that the deletion of Tgfbr2 expression in myeloid cells is associated with decreased tumor growth suggesting pro-tumorigenic effect of TGFβ/TGFβRII signaling (22). It has been suggested that this pro-tumorigenic effect may be associated with accumulation of MDSCs (38). In the tumor microenvironment, however, MDSCs rapidly maturate into terminally differentiated myeloid cells (25). In the current study, we demonstrated the direct effect of TGFβ on differentiation of myeloid cells into CD39+CD73+ TDMMCs in vitro. Using genetic and pharmacological approaches, we found that TGFβ signaling skews maturation of MDSCs into TDMMCs expressing high levels of CD39 and CD73. Several lines of evidence support our conclusion. First, TGFβ induced rapid (within 24 hr) accumulation of CD39+CD73+ TDMMCs at the expense of Gr1+ MDSCs generated from bone marrow HPC in vitro. Second, this effect was inhibited by potent inhibitor of the Tgfbr1 activin receptor-like kinase (ALK5) at a selective concentration (48). Finally, TGFβ had no effect on MDSCs derived from mice lacking Tgfbr2 expression in myeloid cells. In agreement with in vitro data, we observed that the number of CD39+CD73+ TDMMCs was significantly reduced in vivo after myeloid cell-specific deletion of Tgfbr2. In two different mouse models, including subcutaneous Lewis Lung carcinoma and MMTV-PyMT spontaneous mammary carcinomas, we have found that the reduction in the cell number of TDMMCs was accompanied by accumulation of MDSCs. Thus, in addition to previously reported properties of TGFβ as a potent inducer of CD39 and CD73 expression on cell surface of T lymphocytes and dendritic cells (49), our data demonstrated for the first time that the effects of TGFβ on myeloid cell differentiation can also lead to increased generation of CD39+CD73+ TDMMCs capable of increasing extracellular adenosine in tumor microenvironment, which in turn can promote angiogenesis and the tumor-associated immunosuppression. Furthermore, our study demonstrated that TGFβ signaling plays an important role in generation of TDMMCs that can contribute to stimulation of tumor angiogenesis not only through the production of high levels of extracellular adenosine but also through autocrine adenosine-dependent upregulation of their VEGF secretion. The latter is in agreement with our previous data on the significant role of A2B adenosine receptor-dependent VEGF secretion from immune cells in tumor angiogenesis (41).
Our study clearly demonstrated that the loss of TGFβ signaling in myeloid cells led not only to a decrease in number of TDMMC expressing the adenosine-generating enzymes CD39 and CD73 but also to increased tumoral T lymphocyte infiltration and their activation status, reduced VEGF levels and density of blood vessels, and slow progression of both Lewis Lung carcinoma and mammary carcinomas. In agreement with our findings, Pang et al (50) have recently reported that the conditional deletion of Tgfbr2 gene expression in myeloid cells decreased tumor metastasis in implanted tumor models. The authors explained this phenomenon by impaired productions of type II cytokines. We and others have previously shown that adenosine is a potent inducer of IL-4, IL-5, IL-6, IL-10, IL-13 and arginase in immune cells (9, 41, 51, 52). Therefore, it is possible that the effects observed by Pang et al. can be explained by reduced contribution of adenosine in stimulation of type II cytokines in their model.
Of interest, we found that the TGFβ signaling in myeloid cells is also involved in regulation of the expression of class A scavenger receptor (SRA/CD204). Analysis of cell markers revealed an association between the expression of CD73 and scavenger receptor SRA/CD204 on TDMMCs in tumors from MMTV-PyMT mice. Furthermore, CD39+CD73+ but not CD39+CD73− TDMMCs, generated in the presence of TGFβ in vitro, also were characterized by high levels of CD204 expression. We have previously shown that upregulation of CD204 increases lipid accumulation in dendritic cells leading to their reduced capacity to process antigens (53). Recent studies have reported a strong correlation between numbers of CD204+ tumor-associated macrophages and tumor aggressiveness suggesting a tumor-promoting role of these macrophages (54–56). Therefore, it would be interesting in the future to determine whether the pro-tumorigenic effect of CD204 positive macrophages is mediated, at least in part, via action of CD39+ and CD73+.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01HL095787 and R01CA138923 to IF, the CA085492 and CA102162 to HLM, the T.J. Martell Foundation, the Vanderbilt-Ingram Cancer Center, and the Robert J. and Helen C. Kleberg Foundation. NIH grant CA068485 provided core laboratory support.
