Abstract
Unlike most pathogens many of the immunodominant epitopes from Mycobacterium tuberculosis (Mtb) are under purifying selection. This startling finding suggests that Mtb may gain an evolutionary advantage by focusing the human immune response against selected proteins. Although the implications of this to vaccine development are incompletely understood, it has been suggested that inducing strong TH1 responses against antigens that are only weakly recognized during natural infection may circumvent this evasion strategy and increase vaccine efficacy. To test the hypothesis that subdominant and/or weak Mtb antigens are viable vaccine candidates and to avoid complications due to differential immunodominance hierarchies in humans and experimental animals we defined the immunodominance hierarchy of 84 recombinant Mtb proteins in experimentally infected mice. We then combined a subset of these dominant or subdominant antigens with a TH1 augmenting adjuvant, GLA-SE to assess their immunogenicity in Mtb-naïve animals and protective efficacy as measured by a reduction in lung Mtb burden of infected animals following prophylactic vaccination. We observed little correlation between immunodominance during primary Mtb infection and vaccine efficacy, confirming the hypothesis that subdominant and weakly antigenic Mtb proteins are viable vaccine candidates. Finally we developed two fusion proteins based on strongly protective subdominant fusion proteins. When paired with the GLA-SE adjuvant these fusion proteins elicited robust TH1 responses and limited pulmonary Mtb for at least six weeks after infection with a single immunization. These finding expand the potential pool of Mtb proteins that can be considered as vaccine antigen candidates.
Keywords: Tuberculosis, Vaccination, Antigen, Subdominant, TH1 Cells
Introduction
Mycobacterium tuberculosis (Mtb) the causative agent of tuberculosis (TB) is responsible for approximately 9 million new cases of active TB and 1-1. 5 million deaths annually (1). It is estimated that nearly 2 billion people are latently infected with Mtb worldwide, creating a large reservoir of carriers from which new cases of active TB disease may arise. The only approved vaccine for Mtb, Bacillus Calmette–Guérin (BCG) developed nearly a century ago, is routinely given shortly after birth. However the efficacy of BCG wanes in adolescents and the vaccine does not consistently prevent the development of active pulmonary TB in adults (efficacy estimated between 0 and 80% with the lowest efficacy rates often found in countries with the highest burden of TB) (2-4). Thus, there is an urgent need for a new TB vaccine to either boost immunity primed by BCG or replace BCG (5, 6).
Development of an effective vaccine against TB requires optimization of target antigens capable of inducing immunity against a broad range of Mtb isolates, delivered in a manner capable of inducing durable and protective immune responses (5). Most Mtb-infected individuals develop long-lived immunity, which can control and contain the bacilli in a T cell-dependent manner, with only 5-10% of latently infected individuals developing the disease over the course of their lifetime. CD4 T cells producing IFN-γ and TNF are essential for immunity to Mtb. Some studies have found that the frequency of TB-specific multifunctional CD4 T helper 1 (TH1) cells, i.e. cells that make a combination of IFN-γ, TNF, and/or IL-2 upon stimulation, correlates with vaccine efficacy, although this is not always the case. Under some conditions CD8 T cells also contribute to Mtb control, although not to the same degree as CD4 T cells (7, 8).
The selection of optimal antigens for inclusion into a subunit vaccine continues to be an important research question. Much of the effort over the past 25 years has focused on T cell responses to immunodominant antigens of Mtb. Several of these defined antigens delivered as plasmid DNA, vectored DNA, or as recombinant protein in adjuvant, have proven to be effective in animal models (9-19). Subunit TB vaccines are currently under development by multiple groups including our own, and several have entered clinical trials. However, the selection of Mtb vaccine candidates has primarily focused on a small subset of immunodominant proteins, which may have limited the pipeline needed for development of a successful vaccine. Recent studies have found that T cell epitopes of known immunodominant antigens of Mtb are hyper-conserved, implying that immune responses against them may be in some cases more beneficial to the bacilli than to the host (20, 21). Additionally T cell responses to TB antigens have been shown to be significantly higher in active TB than latent Mtb infection suggesting that increased immunity may promote lung pathology and subsequently transmission (22-24). These observations have led to the hypothesis that Mtb has evolved to subvert the immune response against it by focusing the response against antigens in ways that are beneficial for its survival (22, 25). In the present paper we test the hypothesis that antigens that are weakly antigenic during primary Mtb infection can be turned into protective vaccines when properly adjuvanted. If successful this approach may circumvent the benefits of the T cell response to Mtb by altering the antigenic focus.
Materials and Methods
Expression and purification of recombinant Mtb proteins
DNA encoding selected Mtb genes were PCR amplified from HRv37 genomic DNA using Pfx DNA polymerase (Invitrogen). PCR primers were designed to incorporate specific restriction enzyme sites 5′ and 3′ of thegene of interest for directional cloning into the expression vector pET28a (Novagen). Purified PCR products were digested with restriction enzymes, ligated into pET28a using T4 DNA ligase (NEB), and transformed into XL10G cells (Stratagene). Recombinant pET28a plasmid DNA was recovered from individual colonies and sequenced to confirm the correctly cloned coding sequence. The recombinant clones contained an N-terminal six-histidine tag followed by a thrombin cleavage site and the Mtb gene of interest.
