Abstract
The role of cellular proteases and endosome maturation in the entry of caliciviruses including porcine enteric calicivirus (PEC), murine norovirus (MNV)-1 and feline calicivirus (FCV) were investigated. Treatment with chloroquine or cathepsin L inhibitors, but not cathepsin B inhibitors, significantly reduced the replication of PEC, MNV and FCV. When concentrated PEC, MNV or FCV were incubated with recombinant cathepsin L, the minor capsid protein VP2 of PEC and the major capsid protein VP1 of MNV and FCV were cleaved by the protease based on the Western blot analysis. Confocal microscopy analysis of PEC and MNV-1 showed that viral capsid proteins were retained in the endosomes in the presence of a cathepsin L inhibitor or chloroquine during virus entry. The results of this study suggest the important role of endosome maturation and cathepsin L in the entry of caliciviruses, and cathepsin L as a potential therapeutic target for calicivirus infection.
Keywords: Calicivirus, Entry, Endosome maturation, Cathepsin L
Highlights
-
•
Endosome maturation and/or cathepsin L are important in the replication of caliciviruses.
-
•
Inhibition of endosome maturation blocked viral entry by retaining viruses in the endosomes.
-
•
Cathepsin L facilitates the viral escape from endosome by cleaving calicivirus capsid protein.
Introduction
Caliciviruses are nonenveloped viruses of 35–40 nm in diameter and possess a single-stranded, positive-sense RNA genome of approximately 7–8 kb (Green, 2007). Caliciviruses have a T=3 icosahedral capsid assembled with 90 dimers of VP1 which is composed of three domains; the N-terminal arm (NTA), the S (shell) and the P (protruding) domains (Ng and Parra, 2010, Prasad et al., 1999). The P domain of VP1 forms the spike on the virion and is composed of P1 and P2 subdomains. The P2 subdomain is located within the P1 subdomain and contains a conserved region flanked by the hypervariable domains (Prasad et al., 1999). The hypervariable domains are mostly involved in receptor binding (Bhella et al., 2008, Bhella and Goodfellow, 2011, Chen et al., 2006). The function of VP2, a minor structural protein, is not well known but it was reported that VP2 is required for the production of infectious feline calicivirus (FCV) (Sosnovtsev et al., 2005), interacts with VP1 to increase stability of norovirus capsid and is likely to be associated with packaging of RNA genome (Bertolotti-Ciarlet et al., 2003, Bertolotti-Ciarlet et al., 2002.
Caliciviruses belong to the family Caliciviridae which comprises at least five genera including Norovirus, Sapovirus, Lagovirus, Vesivirus, and Nebovirus (Green, 2007). Noroviruses and sapoviruses cause enteric infections in humans and animals (Green et al., 2001). Lagoviruses and vesiviruses mainly cause oral, respiratory and sometimes systemic infections in animals (Green et al., 2001). Noroviruses account for about 58% of foodborne human illnesses causing about 21 million cases of gastroenteritis and 800 deaths annually in the United States alone (http://www.cdc.gov/norovirus/about/overview.html). Despite the importance of norovirus in public health, research on understanding norovirus biology and development of antiviral drugs has been greatly hindered due to the inability to grow human noroviruses in cell culture. Therefore, easily cultivable murine norovirus (MNV) (Wobus et al., 2004) and FCV (Luttermann and Meyers, 2010), in the family Caliciviridae, have been used as surrogate viruses for studying noroviruses.
Virus entry is a multistep process that involves consecutive interactions of cellular and viral factors including binding of virus to cellular receptors, virus uncoating and release of viral genome to initiate virus replication (Grove and Marsh, 2011, Marsh and Helenius, 2006). Virus uncoating may occur at the plasma membrane for direct penetration, or at the endosomes or other cellular compartments (Marsh and Helenius, 2006). Viruses utilize a number of different entry processes, including clathrin-dependent endocytic pathway, clathrin-independent endocytic pathway that involves caveolar or lipid, or other yet poorly defined entry pathways (Mercer et al., 2010). In clathrin-dependent endocytosis, viruses taken up by clathrin-coated vesicles travel through the endocytic compartments from where virus must escape before the increasingly harsher environment of maturing endosomes irreversibly degrade viruses. The journey of viruses following virus uptake to virus uncoating and genome release is less well understood for viruses that utilize clathrin-independent endocytosis (Grove and Marsh, 2011, Marsh and Helenius, 2006). However, there are reports that subsequent sorting of endosomal cargo such as viruses in different endocytic pathways may overlap in the early or late endosomes (Naslavsky et al., 2004, Sharma et al., 2003).
In the endosomal compartments, host enzymes including cathepsins are reported to be involved in virus fusion and/or uncoating of virus capsid for some viruses (Grove and Marsh, 2011). The cathepsin family of proteolytic enzymes contains several diverse classes of proteases including cysteine (cathepsins B, L, H, K, S, and O), aspartyl (cathepsin D and E) and serine (cathepsin G) proteases (Vasiljeva et al., 2007). Among them, cathepsin L, B, or S have been reported to be associated with entry and replication of some viruses including severe acute respiratory syndrome (SARS) coronavirus, murine hepatitis virus, reovirus and Ebola virus (Bosch et al., 2008, Brecher et al., 2012, Ebert et al., 2002, Mainou and Dermody, 2012, Qiu et al., 2006, Schornberg et al., 2006). Cathepsin L and B are shown to cleave Ebola virus glycoprotein, leading to exposure of putative fusion domain required for fusion of viral and endosomal membranes (Schornberg et al., 2006). Cathepsin L is also reported to cleave the spike protein of SARS coronavirus for fusion competence (Bosch et al., 2008). In addition to the enveloped viruses that require cathepsin for virus fusion and uncoating, some non-enveloped viruses are also shown to rely on host cell proteases residing in the endosomes for uncoating of virus capsid. Reovirus disassembly in the endosomes is reported to be mediated predominantly by cathepsin L and less efficiently by cathepsin B, generating infectious subvirion particles that are capable of penetrating membranes and deliver core particles into cytoplasm (Ebert et al., 2002, Mainou and Dermody, 2012). Virus entry mechanisms for caliciviruses including MNV-1, and porcine enteric calicivirus (PEC) or human norovirus are not well understood to date, although it has been shown that clathrin-dependent endocytosis and pH-dependent entry is important in FCV replication (Kreutz and Seal, 1995, Stuart and Brown, 2006). For MNV-1, it was reported that MNV-1 entry is not mediated by clathrin or caveolae, but dependent on dynamin and cholesterol (Gerondopoulos et al., 2010, Perry et al., 2009, Perry and Wobus, 2010), but detailed entry mechanism still needs further elucidation.
Here we demonstrated the importance of cathepsin L activity and endosome maturation during the entry stage of caliciviruses using PEC, FCV and MNV-1. We found that cathepsin L inhibitors, but not a B inhibitor, and chloroquine significantly reduced the replication of PEC, FCV and MNV-1. We also found that recombinant cathepsin L cleaved VP1 of FCV and MNV-1, and VP2 of PEC based on the Western blot analysis. Confocal microscopy analysis of PEC and MNV-1 showed that virus was retained in the endosomes in the presence of a cathepsin L inhibitor or chloroquine during virus entry. Our results suggest a crucial role of cathepsin L in the replication of caliciviruses, and cathepsin L as a potential therapeutic target for calicivirus infections.
