Abstract
Signals mediated by members of the tumor necrosis factorreceptor superfamily modulate a network of diverse processes including initiation of inflammatory responses and altering cell fate between pathways favoring survival and death. Although such pathways have been well-described for the TNF-αreceptor, less is known about signalinginduced by the TNF superfamily member LIGHT and how it is differentially altered by expression of its two receptors LTβR and HVEM in the same cell.We used cell lines with different relative expression of HVEM and LTβR to show that LIGHT-induced signals mediated by these receptors were associated with altered TRAF2 stability andRelA nuclear translocation. Production of the inflammatory chemokine CXCL10 was primarily mediated by LTβR. Higher expression of HVEM was associated with cell survival, while unopposed LTβR signaling favored pathways leading to apoptosis. Importantly, restoring HVEM expression in cells with low endogenous expression recapitulated the phenotype of cells with higher endogenous expression. Together, our data provide evidence that relative expression of HVEM and LTβR modulatescanonical NF-κB and pro-apoptotic signals stimulated by LIGHT.
Keywords: LIGHT, herpesvirus entry mediator (HVEM), lymphotoxin beta receptor (LTβR), TNF receptor associated factor (TRAF), apoptosis, NF-kappa B (NF-κB)
1. Introduction
TheTNF receptor superfamily (TNFRSF) includes more than 25 receptors that interact with nearly 20 ligands to influence cellular responses[1]. The best studied TNFRSF member, TNF-R1, can form at least two distinct signaling complexes after interacting with the ligand TNF-α, with functional outcomes in a cell dependent on a web of complex downstream interactions that may lead to diverse influences on cell survival[2]. Other TNFRSF members have also been found to alter the balance of inflammatory and survival responses in certain cells, often in response to stimulation by different ligands [3].
The TNFRSF memberslymphotoxin β receptor (LTβR) and herpesvirus entry mediator (HVEM) each interact with the pro-inflammatory moleculeLIGHT (Lymphotoxin-related inducible ligand that competes for glycoprotein D binding to HVEM on T cells)[4, 5].LTβR and HVEM may also interact with different isoforms of lymphotoxinα (LTα), LTα1β2 or LTα3, respectively [5, 6], while HVEM but not LTβR also binds B- and T-lymphocyte attenuator (BTLA) and CD160 [7, 8].LTβR and HVEM are expressed in similar cell types, including epithelial cells and certain immune cells[9]. LIGHT,LTα, LTβ, BTLA, and CD160 are produced by a variety of immune cells including macrophages, T cells, B cells, and NK cells[7, 8, 10-14].
Studies of functional outcomes in cells after LTβR or HVEM engagement have generally focused on the individual receptors. Use of LTα1β2 or agonist antibodies to activate LTβR signalingleads to NF-κBactivation, inflammatory cytokine production, and growth arrest or cell death in some but not all LTβR-positive cells [15-17]. Similarly, a mutant form of LIGHT capable of binding HVEM but not LTβR does not activate cell death pathways [18], while an analogous mutant capable of binding LTβR but not HVEM induces cell death [19]. Using HVEM-specific agonists, signaling through this receptor promotes survival in epithelial cell lines [20]. These studies generally used specific agonists of either LTβR or HVEM, and did not focus on the combined effect of signalingthrough both molecules on the responding cell at the same time with the same agonist.
Upon ligand interaction, the intracellular domains of LTβR and HVEM bind TNF receptor associated factor (TRAF) family members [21], specifically TRAF2[20], which acts as a central hub for activation and inhibition of NF-κB, JNK, and caspase 8 [22, 23].While TRAF2signaling itselfmay not have a strong biological effect, TRAF2 activation or degradation can synergize with other signals, such as those stimulated by IFN-γor TNF-α. For example, TRAF2-activated NF-κB binds the NF-κB promoter element of CXCL10, but does not itself drive CXCL10 production. The CXCL10 promoter contains two elements, an NF-κB binding element and interferon stimulated response element (ISRE)[24]. After TNF-α and IFN-γ treatment, STAT1 and TRAF2-activated NF-κB bind the promoter of CXCL10 and synergistically activate transcription of CXCL10 [25]. Similarly, degradation of TRAF2 is insufficient to activate caspase 8 topromote apoptosis; other signals, such as those mediated by TNF-α, are required [26].
Given the complexities of TNFRSF signaling and the overlapping ligands and signal transduction pathways used by LTβR and HVEM, we studied the effect of co-expression of these receptors on LIGHT-induced signals in human cell lines. We show here that, consistent with prior studies, LIGHT induces chemokine production and pro-inflammatory signals in cells in which LTβR expression dominates that of HVEM, leading to chemokine production, TRAF2 degradation, caspase 8 activation, and polyADP ribose polymerase (PARP) cleavage. In cells with balanced LTβR and HVEM expression, TRAF2 stability is increased, RelA nuclear translocation is decreased, and there is less caspase cleavage, favoring cell survival. Thus, cells may vary expression of the different surface receptors detecting LIGHT to regulateoverlapping signaling pathways that modulate cell fate during inflammatory responses.
2. Materials and methods
2.1 Cell lines, media and reagents
HeLa, HT-29, 293T, and U937 cells were maintained in 1× DMEM with 10% FCS. Cells were treated with 10 ng/mL recombinant human IFN-γ, 10 ng/mL TNF-α, 100 ng/mL recombinant human LIGHT (Peprotech), or no stimulation. Plasmids used included the pBEC10 plasmid expressing HVEM [27], NF-κBand ISRE luciferase reporter plasmids (pNF-κB-Luc and pISRE-Luc, Agilent Technologies), and the full ORF of LIGHT cloned into pcDNA3 [5]. Mutations in the LIGHT ORF (G119E, R228E, and G119E/R228E) were introduced by quick-change mutagenesis.