Recombinant plasmids were transformed into the Escherichia coli BL21 derivative Rosetta2 (DE3) pLysS (Novagen). Recombinant strains were cultured overnight at 37°C in 2× yeast tryptone containing appropriate antibiotics, diluted 1/25 into fresh culture medium, grown to mid-log phase (OD at 600 nm of 0.5– 0.7), and induced by the addition of 1 mM isopropyl β-D-thiogalactoside. Cultures were grown for an additional 3–4 h, cells were harvested by centrifugation, and bacterial pellets were stored at-20°C. Bacterial pellets were thawed and disrupted by sonication in 20 mM Tris (pH 8.0), 150 mM NaCl, and 1 mM PMSF, followed by centrifugation to fractionate the soluble and insoluble material. Recombinant His-tagged protein products were isolated under native (soluble recombinant proteins) or denaturing (8 M urea) conditions using Ni-nitrilotriacetic acid metal ion affinity chromatography according to the manufacturer's instructions (Qiagen). Further purification by ion exchange and/or size exclusion chromatography was used as necessary to obtain greater than 95% purity of Mtb proteins, as determined by visualization using SDS-PAGE. Purified protein fractions were combined and dialyzed against 20 mM Tris (pH 8.0), concentrated using Amicon Ultra 10-kDa-molecular mass cutoff centrifugal filters (Millipore), and quantified using a bicinchoninic acid protein assay (Pierce). LPS contamination was evaluated by the Limulus amoebocyte lysate assay (Cambrex). All the recombinant proteins used in this study showed residual endotoxin levels <100 EU/mg of protein.
The fusion proteins ID91 and ID97 were constructed in the same manner as we previously reported for ID93 (9). Briefly the genes for Rv1886, Rv3478, and Rv3619 were attached to the genes for either Rv2389 (ID91) or Rv2875 (ID97) using restriction site linkers and cloned into the pET28a vector. Fusion proteins were expressed in E. coli, purified under denaturing conditions by chromatography on DEAE and Q Sepharose columns, and analyzed by SDS-PAGE on a 4 – 20% Tris glycine gel (Invitrogen). The absence of E. coli contamination was confirmed by immunoblotting with horseradish peroxidase-conjugated rabbit polyclonal anti-E. coli antibody (1:1000, ViroStat, Inc.). Residual LPS contamination was determined to be less than 15 EU/mg of protein by the Limulus amoebocyte lysate assay (Cambrex Corp.).
Experimental animals and infection
6-8 week old female CB6F1 mice were purchased from The Jackson Laboratory and maintained in Specific Pathogen Free conditions. After infection animals were maintained in BL3 containment according to the regulations and guidelines of the IDRI Institutional Animal Care and Use Committee. For vaccine efficacy studies mice were immunized one, two, or three times three weeks apart by intramuscular injection. Each immunization contained 5 pmol of recombinant protein and 5 μg of GLA-SE.
Four weeks after the last immunization, mice (n = 7/group) were aerogenically infected with Mtb strain H37Rv (ATCC No. 35718; American Type Culture Collection) using a GlasCol aerosol generator calibrated to deliver 50–100 bacteria into the lungs. To confirm the amount of bacteria delivered an additional three unimmunized animals per infection were euthanized one day later and bacterial burden in the lungs was enumerated. Protection was determined three to six weeks after challenge by harvesting the lungs and spleens from the infected mice, homogenizing the tissue in 0.1% PBS–Tween 80, and plating 5-fold serial dilutions on7H10 agar plates (Molecular Toxicology) for bacterial growth. Bacterial colonies were counted after incubation at 37°C for 14-21 days.
ELISpots
Four, eight, or twelve weeks after infection splenocytes were isolated from four non-immunized mice. Red blood cells were lysed using Red Blood Cell Lysis Buffer (eBioscience) and resuspended in RPMI 1640 and 10% FBS. A MultiScreen 96-well filtration plate (Millipore) was coated with 10 μg/ml rat anti-mouse IFN-γ or TNF capture antibody (eBioscience and R&D Systems, respectively) and incubated overnight at 4°C. Plates were washed with PBS, blocked with RPMI 1640 and 10% FBS for at least 1 h at room temperature, and washed again. Splenocytes were plated at 2 × 105 cells/well and stimulated with media or recombinant protein (10 μg/ml) for 48 h at 37°C. Plates were developed according to the manufacturer's protocol and fixed with 4% paraformaldehyde. Spots were counted on an automated ELISPOT reader (C.T.L. Series 3A Analyzer; Cellular Technology) and analyzed with ImmunoSpot software (CTL Analyzer).
Intracellular cytokine staining
Four weeks after the final immunization splenocytes were isolated from three to five animals per group. Red blood cells were lysed using Red Blood Cell Lysis Buffer (eBioscience) and resuspended in RPMI 1640 and 10% FBS. Cells were plated at 2×106 cells/well in 96-well plates and were stimulated for 1 hour with media or recombinant protein (10 μg/mL) at 37°C. GolgiPlug (BD Biosciences) was added and the cells were incubated for an additional 8 hours at 37°C. Cells were washed and surface stained with fluorochrome labeled antibodies to CD4 (clone GK1.5) and CD8 (clone 53-6. 7) (BioLegend and eBioscience) in the presence of anti-CD16/32 (clone 2.4G2) for 20 minutes at 4°C. Cells were washed and permeabilized with Cytofix/Cytoperm (BD Biosciences) for 20 minutes at room temperature. Cells were washed twice with Perm/Wash (BD Biosciences) and stained intracellularly with fluorochrome labeled antibodies to CD154 (clone MR1), IFN-γ (clone XMG-1.2), IL-2 (JES6-5H4), and TNF (MP6-XT22) (BioLegend and eBioscience) for 20 minutes at room temperature. Cells were washed and resuspended in PBS. Up to 106 events were collected on a four laser LSRFortessa flow cytometer (BD Biosciences). Data were analyzed with FlowJo. Cells were gated as singlets > live > lymphocytes > CD4+ CD8- > response positive. Analysis and presentation of distributions was performed using SPICE version 5.2, downloaded from <http://exon.niaid.nih.gov/spice.