Results
Inhibitors of cathepsin L, but not cathepsin B, significantly reduced the replication of caliciviruses
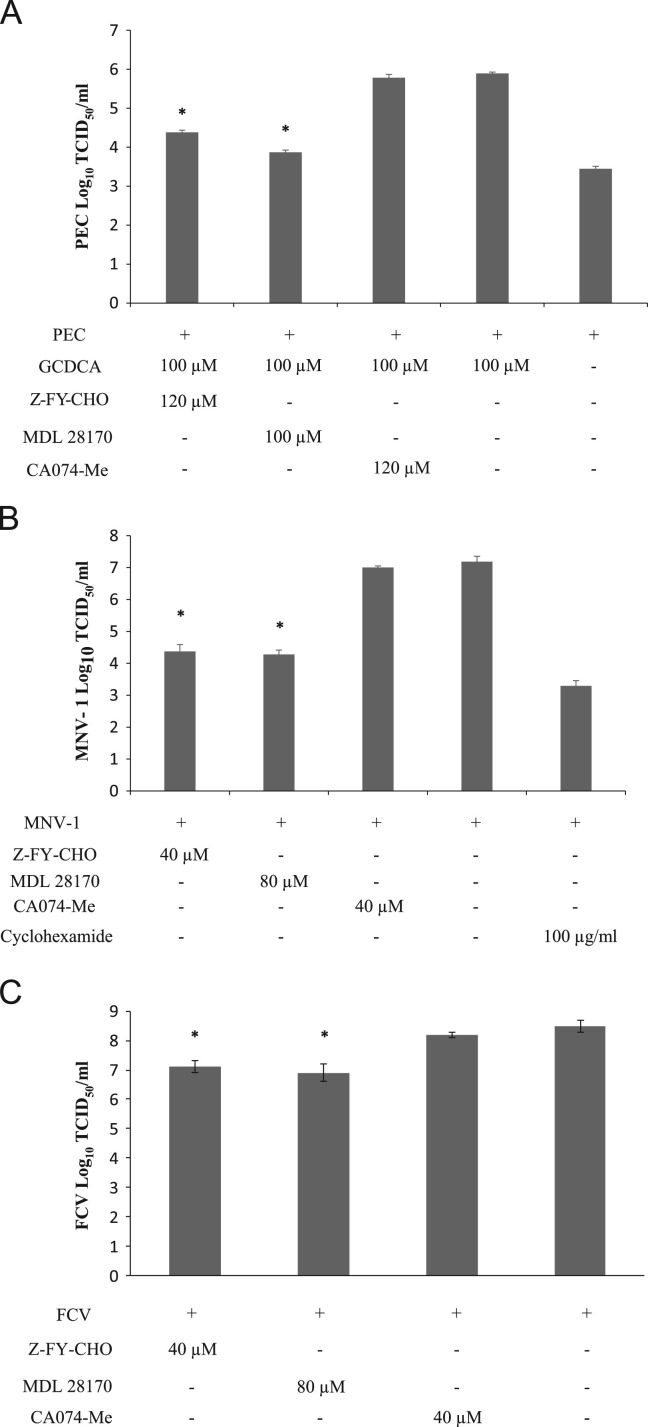
The role of cathepsin B and L in PEC, MNV-1 and FCV replication was studied using inhibitors of cathepsin L and cathepsin B. Our results showed that treatment of cells with inhibitors of cathepsin L (Z-FY-CHO and MDL 28170) reduced PEC titers by 32.6–106-fold, MNV-1 titers by 649.4–801.2-fold, and FCV titers by 25.1–39.8-fold ( Fig. 1A–C). However, cathepsin B inhibitor CA074-Me at up to 120 µM did not lead to a significant reduction of viral replication (Fig. 1A–C). Glycochenodeoxycholic acid (GCDCA), a bile acid, was added to culture media for PEC replication, since PEC does not grow in cell culture without bile acids (Chang et al., 2005, Chang et al., 2004) (Fig. 1A). The PEC levels in absence of bile acid and MNV-1 levels in cyclohexamide-treated cells indicate the levels of internalized viruses (Fig. 1A and B). As reported previously, CA074-Me significantly reduced the replication of feline coronavirus 1146 strain in CRFK cells (Kim et al., 2013, Regan et al., 2008) (data not shown).
Fig. 1.
Effects of cathepsin inhibitors in the replication of PEC, MNV-1 or FCV. LLC-PK, RAW267.4 or CRFK cells were incubated with cathepsin L inhibitors, Z-FY-CHO and MDL 28170, or a cathepsin B inhibitor, CA074-Me, for 1 h, then infected with (A) PEC (MOI 50) (B) MNV-1 (MOI 10) or (C) FCV (MOI 50) in the presence of each inhibitor. For PEC, GCDCA (100 µM) was present in the media to support virus replication during virus infection. Following virus infection for 1 h, cells were washed and the media was replaced with fresh media containing an inhibitor. Cells were further incubated at 37 °C and collected at 12 h PI. Viral replication was quantified by the TCID50 assay. Asterisk indicates that virus titer was significantly reduced by an inhibitor compared to the control (P<0.05).
