Abstract
Cognition is modulated by circadian rhythms, in both nocturnal and diurnal species. Rhythms of clock gene expression occur in brain regions that are outside the master circadian oscillator of the suprachiasmatic nucleus and that control cognitive functions, perhaps by regulating the expression neural-plasticity genes such as brain derived neurotrophic factor (BDNF) and its high affinity receptor, tyrosine kinase B (TrkB). In the diurnal grass rat (Arvicanthis niloticus), the hippocampus shows rhythms of clock genes that are 180° out of phase with those of nocturnal rodents. Here, we examined the hypothesis that this reversal extends to the optimal phase for learning a hippocampal-dependent task and to the phase of hippocampal rhythms in BDNF/TrkB expression. We used the Morris water maze (MWM) to test for time of day differences in reference memory and monitored daily patterns of hippocampal BDNF/TrkB expression in grass rats. Grass rats showed superior long-term retention of the MWM, when the training and testing occurred during the day as compared to the night, at a time when nocturnal laboratory rats show superior retention; acquisition of the MWM was not affected by time of day. BDNF/TrkB expression was rhythmic in the hippocampus of grass rats, and the phase of the rhythms was reversed compared to that of nocturnal rodents. Our findings provide correlational evidence for the claim that the circadian regulation of cognition may involve rhythms of BDNF/TrkB expression in the hippocampus and that their phase may contribute to species differences in the optimal phase for learning.
Keywords: Circadian rhythm, BDNF/ TrkB, cognition, hippocampus, Morris water maze, plasticity genes
1. Introduction
In mammals, circadian rhythms depend upon the integrity of a master circadian oscillator residing within the hypothalamic suprachiasmatic nucleus (Stephan and Zucker, 1972). Within neurons of the SCN circadian rhythmic functions are generated by molecular oscillations described as an autoregulatory transcriptional and translational feedback loop; the genes responsible for this feedback loop are labeled clock genes (Ko and Takahashi, 2006). There is evidence of local rhythmic expression of clock genes in areas of the brain outside of the SCN,(Abe et al., 2002) including the hippocampus (Duncan et al., 2013; Gilhooley et al., 2011; Ikeno et al., 2013; Lamont et al., 2005; Li et al., 2013; Otalora et al., 2013; Ramanathan et al., 2010b; Wang et al., 2009) These observations bring up the question of the role of hippocampal clock-gene expression in the regulation of hippocampal-dependent cognitive functions.
The phases of clock–gene rhythms of extra-SCN oscillators, such as the one in the hippocampus, are 180° out of phase when diurnal grass rats (Arvicanthis niloticus) are compared to nocturnal rodents. Specifically, Ramanathan et al (Ramanathan et al., 2008a; Ramanathan et al., 2008b; Ramanathan et al., 2010a; Ramanathan et al., 2010b) reported that Period (PER) 2 expression peaks during the late light phase in diurnal grass rats and in the late night in nocturnal species (Amir et al., 2006; Amir and Robinson, 2006; Lamont et al., 2005) A similar pattern emerges when other diurnal species, including humans, are compared to nocturnal species (Li et al., 2013; Otalora et al., 2013). Thus, species differences related to the circadian control of hippocampal functions may be determined by the phase of this extra-SCN oscillator.
Possibly related to the phase of extra-SCN oscillators, there is evidence from both animal and human studies that time of training and or/testing can affect learning, retention and performance (Folkard et al., 1985; Folkard, 1990; Gerstner et al., 2009; Smarr et al., 2014). While there is no consensus about the optimal phase for performance across learning tasks, the trend is that both nocturnal and diurnal species perform best when trained and tested during their active phase, and the poorest performance is observed when tested or trained during the inactive phase. In rats, the data support a positive nocturnal bias for the acquisition and retention of a signal-detection task and for retention of the MWM (Gritton et al., 2012), even when time of day differences in acquisition of the MWM task are absent (Valentinuzzi et al., 2004). With some exceptions (see Smarr, Jennings et al. 2014 for a recent review), hippocampal dependent tasks in both rodent and human studies feature optimal performance when individuals are tested during the active phase of the species (Folkard and Monk, 1985; Furnham and Rawles, 1988; Gritton et al., 2012; Hoffmann, 1992; Payne, 1989; Testu, 1986; Valentinuzzi et al., 2004).
In the hippocampus, several gene products contribute to neural plasticity and the process of learning and retention (Minichiello, 2009; Bekinschtein, Kent et al. 2013; Callaghan and Kelly 2013; Bekinschtein, Cammarota et al. 2014, Berchtold et al., 1999; Bova et al., 1998; Cirelli and Tononi, 2000a; Cirelli and Tononi, 2000b; Dolci et al., 2003; Eckel-Mahan et al., 2008; Hamatake et al., 2011; Ikeno et al., 2013; Katoh-Semba et al., 2008; Roth and Sweatt, 2008; Selcher et al., 1999). Prominent among such plasticity gene products are brain derived neurotrophic factor (BDNF) and its high affinity tyrosine kinase receptor (TrkB). Thus, once the TrkB receptor is occupied by BDNF, a number of signaling cascades, including phosphatidylinositol 3- kinase (PI3-K), mitogen-activated protein kinase (MAPK) and phospholipase C-y(PLC-y) are activated (see Tapia-Arancibia et al., 2004 for review). Activation of these signaling cascades has been shown to be involved in many processes important for learning and neural plasticity, including long-term potentiation (Hall et al., 2000; Tyler et al., 2002; Yamada and Nabeshima, 2003). BDNF/TrkB expression in the hippocampus is important for hippocampal dependent learning and memory (Bekinschtein et al., 2013; Bekinschtein et al., 2014; Callaghan and Kelly, 2013; Minichiello et al., 1999; Minichiello, 2009; Yamada and Nabeshima, 2003). Reduction or elimination of BDNF/TrkB expression in the hippocampus results in deficits in both acquisition and retention of hippocampal dependent tasks (Gorski et al., 2003; Korte et al., 1995; Pang and Lu, 2004; Tyler et al., 2002). Studies using late-onset forebrain-specific BDNF knockout mice serve to show the importance of BDNF production in adulthood for normal spatial learning abilities (Vigers et al., 2012). Additionally when the expression of BDNF/TrkB is restored via exercise or pharmacological means, hippocampal dependent memory is rescued (Erickson et al., 2013; Minichiello et al., 1999; Patterson et al., 1996).