Statistical analysis
Bacterial burdens were normalized by log10 transformation. Statistically significance differences in bacterial burden were determined using one-way analysis of variance. Differences relative to saline immunized animals were calculated using Dunnett's posttest using Prism 5 (GraphPad Software).
Results
Immunodominance hierarchy of Mtb proteins in mice
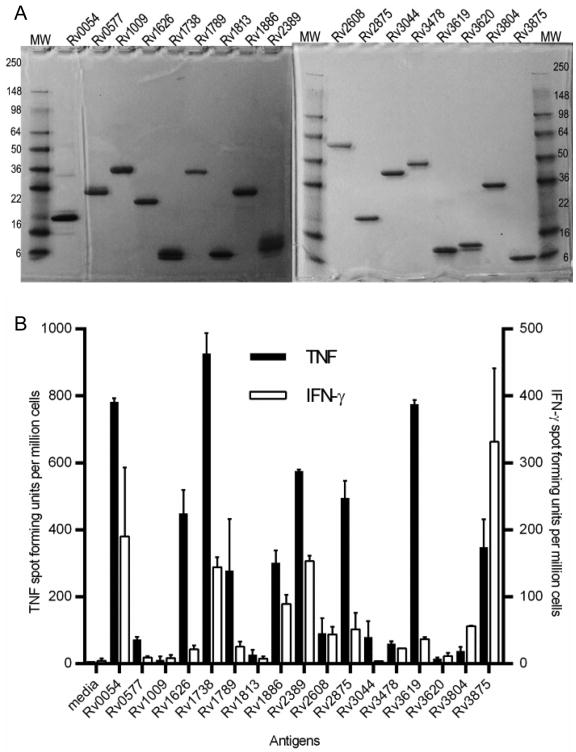
To establish the immunodominance hierarchy of a large pool of recombinant Mtb proteins (Figure 1A and (26)), we infected a cohort of CB6F1 mice with a low dose of aerosolized Mtb. Four, eight, and twelve weeks after infection we assessed the immune response to 84 recombinant Mtb proteins by ELISpot assay for IFN-γ and TNF (Figure S1). The antigens selected for analysis include a number incorporated in current vaccine and diagnostic candidates hey include proteins from several Mtb protein families including PE/PPE, ESAT-like, membrane/secreted proteins, proteins associated with hypoxia and/or resuscitation, and virulence factors. There was a tight correlation between the number of TNF and IFN-γ producing cells specific for a given antigen, with TNF responses always being more frequent, except for Rv3875 (ESAT-6) to which the IFN-γ and TNF responses were similar (Figure S1). There was a wide spectrum of response frequencies in this mouse strain including a number of known dominant antigens such as Rv3875,subdominant antigens such as Rv1886 (Ag85B), and poorly immunogenic antigens such Rv3804 (Ag85A) all of which are components of candidate vaccines against TB (27, 28). The responses over time changed with different patterns, but in general antigens that were recognized at four weeks continued to be recognized at eight and twelve weeks post-infection. At four weeks post-infection the most frequent TNF responses were to Rv1738, Rv1253, Rv0054, Rv3619, and Rv1246. At this time the most frequent IFN-γ responses were against Rv3875, Rv0125, Rv2389, Rv1738, and Rv0054 (Figure S1).
Figure 1. Diversity of T cell response magnitudes to select Mtb proteins during infection.
A) 2 μg of each purified recombinant Mtb protein was run on a denaturing 4-20% SDS-PAGE and stained by Coomassie to determine relative size and purity. Individual Mtb proteins are listed by their H37Rv gene number; MW= reference molecular weight size standards (kDa) are noted on the y-axes. (B) IFN-γ and TNF responses to recombinant Mtb proteins by splenocytes from mice four weeks after aerosolized Mtb infection. Data show results from one of two experiments with similar results (N=4 animals).
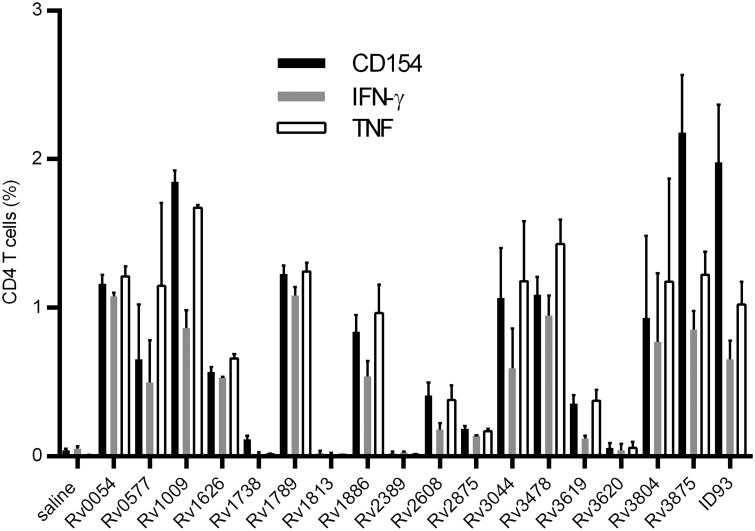
To assess how antigen dominance during primary infection may impact suitability of antigens for vaccine development we chose Rv1738 and Rv0054 as antigens that were dominant by both TNF and IFN-γ production, Rv3619 that was dominant by TNF responses, Rv2389 that was dominant by IFN-γ responses and Rv3875 that was unique in the equal IFN-γ and TNF response induction at four weeks. Based on the response magnitude at four weeks after infection we also selected four subdominant antigens (Rv1626, Rv1789, Rv1886, and Rv2875) and eight antigens that were weakly antigenic at this time (Rv0577, Rv1009, Rv1813, Rv2608, Rv3044, Rv3478, Rv3620, and Rv3804). The IFN-γ and TNF responses to these antigens at four weeks after Mtb exposure are shown in Figure 1B.