Endosomal acidification is required for calicivirus replication
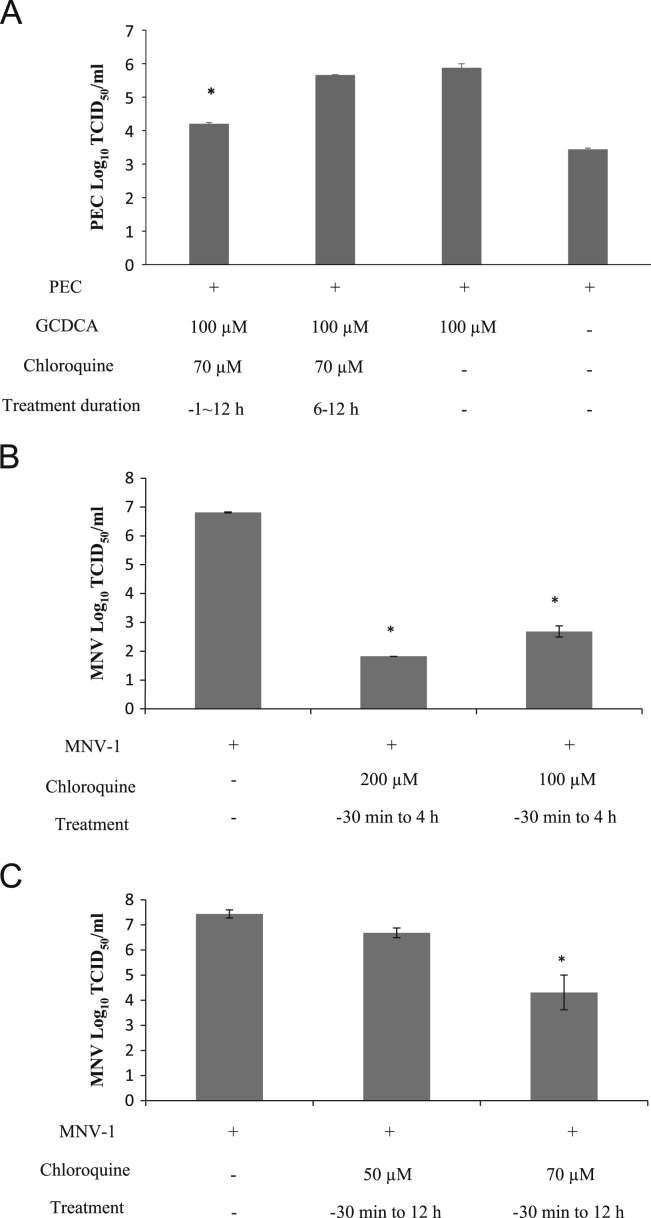
Since the maximal activity of cathepsin L requires acidic condition at around pH 5 (Turk et al., 1993, Vasiljeva et al., 2007), we studied the consequence of blocking endosomal acidification in calicivirus replication using chloroquine. Our findings indicate that the presence of chloroquine during −1 to 12 h post infection (PI) resulted in a marked reduction in PEC replication by 46.3-fold, determined by the the 50% tissue culture infectious dose (TCID50) assay ( Fig. 2A). However, no statistically significant inhibitory effect was observed when chloroquine was added during the later phase of virus replication cycle (at 6 h PI) (Fig. 2A). Similar inhibitory effect of chloroquine was observed for MNV-1: a 1334.9-fold reduction in viral load was observed when chloroquine 70 µM was present continuously in the media, but chloroquine 50 µM did not significantly reduce MNV-1 replication (Fig. 2C). When chloroquine was present in the media at high concentrations (100 or 200 µM) for a short time period, greater reduction of virus replication was observed (Fig. 2B and C). Fig. 1, Fig. 2 contain only the TCID50 data, but the results obtained by real time qRT-PCR (MNV or PEC genome copy numbers) were in line with those with MNV or PEC TCID50 assay.
Fig. 2.
Effects of endosomal acidification in the replication of PEC or MNV-1. (A) LLC-PK cells were pre-treated with mock (medium) or chloroquine (70 µM) for 1 h, and infected with PEC (MOI 50) in the presence of mock or cholorquine. In all cells, GCDCA (100 µM) was present in the media to support virus replication during virus infection. Following virus infection, cells were thoroughly washed with PBS and fresh medium containing mock or chloroquine was added to the cells. Cells were then further incubated and collected at 12 h PI. In a separate experiment, chloroquine was added to the PEC-infected cells at 6 h PI and cells were collected at 12 h PI for the determination of viral replication by the TCID50 assay. (B) RAW267.4 cells were pre-treated with mock (medium) or chloroquine (200 or 100 µM) for 30 min then infected with MNV-1 (MOI 10) in the presence of mock or chloroquine at 4 °C for 1 h. Cells were then washed with PBS and fresh medium containing mock or chloroquine was added to the cells for further incubation at 37 °C. At 4 h PI, cells were thoroughly washed again with PBS and fresh medium without chloroquine was added to the cells. Cells were collected at 12 h PI for the determination of virus replication by the TCID50 assay. (C) Cells were pretreated with chloroquine (50 or 70 µM) for 30 min , and infected with MNV-1 (10 MOI) in the presence of mock or chloroquine at 4 °C for 1 h. Cells were then washed with PBS and fresh medium containing chloroquine was added to the cells. Cells were further incubated and collected at 12 h PI for determination of virus replication by the TCID50 assay. (A–C) Asterisk indicates a significant reduction of viral titers in the treatment group compared to the control (P<0.05).
Cathepsin L, but not cathepsin B, cleaves calicivirus structural (major or minor) protein
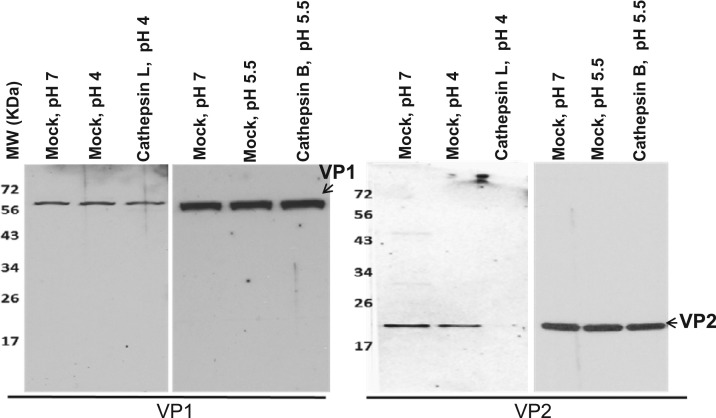
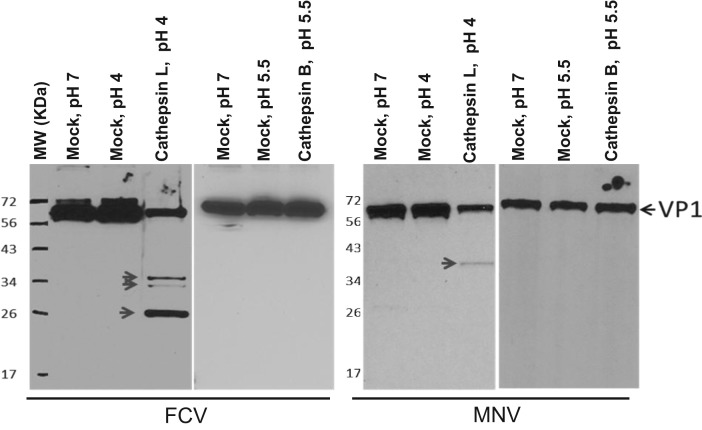
To study if cathepsin L and cathepsin B processes calicivirus capsid proteins VP1 and VP2, concentrated PEC, MNV-1 and FCV were incubated with recombinant cathepsin L or cathepsin B for 2 h at 37 °C. In Western blots, PEC VP1 and VP2 proteins were detected as a single band of approximately 58 kDa and 18 kDa in mock-incubated samples ( Fig. 3, Fig. 4). The Western blot analysis on the samples incubated with cathepsin L or B showed that neither enzyme cleaved PEC VP1 (Fig. 3). However, a band that corresponds to a full length PEC VP2 (18 kDa) disappeared only in the samples incubated with cathepsin L, suggesting cleavage by the protease (Fig. 3). For MNV-1 and FCV, incubation of virus and cathepsin L produced multiple bands in the Western blot analysis: antibodies against FCV VP1 detected multiple fragments of approximately 59 kDa (corresponding to a full length VP1), 36 kDa, 34 kDa and 23 kDa (Fig. 4), and antibodies against MNV-1 VP1 detected two bands of approximately 59 kDa (corresponding to a full length VP1) and 40 kDa (Fig. 4). However, cathepsin B did not cleave FCV and MNV-1 VP1 (Fig. 4). Cleavage of virus proteins was prevented by the presence of cathepsin L inhibitors (Z-FY-CHO or MDL 28170) in the cathepsin L cleavage assay (data not shown). FCV and MNV-1 VP2 cleavage by cathepsin L or B was not studied due to unavailability of antibodies against VP2 of FCV or MNV-1.