Although some discrepancies exist with respect to rhythms of BDNF/TrkB mRNAs in the hippocampus of nocturnal species (Berchtold et al., 1999; Bova et al., 1998; Cirelli and Tononi, 2000a; Ikeno et al., 2013; Schaaf et al., 2000; Dolci et al., 2003;Golini et al., 2012; Liang et al., 1998; Schaaf et al., 2000), measures of protein abundance show peak hippocampal expression of BDNF/TrkB during the dark phase in adult nocturnal rats (Dolci et al., 2003; Hamatake et al., 2011; Katoh-Semba et al., 2008). Rhythmic expression of BDNF and TrkB may mediate the circadian modulation of hippocampal-dependent memory, and differences in the phase of those rhythms could correlate with differences in the optimal times for learning and/or retention when diurnal and nocturnal species are compared. However, the evaluation of the second part of that claim is hampered by the fact that nothing is known about the features of BDNF/TrkB rhythmic expression in the hippocampus of diurnal mammals.
Here we use the diurnal grass rats to determine if their phase reversal in the rhythmic expression of PER1 and 2 in the hippocampus, when compared with nocturnal rodents, predicts similar phase reversals in the acquisition and retention of a hippocampal-dependent task and in the pattern of expression of BDNF/TrkB in the hippocampus. First, we used the reference memory version of the MWM to determine if time of training affects performance and retention of a spatial navigation task in this diurnal species. Second, we determined expression patterns of BDNF and TrkB protein in the hippocampus of these animals. Since there is evidence of functional heterogeneity and possible differential rhythmic expression of both plasticity and clock genes across the hippocampal formation (Berchtold et al., 1999; Bova et al., 1998; Cirelli and Tononi, 2000a; Ikeno et al., 2013; Schaaf et al., 2000), we evaluated the patterns of protein expression in three distinct regions of the hippocampus, the CA1 area, the dorsal blade of the DG and the hilus.
2. Results
Experiment 1
Learning Curve
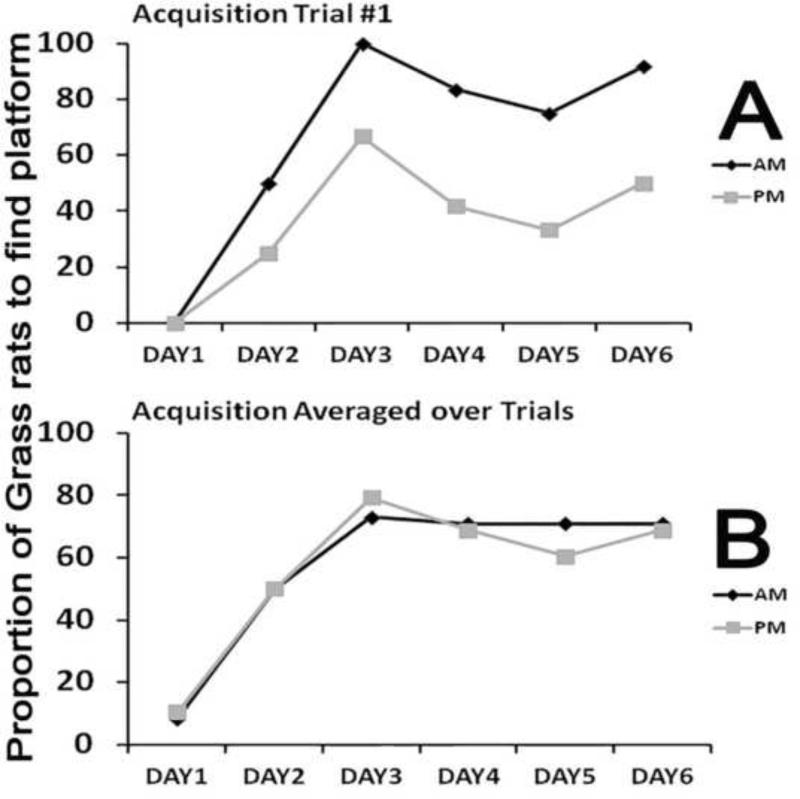
Figure 1 shows the proportion of animals that reached the platform on the first trial of each day of training (Panel A) and for the average of the four trials for each day of training (Panel B). For both the AM and PM groups, Cochran's Q analyses detected a significant effect of days of training, for the data shown on both panels A and B (See Figure 1 legend for statistical details). Comparisons between the AM and PM groups for each day of training found no significant differences for the data shown in panels A and B (all χ2s (1, N=24) = 0.00- 3.22, all Ps> 0.07). Table 1 summarizes the results of the 2×6 ANOVAs for the other three dependent variables using the averages of trials 1-4. Although there was a significant effect of training day for all of the dependent variables, there was not a significant effect of time of training and no significant interaction effects. The same results (data and analyses not shown) were obtained when the analysis was limited to the first trial of each training day.
Figure 1. Time of training effects during acquisition trials.
Cochran's Q analyses showed a significant effect of training days for both trial 1 data (panel A; AM group: Q(5)=31.957 and PM group: Q(5)=14.412, ps < .001) and trials 1-4 averaged data showing the averaged data (+ SEM) for each day (panel B; AM group: Q(5)=69.985, and PM group: Q(5)=60.255, p <.001), but Chi squared analysis showed no effect of time of training on proportion of grass rats to find the platform each day for both data sets (χ2(1,N=24) = 0.00-3.227, all Ps> 0.07). The SEM values in panel B are so small that they are obscured by the symbols demarking the value of each data point.
Table 1.
Tests for Time of Day Effects over Acquisition Trials
| Dependent Variable | Main effect of time of training | Main effect of days | Interaction |
|---|---|---|---|
| Swim Path | F=0.516, df=1, P>0.05 | F=7.357,df=1, P<0.05 | NS |
| Swim Velocity | F=0.063, df=1, P>0.05 | F=6.655,df=1, P<0.05 | NS |
| Latency to Platform Quadrant | F=0.564, df=1, P>0.05 | F=37.553,df=1, P<0.05 | NS |
Acquisition Probe
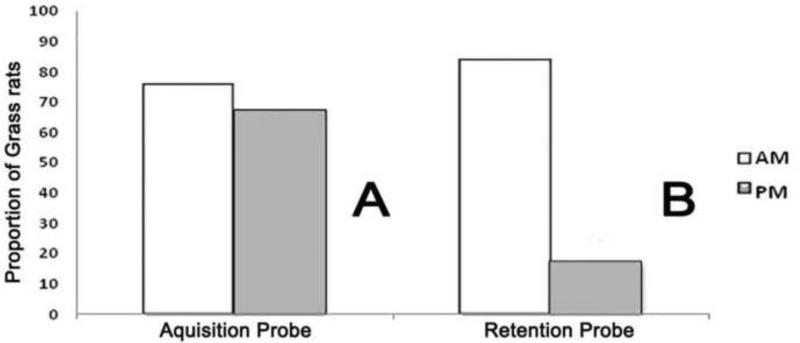
Figure 2 panel A shows the proportion of animals that reached the platform during the acquisition probe. Chi squared analysis found no significant difference between the AM and PM groups (χ2 (1, N=24) = .202, P> 0.05) (Figure 2A). Table two summarizes the analyses for the other four dependent variables. No significant differences between the AM and PM groups were detected by the t-tests for swim path, swim velocity, or latency to platform quadrant. Similarly the proportion of animals that displayed thigmotaxis did not differ between the AM and PM groups.