Abbreviation
- HPC
hematopoietic progenitors cell
- LLC
Lewis Lung carcinoma
- MDSC
myeloid-derived suppressor cell
- MMTV
Mouse mammary tumor virus
- MMTV-PyMT/TGFβRIIKO
mice with spontaneous tumor formation of mammary gland and conditional deletion of the type II TGFβ receptor in mammary epithelium
- MMTV-PyMT/TGFβRIIMeyKO
mice with spontaneous tumor formation of mammary gland and conditional deletion of the type II TGFβ receptor in myeloid cells (LysM+)
- MMTV-PyMT/TGFβRIIWT/floxed (or MMTV-PyMT)
mice with spontaneous tumor formation of mammary gland with intact TGFβ signaling
- NECA
5′-N-ethylcarboxamido adenosine
- TDMMC
terminally differentiated myeloid mononuclear cell
- PyMT
polyoma middle T antigen
- TGFβ
Transforming growth factor beta
- TβRII
TGFβ receptor II
- TβRI
TGFβ receptor I
- TGFβRIIMyeKO (or LysM-Cre/Tgfbr2KO)
mice with conditional deletion of the type II TGFβ receptor in myeloid cells (LysM+)
- TGFβRIIMyeWT
normal mice (intact TGFβ signaling) as a control for TGFβRIIMyeKO mice
- VEGF
vascular endothelial growth factor
References
- 1.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia. 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 4.Beigi RD, Kertesy SB, Aquilina G, Dubyak GR. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br J Pharmacol. 2003;140:507–519. doi: 10.1038/sj.bjp.0705470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canaday DH, Beigi R, Silver RF, Harding CV, Boom WH, Dubyak GR. ATP and control of intracellular growth of mycobacteria by T cells. Infect Immun. 2002;70:6456–6459. doi: 10.1128/IAI.70.11.6456-6459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- 7.Huang SP, Shieh GJ, Lee L, Teng HJ, Kao ST, Lin JG. Inhibition effect of shengma-gegen-tang on measles virus in Vero cells and human peripheral blood mononuclear cells. Am J Chin Med. 1997;25:89–96. doi: 10.1142/S0192415X97000123. [DOI] [PubMed] [Google Scholar]
- 8.Desai A, Victor-Vega C, Gadangi S, Montesinos MC, Chu CC, Cronstein BN. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67:1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- 9.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host A2B adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 12.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koziak K, Kaczmarek E, Kittel A, Sevigny J, Blusztajn JK, Schulte Am Esch J, 2nd, Imai M, Guckelberger O, Goepfert C, Qawi I, Robson SC. Palmitoylation targets CD39/endothelial ATP diphosphohydrolase to caveolae. J Biol Chem. 2000;275:2057–2062. doi: 10.1074/jbc.275.3.2057. [DOI] [PubMed] [Google Scholar]
- 15.Stella J, Bavaresco L, Braganhol E, Rockenbach L, Farias PF, Wink MR, Azambuja AA, Barrios CH, Morrone FB, Oliveira Battastini AM. Differential ectonucleotidase expression in human bladder cancer cell lines. Urol Oncol. 2010;28:260–267. doi: 10.1016/j.urolonc.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Jin Q, Yuan LX, Boulbes D, Baek JM, Wang YN, Gomez–Cabello D, Hawke DH, Yeung SC, Lee MH, Hortobagyi GN, Hung MC, Esteva FJ. Fatty acid synthase phosphorylation: a novel therapeutic target in HER2-overexpressing breast cancer cells. Breast Cancer Res. 2010;12:R96. doi: 10.1186/bcr2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 18.Teicher BA. Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer Metastasis Rev. 2001;20:133–143. doi: 10.1023/a:1013177011767. [DOI] [PubMed] [Google Scholar]
- 19.Dong M, Blobe GC. Role of transforming growth factor-beta in hematologic malignancies. Blood. 2006;107:4589–4596. doi: 10.1182/blood-2005-10-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, Wolfraim L, Hong S, Mushinski E, Potter M, Kim SJ, Fu XY, Deng C, Letterio JJ. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 21.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novitskiy SV, Pickup MW, Chytil A, Polosukhina D, Owens P, Moses HL. Deletion of TGF-beta signaling in myeloid cells enhances their anti-tumorigenic properties. J Leukoc Biol. 2012;92:641–651. doi: 10.1189/jlb.1211639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M, Wu H, Shyr Y, Moses HL. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011;1:430–441. doi: 10.1158/2159-8290.CD-11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 25.