2.2 Receptor Quantitation
Cells and anti-mouse calibration beads (Bang Laboratories) were incubated with anti-trinitrophenyl (anti-TNP), anti-HVEM (Santa Cruz) or anti-LTβR (Biolegend) for 45 minutes in 1%BSA in PBS. Following incubation, the cells were washed and incubated with anti-mouse Alexafluor 647 (Invitrogen) for 45 minutes in 1% BSA in PBS. The cells were washed with PBS and fluorescence measured per cell. Receptor number per cell was calculated using a standard curve generated from calibration beads, according to the manufacturer instructions. This method provides quantitative assessment of each receptor from individual calibration curves, allowing direct comparison of surface levels for different proteins, and is not influenced by differences in fluorescence of antibodies used for detection of different proteins (as might occur with measurements of direct fluorescence intensity). Similar methods have been previously applied to determination of surface levels of viral entry receptors [28].
2.3 Luciferase reporter assay
HT-29 or HeLa cells were plated into a 6 well plate at 1×106 cell per well. Cells were transfected with either pNF-κB-Luc or pISRE-Luc using Lipofectamine 2000 (Invitrogen) according to manufacturer instructions and incubated overnight. The following day the cells were split into 12 well plates and incubated overnight. After incubation, the cells were treated with either 10 ng/mL IFN-γ, 100 ng/mL LIGHT, both in combination, or left untreated. After 24 hours, the cells were washed with PBS containing 0.1 g/L MgCl2 and 0.1 g/L CaCl2 (PBS-ABC)and lysed in 1× passive lysis buffer (Promega). Firefly and Renilla luciferase activity was measured from lysates using the Promega Dual Luciferase Reporter Assay.
2.4 Cytokine measurements
HeLa, and HT-29 cells were plated in 12 well plates at a density of 3×105 cells per well overnight. The following day the cells were treated with either 10 ng/mL IFN-γ, 100 ng/mL LIGHT, both in combination, or left untreated. After 24 hours, the supernatants were harvested and spun down at 14,000×g for 5 minutes to pellet cell debris. CXCL10 (R&D systems or Peprotech) was detected from supernatants by ELISA according to manufacturer instructions.
2.5 Nuclear fractionation
1×107 HT-29 or HeLa cells were treated with or without 100 ng/mL LIGHT for 24 hours. The cells were washed with PBS-ABC and lysed in 500 μL of cytoplasmic extraction buffer (10 mMKCl, 10 mM HEPES, pH 7.9, 0.1 mM EDTA, 1 mM EGTA, 1mMdithiothreitol) and placed on ice for 20 minutes. 25 μL of 10% NP-40 was added to each lysate, and samples were vortexed and then centrifuged at 14,000×g for 10 minutes. The supernatants were removed and the pellet containing the nuclei resuspended in 100 μL of nuclear extraction buffer (0.4M NaCl, 20 mM HEPES, pH 7.9, 1 mM EDTA, 1 mM EGTA) and incubated for 30 minutes on ice. The samples were spun at 14,000×g for 10 minutes and the supernatants were collected. To concentrate the nuclear fraction, 400 μL dH20, 500 μL methanol, and 200 μL chloroform were added and samples were vortexed, centrifuged for 10 minutes at 14,000×g, and the upper layer discarded above the phase separation. 400 μL of methanol was added, samples were again vortexedand centrifuged at 14,000×g for 10 minutes, and supernatants were discarded and the pellet resuspended in 50 μL of lysis buffer.
2.6 Western blot analysis
Nuclear fractions were electrophoresed on a 10% polyacrylamide gel. Samples were transferred to nitrocellulose and blocked in 5% milk in 1× TBST (50 mMTris.HCl, pH 7.4, 150 mMNaCl, 0.1% Tween 20) for 1 hour at room temperature. Blots for RelAwere incubated in 5% milk in1× TBST with 1:500 rabbit anti-RelA (Biolegend). Blots for JNK were incubated with 5% BSA in 1×TBST with 1:250 rabbit total JNK (Cell Signaling) or 1:250 rabbit anti-phosphorylated JNK (Cell Signaling). IKBα and TRAF2 antibodies(Cell Signaling) were used at a concentration of 1:500 in 5% BSA in TBST. Blots for caspase8 or PARP were incubated with 5% BSA in 1×TBST with 1:1000 mouse anti-caspase 8 or 1:1000 rabbit anti-PARP (Cell Signaling), respectively. Blots were incubated overnight at 5 °C on a shaker, washed 3 times with 1×TBST, and incubated with anti-rabbit HRP-conjugated secondary antibody for 45 minutes at room temperature. Blots were then washed 3 times with 1×TBST and developed using enhanced chemiluminescence(ECL).An Odyssey Infrared Imaging System (LI-COR Biosciences) was used for quantification of band density.