Figure 2. Differential effects of time of training on acquisition and retention probe test performance.
Chi squared analyses revealed that although there was no effect of ZT on the proportion of grass rats to find the platform during the acquisition probe, (χ2 (1, N=24) = .202, P> 0.05) (panel A), during the retention probe (panel B) there was an effect of ZT (χ2 (1, N=24) =10.667, P< 0.001).
Retention Probe
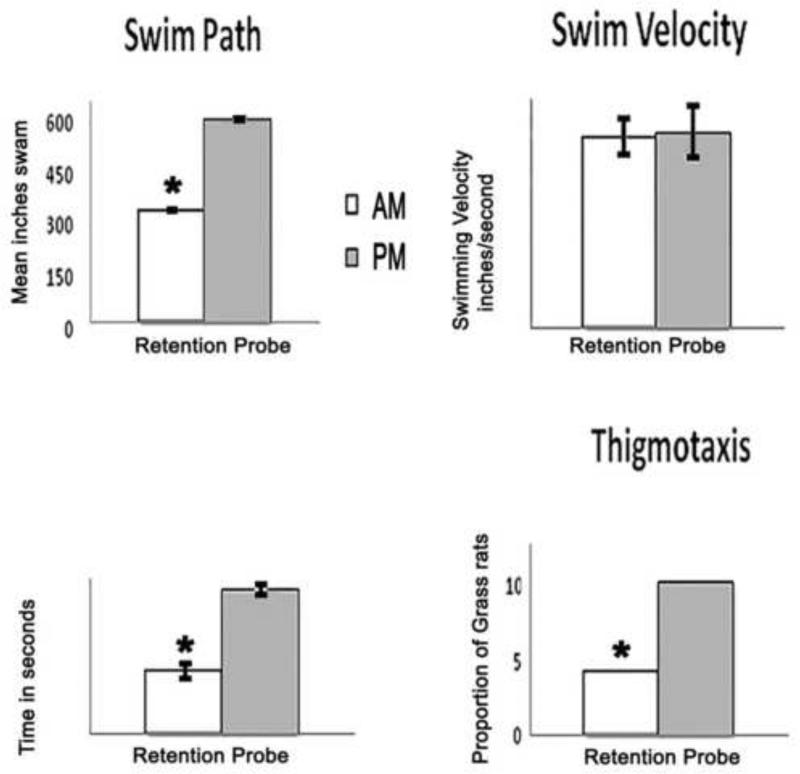
Figure 2 A and B shows the proportion of animals that reached the platform during the retention probe. Chi squared analysis showed a significant effect of time of training on this measure. The mean (+/− SEM) latency to reach the platform was 45.0 +/− 7.0 seconds for the AM group; the two animals from the PM group that reached the platform during the retention probe had latencies of 60 seconds. Figure 3 shows the data for the other four dependent variables. For swim path and latency to platform quadrant there was a significant effect of training time with superior performance for the AM group. No significant differences were found for swim velocity, but thigmotaxis was significantly more frequent for the PM-trained animals. See Figure 3 legend for statistical details.
Figure 3. Time of day effects on retention probe.
There was a significant effect of time of training on all measures except swim velocity, (t(22) =.256, P > 0.05) during the retention probe. Significant time of training effects were seen in swim path, (t (22) =50.30, P < 0.001) and latency to platform quadrant, ( t (22) =9.602, P < 0.001). AM trained grass rats were faster to the platform quadrant and had shorter swim paths than PM trained grass rats. Bar charts showing the mean (+ SEM) inches (swim path, upper left), inches/second (swim velocity, upper right) and seconds (latency to platform quadrant, lower left). Chi squared analysis revealed a significant effect of training time on the proportion of grass rats to engage in thigmotaxis (χ2(1,N=24) = 6.171, P< 0.01). Fewer AM trained grass rats engaged in thigmotaxic behavior than PM trained grass rats. Significant differences between AM and PM groups are denoted by asterisks.
Experiment 2
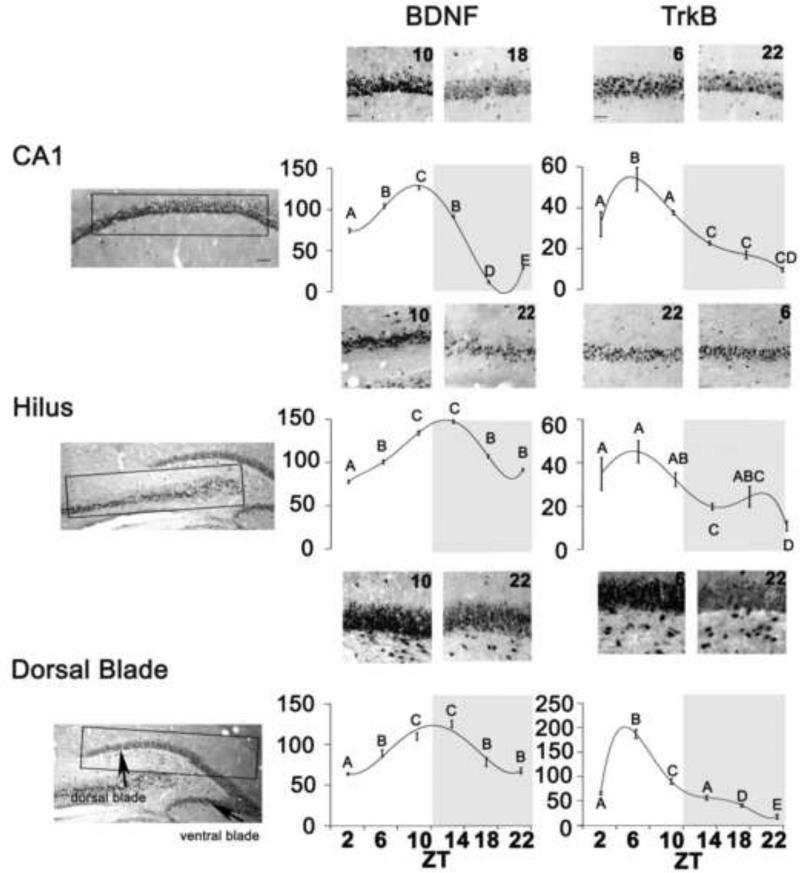
For the number of cells expressing BDNF (Fig. 4 Center Panels) ANOVAs revealed a significant main effect of ZT in the CA1(F=427.27, df=5,P<0.0001), hilus (F=161.140, df=5,P<0.0001), and dorsal blade of the DG (F=57.343, df=5,P<0.0001). In the CA1, BDNF expression peaked at ZT10. In the hilus and dorsal blade of the DG, BDNF expression was elevated at both ZT10 and ZT 14. For the number of cells expressing TrkB (Fig. 4 Right Panels), ANOVAs revealed a significant main effect of ZT in the CA1 (F=28.81, df=5, P<0.0001), hilus (F=7.21, df=5,P<0.0001), and the dorsal blade of the DG (F=1108.716, df=5, P<0.001). In the CA1, hilus and dorsal blade of the DG, TrkB expression peaked at ZT6. Fig 4. shows representative examples of BDNF (Center Panels) and of TrkB (Right Panels) staining in the three brain regions. For both BDNF/TrkB, cellular staining resembles what has been shown in nocturnal species (Cirelli and Tononi, 2000a; Dolci et al., 2003).