Fig. 3.
The effects of cathepsins on the cleavage of PEC VP1 and VP2. Concentrated PEC was incubated with a recombinant cathepsin L or cathepsin B in a cathepsin reaction buffer at pH 4.0 or pH 5.5, respectively, for 2 h at 37 °C. Mock-treatment samples were incubated in PBS at pH 4, 5.5 or 7.0. The samples were then subjected to SDS-PAGE, transferred to nitrocellulose membrane, probed with antibodies against PEC VP1 or VP2 and visualized by chemiluminescence. Uncleaved or cleaved VP1 and VP2 are indicated by arrows.
Fig. 4.
The effects of cathepsins on the cleavage of VP1 of FCV or MNV-1. Concentrated FCV (left panel) or MNV-1 (right panel) was incubated with a recombinant cathepsin L or cathepsin B in a cathepsin reaction buffer at pH 4.0 or pH 5.5, respectively, for 2 h at 37 °C. Mock-treatment samples were incubated in PBS at pH 4, 5.5 or 7.0. The samples were then subjected to SDS-PAGE, transferred to nitrocellulose membrane, probed with antibodies against FCV or MNV-1 VP1 visualized by chemiluminescence. Uncleaved or cleaved VP1 is indicated by an arrow.
PEC or MNV-1 accumulated in the endosomes in the presence of a cathepsin L inhibitor or chloroquine
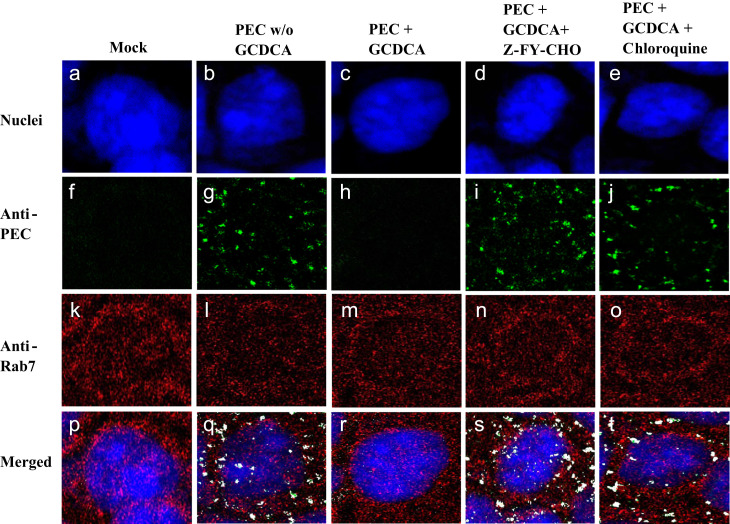
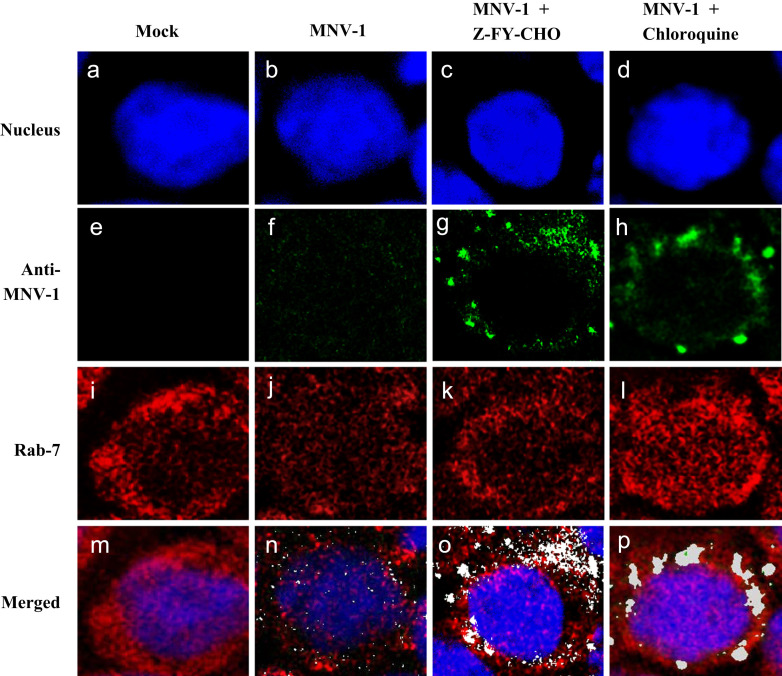
PEC replication in cell culture is dependent on the presence of bile acids in the media (Chang et al., 2005, Chang et al., 2004). However, the mechanism of bile acid-supported PEC replication has not been understood. In our recent report, we demonstrated that the absence of bile acids (GCDCA) in cell culture or inhibition of endosomal acidification by chloroquine led to accumulation of PEC in the endosomes, while PEC disappeared from the endosomes within 1 h of virus infection in bile acid-supplemented cell culture (Shivanna et al., 2014). These results suggest that PEC failed to escape from the endosomes in the absence of bile acids or when endosomal maturation is inhibited. In this study, we observed the similar phenomenon with cathepsin L inhibitor treatment of the cells, indicated by colocalization of fluorescently labeled antibodies against PEC with Rab7, (Fig. 5). Rab7 is a GTPase of a low molecular weight and mainly localized in the late endosomes/lysosomes in mammalian cells, although it is also associated with the early endosomes (Chavrier et al., 1990, Soldati et al., 1995). The confocal images of PEC in cell culture in the absence or presence of bile acids and chloroquine were included as a reference in Fig. 5 (Shivanna et al., 2014). The cells infected with PEC in the presence of GCDCA showed no or little fluorescence staining of PEC VP1 at 1 h PI, while relatively abundant fluorescence for PEC VP1 was detected in the cells infected by PEC without GCDCA and in the cells treated with chloroquine or a cathepsin L inhibitor Z-FY-CHO (Fig. 5). Confocal microscopic analysis using MNV-1 also revealed similar results to those shown with PEC infection. In RAW267.4 cells, no or little MNV-1 were stained in the cytoplasm at 1 h PI ( Fig. 6). However, in the presence of Z-FY-CHO or chloroquine, MNV-1 VP1 was detected at high abundance in the endosomes at 1 h PI (Fig. 6), a similar observation made in PEC-infected cells (Fig. 5). These results suggest that MNV-1, as well as PEC, failed to escape from the endosomes in the presence of a cathepsin L inhibitor or chloroquine. For quantitative statistical colocalization analysis, single channel images were auto thresholded by Costes׳ auto threshold method and Mander׳s split correlation coefficients and Pearson׳s correlation coefficients for the endosomal marker Rab7 and MNV-1 or PEC were calculated to quantify the degree of colocalization of viral proteins with Rab7 for all fluorescence confocal microscopy images. The calculated Mander׳s split correlation coefficients and Pearson׳s correlation coefficients were higher than 0.90 for all analyzed images.