3. Results
3.1 Relative surface expression of the LIGHT receptors HVEM and LTβR is associated with altered TRAF2 stability and RelA nuclear translocation
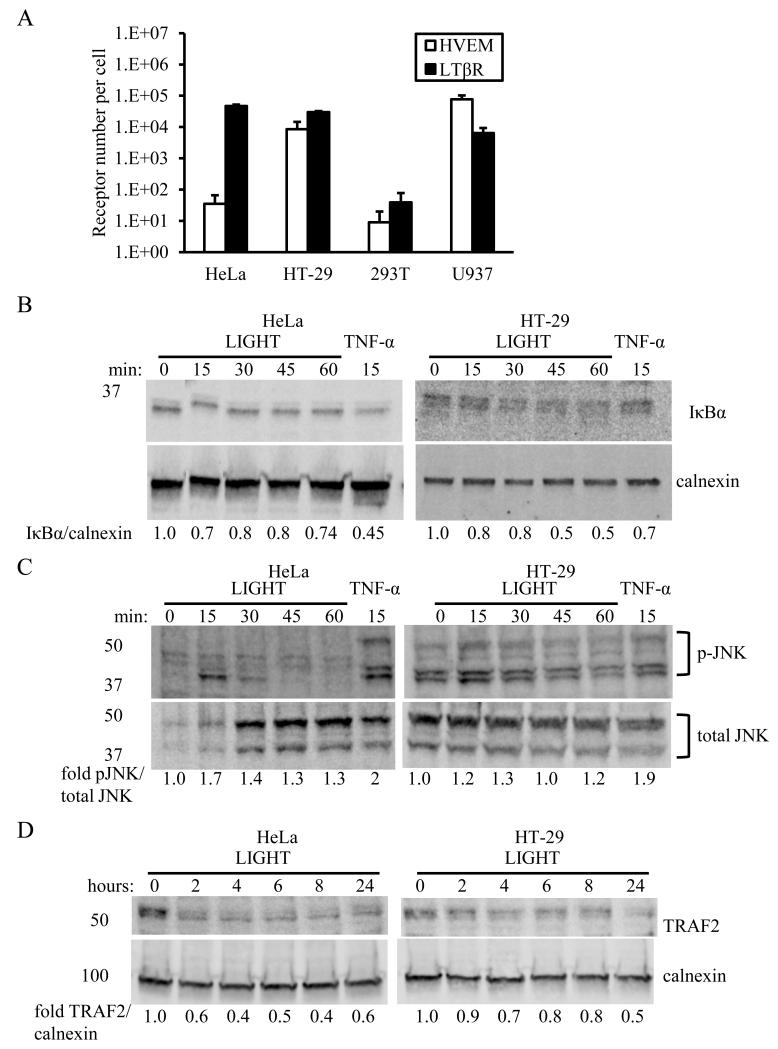
Epithelial cells and certain immune cells including dendritic cells and monocytes express the LIGHT receptors LTβR and HVEM [29-33]. We measured receptor numbers of LTβR and HVEM on the plasma membrane of different relevant human cell lines (Fig. 1A). The epithelial cell lines HeLa and HT-29 had similar amounts of LTβR expressed at the cell surface, about a log higher in receptor number than the U937 monocyte cell line expected to express both receptors. In contrast, variable amounts of HVEM were expressed on HeLa and HT-29 cells, with levels on HT-29 cells more than 2 logs higher. The human embryonic kidney cell line 293T expressed near-background levels of both HVEM and LTβR. We used the HeLa and HT-29 cell lines to study the influence of variable HVEM expression on LIGHT-induced signals.
Figure 1.
Differential expression of HVEM and LTβR onHeLa and HT-29 cells is associated with altered TRAF2 stability.A. Expression of HVEM and LTβR on different cell lines, measured by flow cytometric bead assay as described in the Methods. Experiment was repeated three times and data averaged.HeLa and HT-29 cells express similar levels of LTβR but different levels of HVEM. B. HeLa and HT-29 cells degrade IκBα with similar kinetics after stimulation with LIGHT. TNF-α was used as a positive control. C. Transient activation of JNK in HeLa and HT-29 cells is observed in response to LIGHT stimulation. TNF-α was used as a positive control. D. TRAF2 stability in HT-29 cells after LIGHT stimulation is increased relative to that in HeLa cells. B-D show representative blots from experiments in duplicate.
NF-κB is activated by LIGHT-induced signals via either HVEM [20] or LTβR[34], mediated by the adaptor molecule TRAF2 [21, 35]. Both HT-29 cells and HeLa cells responded to LIGHT stimulation with activation of early signals consistent with canonical NF-κBsignaling, evident by degradation of IκBα (Fig. 1B).We also observed transient phosphorylation of JNK in both cell types (Fig. 1C), as has been previously observed for LIGHT signaling through LTβR [36]. However, the stability of TRAF2, which acts as a hub for a variety of signals mediated byHVEM and LTβR [21, 35], was enhanced in HT-29 cells compared with HeLa cells after stimulation with LIGHT (Fig. 1D).
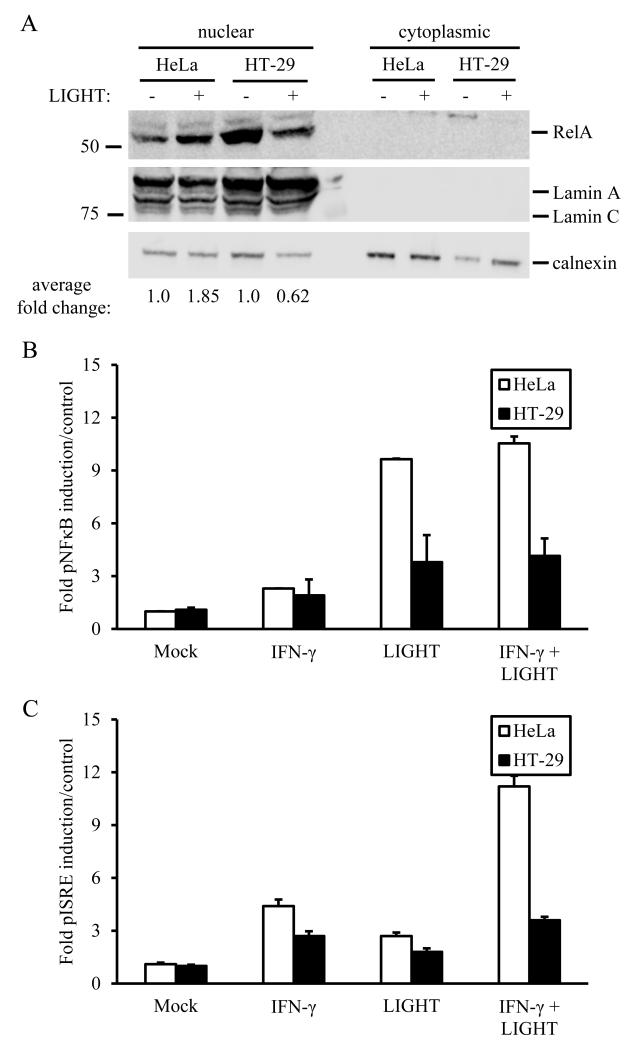
Induction of canonical NF-κBsignalingby LIGHT was investigated further in both cell types by measuring RelA nuclear translocation, which was increased in HeLa cells relative to HT-29 cells (Fig. 2A). Since stimulation of cells with LIGHT has been shown to enhance production of IFN-γ-induced chemokines[37], we also tested induction of promoter activity by NF-κB and the interferon-sensitive response element (ISRE). Similarly to RelA translocation, LIGHT-stimulated promoter activity by both NF-κB and ISRE was increased in HeLa cells relative to HT-29 cells (Fig 2B,C).