Figure 4. Rhythms in Plasticity gene products.
Left Panels: Sampling boxes (rectangles) were used for cell counts in of the CA1, dorsal blades of the DG, and hilus as described in Materials and Methods. Anatomical boundaries are based on Paxinos and Watson (1997). CA1, DG, dentate gyrus; ZT, Zeitgeber Time. Center and Right Panels: Photomicrographs depicting BDNF and Trk B expression at two different ZTs. Scale bar represent 200μm. Line charts showing the mean (+ SEM) number of BDNF (Center) and TrkB (Right) expressing cells for each ZT in the CA1, dorsal blade of the DG, and hilus of male grass rats. Significant differences between ZTs (p<0.001) are noted by different letters. Grey region on each chart indicates the dark phase of the cycle.
3. Discussion
Our results provide evidence for an effect of time of training on the retention of a hippocampal dependent task and document the presence of rhythms in hippocampal protein expression of two plasticity gene products in a diurnal species. Here, diurnal grass rats showed remarkably better retention of the MWM when trained and tested during the day, which is the opposite of what is seen in laboratory rats (Gritton et al., 2012); in that nocturnal species better retention is associated with night-time training and testing under conditions similar to those of the present study (Gritton et al., 2012). Thus, there is a positive bias for both nocturnal and diurnal species for optimal retention of this task when the learning occurs during the active phase of the species. Interestingly, the rhythms of hippocampal BDNF and TrkB protein production of grass rats appear to be out of phase from those reported for laboratory rats (Cirelli and Tononi, 2000b; Dolci et al., 2003; Hamatake et al., 2011; Ikeno et al., 2013; Katoh-Semba et al., 2008). These data provide at least correlational evidence for the claim that differences in optimal phase for retention of hippocampal dependent tasks between nocturnal and diurnal species stem from phase differences in the rhythmic expression of plasticity genes in the hippocampus. Information about differences in the rhythmic expression of other plasticity genes, such as MAPK (Eckel-Mahan et al., 2008) between diurnal and nocturnal species would be of interest, however, direct manipulations of the rhythmic expressions of these gene products are needed to establish a causal link between them and the circadian regulation of learning and memory in species-specific ways.
In grass rats there was no time of day difference in the acquisition of the MWM. These animals showed no time of training differences in their ability to learn the location of the hidden platform and this insensitivity to time of training extended to all other measures that serve to assess motivation, motor functions (swim speed) and anxiety (thigmotaxis) (Clark et al., 2005b; D'Hooge and De Deyn, 2001; Jeltsch et al., 2001; Morris, 1984). Also, the behavior of the two groups was not statistically different for the first trials of each training day, thus showing that all animals were able to remember over periods of 24 hours. These findings and those previously reported for laboratory rats (Gritton et al., 2012), may be indicative of a selective role for the circadian system in affecting the long-term retention of hippocampal tasks. An explanation for the insensitivity to time of day for the acquisition of MWM task could be due to the moderate cognitive load of the MWM. Studies in both human and animals have shown that tasks that are not very cognitively demanding show little or no time of day differences during acquisition, whereas those that are highly demanding show more salient time of day differences (Folkard et al., 1983; Gritton et al., 2012). Work using more challenging hippocampal-dependent learning tasks (Eichenbaum et al., 1987; Eichenbaum et al., 1989; Eichenbaum, 1992; Ergorul and Eichenbaum, 2006; Otto et al., 1991) could serve to clarify that issue.
Our results of time of training on long-term retention could be interpreted as a cognitive deficit in the animals trained and tested during the normal rest phase, but other possibilities exist. For example on the retention probe, the pm animals displayed thigmotaxis, a measure of anxiety (Hostetter and Thomas, 1967; Huang et al., 2012; Simon et al., 1994; Treit and Fundytus, 1988), more frequently than the am group. Thus, the poor performance of the animals tested at night could have been due to non-cognitive factors such as enhanced susceptibility to stress at that phase of the circadian cycle. However, counter to that interpretation is the fact that during all the other tests the pm animals did not differ from the am ones in the display of thigmotaxis. Thus, it is possible that the apparent enhanced anxiety of the pm group during the retention probe was secondary to a memory deficit that made the situation appear less familiar to those animals.
Our testing procedure resulted in different light intensities prevailing during the two testing times, raising the possibility that the poor performance of the pm group during the retention probe was caused by limited visual acuity. This is unlikely for two reasons. First, the pm animals did not show deficits during acquisition although the same lighting conditions were present. Second, there is evidence that rats and mice are able to navigate the MWM under both red light and complete darkness (Klapdor and VanderStaay, 1996; Valentinuzzi et al., 2004). Further, blind or visually impaired rodents are still able to learn and remember the location of the hidden platform in the MWM (Lindner et al., 1997; O'Steen et al., 1995; Spencer et al., 1995). Other non-cognitive deficits such as lack of motivation or diminished motor competence do not appear to be responsible for the differences seen in the retention probe, since swimming speed was not different between the two groups.
For experiment two, our main finding was the presence, in a diurnal mammal, of rhythmic expression of plasticity-gene products in three hippocampal regions known to play distinct roles in cognition (Clark et al., 2005a; Clark et al., 2005b; Dees and Kesner, 2013; Jeltsch et al., 2001; Kesner, 2013; McDonald and White, 1993; Morris et al., 2013; Morris et al., 1986; Packard and Knowlton, 2002; Sweatt, 2004). For all the hippocampal areas, the rhythms were about 180° out of phase with those reported in nocturnal laboratory rats. For nocturnal rodents, peaks in both BDNF and TrkB expression occur late in the dark period (Bova et al., 1998; Dolci et al., 2003), whereas, they occurred much earlier in our diurnal grass rats. Little is known about the relationship between the rhythms of BDNF and TrkB expression. Once the TrkB receptor is occupied by BDNF, a number of signaling cascades are activated (as reviewed in Tapia-Arancibia et al., 2004) ,which are involved in several processes important for learning and neural plasticity, including hippocampal long-term potentiation (Hall et al., 2000; Tyler et al., 2002; Yamada and Nabeshima, 2003). In the diurnal grass rats of this experiment, the peak of TrkB expression within the hippocampus preceded that of BDNF by four hours, and a similar relationship between the two rhythms appears to exist for laboratory rats (Bova et al., 1998). Thus, the daily increase in receptor expression before the peak of BDNF may be a common feature of the mammalian hippocampus that may influence the optimal time of day for learning in a species-specific fashion.