Fig. 5.
The effects of a cathepsin L inhibitor or chloroquine in PEC entry into the cells. Confluent LLC-PK cells were pre-treated with mock (medium), Z-FY-CHO or chloroquine for 30 min before virus inoculation. Cells were then inoculated with PEC (MOI 50) in the presence of mock, Z-FY-CHO or chloroquine. GCDCA (100 µM) was present in cell culture during virus infection to support PEC replication, with the exception of mock-infected cells and PEC-infected negative control cells (PEC w/o GCDCA) . Following virus infection for 1 h, cells were fixed and probed with rabbit polyclonal anti-Rab7 or swine anti-PEC polyclonal antibodies, followed by secondary antibodies of PerCP-Cy5.5 labeled secondary antibody against Rab7 (red, k–o) or FITC-labeled secondary antibody against PEC (green, f–j). Nuclei were stained with sytox orange (pseudo colored blue, a–e). Confocal images on the prepared samples were obtained and colocalization analysis of PEC and Rab7 was performed using ImageJ software. In the merged images (p–t), colocalization of PEC (green) and Rab7 (red) appears in white color.
Fig. 6.
The effects of cathepsin L inhibitor or chloroquine in MNV-1 entry into the cells. RAW267.4 cells were pre-treated with mock (medium), Z-FY-CHO or chloroquine for 30 min before MNV-1 inoculation (MOI 10). Following virus infection at 37 °C for 1 h, cells were fixed and probed with rabbit polyclonal anti-Rab7 or guinea pig polyclonal antibody against MNV-1, followed by PerCP-Cy5.5 labeled secondary antibody against Rab7 (red, i–l) or FITC-labeled secondary antibody against MNV-1 (green, e–h). Nuclei were stained with sytox orange (pseudo colored blue, a–d). Confocal images on the prepared samples were obtained and colocalization analysis of Rab7 and MNV-1 was performed by ImageJ software. In the merged images (p–t), colocalization of MNV-1 (green) and Rab7 (red) appears in white color.
Discussion
Virus entry is the first step in virus replication cycle and is characterized by complex interactions of cellular and viral factors that subsequently lead to virus uncoating and release of viral genome to initiate virus replication (Grove and Marsh, 2011, Marsh and Helenius, 2006). In the limited number of studies available on the entry process of caliciviruses, FCV entry has been relatively well known, but the studies on the entry of other caliciviruses such as human norovirus, PEC or MNV-1 have been limited (Kreutz and Seal, 1995, Perry et al., 2009, Perry and Wobus, 2010, Shivanna et al., 2014, Stuart and Brown, 2006). In this study, we demonstrated that cathepsin L plays an important role in the replication of PEC, FCV and MNV-1 by showing that inhibition of cathepsin L, but not cathepsin B, led to a significant reduction in the replication of PEC, FCV and MNV-1 in cell culture. It has been shown that cathepsin enzymes are involved in entry and replication of some enveloped and non-enveloped viruses, including SARS coronavirus, murine hepatitis virus, reovirus and Ebola virus (Bosch et al., 2008, Brecher et al., 2012, Mainou and Dermody, 2012, Qiu et al., 2006, Schornberg et al., 2006). However, the function of cathepsin in calicivirus entry has not been previously reported. Once we found that inhibition of cathepsin L function significantly reduce the replication of the tested caliciviruses, we determined if endosomal acidification is important in the replication of PEC and MNV-1, since cathepsin L requires acidic pH for optimal activity, which may reflect their primary location in the late endosomes/lysosomes (Honey and Rudensky, 2003, Turk et al., 2000). For FCV, it was previously reported that maturation of the endosomes is important in FCV replication (Kreutz and Seal, 1995, Stuart and Brown, 2006), and we confirmed that FCV entry is dependent on acidic pH (data not shown). PEC entry has not been previously studied for chloroquine susceptibility and this is the first report that PEC entry requires acidic pH. Of note, MNV-1 entry was previously reported to be pH-independent by other researchers (Gerondopoulos et al., 2010, Perry et al., 2009). Our finding of the dependency of MNV-1 replication on acidification of the endosomes is contrast to previous reports, and the reason for this discrepancy between our finding and others is not clear, although thoroughly removing residual viruses after virus infection was important for observing the inhibitory effects of chloroquine.
Since it is a new finding that inhibition of cathepsin L function during the early phase of virus replication causes a significant reduction of calicivirus replication in cell culture, we studied if cathepsin L is capable of cleaving the structural proteins of PEC, FCV and MNV-1. Based on Western blot analysis, cathepsin L cleaved VP2 of PEC and VP1 of FCV and MNV-1 (Fig. 3, Fig. 4). Although we do not know the significance of FCV and MNV-1 VP1 cleavage by cathepsin L at the present moment, we may speculate that VP1 cleavage by cellular enzymes such as cathepsin L contributes to virus capsid uncoating process to allow release of viral genome for virus replication. Our finding of cleavage of VP2 of PEC by cathepsin L was an unexpected finding. The role of the minor capsid protein VP2 is not clearly understood so far, however, VP2 is implicated in the assembly of virus particle by correctly forming capsid and/or increasing the stability of VP1 of FCV and human noroviruses (Bertolotti-Ciarlet et al., 2003, Di Martino and Marsilio, 2010). Therefore, it prompts us to speculate that VP2 cleavage by cathepsin L may contribute to destabilization of capsid. Although the uncoating process of non-enveloped viruses is poorly understood, reovirus, non-enveloped virus in the Reoviridae family, has been studied well. Reovirus entry and productive replication is dependent on cathepsin L and B for cleavage of outer capsid proteins during entry, which leads to virus disassembly and productive replication (Ebert et al., 2002, Mainou and Dermody, 2012). We were not able to study the effects of cathepsin enzymes on VP2 of FCV and MNV-1 for the lack of specific antibodies, thus it remains to be determined if FCV and MNV-1 VP2 is also processed by cathepsin L or other cellular enzymes. It also needs to be determined if PEC VP1 is processed by other cellular enzymes.
Since cathepsin L primarily resides in the endosomes, we then next studied the effects of cathepsin L inhibition on virus entry using confocal microscopy. PEC replication is known to be dependent on the presence of bile acids in cell culture, although the role of bile acid in PEC replication has not been clear (Chang et al., 2005, Chang et al., 2004). In our recent report, we demonstrated that bile acids are required for PEC escape from the endosomes, and endosomal acidification/maturation is also essential for the function of bile acid in the endosomes and/or is independently contribute to virus endosomal escape (Shivanna et al., 2014). In this study, we observed similar phenomenon of virus entrapment of MNV-1 and PEC on confocal microscopy: inhibition of cathepsin L or endosomal acidification led to accumulation of MNV-1 or PEC in the endosomes, preventing virus endosomal escape to initiate virus replication (Fig. 5, Fig. 6). These results demonstrate that cathepsin L activity in the low pH environment is important for calicivirus entry and subsequent replication, and are consistent with the findings of a significant reduction of virus titers by treatment of cells with cathepsin L inhibitors or chloroquine in the cell culture.