Figure 2.
Canonical NF-κBsignaling is altered in association with differential expression of HVEM and LTβR. A. RelA nuclear translocation is increased in HeLa cells relative to HT-29 cells. Representative blot shown from duplicate experiments. B. NF-κB reporter activity is increased in HeLa cells stimulated with LIGHT +/− IFN-γ as compared with HT-29 cells. C. ISRE reporter activity is increased in HeLa cells stimulated with LIGHT +/− IFN-γ as compared with HT-29 cells, with LIGHT and IFN-γ acting synergistically in HeLa cells. For both B and C, experiments were done in triplicate twice, and firefly luciferase reporter luminescence was divided over renilla control and results averaged as fold over mock.
3.2 Synergistic production of the IFN-γ-induced chemokine CXCL10 by LIGHT is primarily mediated by LTβR
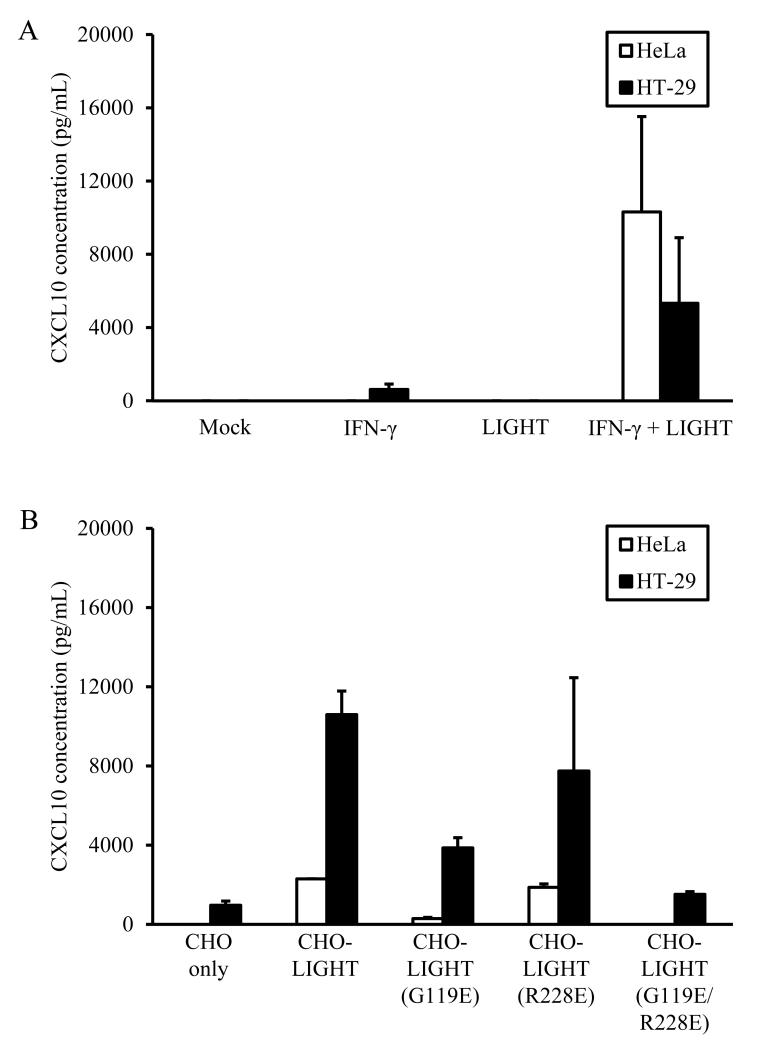
LIGHT induces signals through pathways that influence a variety of immune processes including chemokine and cytokine production [38], and it is not known whether the synergistic effects of LIGHT on IFN-γ-induced chemokine responses [37]are preferentially mediated by signaling through HVEM, LTβR, or both. We measured production of the IFN-γ-induced chemokine CXCL10 after LIGHT stimulation of HeLa or HT-29 cells in the presence and absence of IFN-γ. Neither cell line produced measureable levels of CXCL10 in response to soluble LIGHT alone, andsynergistic enhancement of IFN-γ-induced CXCL10 production by soluble LIGHT was observed in both HeLa and HT-29 cells (Fig 3A).
Figure 3.
LIGHT-enhanced CXCL10 production in response to IFN-γ stimulation is primarily mediated by signaling through LTβR. A. Soluble LIGHT synergizes with IFN-γ to promote CXCL10 production by both HeLa and HT-29 cells. B. Mutant forms of membrane-bound LIGHT which interact preferentially with HVEM (G119E) or LTβR (R228E) suggest that the majority of LIGHT-induced signaling is through LTβR in both cell types. Experiments were done with triplicate samples twice, with representative data shown.