The hippocampal data suggest that the phases of rhythms of BDNF/TrkB expression correspond to the phase preference for the display of activity of each species, and thus may contribute to species differences in the circadian regulation of cognitive functions. However, for both nocturnal and diurnal species, we do not know if the daily fluctuations in the expression of BDNF/TrkB are under the control of circadian oscillators, or are dependent upon either environmental influences or upon the prevalent stage of vigilance of the organism at different times of day. Data on the rhythmic expression of these products obtained under constant environmental conditions and under experimental conditions that control for the influences of sleep and wakefulness are needed to test these alternative hypotheses. Further, if the circadian nature of these rhythms is established, then determining the neural basis of such rhythmicity becomes an important question. Interestingly, some aspects of the circadian regulation of cognition appear to be independent of the SCN in hamsters (Cain et al., 2012; but see Ruby et al. 2008).
Our results represent an important first step in the developing of the grass rat as a diurnal animal model, which is needed to test the species generality of the observations made using traditional laboratory rodents with nocturnal activity profiles. Future directions of this research with our diurnal model could include the examination of circadian regulation of hippocampal-independent memory that require the integrity of other brain regions such as the amygdala and the dorsal striatum (McDonald and White, 1993), which also exhibit a phase reversal in PER 1 and 2 rhythms in grass rats compared to nocturnal mammals (Ramanathan et al., 2008a; Ramanathan et al., 2008b; Ramanathan et al., 2010a; Ramanathan et al., 2010b). Another direction for future studies could use grass rats to develop a diurnal animal model for understanding the cognitive deficits sometimes observed in human shift- and night-workers (Folkard, 1989; Gold et al., 1992). Interestingly, although strongly diurnal in the wild and under standard laboratory conditions (Blanchong et al., 1999; McElhinny et al., 1997), when given access to running wheels a subset of grass rats voluntarily shift to a predominantly nocturnal display of activity (Blanchong et al., 1999). This remarkable shift in activity phase is accompanied by a phase reversal in the rhythmic expression of PER 1 and 2 in many extra-SCN oscillators, including the hippocampus (Ramanathan et al., 2010b).
Understanding what effects these changes in the phase of activity rhythms and clock-gene rhythmic expression might have on rhythms of BDNF/TrkB expression and on the animals’ cognitive functions could be instrumental in furthering our understanding of deficits seen in human night-shift workers and others who voluntary become active at night (Barnard and Nolan, 2008; Czeisler, 2009) and in the development of a diurnal animal model to explore the mechanisms responsible for interactions between chronotype and time of day in human cognition (Hahn et al., 2012).
In summary, our results represent the first descriptions of time of day differences in hippocampal memory using a diurnal rodent model and of rhythms in the expression of plasticity-gene products in a diurnal brain. This information serves to help us understand species differences in the circadian regulation of cognitive functions as well as time of day effects on human memory, and the cognitive deficits seen when circadian rhythms are disrupted, as in the case of human shift- or night- workers (Barnard and Nolan, 2008; Gold et al., 1992).
4. Experimental Procedures
Animals
Male grass rats (3 – 5 months old; n = 24 for Experiment 1; n = 36 for Experiment 2) from our breeding colony at Michigan State University were housed individually in Plexiglas cages (34 × 28 × 17 cm), under a 12:12 h light/dark (LD) cycle, with lights on at Zeitgeber time (ZT) 0, with dim red lights on at all times 7lux) and ab libitum access to food (PMI Nutrition Prolab RMH 2000, Brentwood, MO, USA) and water. All experiments were performed in compliance with guidelines established by the Michigan State University All University Committee on Animal Use and Care, and the National Institute of Health guide for the Care and Use of Laboratory Animals.
Experiment 1
Morris Water Maze
Handling
Grass rats, even after many generations in captivity, are not domesticated and are more reactive than common laboratory rodents. Therefore, the animals were gently handled daily for at least two weeks prior to the start of water maze training to reduce the effects of handling stress on learning. To transfer the animals to and from the water maze we used clear-plastic salad tongs as previously described (Walker, 2011). The animals were habituated to contact with the plastic tongs during the two weeks of daily handling before the start of behavioral testing.
Testing Room Conditions and Apparatus
Behavioral testing took place in a room with the same LD cycle and illumination conditions of the animals’ colony room. Black images (i.e. star, circle, square, and a triangle) were fixed to white walls to serve as high-contrast extra-maze cues. The location of these extra maze cues remained constant throughout the experiment. The testing apparatus was a circular pool, 140cm in diameter, which was filled with 22° ± 5° water. Pool wall height was 37.46 cm; the pool was filled with 19.05cm of water. The water was made opaque with nontoxic paint (ArtMinds ™). A clear 15.24 cm wide platform was placed in the center of the SW quadrant. The hidden platform was approximately 1.5cm below the surface of the water and invisible to grass rats.
Training
Animals were randomly assigned to either the AM or PM groups (n = 12/ZT) and trained for 6 consecutive days. The AM group was trained and tested at ZT4 while the PM group was tested and trained at ZT 16. Daily training sessions consisted of 4 trials. Each trial started when the grass rat entered the water and ended when it found the platform or after 120 seconds had lapsed. Grass rats were placed in the water facing the walls of the pool at randomly assigned locations, except the location that that would later be used for the acquisition and retention probes (see below). Grass rats that failed to find the platform in 120 seconds were gently guided to the platform and allowed to rest for 15 seconds before the start of the next trial. At the end of the fourth trial, the animals were dried off manually with an absorbent towel and returned to their home cage.
Acquisition and Retention Probe Test
An acquisition probe test was given on the 7th day, following the 6 days of training. Grass rats were placed in the pool in a location, that had not been used during training and given 60 seconds to find the hidden platform. After the acquisition probe, grass rats were returned to their home cage. Fourteen days later the retention probe test was given in an identical manner to that of the acquisition probe.
Behavioral Quantification and Statistical Analysis
All training trials and probe tests were recorded and analyzed using the Noldus EthoVision-system (version 8.5; Noldus®). The proportion of grass rats to reach the platform, swim path lengths, swim velocity and latency to platform quadrant, were calculated. For the acquisition and retention probes, the proportion of animals showing thigmotaxis was also determined. In order to monitor thigmotaxic behavior, the pool was divided during analysis into two rings. Time spent swimming in the 10.5cm wide ring closest to the edge of the pool was quantified, and grass rats that spent more than 30secs swimming in the thigmotaxic zone were characterized as displaying thigmotaxis. The latencies to reach the platform were recorded, but were not used for group comparisons since not all animals reached the platform within the 2 minutes of each trial.