Induction of CD4 T cell responses by vaccination with Mtb proteins
To assess whether we could induce a TH1 response against the selected proteins, we immunized cohorts of mice with the recombinant proteins combined with the TH1 skewing adjuvant GLA-SE (29, 30). We used the recombinant fusion protein ID93 (a fusion of Rv1813, Rv2608, Rv3619, and Rv320) as a positive control for elicitation of a robust TH1 response with GLA-SE (9). One month after the third immunization we assessed IFN-γ and TNF responses as well as up-regulation of CD154 by CD4 T cells upon cognate antigen stimulation (Figure 2). Four of the antigens, Rv1738, Rv1813, Rv2389, and Rv3620 did not elicit a detectable TH1 response. Rv1738 and Rv2389 were two of the dominant antigens during primary infection, thus we hypothesize that cytokine responses to these proteins observed during primary infection (Figure 1B) may be produced by CD8 T cells, which are only weakly induced with a recombinant protein immunization adjuvanted with GLA-SE. All of the other antigens elicited measurable TH1 responses although there was considerable heterogeneity in the response magnitude. Importantly a number of the subdominant and weak antigens induced robust TH1 responses including Rv0577, Rv3044, Rv3478, and Rv3804. Antigen specific CD8 T cell responses were not detected in any of the immunized groups. These data demonstrate that it is feasible to augment the TH1 response to weak antigens by vaccination.
Figure 2. Immunogenicity of Mtb proteins adjuvanted with GLA-SE.
Cohorts of mice were immunized three times with Mtb proteins adjuvanted with GLA-SE. One month after the final immunization antigen specific CD4 T cells were identified by intracellular IFN-γ, TNF and/or CD154 expression following ex-vivo stimulation with the cognate antigen as determined by flow cytometry. Data show results from one of three experiments with similar results (N=3 animals/group).
Protective efficacy of select Mtb proteins with adjuvant
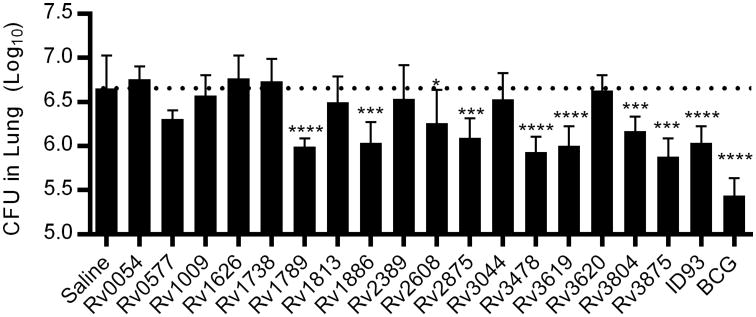
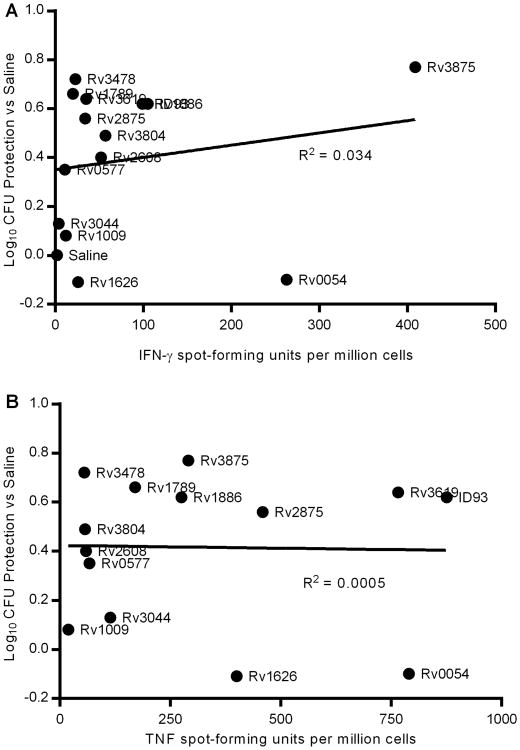
To determine whether the protective efficacy of vaccine antigens correlates to immune dominance during primary infection we challenged cohorts of immunized mice with aerosolized Mtb and determined pulmonary Mtb burden three weeks later (Figure 3). Of the dominant antigens both Rv3875 and Rv3619 were strongly protective, whereas Rv1738 and Rv2389 elicited minimal protection as might be expected by the lack of vaccine induced TH1 responses to these antigens (Figure 2). Surprisingly the dominant antigen Rv0054 induced a strong TH1 response to vaccination but produced little control of pulmonary Mtb. Of the subdominant antigens studied Rv1886 was the most strongly protective, in line with many other studies of the protective efficacy of this antigen in animal challenge models. Additionally both Rv1789 and Rv2875 were highly protective whereas Rv1626 elicited minimal protection despite robust TH1 responses following vaccination. Surprisingly several of the antigens that were only weakly antigenic during primary infection were protective when included in a vaccine. Specifically Rv3478, and to a lesser extent Rv2608 and Rv3804, limited pulmonary Mtb burden. Three of the weak antigens, Rv0577, Rv1009, and Rv3044, that elicited robust TH1 responses following vaccination failed to provide substantial protection. None of the antigens that failed to elicit TH1 response upon vaccination were protective (Rv1738, Rv1813, Rv2389, and Rv3620), suggesting that CD4 T cells were necessary for vaccine efficacy in this model. Taken together these data demonstrate that there is little correlation between the magnitude of the TNF or IFN-γ response to an antigen during primary infection and that antigen's potential to make an effective vaccine antigen (Figure 4). Even when the four antigens that did not elicit a CD4 T cell response by vaccination were excluded from analysis there was little correlation between vaccine efficacy and IFN-γ or TNF ELISpot magnitude during primary infection of unimmunized animals (R2 <0.1 in both cases).