The production of similar levels of CXCL10 by HeLa and HT-29 cells after costimulation with IFN-γ and LIGHT, combined with the observation that LTβR is expressed at similar levels by both HeLa and HT-29 cells but HVEM is poorly expressed by HeLa cells, suggests that LIGHT signaling through LTβR may be more important in mediating synergistic chemokine production. To test this more directly, we generated receptor-specific mutants of membrane-bound LIGHT. Prior studies have shown that an R228E mutation in LIGHT leads to preferential binding to LTβR [19], while a G119E mutant preferentially binds HVEM [18]. These constructs were expressed in CHO cells, which do not express HVEM or LTβR and do not respond to LIGHT. Incubation of either HeLa cells or HT-29 cells (Fig. 3B) in the presence of IFN-γ with CHO cells expressing different forms of LIGHT led to CXCL10 production at comparable levels between wild-type LIGHT and mutant LIGHT-R228E, with significantly lower levels measured when these epithelial cells were costimulated with mutant LIGHT-G119E. Co-incubation of HeLa or HT-29 cells with CHO cells either not expressing LIGHT or expressing LIGHT with both mutations (G119E/R228E) led to detection of only background levels of CXCL10. CXCL10 was not produced when HeLa or HT-29 cells were exposed to the different LIGHT constructs in the absence of IFN-γ costimulation (not shown).Together, these experiments support the conclusion that synergistic production of the IFN-γ-induced chemokine CXCL10 by LIGHT in epithelial cells is primarily mediated by signals via LTβR.
3.3 LIGHT stimulation of cell death pathways is associated with low HVEM expression
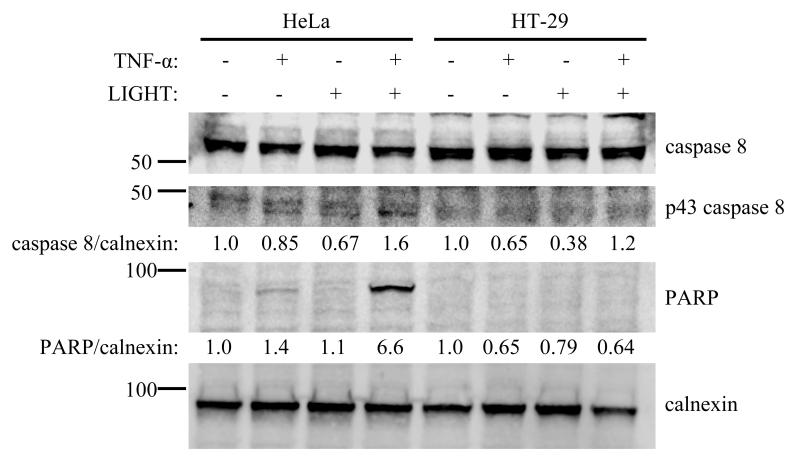
Ligands in the TNF superfamily often mediate context-dependent signaling which leads to differing biological effects affecting cell survival; for example, stimulation of cells with TNF-α can promote NF-κB activation and cell survival[39, 40] or lead to activation of caspase 8, resulting in cleavage of PARP and apoptosis [39, 41]. We tested the effect of LIGHT on induction of cell death pathways by TNF-α in HeLa and HT-29 cells. When LIGHT is present, caspase 8 activation is more pronouncedin HeLa cells relative to HT-29 cells, leading to significantly more PARP cleavage (Fig 4).
Figure 4.
HeLa cells activate caspase 8 in response to costimulation with TNF-α and LIGHT, cleaving full-length caspase 8 to the active p43 form, leading to PARP cleavage. HT-29 cells treated similarly do not activate caspase 8 to cleave PARP. Cells were treated for 24 hours with 10 ng/mL TNF-α, 100 ng/mL LIGHT, or both in combination and cell lysates analyzed by Western blot for the indicated proteins. Representative blot shown from duplicate experiments.
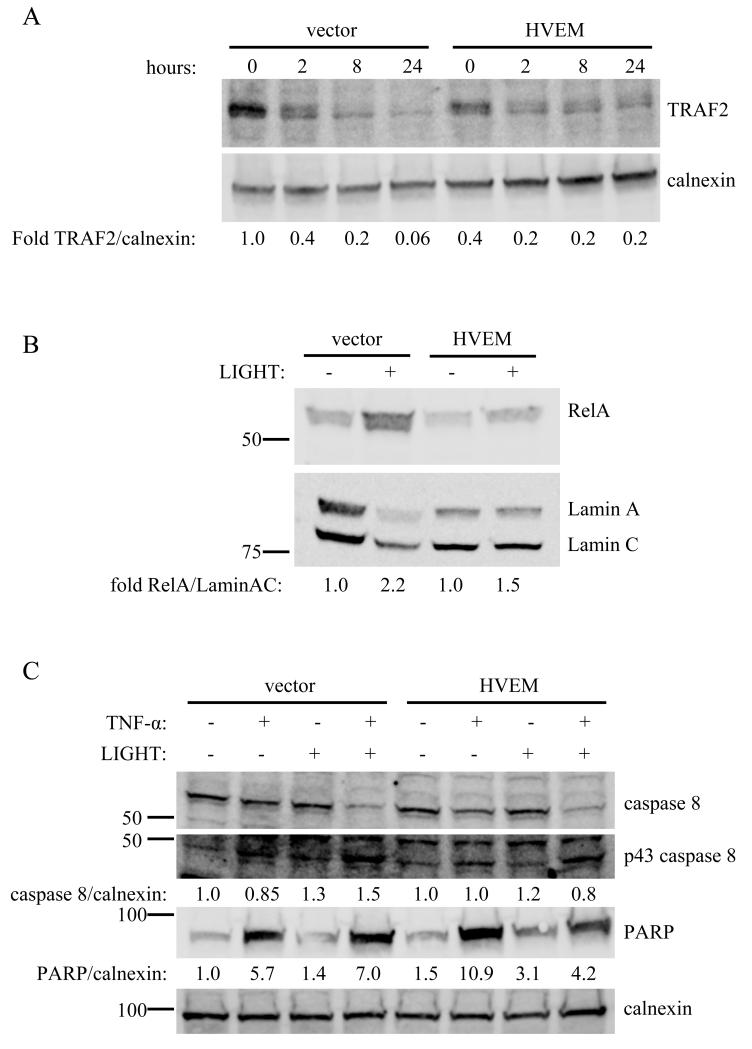
Comparisons of HT-29 and HeLa cells could be confounded by other signaling differences between these cells. Therefore, we additionally studied HeLa cells in which HVEM was exogenously expressed by transfection. Importantly, transfection recapitulates the phenotype of HT-29 cells in HeLa cells, supporting a role for HVEM in attenuating apoptosis-inducing signals stimulated by TNF-α. In the HVEM-transfected HeLa cells compared with vector transfection, TRAF2 stability is enhanced (Fig 5A), RelA nuclear translocation is decreased (Fig 5B), and caspase 8 activation and PARP cleavage are attenuated (Fig 5C). Expression of HVEM in HeLa cells did not affect JNK phosphorylation or IκBα degradation (not shown).