The Cochrans Q test (Cochran and Cox, 1957; Conover, 1999) was used to examine the effects of training days on the proportion of grass rats that found the platform over the course of the training. This analysis was performed for the AM and PM groups separately using both the average of the four trials per day or just for the first trial of each training day. Chi squared tests were used to compare the AM and PM groups with respect to proportion of the animals reaching the platform (all trials) or showing thigmotaxic behavior during acquisition and retention probes. Two way analyses of variance (ANOVA) were used to examine the main effects of training day and time of training and the interaction of these two factors on swim path lengths, swim velocity and latency to platform quadrant during trial 1 of each training day and for the average of trials 1-4 for each training day. Student's t tests for independent samples were used to determine the effect of the time of training on swim path lengths, swim velocity and latency to platform quadrant, for both acquisition and retention probes. All statistical analyses for both experiments used SPSS version 17 software, and all differences were considered statistically significant when P was equal to or less than 0.05, using two-tailed probabilities when pertinent.
Experiment 2
Tissue collection and immunocytochemistry
At 4-h intervals from ZT2 to ZT22, animals (n = 6/ZT) were deeply anesthetized with an intraperitoneal injection of 400mg/kg of sodium pentobarbital (Ovation Pharmaceutical, Deerfield, IL, USA) and perfused transcardially with 0.01 M phosphate buffered saline (PBS; pH 7.2) followed by 4% paraformaldehyde –lysine–sodium periodate (PLP) in 0.1 M phosphate buffer (Sigma, St Louis, MO USA). Brains were removed, post-fixed for 4-8 h and then transferred to 20% sucrose for 24h, before storing them in cryoprotectant at –20 °C. Sections (30 m; coronal plane) were obtained using a freezing sliding microtome and stored in cryoprotectant until the immunocytochemical (ICC) procedure for either BDNF or TrkB detection was performed on every other section.
Sections were taken out of cryoprotectant and stored overnight in 0.01M PBS at 4°C. Just prior to undergoing ICC procedures, sections were rinsed three times (10 min/rinse) in fresh 0.01 M PBS. Unless otherwise noted, the sections were rinsed three times (10 min/rinse) in 0.01 M PBS between all steps of the ICC procedures. All steps were carried out at room temperature unless noted otherwise. Free-floating sections containing the hippocampus (CA1, hilus, and dorsal dentate blade) were rinsed in 0.01 M PBS, blocked for 1 h using 5% normal goat serum (NGS; Vector Laboratories, Burlingame, CA, USA) in PBS and incubated for 48 h in a rabbit anti-BDNF antibody at 4°C (Chemicon/Millipore, Temecula, CA, USA; diluted 1:10,000 in PBS and 3% NGS). For detecting TrkB immunoreactivity, the same procedure was followed (rabbit polyclonal antibody against the carboxyl terminal domain of TrkB receptor (trkB794) commercially available from Santa Cruz Biotechnologies, Santa Cruz, CA. U.S.A). All sections were then incubated for 1 h in a goat anti-rabbit biotinylated antibody (Vector Labs, Burlingame, CA, USA; diluted 1:200 in PBS and 3% NGS), and then for 1 h in avidin–biotin peroxidase complex (AB complex, Vector Laboratories, Burlingame, CA, USA; in PBS). After three rinses (10 min/rinse) in Acetate buffer (pH=7.2), the sections were reacted with 0.025% diaminobenzidine (DAB; Sigma-Aldrich) enhanced with 2.5% nickel sulfate (Sigma-Aldrich) in Tris buffer with 3% hydrogen peroxide (J.T. Baker, Phillipsburg, NJ, USA) for 12 min. The reaction was followed by three 10 min rinses in Acetate buffer (pH=7.2), All sections were mounted onto gelatin-coated slides, dehydrated, and coverslipped with Permount (Sigma-Aldrich). A set of control sections was selected and incubated in PBS and 3% NGS without anti-BDNF or anti-TrkB antibody at 4°C. Immunoreactivity was undetectable in sections that were processed in the absence of primary antibody.
Quantitative and Statistical Analysis
A single section containing the three targeted hippocampal regions, i.e., CA1 and the hilus and dorsal blade of the DG, was identified for each animal using the rat brain atlas of Paxinos and Watson (Paxinos and Watson, 2007). The approximate level of the sections was –2.80 mm from bregma. For each region, BDNF and TrkB labeled cells were counted bilaterally within an area defined by a 500×100 m2 counting box by an investigator unaware of the sampling time associated with each section. All BDNF counts were done using a light microscope (Leitz, Laborlux S, Wetzlar, Germany; 25X objective). Camera lucida drawings were made of visually identified labeled cells within the boxes in each area. TrkB counts were performed using photomicrographs that were taken of each region with a digital camera (MBF Bioscience Inc, 2007) attached to a Zeiss light microscope (Carl Zeiss, Göttingen, Germany). Photomicrographs were arranged in Photoshop CS5. For every region, TrkB labeled cells were counted within the region specific counting boxes. Bilateral cell counts were obtained using NIH Image J software (National Institute of Health, Bethesda, MD, USA). For each region counted for TrkB staining, circularity (0-10) and contrast intensity thresholds were used to distinguish immunoreactive labeled cells from background staining.
Total bilateral counts of each area were subjected to one-way ANOVAs to assess the effect of ZT on BDNF and TrkB expression in each region. Significant F ratios were followed by individual comparisons using Fisher's Least Significant Difference (LSD) tests.
highlights.
Cognitive functions show daily fluctuations
Diurnal grass rats show superior retention when trained and tested during the day
The phase of plasticity genes rhythms differ between grass rats and that reported for nocturnal rats.
The phase of hippocampal rhythms may dictate optimal phase for retention
Effects of time of training were restricted to long-term retention
Table 2.
Tests for Time of Day Effects on the Acquisition Probe
| Dependent Variable | t-test or χ2 |
|---|---|
| Swim Path | (t(22)=1.959, P>0.05) |
| Swim Velocity | (t(22)=1.473, P>0.05) |
| Latency to Platform Quadrant | (t(22)=0.627, P>0.05) |
| Thigmotaxis | (χ2(1,N=24) = 1.510, P> 0.05) |
Acknowledgements
We thank Dr. L. Yan, D. Fairey, B. Bostic, B. Cavanaugh, Dr. L Smale, Dr. B. Fermin, M. Coleman, C. Tucker, A. Stowie, S. Fairey, T. Groves, Dr. Castillo-Ruiz, and Dr. C. Ramanathan for technical assistance and support. This work was supported by NIMH RO1 MH53433 to Drs. L. Smale, A. Nunez and D. Weaver. The first author was a Michigan State University King-Chavez-Park fellow during the preparation of this paper.