Figure 3. Protective efficacy of Mtb proteins adjuvanted with GLA-SE.
Cohorts of mice were immunized three times with Mtb proteins adjuvanted with GLA-SE or once with BCG. One month after the final immunization animals were challenged with a low dose of aerosolized Mtb. Lung burdens were determined three weeks after infection. *, ***, and **** indicate P<0.05, 0.001, and 0.0001 relative saline immunized controls, respectively. Data show results from one of five experiments with similar results (N=7 animals/group). The dashed line indicates the mean burden in saline immunized animals.
Figure 4. Vaccine efficacy of Mtb proteins does not correlate with immunodominance.
Protective efficacy against pulmonary Mtb burden for recombinant protein antigen vaccines (mean CFU immunized – mean CFU saline) is plotted against (A) IFN-γ or (B) TNF ELISpot magnitude from 4 weeks after primary infection. Data show results from one of two ELISpot experiments (x-axis; N=4 animals) and one of five protection experiments (y-axis; N=7 animals/group) with similar results.
Fusion proteins of subdominant antigens are strongly protective against Mtb
Based on the protective efficacy of several of the subdominant antigens (defined by IFN-γ production during primary infection) demonstrated above we developed two fusion protein antigens that included the three most protective subdominant antigens, Rv1886, Rv3478, and Rv3619 combined with either Rv2389 (also known as resuscitation factor D or RpfD) or another subdominant antigen Rv2875, designated ID91 and ID97 respectively. Rv2875 demonstrated moderate levels of protection on its own (Figure 3). Rv2389 was chosen due to its expression during reactivation from latency, which may be important for developing a multi-stage targeted vaccine against active TB (31). Immunization with ID91, ID93, or ID97 adjuvanted with GLA-SE elicited similar frequencies of TH1 cells after one or two immunizations. Surprisingly both ID91 and ID97 showed a dramatic increase in the frequency of TH1 cells after a third immunization that was more substantial than for ID93 (Figure 5A). Although ID91 and ID97 elicited a more extensive tertiary response than ID93,these antigens were at least as effective as ID93 in eliciting multifunctional TH1 cells that co-produced IFN- γ, TNF and IL-2 or IFN-γ and TNF alone suggesting we have not driven these cells to terminal exhaustion (Figure 5B). For all three fusion proteins the majority of the CD4 T cell response was contributed by only one of the component proteins, Rv1886 for ID91 and ID97 and Rv2608 for ID93 (Figure S2). Rv2875, Rv3478, and Rv3619 specific responses were raised with those fusion proteins that contained these antigens, although these responses were less frequent than those for Rv1886 or Rv2608. Not surprisingly ID91 and ID93 elicited only very minor Rv2389 and Rv3620 specific TH1 responses and no response to Rv1813, respectively, as immunization with these individual components did not elicit robust TH1 responses (Figure S2 and 2).
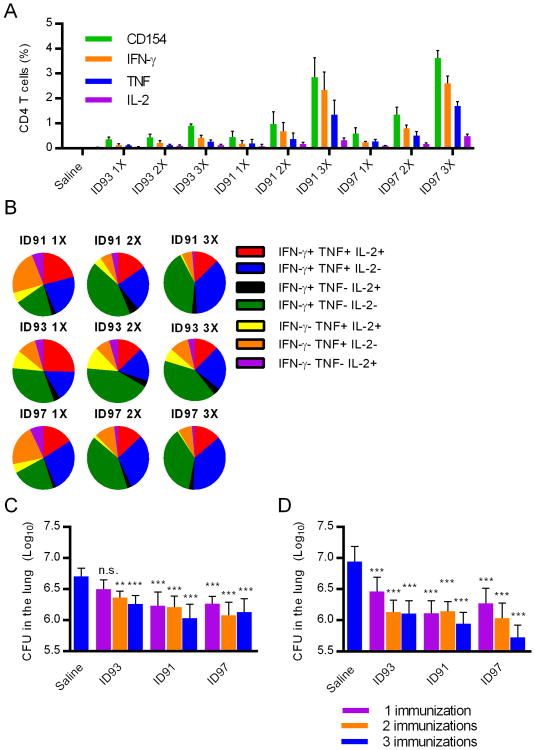
Figure 5. Fusion proteins of subdominant antigens are highly immunogenic and limit pulmonary Mtb.
Cohorts of seventeen mice per group were immunized one, two, or three times with saline or the fusion proteins ID93, ID91, or ID97 adjuvanted with GLA-SE. (A) One month after the final immunization antigen specific CD4 T cells were identified by CD154, IFN-γ, TNF and/or IL-2 expression following ex-vivo stimulation with the cognate antigen. (B) The frequency of cells co-expressing IFN-γ, TNF and/or IL-2 was analyzed using SPICE visualization software. One month after the final immunization animals were challenged with a low dose of aerosolized Mtb. Lung burdens were determined at (C) three or (D) six weeks after infection. ** and *** indicate P< 0.01 and 0.001 relative saline immunized controls, respectively. Data show results from one of two experiments with similar results (N=3 animals/group for A and B; N=7 animals/group for C and D).