Figure 5.
Transfection of HVEM into HeLa cells recapitulates the signaling phenotype of HT-29 cells. A. HeLa cells were transfected with HVEM, and TRAF2 levels measured by Western blot at different times after stimulation with 100 ng/mL LIGHT. TRAF2 stability is enhanced in association with exogenous expression of HVEM. B. RelA nuclear translocation is decreased after exogenous expression of HVEM in HeLa cells. Nuclear extracts were prepared 24 hours after stimulation of HVEM-transfected or vector-transfected HeLa cells with 100 ng/mL LIGHT, and analyzed by Western blot for RelA nuclear translocation. C. Caspase 8 activation and PARP cleavage are decreased in HeLa cells after exogenous expression of HVEM. Transfected HeLa cells were treated for 24 hours with 10 ng/mL TNF-α, 100 ng/mL LIGHT, or both in combination and cell lysates analyzed by Western blot for the indicated proteins. For A-C, experiments were done twice with representative blots shown.
4. Discussion
We used cell lines differing in relative expression of HVEM and LTβR to study intracellular signaling after interaction of LIGHT with its two known receptors. Our major finding is that HVEM acts to modulate canonical NF-κB and pro-apoptotic signals mediated by LTβR, in association with increased stability of TRAF2. The presence of HVEM did not have obvious effects on JNK phosphorylation or IκBα degradation, and signaling through HVEM did not appreciably alter LIGHT-stimulated CXCL10 production via LTβR.Our results describe a situation in which cells may employ differential expression of receptors which recognize identical ligands to modulate specific responses.
Opposing cell-survival signals in the context of cell stimulation by a single ligand have been described for TNF-α, which in addition to engaging the different receptors TNFR1 and TNFR2, may also trigger formation of different complexes after activating TNFR1 [39]. The context of TNFR1 activation determines the response of the cell, which may include proliferation, apoptosis, or necroptosis[42]. Engagement of TNFR2 stimulates anti-apoptotic signals [43], but may also alter TRAF2 localization, leading to its degradation [44]. The net result is an autoregulatorysignaling loop that in some contexts may promote cell death [45]. As understanding of the complexities of cellular responses to TNF-α signaling increases, the similarly complex pathways stimulated by engagement of other TNFRSF members have also begun to be clarified[3]. Our results add to the understanding of the overlapping and potentially competing HVEM-LTβR responses to LIGHT in the same cell, demonstrating that HVEM expression can counteract pro-apoptotic signals mediated by LTβR which lead to PARP cleavage.
Our observation that the presence of HVEM improves TRAF2 stability is also consistent with the signaling paradigm described for TNFR1 and TNFR2[39]. Degradation of TRAF2 induced by signaling through TNFR2 alters the balance of signals mediated by TNFR1, which must bind TRAF2 to activate NF-κB. For the TNF-α/TNFR system, this generally leads to enhancement of cell death pathways when both TNFR1 and TNFR2 are present in the cell; in the case of LIGHT signaling through HVEM/LTβR, our data suggest that by stabilizing cytoplasmic TRAF2 levels, HVEM dampens signals favoring cell death.
We additionally observed that unopposed LIGHT signaling through LTβR led to enhanced RelA nuclear translocation and NF-κB reporter activity when compared with cells expressing both HVEM and LTβR. The modulation in RelA translocation observed when both receptors are present deserves further investigation, but it is of interest to note a recent report suggesting TRAF3 involvement in inhibition of LTβR-mediated activation of canonical NF-κBsignaling[46]. This study used agonist antibodies to activate LTβRsignaling, such that any co-signaling effect of HVEM activation would not be observed. The cytoplasmic regions of both HVEM [35] and LTβR [47] bind TRAF3, so it is reasonable to speculate involvement of TRAF3 in our observation of RelA modulation. TRAF3 has also been reported to negatively regulate non-canonical NF-κBsignaling induced via LTβR [46] and other TNFRSF members [48]; we did not investigate non-canonical NF-κBsignaling in our study.
The observation that HVEM acts to promote cell survival is supported by prior data using agonist antibodies and HVEM ligands other than LIGHT [20]. Similarly and as noted above, much of our understanding of LTβR signaling is derived from studies using specific agonists that do not also bind HVEM [15-17]. Prior reports in which both receptors were present and engaged in the same cell did not elucidate the overlapping downstream signals that may be mediated by both HVEM and LTβR interactions with LIGHT [18, 19], as we have done here. Our data therefore extend on these prior studies. Interestingly, studies in which HVEM was the primary available LIGHT receptor led to significantly different conclusions, with induction of endogenous TNF-α and promotion of cell death in cells from patients with chronic lymphocytic leukemia [49]. Although this suggests that at least in some contexts signals mediated by HVEM may promote inflammation and cell death, an imbalance of LIGHT-promoted signals and their interaction with overlapping pathways, including those mediated by TNF-α, may also contribute to the outcomes within a given cell.