Abbreviations
- BDNF
brain derived neurotrophic factor
- TrkB
tyrosine kinase B
- ANOVA
analysis of variance
- LD
light/dark
- LSD
least significant difference
- PER
Period
- SCN
suprachiasmatic nucleus
- ZT
Zeitgeber time
- DG
dentate gyrus
- CA1
cornus Ammon 1
- ICC
Immunocytochemical
- MWM
Morris water maze
- MAPK
mitogen activated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe M, et al. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–6. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S, Harbour VL, Robinson B. Pinealectomy does not affect diurnal PER2 expression in the rat limbic forebrain. Neurosci Lett. 2006;399:147–50. doi: 10.1016/j.neulet.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Amir S, Robinson B. Thyroidectomy alters the daily pattern of expression of the clock protein, PER2, in the oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neurosci Lett. 2006;407:254–7. doi: 10.1016/j.neulet.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Barnard AR, Nolan PM. When clocks go bad: neurobehavioural consequences of disrupted circadian timing. PLoS Genet. 2008;4:e1000040. doi: 10.1371/journal.pgen.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, et al. BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep. 2013;5:759–68. doi: 10.1016/j.celrep.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology. 76 Pt. 2014;C:677–83. doi: 10.1016/j.neuropharm.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, et al. Hippocampal BDNF mRNA shows a diurnal regulation, primarily in the exon III transcript. Brain Res Mol Brain Res. 1999;71:11–22. doi: 10.1016/s0169-328x(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Blanchong JA, et al. Nocturnal and diurnal rhythms in the unstriped Nile rat, Arvicanthis niloticus. J Biol Rhythms. 1999;14:364–77. doi: 10.1177/074873099129000777. [DOI] [PubMed] [Google Scholar]
- Bova R, et al. BDNF and trkB mRNAs oscillate in rat brain during the light-dark cycle. Brain Res Mol Brain Res. 1998;57:321–4. doi: 10.1016/s0169-328x(98)00092-8. [DOI] [PubMed] [Google Scholar]
- Cain SW, Chalmers JA, Ralph MR. Circadian modulation of passive avoidance is not eliminated in arrhythmic hamsters with suprachiasmatic nucleus lesions. Behav Brain Res. 2012;230:288–90. doi: 10.1016/j.bbr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Callaghan CK, Kelly AM. Neurotrophins play differential roles in short and long-term recognition memory. Neurobiol Learn Mem. 2013;104:39–48. doi: 10.1016/j.nlm.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000a;885:303–21. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000b;20:9187–94. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Impaired remote spatial memory after hippocampal lesions despite extensive training beginning early in life. Hippocampus. 2005a;15:340–6. doi: 10.1002/hipo.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005b;15:260–72. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran WG, Cox GM. Experimental designs. Wiley; New York: 1957. [Google Scholar]
- Conover WJ. Practical nonparametric statistics. Wiley; New York: 1999. [Google Scholar]
- Czeisler CA. Medical and genetic differences in the adverse impact of sleep loss on performance: ethical considerations for the medical profession. Trans Am Clin Climatol Assoc. 2009;120:249–85. [PMC free article] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dees RL, Kesner RP. The role of the dorsal dentate gyrus in object and object-context recognition. Neurobiol Learn Mem. 2013;106:112–7. doi: 10.1016/j.nlm.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Dolci C, et al. Circadian variations in expression of the trkB receptor in adult rat hippocampus. Brain Res. 2003;994:67–72. doi: 10.1016/j.brainres.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, et al. Influence of aging on Bmal1 and Per2 expression in extra-SCN oscillators in hamster brain. Brain Res. 2013;1491:44–53. doi: 10.1016/j.brainres.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Mahan KL, et al. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci. 2008;11:1074–82. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, et al. Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. J Neurosci. 1987;7:716–32. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Mathews P, Cohen NJ. Further studies of hippocampal representation during odor discrimination learning. Behav Neurosci. 1989;103:1207–16. doi: 10.1037//0735-7044.103.6.1207. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampal system and declarative memory in animals. J Cogn Neurosci. 1992;4:217–31. doi: 10.1162/jocn.1992.4.3.217. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. Essential role of the hippocampal formation in rapid learning of higher-order sequential associations. J Neurosci. 2006;26:4111–7. doi: 10.1523/JNEUROSCI.0441-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, et al. The brain-derived neurotrophic factor Val66Met polymorphism moderates an effect of physical activity on working memory performance. Psychol Sci. 2013;24:1770–9. doi: 10.1177/0956797613480367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkard S, Wever RA, Wildgruber CM. Multi-oscillatory control of circadian rhythms in human performance. Nature. 1983;305:223–6. doi: 10.1038/305223a0. [DOI] [PubMed] [Google Scholar]
- Folkard S, et al. Circadian rhythms in human performance and affective state. Acta Psychiatr Belg. 1985;85:568–81. [PubMed] [Google Scholar]
- Folkard S, Monk TH. Hours of work : temporal factors in work scheduling. Wiley. Chichester West Sussex ; New York: 1985. [Google Scholar]
- Folkard S. Circadian performance rhythms: some practical and theoretical implications. Philos Trans R Soc Lond B Biol Sci. 1990;327:543–53. doi: 10.1098/rstb.1990.0097. [DOI] [PubMed] [Google Scholar]
- Furnham A, Rawles R. Spatial ability at different times of day Personality and Individual Differences. 1988;9:937–939. [Google Scholar]
- Gerstner JR, et al. Cycling Behavior and Memory Formation. Journal of Neuroscience. 2009;29:12824–12830. doi: 10.1523/JNEUROSCI.3353-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhooley MJ, Pinnock SB, Herbert J. Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: implications for neurogenesis. Neurosci Lett. 2011;489:177–81. doi: 10.1016/j.neulet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Gold DR, et al. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am J Public Health. 1992;82:1011–4. doi: 10.2105/ajph.82.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golini RS, et al. Daily patterns of clock and cognition-related factors are modified in the hippocampus of vitamin A-deficient rats. Hippocampus. 2012;22:1720–1732. doi: 10.1002/hipo.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, et al. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–54. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- Gritton HJ, et al. Bidirectional interactions between circadian entrainment and cognitive performance. Learn Mem. 2012;19:126–41. doi: 10.1101/lm.023499.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–5. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hamatake M, et al. Phase advance of the light-dark cycle perturbs diurnal rhythms of brain-derived neurotrophic factor and neurotrophin-3 protein levels, which reduces synaptophysin-positive presynaptic terminals in the cortex of juvenile rats. J Biol Chem. 2011;286:21478–87. doi: 10.1074/jbc.M110.195859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann HJ. Circadian differences in maze performance of C57Bl/6 Ola mice. Behavioral Processes. 1992;27:77–84. doi: 10.1016/0376-6357(92)90017-8. [DOI] [PubMed] [Google Scholar]