Here we report a new finding that cathepsin L activity is important in virus replication and endosomal escape of caliciviruses, including FCV, MNV-1, and PEC, based on the cathepsin inhibitor study, capsid cleavage assay, and confocal microscopy study. These results allow us to speculate that the entry pathways of caliciviruses may share a common step of interactions of virus and cathepsin for virus uncoating and/or viral escape from the endosomes, and may also shed an important insight to the entry of other caliciviruses, including human noroviruses which are major public health concerns.
Materials and methods
Cell culture and viruses
Cell culture adapted PEC Cowden strain was propagated in pig kidney epithelial cells (LLC-PK) in the presence of GCDCA ( 100 µM) in Eagle׳s Minimal Essential Medium (MEM) supplemented with 5% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin. FCV Urbana strain was propagated in Crandell Rees feline kidney (CRFK) cells in MEM containing 5% FBS, penicillin (100 U/ml) and streptomycin (100 µg/ml). MNV-1 was propagated in RAW 264.7 cells (Wobus et al., 2004) with Dulbecco׳s modified Eagle׳s medium (DMEM) supplemented with 5% FBS, penicillin (100 U/ml) and streptomycin (100 µg/ml). Each virus was concentrated prior to infection by centrifuging viruses at 27,000 RPM through a 40% w/v sucrose cushion at 4 °C for 2 h. The pellet was reconstituted in serum free MEM and stored at −80 °C. Feline coronavirus WSU-1146 strain, which is reported to be susceptible to a cathepsin B inhibitor, was obtained from ATCC.
Reagents and antibodies
GCDCA and chloroquine were purchased from Sigma-Aldrich (St Louis, MO). Cathepsin L inhibitors, Z-FY-CHO and MDL 28170, were purchased from Santa Cruz Biotech (Santa Cruz, CA), and a cathepsin B inhibitor CA074-Me was purchased from Sigma-Aldrich. Recombinant human cathepsin L and cathepsin B were purchased from R & D systems (Minneapolis, MN). The primary antibodies used in this study were anti-PEC/Cowden antibodies raised in swine (Chang et al., 2005), anti-PEC VP2 antibodies raised in guinea pig (Chang et al., 2005), anti-FCV antibodies raised in guinea pig (Sosnovtsev and Green, 1995), anti-MNV-1 antibodies raised in guinea pig (Wobus et al., 2004) and anti-Rab7 antibodies (Santa Cruz Biotech, CA). The secondary antibodies for Western blot analysis included horseradish peroxidase-conjugates of anti-swine Ig and anti-guinea pig Ig antibody (Thermo Scientific, Pittsburgh, PA). The secondary antibodies for confocal microscopy were FITC-labeled anti-swine IgG for PEC (Kirkegaard & Perry Lab Inc, MD), FITC-labeled anti-guinea pig IgG for MNV-1 (Sigma-Aldrich, MO) and anti-rabbit PerCP-Cy5.5 (Santa Cruz Biotech, CA). Other basic chemicals for confocal microscopy and other studies were purchased from various sources including Sigma-Aldrich.
Effects of inhibition of cathepsin B or L activities in calicivirus replication
Confluent LLC-PK cells cultured in 12 well plates were pre-treated with inhibitors of cathepsin L (Z-FY-CHO 120 µM; MDL 28170, 100 µM) or cathepsin B (CA074-Me, 120 µM) for 1 h. Cells were then infected with PEC Cowden strain at a multiplicity of infection (MOI) of 50 in the presence of 100 µM GCDCA and a cathepsin inhibitor L or B at 37 °C. After 1 h, cell monolayer was washed twice with PBS and the medium was replaced with fresh medium containing a cathepsin L or B inhibitor at the same concentration. The cells were further incubated at 37 °C. At 12 h PI, cells were subjected to repeated freezing and thawing for virus titration using the TCID50 assay (Reed and Muench, 1938), or total RNA was extracted from the cells for real time qRT-PCR as described previously (Shivanna et al., 2014). The primers and probe sets used for real time qRT-PCR are listed in Table 1. Viral genome copy number was calculated from an external standard curve that was generated by plotting the Ct values vs. ten-fold dilutions of a known concentration of PEC RNA (Chang et al., 2005).
Table 1.
The primers and probes used for real time qRT-PCR for PEC and MNV-1.
| Name | Sequences (5׳→3׳) |
|---|---|
| PEC qRT-PCRa | F: ATTCCAGAGTTGACCCACAG |
| R: CTACTGGGTTGATGGCGAC | |
| P: 6-FAM/TGGTATAGATTAAGAGGCACAGCGGC/IABkFQ | |
| MNV-1 qRT-PCR | F: CATAGATGCCCCTGGAGTAAAG |
| R: CTCTCCAACATCTTCGCTCTG | |
| P: 6-FAM/CATGATGACCACCTGCTCCACCTT/IABkFQ |
F: forward primer; R: reverse primer; P: probe.
The effect of each inhibitor on the replication of FCV and MNV-1 was also studied following a similar experimental method. CRFK and RAW267.4 cells were preincubated with Z-FY-CHO (40 µM), MDL 28170 (80 µM), or CA074-Me (40 µM) for 1 h prior to inoculation of FCV (MOI 50) or MNV-1 (MOI 10). Following virus infection for 1 h, cells were washed with PBS and fresh medium containing an inhibitor was added to the cells. In a separate experiment, cells were preincubated with cyclohexamide (100 µg/ml) for 1 h prior to MNV-1 infection to monitor the levels of internalized virus without further virus replication. At 12 h PI, virus replication was monitored by the TCID50 assay (MNV-1 and FCV) or real time qRT-PCR (MNV-1). The inhibitors at the concentrations used in this study showed minimal cytotoxicity at 12 h PI in LLC-PK, CRFK and RAW267.4 cells. Because cathepsin B inhibitor CA074-Me blocks the replication of feline coronavirus (Kim et al., 2013, Regan et al., 2008), feline coronavirus 1146 strain was included in this study as a control and virus replication was monitored by the TCID50 assay (Kim et al., 2013).
Effects of inhibition of endosomal acidification in calicivirus replication
Confluent LLC-PK cells cultured in 12 well plates were pre-treated with mock (medium) or an inhibitor of endosomal acidification (chloroquine, 70 µM) for 1 h. Cells were then infected with PEC at an MOI of 50 in the presence of chloroquine or mock at 37 °C. Since PEC does not replicate without bile acid, GCDCA (100 µM, final concentration) was added to the media during virus infection. Following virus infection for 1 h, cell monolayer was thoroughly washed three times with PBS and the medium was replaced with fresh medium containing the same concentration of chloroquine or mock. In other experiments, chloroquine was added at 6 h PI to determine its effect at later stages of virus replication. Chloroquine at this concentration (70 µM) was minimally cytotoxic in LLC-PK cells. At 12 PI, cells were subjected to repeated freezing and thawing for virus titration using the TCID50 assay, or total RNA was extracted for real time qRT-PCR.