It is worth noting that a complete picture of the contributions of LTβR and HVEM to inflammatory responses in tissues needs to account for differential interactions of both receptors with isoforms of LT-α and of HVEM with the additional receptors BTLA and CD160[7, 8]. These latter two ligands, in addition to promoting NF-κB activation via HVEM, may dampen inflammatory responses in some cell types via intracellular signals mediated by BTLA or CD160. In this regard, varying observations have been reported on the role of HVEM in specific models of infection. Murine studies in a systemic model of bacterial infection suggest that the HVEM-BTLA interaction may predominate in some situations to inhibit early innate immune responses through effects on proinflammatory cytokine production [50]. HVEM interaction with CD160 was necessary for effective control of bacterial infection at gastrointestinal and pulmonary mucosal barriers [32]. In these latter studies, LIGHT KO mice cleared pathogenic intestinal bacteria similarly to WT mice. Clearly, multiple interacting and overlapping signaling pathways determine the ultimate response to inflammation from various causes.
In summary, the studies reported here further our understanding of the complex interactions between LIGHT and its receptors LTβR and HVEM.The major effect of differential receptor expression resulted in an altered balance between cell survival and cell death, in a manner analogous to TNF-α signaling. Future studies on signaling induced by these receptors should account for both the shared and distinct ligands that may activate their respective signaling pathways.
5. Conclusions
Expression of both LIGHT receptors LTβR and HVEM in the same cell leads to alterations in signaling compared to LTβR alone:
LIGHT stimulates canonical NF-κB and pro-apoptotic signals via engagement of LTβR which are attenuated in the presence of HVEM.
LTβR-mediated pro-inflammatory signals promoted by LIGHT, including chemokine production and JNK signaling, are not obviously altered by HVEMsignaling.
HVEM-mediated attenuation of LTβR signaling is associated with increased TRAF2 stability.
Together, these data suggest that combinatorial LIGHT signals influence cell fate.
Highlights.
LIGHT signalling through its two receptors depends on their differential expression.
LTβR mediates pro-inflammatory and pro-apoptotic signals when engaged by LIGHT.
HVEM attenuates pro-apoptotic signals when both receptors are present.
Acknowledgments
The authors thank Richard Longnecker and members of the Longnecker laboratory for critical feedback on data and the manuscript.This work was supported by NIH grant K08 AI089942
Abbreviations
- LIGHT
lymphotoxin-related inducible ligand that competes for glycoprotein D binding to HVEM on T cells
- HVEM
herpesvirus entry mediator
- LTβR
lymphotoxin beta receptor
- TNFRSF
TNF receptor superfamily
- BTLA
B- and T-lymphocyte attenuator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schrofelbauer B, Hoffmann A. How do pleiotropic kinase hubs mediate specific signaling by TNFR superfamily members? Immunol Rev. 2011;244(1):29–43. doi: 10.1111/j.1600-065X.2011.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silke J. The regulation of TNF signalling: what a tangled web we weave. Curr Opin Immunol. 2011;23(5):620–6. doi: 10.1016/j.coi.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 4.Harrop JA, et al. Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem. 1998;273(42):27548–56. doi: 10.1074/jbc.273.42.27548. [DOI] [PubMed] [Google Scholar]
- 5.Mauri DN, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1998;8(1):21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 6.Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 7.Cai G, et al. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9(2):176–85. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 8.Sedy JR, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6(1):90–8. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 9.Browning JL, French LE. Visualization of lymphotoxin-beta and lymphotoxin-beta receptor expression in mouse embryos. J Immunol. 2002;168(10):5079–87. doi: 10.4049/jimmunol.168.10.5079. [DOI] [PubMed] [Google Scholar]
- 10.Morel Y, et al. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol. 2000;165(8):4397–404. doi: 10.4049/jimmunol.165.8.4397. [DOI] [PubMed] [Google Scholar]
- 11.Paya CV, et al. Tumor necrosis factor and lymphotoxin secretion by human natural killer cells leads to antiviral cytotoxicity. J Immunol. 1988;141(6):1989–95. [PubMed] [Google Scholar]
- 12.Ware CF, et al. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J Immunol. 1992;149(12):3881–8. [PubMed] [Google Scholar]
- 13.Gramaglia I, et al. Lymphotoxin alphabeta is expressed on recently activated naive and Th1-like CD4 cells but is down-regulated by IL-4 during Th2 differentiation. J Immunol. 1999;162(3):1333–8. [PubMed] [Google Scholar]
- 14.Gonzalez LC, et al. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A. 2005;102(4):1116–21. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackay F, et al. Lymphotoxin beta receptor triggering induces activation of the nuclear factor kappaB transcription factor in some cell types. J Biol Chem. 1996;271(40):24934–8. doi: 10.1074/jbc.271.40.24934. [DOI] [PubMed] [Google Scholar]
- 16.Degli-Esposti MA, et al. Activation of the lymphotoxin beta receptor by cross-linking induces chemokine production and growth arrest in A375 melanoma cells. J Immunol. 1997;158(4):1756–62. [PubMed] [Google Scholar]
- 17.Browning JL, et al. Signaling through the lymphotoxin beta receptor induces the death of some adenocarcinoma tumor lines. J Exp Med. 1996;183(3):867–78. doi: 10.1084/jem.183.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rooney IA, et al. The lymphotoxin-beta receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J Biol Chem. 2000;275(19):14307–15. doi: 10.1074/jbc.275.19.14307. [DOI] [PubMed] [Google Scholar]