- Hostetter G, Thomas GJ. Evaluation of enhanced thigmotaxis as a condition of impaired maze learning by rats with hippocampal lesions. J Comp Physiol Psychol. 1967;63:105–10. doi: 10.1037/h0024144. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhou W, Zhang Y. Bright lighting conditions during testing increase thigmotaxis and impair water maze performance in BALB/c mice. Behav Brain Res. 2012;226:26–31. doi: 10.1016/j.bbr.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Ikeno T, Weil ZM, Nelson RJ. Photoperiod affects the diurnal rhythm of hippocampal neuronal morphology of siberian hamsters. Chronobiol Int. 2013;30:1089–100. doi: 10.3109/07420528.2013.800090. [DOI] [PubMed] [Google Scholar]
- Jeltsch H, et al. Cognitive performances and locomotor activity following dentate granule cell damage in rats: role of lesion extent and type of memory tested. Neurobiol Learn Mem. 2001;76:81–105. doi: 10.1006/nlme.2000.3986. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, et al. A phase advance of the light-dark cycle stimulates production of BDNF, but not of other neurotrophins, in the adult rat cerebral cortex: association with the activation of CREB. J Neurochem. 2008;106:2131–42. doi: 10.1111/j.1471-4159.2008.05565.x. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Role of the hippocampus in mediating interference as measured by pattern separation processes. Behav Processes. 2013;93:148–54. doi: 10.1016/j.beproc.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Klapdor K, VanderStaay FJ. The Morris water-escape task in mice: Strain differences and effects of intra-maze contrast and brightness. Physiology & Behavior. 1996;60:1247–1254. doi: 10.1016/s0031-9384(96)00224-7. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 15 Spec No. 2006;2:R271–7. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Korte M, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–60. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont EW, et al. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci U S A. 2005;102:4180–4. doi: 10.1073/pnas.0500901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JZ, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A. 2013;110:9950–5. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Walline R, Earnest DJ. Circadian rhythm of brain-derived neurotrophic factor in the rat suprachiasmatic nucleus. Neurosci Lett. 1998;242:89–92. doi: 10.1016/s0304-3940(98)00062-7. [DOI] [PubMed] [Google Scholar]
- Lindner MD, et al. Blind rats are not profoundly impaired in the reference memory Morris water maze and cannot be clearly discriminated from rats with cognitive deficits in the cued platform task. Brain Res Cogn Brain Res. 1997;5:329–33. doi: 10.1016/s0926-6410(97)00006-2. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Minichiello L, et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–14. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–60. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Morris AM, et al. The role of the dentate gyrus in the formation of contextual representations. Hippocampus. 2013;23:162–8. doi: 10.1002/hipo.22078. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial-learning in the rat. Journal of Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Hagan JJ, Rawlins JN. Allocentric spatial learning by hippocampectomised rats: a further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q J Exp Psychol B. 1986;38:365–95. [PubMed] [Google Scholar]
- O'Steen WK, et al. Analysis of severe photoreceptor loss and Morris water-maze performance in aged rats. Behav Brain Res. 1995;68:151–8. doi: 10.1016/0166-4328(94)00168-f. [DOI] [PubMed] [Google Scholar]
- Otalora BB, et al. Period gene expression in the brain of a dual-phasing rodent, the Octodon degus. J Biol Rhythms. 2013;28:249–61. doi: 10.1177/0748730413495521. [DOI] [PubMed] [Google Scholar]
- Otto T, et al. Hippocampus and olfactory discrimination learning: effects of entorhinal cortex lesions on olfactory learning and memory in a successive-cue, go-no-go task. Behav Neurosci. 1991;105:111–9. doi: 10.1037//0735-7044.105.1.111. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–93. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–30. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Patterson SL, et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–45. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR. Psychomotor performance as a function of time of day Perceptual and Motor Skills. Vol. 68. Academic Press; San Diego, CA. Payne, R.B.: 2007. 1989. The rat brain in stereotaxic coordinates; pp. 455–461. [DOI] [PubMed] [Google Scholar]
- Ramanathan C, Nunez AA, Smale L. Daily rhythms in PER1 within and beyond the suprachiasmatic nucleus of female grass rats (Arvicanthis niloticus). Neuroscience. 2008a;156:48–58. doi: 10.1016/j.neuroscience.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Smale L, Nunez AA. Rhythms in expression of PER1 protein in the amygdala and bed nucleus of the stria terminalis of the diurnal grass rat (Arvicanthis niloticus). Neurosci Lett. 2008b;441:86–9. doi: 10.1016/j.neulet.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, et al. PER2 rhythms in the amygdala and bed nucleus of the stria terminalis of the diurnal grass rat (Arvicanthis niloticus). Neurosci Lett. 2010a;473:220–3. doi: 10.1016/j.neulet.2010.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, et al. Phase preference for the display of activity is associated with the phase of extra-suprachiasmatic nucleus oscillators within and between species. Neuroscience. 2010b;170:758–72. doi: 10.1016/j.neuroscience.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sweatt JD. Rhythms of memory. Nat Neurosci. 2008;11:993–4. doi: 10.1038/nn0908-993. [DOI] [PubMed] [Google Scholar]
- Ruby NF, et al. Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci U S A. 2008;105:15593–8. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf MJ, et al. Circadian variation in BDNF mRNA expression in the rat hippocampus. Brain Res Mol Brain Res. 2000;75:342–4. doi: 10.1016/s0169-328x(99)00314-9. [DOI] [PubMed] [Google Scholar]
- Selcher JC, et al. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–90. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Smarr BL, et al. A time to remember: The role of circadian clocks in learning and memory. Behav Neurosci. 2014 doi: 10.1037/a0035963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RL, O'Steen WK, McEwen BS. Water maze performance of aged Sprague-Dawley rats in relation to retinal morphologic measures. Behav Brain Res. 1995;68:139–50. doi: 10.1016/0166-4328(94)00167-e. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Hippocampal function in cognition. Psychopharmacology (Berl) 2004;174:99–110. doi: 10.1007/s00213-004-1795-9. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, et al. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25:77–107. doi: 10.1016/j.yfrne.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Testu F. Diurnal-variations of performance and information-processing Chronobiologia. 1986;13:319–326. [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–62. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, et al. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–37. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinuzzi VS, Menna-Barreto L, Xavier GF. Effect of circadian phase on performance of rats in the Morris water maze task. J Biol Rhythms. 2004;19:312–24. doi: 10.1177/0748730404265688. [DOI] [PubMed] [Google Scholar]
- Vigers AJ, et al. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience. 2012;212:1–18. doi: 10.1016/j.neuroscience.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. A Simple and Safe Handling Technique for African Grass Rats. TechTalk. 2011:4. ed.^eds. AALAS. [Google Scholar]
- Wang LM, et al. Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro. 2009;1 doi: 10.1042/AN20090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–70. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]