The effect of chloroquine on the replication of MNV-1 was also studied following the similar experimental design using RAW267.4 cells. Cells were pre-treated with chloroquine at 50 or 70 µM at 37 °C for 30 min, and infected with MNV-1 at an MOI of 10 in the presence of chloroquine or mock (medium) at 4 °C. Following 1 h incubation, cells were thoroughly washed three times with PBS, and the same concentration of chloroquine or mock was added into the media. In another set of experiments, high concentrations of chloroquine (100 or 200 µM) were added to the media for 30 min prior to MNV-1 infection at an MOI of 10, and the media was removed at 4 h PI by thoroughly washing the cells with PBS to minimize cytotoxicity (Perry et al., 2009). In all experiments, cells were subjected to repeated freezing and thawing at 12 h PI for virus titration using the TCID50 assay and real time qRT-PCR.
In-vitro cleavage of viral proteins by cathepsin
Concentrated PEC, FCV or MNV-1 was incubated with or without recombinant human cathepsin L (0.64 µg) or cathepsin B (0.64 µg) in reaction buffer containing 100 mM Na acetate, 1 mM EDTA, and 4 mM DTT at pH 4.0 (for cathepsin L) or pH 5.5 (for cathepsin B), or concentrated viruses were incubated in PBS (pH 7.0) for 2 h at 37 °C. To assess the cathepsin L inhibitors in in-vitro cathepsin L cleavage of PEC, FCV or MNV-1 capsid proteins, Z-FY-CHO or MDL 28170 was added to the cathespin L cleavage assay at 200 µM (final concentration). The samples were then mixed with 1× LDS sample buffer (Invitrogen) containing 2-mercaptoethanol, and heated to 95 °C for 5 min. Following heat treatment, the samples were subjected to SDS-PAGE (12% Tris-glycine) and proteins were transferred to nitrocellulose membranes. Membranes were probed with primary antibodies followed by horseradish peroxidase-conjugated species-specific secondary antibodies. Proteins were visualized by chemiluminescence according to the manufacturer׳s instructions (Thermo Scientific).
Confocal laser scanning microscopy
To study the effects of a cathepsin L inhibitor (Z-FY-CHO) or chloroquine during the entry of PEC or MNV-1, virus trafficking was monitored using confocal microscopy (Shivanna et al., 2014). LLC-PK or RAW267.4 cells were seeded onto a Lab-Tek II CC2 chamber slide (Fisher Scientific) treated with FBS and grown to 90% confluency. Confluent cells on the chamber slides were treated with mock (medium), chloroquine (100 µM) or Z-FY-CHO (100 µM) for 30 min. PEC or MNV-1 was then inoculated to the cells at an MOI of 50 or 10, respectively, and the cells were further incubated at 37 °C for 1 h. In LLC-PK cells, GCDCA (100 µM) was added to the media at the time of virus inoculation. At 1 h PI, cells were fixed in 4% paraformaldehyde (Sigma-Aldrich) in PBS (pH 7.4) for 15 min at room temperature (RT), permeabilized with 0.1% Triton-100× in PBS for 10 min at RT, washed three times with PBS, and incubated in blocking buffer [PBS containing 0.5% bovine serum albumin] for 15 min. The cells were then incubated with primary antibodies specific to PEC (1:200), MNV-1 (1:200) or Rab7 (1:200) at 37 °C to probe PEC, MNV-1 and the endosomes, respectively. After 2 h incubation, cells were washed three times with PBS and further incubated at 37 °C for 2 h with FITC-labeled anti-swine IgG or anti-guinea pig IgG against PEC or MNV-1, respectively. For Rab7, PerCP-Cy5.5 labeled anti-rabbit IgG was used as a secondary antibody. The same cells were also stained with sytox orange (Life technologies) DNA stain (0.5 µM in 0.9% NaCl). Coverslips were mounted in Prolong Gold antifade reagent (Molecular Probes), and the cells were scanned with a confocal microscope LSM 510 (Zeiss, Oberkochen, Germany) using a 100× oil-immersion objective. The images were analyzed by ImageJ software 1.47 (http://imagej.nih.gov/ij/), and colocalization analysis was performed using JACoP and colocalization-MBF plugins for ImageJ software. Single channel images were thresholded by Costes׳ auto threshold method and the Mander׳s split correlation coefficient and Pearson׳s correlation coefficient for colocalization was then determined for each image.
Statistical analysis
The effects of cathepsin or endosomal acidification inhibitors in virus replication were compared to the mock treatment using a two-tailed Student׳s t-test. P value<0.05 was considered statistically significant. Data were from at least three independent experiments.
Acknowledgments
We would like to thank David George and Dr. Daniel L. Boyle for technical assistance. This work was supported by the NIH Grant U01 AI081891 and the Morris Animal Foundation D14FE-012.
Contributor Information
Yunjeong Kim, Email: ykim@vet.ksu.edu.
Kyeong-Ok Chang, Email: kchang@vet.ksu.edu.