- 19.Chen MC, et al. The role of apoptosis signal-regulating kinase 1 in lymphotoxin-beta receptor-mediated cell death. J Biol Chem. 2003;278(18):16073–81. doi: 10.1074/jbc.M208661200. [DOI] [PubMed] [Google Scholar]
- 20.Cheung TC, et al. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A. 2009;106(15):6244–9. doi: 10.1073/pnas.0902115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YS, Nedospasov SA, Liu ZG. TRAF2 plays a key, nonredundant role in LIGHT-lymphotoxin beta receptor signaling. Mol Cell Biol. 2005;25(6):2130–7. doi: 10.1128/MCB.25.6.2130-2137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ea CK, et al. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22(2):245–57. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 23.DiDonato JA, et al. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388(6642):548–54. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 24.Clarke DL, et al. TNFalpha and IFNgamma synergistically enhance transcriptional activation of CXCL10 in human airway smooth muscle cells via STAT-1, NF-kappaB, and the transcriptional coactivator CREB-binding protein. J Biol Chem. 2010;285(38):29101–10. doi: 10.1074/jbc.M109.0999952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeruva S, Ramadori G, Raddatz D. NF-kappaB-dependent synergistic regulation of CXCL10 gene expression by IL-1beta and IFN-gamma in human intestinal epithelial cell lines. Int J Colorectal Dis. 2008;23(3):305–17. doi: 10.1007/s00384-007-0396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vince JE, et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol. 2008;182(1):171–84. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery RI, et al. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87(3):427–36. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 28.Krummenacher C, et al. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology. 2004;322(2):14. doi: 10.1016/j.virol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 29.De Trez C. Lymphotoxin-beta receptor expression and its related signaling pathways govern dendritic cell homeostasis and function. Immunobiology. 2012;217(12):1250–8. doi: 10.1016/j.imbio.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy DD, et al. The lymphotoxin pathway: beyond lymph node development. Immunol Res. 2006;35(1-2):41–54. doi: 10.1385/IR:35:1:41. [DOI] [PubMed] [Google Scholar]
- 31.Force WR, et al. Mouse lymphotoxin-beta receptor. Molecular genetics, ligand binding, and expression. J Immunol. 1995;155(11):5280–8. [PubMed] [Google Scholar]
- 32.Shui JW, et al. HVEM signalling at mucosal barriers provides host defence against pathogenic bacteria. Nature. 2012;488(7410):222–5. doi: 10.1038/nature11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollara G, Katz DR, Chain BM. LIGHTing up dendritic cell activation: Immune regulation and viral exploitation. J Cell Physiol. 2005;205(2):161–2. doi: 10.1002/jcp.20473. [DOI] [PubMed] [Google Scholar]
- 34.Dejardin E, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17(4):525–35. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 35.Marsters SA, et al. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem. 1997;272(22):14029–32. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 36.Chang YH, et al. Lymphotoxin beta receptor induces interleukin 8 gene expression via NF-kappaB and AP-1 activation. Exp Cell Res. 2002;278(2):166–74. doi: 10.1006/excr.2002.5573. [DOI] [PubMed] [Google Scholar]
- 37.Hosokawa Y, et al. TNFSF14 coordinately enhances CXCL10 and CXCL11 productions from IFN-gamma-stimulated human gingival fibroblasts. Mol Immunol. 2010;47(4):666–70. doi: 10.1016/j.molimm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev. 2004;202:49–66. doi: 10.1111/j.0105-2896.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 39.Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell Signal. 2012;24(6):1297–305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 41.Benchoua A, et al. Active caspase-8 translocates into the nucleus of apoptotic cells to inactivate poly(ADP-ribose) polymerase-2. J Biol Chem. 2002;277(37):34217–22. doi: 10.1074/jbc.M203941200. [DOI] [PubMed] [Google Scholar]
- 42.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–90. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 43.Rauert H, et al. Membrane tumor necrosis factor (TNF) induces p100 processing via TNF receptor-2 (TNFR2) J Biol Chem. 2010;285(10):7394–404. doi: 10.1074/jbc.M109.037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez M, et al. NF-kappaB signal triggering and termination by tumor necrosis factor receptor 2. J Biol Chem. 2011;286(26):22814–24. doi: 10.1074/jbc.M111.225631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Depuydt B, et al. Induction of apoptosis by TNF receptor 2 in a T-cell hybridoma is FADD dependent and blocked by caspase-8 inhibitors. J Cell Sci. 2005;118(Pt 3):497–504. doi: 10.1242/jcs.01640. [DOI] [PubMed] [Google Scholar]
- 46.Bista P, et al. TRAF3 controls activation of the canonical and alternative NFkappaB by the lymphotoxin beta receptor. J Biol Chem. 2010;285(17):12971–8. doi: 10.1074/jbc.M109.076091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuai J, et al. Endogenous association of TRAF2, TRAF3, cIAP1, and Smac with lymphotoxin beta receptor reveals a novel mechanism of apoptosis. J Biol Chem. 2003;278(16) doi: 10.1074/jbc.M208672200. [DOI] [PubMed] [Google Scholar]
- 48.Hauer J, et al. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci U S A. 2005;102(8):2874–9. doi: 10.1073/pnas.0500187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasero C, et al. A role for HVEM, but not lymphotoxinβ receptor, in LIGHTinduced tumor cell death and chemokine production. European journal of immunology. 2009;39(9):13. doi: 10.1002/eji.200939069. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y, et al. B and T lymphocyte attenuator tempers early infection immunity. J Immunol. 2009;183(3):1946–51. doi: 10.4049/jimmunol.0801866. [DOI] [PMC free article] [PubMed] [Google Scholar]