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 27.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61:1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novitskiy SV, Pickup MW, Chytil A, Polosukhina D, Owens P, Moses HL. Deletion of TGF-beta signaling in myeloid cells enhances their anti-tumorigenic properties. J Leukoc Biol. 2012;92:641–651. doi: 10.1189/jlb.1211639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M, Wu H, Shyr Y, Moses HL. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011;1:430–441. doi: 10.1158/2159-8290.CD-11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez R, Daniels DV. A separation method for the assay of adenylylcyclase, intracellular cyclic AMP, and cyclic-AMP phosphodiesterase using tritium-labeled substrates. Anal Biochem. 1992;203:76–82. doi: 10.1016/0003-2697(92)90045-9. [DOI] [PubMed] [Google Scholar]
- 35.Jessen KA, Liu SY, Tepper CG, Karrim J, McGoldrick ET, Rosner A, Munn RJ, Young LJ, Borowsky AD, Cardiff RD, Gregg JP. Molecular analysis of metastasis in a polyomavirus middle T mouse model: the role of osteopontin. Breast Cancer Res. 2004;6:R157–169. doi: 10.1186/bcr768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar AR, Muller WJ, Moses HL. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65:2296–2302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell EJ, Lee K, O’Connor-McCourt MD. Characterization of transforming growth factor-beta (TGF-beta) receptors on BeWo choriocarcinoma cells including the identification of a novel 38-kDa TGF-beta binding glycoprotein. Mol Biol Cell. 1992;3:1295–1307. doi: 10.1091/mbc.3.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host A(2B) adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koshiba M, Kojima H, Huang S, Apasov S, Sitkovsky MV. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J Biol Chem. 1997;272:25881–25889. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 43.Raskovalova T, Huang X, Sitkovsky M, Zacharia LC, Jackson EK, Gorelik E. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J Immunol. 2005;175:4383–4391. doi: 10.4049/jimmunol.175.7.4383. [DOI] [PubMed] [Google Scholar]
- 44.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 45.Uhrberg M. The CD107 mobilization assay: viable isolation and immunotherapeutic potential of tumor-cytolytic NK cells. Leukemia. 2005;19:707–709. doi: 10.1038/sj.leu.2403705. [DOI] [PubMed] [Google Scholar]
- 46.Shevchenko I, Bazhin AV, Umansky V. Comment on “Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b(+)Gr1(+) cells”. J Immunol. 2012;188:2929–2930. doi: 10.4049/jimmunol.1290007. author reply 1930. [DOI] [PubMed] [Google Scholar]
- 47.Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 49.Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, Cobbold SP, Waldmann H. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur J Immunol. 2011;41:2955–2965. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 50.Pang Y, Gara SK, Achyut BR, Li Z, Yan HH, Day CP, Weiss JM, Trinchieri G, Morris JC, Yang L. TGF-beta signaling in myeloid cells is required for tumor metastasis. Cancer Discov. 2013;3:936–951. doi: 10.1158/2159-8290.CD-12-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- 53.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, Knight SC, Padhya T, McCaffrey TV, McCaffrey JC, Antonia S, Fishman M, Ferris RL, Kagan VE, Gabrilovich DI. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, Hishida T, Nishimura M, Yoshida J, Takeyoshi I, Ochiai A. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol. 2010;5:1507–1515. doi: 10.1097/JTO.0b013e3181eba692. [DOI] [PubMed] [Google Scholar]
- 55.Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, Takeya M. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011;102:1424–1431. doi: 10.1111/j.1349-7006.2011.01945.x. [DOI] [PubMed] [Google Scholar]
- 56.Shigeoka M, Urakawa N, Nakamura T, Nishio M, Watajima T, Kuroda D, Komori T, Kakeji Y, Semba S, Yokozaki H. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1112–1119. doi: 10.1111/cas.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.