Both ID91 and ID97 were at least as effective as ID93 in limiting pulmonary Mtb burden following an aerosol challenge. To our surprise a single immunization with any of the three fusion proteins adjuvanted with GLA-SE was sufficient to reduce bacterial burden three weeks after challenge (Figure 5C). Typically multiple immunizations with subunit vaccines are necessary to achieve vaccine efficacy in this model, indicating the potency of the ID91, ID93, and ID97 antigens. This vaccine efficacy was further enhanced with additional boosters (P<0.05 and P<0.001 for one vs three immunizations with ID93 and ID97, respectively), particularly for ID97 which reduced lung burden by greater than 90% after three immunizations. Importantly the reductions in bacterial burden were sustainable as two or three immunizations with ID93 or even a single immunization with ID91 or ID97 were sufficient to maintain lower bacterial burden for at least six weeks after challenge (Figure 5D).
Discussion
To test the hypothesis that subdominant and/or weak Mtb antigens are viable vaccine candidates and to avoid complications due to differential immunodominance hierarchies in humans and experimental animals we defined the immunodominance hierarchy of 84 recombinant Mtb proteins in experimentally infected mice. These responses were classified as dominant, subdominant, or weak according to the frequency of antigen-specific cells that produced IFN-γ or TNF after infection. When combined with the TH1 skewing GLA-SE adjuvant the majority of these antigens were immunogenic in Mtb-naïve animals. A subset of these vaccines substantially reduced the Mtb burden in the lungs of infected animals when given prophylactically. There was no correlation between immunodominance during primary Mtb infection and vaccine efficacy confirming the hypothesis that subdominant and weakly antigenic Mtb proteins are viable vaccine candidates. The data suggest that induction of a TH1 response during vaccination is a prerequisite for vaccine efficacy, but vaccine efficacy cannot be predicted by the presence or magnitude of the TH1 response. That is, antigens that did not elicit TH1 responses uniformly failed to protect against pulmonary Mtb, but not all antigens that induced TH1 responses made effective vaccine antigens. This discordance may be due to antigen load and/or presentation, immune subversion, antigen presentation by noninfected cells, or other causes.
In our development of the ID93 antigen we have found that ∼5 pmol of antigen is optimal for induction of maximal TH1 responses and similar to the findings of others that a low dose of antigen may be beneficial for eliciting protective T cell responses (32). Based on this we used an equimolar amounts of each recombinant antigen. For Rv3875 this meant using only 0.05 μg of protein for each immunization, which is at least two orders of magnitude lower than is typically used. Despite this very low dose of antigen we were able to induce robust TH1 response that correlated with protection against pulmonary Mtb. We have found previously that subtle alterations in the adjuvant formulation can modify the adjuvant capacity of the TLR4 agonist GLA molecule (30). In turn this has a profound impact on vaccine efficacy. Some of the subdominant antigens that we found to be strongly protective with the GLA-SE adjuvant were much less protective when combined with a weaker adjuvant such as unformulated CpG ODN1826, which we reported previously (18). Thus the choice of adjuvant or delivery system is critical to the evaluation of candidate vaccine antigens, at least for TB. The current TB vaccine pipeline is filled with candidates that vary not only in antigen composition, but also in adjuvant or delivery system, making direct comparisons between the merits of the different antigens complex (5, 6, 28).
One strategy to increase vaccine HLA coverage over a disparate patient population and to avoid immune evasion by epitope mutation is to include multiple antigens in the vaccine. We have and others have taken this approach in developing ID93, H56, M72, and Aeras-402 (5, 6, 28). Using this approach we combined the three most protective subdominant antigens into the ID91 and ID97 fusion proteins. Additionally ID91 contains the Rv2389 protein, which is involved in resuscitation of Mtb from hypoxia (31). Inclusion of this antigen may more effectively target latent or reactivating bacteria, a condition which cannot be effectively modeled in mouse model of aerosolized Mtb challenge (33). Both of these fusion proteins were at least as immunogenic and protective as the ID93 fusion. We were surprised to find that all three fusion proteins were effective in limiting pulmonary Mtb burden after a single immunization and this was further enhanced by subsequent boosters. For each fusion protein there was a clear antigen dominance hierarchy of the component proteins that was not predicted by vaccinating with each antigen individually (e.g. Rv1886 and Rv3478 were similarly immunogenic as individual proteins, but Rv1886 was the dominant response in ID91 and ID97 vaccinated animals). This dominancy hierarchy may stem from MHCII binding affinity, specific T cell precursor frequency and/or other factors that may vary between mice and humans. Thus the contribution of each fusion component to antigenicity in humans will need to be determined in potential clinical trials. We have previously found that all of the components of these fusions are antigenic in latently infected human volunteers, confirming their compatibility with one or more human MHC molecules (18).
Although the data presented here are limited to the immunodominance profiles in the mouse strain used and thus do not directly point to ideal vaccine candidates(e.g. Rv1886 is a dominant antigen in humans with both active TB and latent infection), there has been substantial efforts made to map the immunodominance hierarchy in latently infected volunteers. Using a large panel of peptides from the Mtb proteome, Sette's group mapped a core of commonly recognized Mtb peptides that bind to multiple HLA alleles (34). By implication there was a large pool of these HLA-promiscuous Mtb epitopes that do not induce T cell responses during natural latent infection. This peptide library could serve as a starting point for selecting subdominant epitopes to test as vaccine candidates under the hypothesis that increasing the Mtb-specific T cell repertoire beyond that induced by infection is an effective vaccine strategy.
One caveat to this approach and indeed to the development of T cell focused subunit vaccines against human diseases in general is the difficulty in translating a protein or peptide's immunogenicity and efficacy from experimental animals to humans due to species differences in MHC alleles. Although it is not feasible to immunize human volunteers with a large array of Mtb proteins it may be reasonable to identify a panel of proteins with diverse immunodominance profiles as mapped by Sette's group (34) or others to identify proteins that can be antigenic in a vaccine setting. Further, this approach to clinical experimentation could be augmented with the recently developed BCG challenge model in which immunized volunteers are intentionally exposed to BCG and followed for control and elimination of BCG from the injection site (35). This approach would allow in human testing of this alternative approach to vaccine antigen selection.