References
- Bertolotti-Ciarlet A., Crawford S.E., Hutson A.M., Estes M.K. The 3׳ end of Norwalk virus mRNA contains determinants that regulate the expression and stability of the viral capsid protein VP1: a novel function for the VP2 protein. J. Virol. 2003;77:11603–11615. doi: 10.1128/JVI.77.21.11603-11615.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti-Ciarlet A., White L.J., Chen R., Prasad B.V., Estes M.K. Structural requirements for the assembly of Norwalk virus-like particles. J. Virol. 2002;76:4044–4055. doi: 10.1128/JVI.76.8.4044-4055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhella D., Gatherer D., Chaudhry Y., Pink R., Goodfellow I.G. Structural insights into calicivirus attachment and uncoating. J. Virol. 2008;82:8051–8058. doi: 10.1128/JVI.00550-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhella D., Goodfellow I.G. The cryo-electron microscopy structure of feline calicivirus bound to junctional adhesion molecule A at 9-angstrom resolution reveals receptor-induced flexibility and two distinct conformational changes in the capsid protein VP1. J. Virol. 2011;85:11381–11390. doi: 10.1128/JVI.05621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., Bartelink W., Rottier P.J. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 2008;82:8887–8890. doi: 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecher M., Schornberg K.L., Delos S.E., Fusco M.L., Saphire E.O., White J.M. Cathepsin cleavage potentiates the Ebola virus glycoprotein to undergo a subsequent fusion-relevant conformational change. J. Virol. 2012;86:364–372. doi: 10.1128/JVI.05708-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.O., Sosnovtsev S.S., Belliot G., Wang Q., Saif L.J., Green K.Y. Reverse genetics system for porcine enteric calicivirus, a prototype sapovirus in the Caliciviridae. J. Virol. 2005;79:1409–1416. doi: 10.1128/JVI.79.3.1409-1416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.O., Sosnovtsev S.V., Belliot G., Kim Y., Saif L.J., Green K.Y. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc. Natl. Acad. Sci. USA. 2004;101:8733–8738. doi: 10.1073/pnas.0401126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Parton R.G., Hauri H.P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chen R., Neill J.D., Estes M.K., Prasad B.V. X-ray structure of a native calicivirus: structural insights into antigenic diversity and host specificity. Proc. Natl. Acad. Sci. USA. 2006;103:8048–8053. doi: 10.1073/pnas.0600421103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B., Marsilio F. Feline calicivirus VP2 is involved in the self-assembly of the capsid protein into virus-like particles. Res. Vet. Sci. 2010;89:279–281. doi: 10.1016/j.rvsc.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Ebert D.H., Deussing J., Peters C., Dermody T.S. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 2002;277:24609–24617. doi: 10.1074/jbc.M201107200. [DOI] [PubMed] [Google Scholar]
- Gerondopoulos A., Jackson T., Monaghan P., Doyle N., Roberts L.O. Murine norovirus-1 cell entry is mediated through a non-clathrin-, non-caveolae-, dynamin- and cholesterol-dependent pathway. J. Gen. Virol. 2010;91:1428–1438. doi: 10.1099/vir.0.016717-0. [DOI] [PubMed] [Google Scholar]
- Green K.Y. fifth ed. Lippincott Williams & Wilkins; Philadelphia: 2007. Caliciviruses: The Noroviruses. [Google Scholar]
- Green K.Y., Chanock R.M., Kapikian A.Z. Lippincott, Williams & Wilkins; Philadelphia: 2001. Fields Virology. [Google Scholar]
- Grove J., Marsh M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011;195:1071–1082. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey K., Rudensky A.Y. Lysosomal cysteine proteases regulate antigen presentation. Nat. Rev. Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- Kim Y., Mandadapu S.R., Groutas W.C., Chang K.O. Potent inhibition of feline coronaviruses with peptidyl compounds targeting coronavirus 3C-like protease. Antivir. Res. 2013;97:161–168. doi: 10.1016/j.antiviral.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutz L.C., Seal B.S. The pathway of feline calicivirus entry. Virus Res. 1995;35:63–70. doi: 10.1016/0168-1702(94)00077-p. [DOI] [PubMed] [Google Scholar]
- Luttermann C., Meyers G. Feline calicivirus. In: Hansman G.S., Jiang X., Green K.Y., editors. Caliciviruses. Molecular and Cellular Virology Caister Academic Press; Norfolk, U.K.: 2010. pp. 145–168. [Google Scholar]
- Mainou B.A., Dermody T.S. In search of cathepsins: how reovirus enters host cells. DNA Cell Biol. 2012;31:1646–1649. doi: 10.1089/dna.2012.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J., Schelhaas M., Helenius A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- Naslavsky N., Weigert R., Donaldson J.G. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol. Biol. Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K.S., Parra F. Calicivirus protein structures. In: Hansman G.S., Jiang X., Green K.Y., editors. Caliciviruses: Molecular and Cellular Virology. Caister Academic Press; Norfolk, U.K.: 2010. pp. 95–110. [Google Scholar]
- Perry J.W., Taube S., Wobus C.E. Murine norovirus-1 entry into permissive macrophages and dendritic cells is pH-independent. Virus Res. 2009;143:125–129. doi: 10.1016/j.virusres.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J.W., Wobus C.E. Endocytosis of murine norovirus 1 into murine macrophages is dependent on dynamin II and cholesterol. J. Virol. 2010;84:6163–6176. doi: 10.1128/JVI.00331-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B.V., Hardy M.E., Dokland T., Bella J., Rossmann M.G., Estes M.K. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- Qiu Z., Hingley S.T., Simmons G., Yu C., Das Sarma J., Bates P., Weiss S.R. Endosomal proteolysis by cathepsins is necessary for murine coronavirus mouse hepatitis virus type 2 spike-mediated entry. J. Virol. 2006;80:5768–5776. doi: 10.1128/JVI.00442-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Regan A.D., Shraybman R., Cohen R.D., Whittaker G.R. Differential role for low pH and cathepsin-mediated cleavage of the viral spike protein during entry of serotype II feline coronaviruses. Vet. Microbiol. 2008;132:235–248. doi: 10.1016/j.vetmic.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornberg K., Matsuyama S., Kabsch K., Delos S., Bouton A., White J. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J. Virol. 2006;80:4174–4178. doi: 10.1128/JVI.80.8.4174-4178.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D.K., Choudhury A., Singh R.D., Wheatley C.L., Marks D.L., Pagano R.E. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 2003;278:7564–7572. doi: 10.1074/jbc.M210457200. [DOI] [PubMed] [Google Scholar]
- Shivanna V., Kim Y., Chang K.O. The crucial role of bile acids in the entry of porcine enteric calicivirus. Virology. 2014;456-457:268–278. doi: 10.1016/j.virol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati T., Rancano C., Geissler H., Pfeffer S.R. Rab7 and Rab9 are recruited onto late endosomes by biochemically distinguishable processes. J. Biol. Chem. 1995;270:25541–25548. doi: 10.1074/jbc.270.43.25541. [DOI] [PubMed] [Google Scholar]
- Sosnovtsev S., Green K.Y. RNA transcripts derived from a cloned full-length copy of the feline calicivirus genome do not require VpG for infectivity. Virology. 1995;210:383–390. doi: 10.1006/viro.1995.1354. [DOI] [PubMed] [Google Scholar]
- Sosnovtsev S.V., Belliot G., Chang K.O., Onwudiwe O., Green K.Y. Feline calicivirus VP2 is essential for the production of infectious virions. J. Virol. 2005;79:4012–4024. doi: 10.1128/JVI.79.7.4012-4024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart A.D., Brown T.D.K. Entry of feline calicivirus is dependent on clathrin-mediated endocytosis and acidification in endosomes. J. Virol. 2006;80:7500–7509. doi: 10.1128/JVI.02452-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk B., Dolenc I., Turk V., Bieth J.G. Kinetics of the pH-induced inactivation of human cathepsin L. Biochemistry. 1993;32:375–380. doi: 10.1021/bi00052a046. [DOI] [PubMed] [Google Scholar]
- Turk B., Turk D., Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim. Biophys. Acta. 2000;1477:98–111. doi: 10.1016/s0167-4838(99)00263-0. [DOI] [PubMed] [Google Scholar]
- Vasiljeva O., Reinheckel T., Peters C., Turk D., Turk V., Turk B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr. Pharm. Des. 2007;13:387–403. doi: 10.2174/138161207780162962. [DOI] [PubMed] [Google Scholar]
- Wobus C.E., Karst S.M., Thackray L.B., Chang K.O., Sosnovtsev S.V., Belliot G., Krug A., Mackenzie J.M., Green K.Y., Virgin H.W. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004;2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]