An alternative or complementary approach would be to employ one or more of the recently developed humanized mouse models (36). Although these models are imperfect in recreating the human immune system, recent advances including the BLT mouse model in which APCs express human HLA and human T cells undergo positive and negative selection to develop a human T cell repertoire as well as recently developed HLA class I and class II transgenic models, provide models for studying human T cell responses in a small animal model (37-39). Importantly, several recent papers have described productive Mtb and BCG infection in these animal models, although the human T cell response repertoire during infection was not assessed in these studies (40-42).
The data presented here do not in any way invalidate the approaches taken to identify the TB vaccine candidates developed to date. Rather these data suggest that TB vaccine development should not be limited to proteins that are the most antigenic during natural infection. Although the first new Phase II clinical trial of a TB vaccine in forty years, the MVA vectored Rv3804 (Ag85A), failed to demonstrate protective efficacy in immunized infants, it provides important information both in clinical trial design and insight into the relationship between vaccine immunogenicity and efficacy (43). Specifically in the study population (infants given MVA85A shortly after BCG immunization) there was only a muted augmentation of the Ag85A specific T cell response compared to the BCG only control. The reasons for this muted response are unclear as previous Phase I studies with this vaccine demonstrated substantial augmentation of Ag85A T cell responses in a variety of volunteer populations (44-46). These findings closely mirror our findings that induction of a vaccine specific CD4 T cell response is a minimum bar that must be achieved if a vaccine has any chance of being effective. Our data support the concept that inducing robust TH1 responses to Ag85A can limit pulmonary Mtb growth, although other single antigens were more protective in the data presented here. Additional Phase I and Phase II data with other antigens and vaccine delivery systems will be crucial to understand what immunological parameters are important for vaccine efficacy. These findings will also be crucial to understand how effective various animal models of Mtb infection, be it mouse, rabbit, guinea pig, non-human primate, or other models, are in predicting vaccine efficacy. In regards to MVA85A the preclinical animal data correctly predicted that in the absence of strong boost of Ag85A specific T cell responses we could not expect the MVA85A vaccine to be efficacious (47).
In summary the results presented here widen, rather than narrow, the pool of potential vaccine antigens. We provide evidence that Mtb proteins that are not antigenic during primary infection may make viable vaccine candidates by broadening the immune repertoire beyond that induced by natural infection.
Supplementary Material
Acknowledgments
We thank Chris Fox for preparing GLA-SE and Thomas Hudson, Tara Evers, and Brian Grainger for technical assistance.
Footnotes
This work was conducted with support from National Institutes of Health grant AI-078054 and contract HHSN272200800045C to R.N.C. and grant AI-044373 to S.G.R..
References
- 1.Global tuberculosis report 2013. World Health Organization; Geneva: 2013. [Google Scholar]
- 2.Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne JA, Fine PE, Smith PG, Lipman M, Elliman D, Watson JM, Drumright LN, Whiting PF, Vynnycky E, Rodrigues LC. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guerin vaccination against tuberculosis. Health technology assessment. 2013;17:1–372. v–vi. doi: 10.3310/hta17370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart PD, Sutherland I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br Med J. 1977;2:293–295. doi: 10.1136/bmj.2.6082.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baily GV. Tuberculosis prevention Trial, Madras. The Indian journal of medical research. 1980;72(Suppl):1–74. [PubMed] [Google Scholar]
- 5.Brennan MJ, Thole J. Tuberculosis vaccines: a strategic blueprint for the next decade. Tuberculosis (Edinb) 2012;92(Suppl 1):S6–13. doi: 10.1016/S1472-9792(12)70005-7. [DOI] [PubMed] [Google Scholar]
- 6.Checkley AM, McShane H. Tuberculosis vaccines: progress and challenges. Trends Pharmacol Sci. 2011;32:601–606. doi: 10.1016/j.tips.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN, Reed SG. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich J, Aagaard C, Leah R, Olsen AW, Stryhn A, Doherty TM, Andersen P. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. 2005;174:6332–6339. doi: 10.4049/jimmunol.174.10.6332. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard RD, Flory CM, Collins FM. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McShane H, Hill A. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 2005;7:962–967. doi: 10.1016/j.micinf.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, Orme IM, Skeiky YA, Alderson MR, Cowgill KD, Prieels JP, Abalos RM, Dubois MC, Cohen J, Mettens P, Lobet Y. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci U S A. 2009;106:2301–2306. doi: 10.1073/pnas.0712077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, Dillon DC, Campos-Neto A, Lobet Y, Dalemans W, Orme IM, Reed SG. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004;172:7618–7628. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 15.Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011;17:189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich J, Billeskov R, Doherty TM, Andersen P. Synergistic effect of bacillus calmette guerin and a tuberculosis subunit vaccine in cationic liposomes: increased immunogenicity and protection. J Immunol. 2007;178:3721–3730. doi: 10.4049/jimmunol.178.6.3721. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen NP, Norskov-Lauritsen S, Dolganov GM, Schoolnik GK, Lindenstrom T, Andersen P, Agger EM, Aagaard C. Tuberculosis vaccine with high predicted population coverage and compatibility with modern diagnostics. Proc Natl Acad Sci U S A. 2014;111:1096–1101. doi: 10.1073/pnas.1314973111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, Laughlin EM, Baldwin SL, Vedvick TS, Coler RN, Reed SG. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181:7948–7957. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171:1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 20.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, Gagneux S. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Copin R, Coscolla M, Seiffert SN, Bothamley G, Sutherland J, Mbayo G, Gagneux S, Ernst JD. Sequence Diversity in the pe_pgrs Genes of Mycobacterium tuberculosis Is Independent of Human T Cell Recognition. MBio. 2014;5:e00960–00913. doi: 10.1128/mBio.00960-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 23.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol. 2009;39:723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 24.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, Sanduzzi A, Franken WP, Ottenhoff TH, Dieli F. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 25.Orme IM. Development of new vaccines and drugs for TB: limitations and potential strategic errors. Future Microbiol. 2011;6:161–177. doi: 10.2217/fmb.10.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ireton GC, Greenwald R, Liang H, Esfandiari J, Lyashchenko KP, Reed SG. Identification of Mycobacterium tuberculosis antigens of high serodiagnostic value. Clin Vaccine Immunol. 2010;17:1539–1547. doi: 10.1128/CVI.00198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lalvani A, Sridhar S, von Reyn CF. Tuberculosis vaccines: time to reset the paradigm? Thorax. 2013;68:1092–1094. doi: 10.1136/thoraxjnl-2013-203456. [DOI] [PubMed] [Google Scholar]
- 28.Orme IM. Vaccine development for tuberculosis: current progress. Drugs. 2013;73:1015–1024. doi: 10.1007/s40265-013-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, Lin S, Phan T, Moon JJ, Vedvick TS, Reed SG, Coler RN. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J Control Release. 2013;172:190–200. doi: 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukamolova GV, Turapov OA, Young DI, Kaprelyants AS, Kell DB, Young M. A family of autocrine growth factors in Mycobacterium tuberculosis. Mol Microbiol. 2002;46:623–635. doi: 10.1046/j.1365-2958.2002.03184.x. [DOI] [PubMed] [Google Scholar]
- 32.Aagaard C, Hoang TT, Izzo A, Billeskov R, Troudt J, Arnett K, Keyser A, Elvang T, Andersen P, Dietrich J. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One. 2009;4:e5930. doi: 10.1371/journal.pone.0005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., 3rd Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindestam Arlehamn CS, Gerasimova A, Mele F, Henderson R, Swann J, Greenbaum JA, Kim Y, Sidney J, James EA, Taplitz R, McKinney DM, Kwok WW, Grey H, Sallusto F, Peters B, Sette A. Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3+CCR6+ Th1 subset. PLoS Pathog. 2013;9:e1003130. doi: 10.1371/journal.ppat.1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris SA, Meyer J, Satti I, Marsay L, Poulton ID, Tanner R, Minassian AM, Fletcher HA, McShane H. Evaluation of a Human BCG Challenge Model to Assess Antimycobacterial Immunity Induced by BCG and a Candidate Tuberculosis Vaccine, MVA85A, Alone and in Combination. J Infect Dis. 2013 doi: 10.1093/infdis/jit647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Billerbeck E, Horwitz JA, Labitt RN, Donovan BM, Vega K, Budell WC, Koo GC, Rice CM, Ploss A. Characterization of human antiviral adaptive immune responses during hepatotropic virus infection in HLA-transgenic human immune system mice. J Immunol. 2013;191:1753–1764. doi: 10.4049/jimmunol.1201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danner R, Chaudhari SN, Rosenberger J, Surls J, Richie TL, Brumeanu TD, Casares S. Expression of HLA class II molecules in humanized NOD.Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PLoS One. 2011;6:e19826. doi: 10.1371/journal.pone.0019826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, Doi T, Sone A, Suzuki N, Fujiwara H, Yasukawa M, Ishikawa F. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J, Brehm MA, Greiner D, Shultz LD, Kornfeld H. Engrafted human cells generate adaptive immune responses to Mycobacterium bovis BCG infection in humanized mice. BMC Immunol. 2013;14:53. doi: 10.1186/1471-2172-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calderon VE, Valbuena G, Goez Y, Judy BM, Huante MB, Sutjita P, Johnston RK, Estes DM, Hunter RL, Actor JK, Cirillo JD, Endsley JJ. A humanized mouse model of tuberculosis. PLoS One. 2013;8:e63331. doi: 10.1371/journal.pone.0063331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heuts F, Gavier-Widen D, Carow B, Juarez J, Wigzell H, Rottenberg ME. CD4+ cell-dependent granuloma formation in humanized mice infected with mycobacteria. Proc Natl Acad Sci U S A. 2013;110:6482–6487. doi: 10.1073/pnas.1219985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ota MO, Odutola AA, Owiafe PK, Donkor S, Owolabi OA, Brittain NJ, Williams N, Rowland-Jones S, Hill AV, Adegbola RA, McShane H. Immunogenicity of the tuberculosis vaccine MVA85A is reduced by coadministration with EPI vaccines in a randomized controlled trial in Gambian infants. Sci Transl Med. 2011;3:88ra56. doi: 10.1126/scitranslmed.3002461. [DOI] [PubMed] [Google Scholar]
- 45.Brookes RH, Hill PC, Owiafe PK, Ibanga HB, Jeffries DJ, Donkor SA, Fletcher HA, Hammond AS, Lienhardt C, Adegbola RA, McShane H, Hill AV. Safety and immunogenicity of the candidate tuberculosis vaccine MVA85A in West Africa. PLoS One. 2008;3:e2921. doi: 10.1371/journal.pone.0002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawkridge T, Scriba TJ, Gelderbloem S, Smit E, Tameris M, Moyo S, Lang T, Veldsman A, Hatherill M, Merwe L, Fletcher HA, Mahomed H, Hill AV, Hanekom WA, Hussey GD, McShane H. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis. 2008;198:544–552. doi: 10.1086/590185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McShane H, Williams A. A review of preclinical animal models utilised for TB vaccine evaluation in the context of recent human efficacy data. Tuberculosis (Edinb) 2014;94:105–110. doi: 10.1016/j.tube.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.