Abstract
Synthesis and secretion of immunomodulatory proteins, such as cytokines and chemokines, controls the inflammatory response within pancreatic islets. When this inflammation does not resolve, destruction of pancreatic islet β-cells leads to diabetes mellitus. Production of the soluble mediators of inflammation, such as TNF-α and IL-1β, from resident and invading immune cells, as well as directly from islet β–cells, is also associated with suboptimal islet transplantation outcomes. In this study, we found that IL-1β induces rapid increases in TNF-α mRNA in rat and human islets and the 832/13 clonal β-cell line. The surge in transcription of the TNF-α gene required the inhibitor of kappa B kinase beta (IκKβ), the p65 subunit of the NF-κB and a signal-specific recruitment of RNA polymerase II to the gene promoter. Of note was the increased intracellular production of TNF-α protein in a manner consistent with mRNA accumulation in response to IL-1β, but no detectable secretion of TNF-α into the media. Additionally, TNF-α specifically induces expression of CD11b, but not CD11c, on neutrophils, which could contribute to the inflammatory milieu and diabetes progression. We conclude that activation of the NF-κB pathway in pancreatic β-cells leads to rapid intracellular production of the pro-inflammatory TNF-α protein through a combination of specific histone covalent modifications and NF-κB signaling pathways.
Keywords: cytokine, diabetes mellitus, inflammation, islet, NF-κB, transcription
1. Introduction
Tumor necrosis factor-α (TNF-α) is a soluble protein linked with many human pathologies, including autoimmune diseases [1], obesity-associated insulin resistance [2, 3], cardiovascular complications [4, 5], and cancer [6]. It was first discovered over 30 years ago as an immune-cell derived molecule with ability to cause necrosis of tumors [7]. The TNF family currently consists of many different ligands with multiple corresponding types of receptors [8]. Most of the biological effects of TNF-α are mediated through the appropriate associated receptor, which is linked to activation of the NF-κB pathway [9, 10].
The NF-κB transcriptional regulators include p65 (RelA), RelB, c-Rel, p50, and p52. These proteins control numerous cellular processes, including proliferation, inflammation, and immune cell responses through signal-induced homo- and hetero-dimerization that lead to specific alterations in gene transcription [10]. In pancreatic β-cells, the activation of NF-κB by cytokines, such as IL-1, leads to increased production of nitric oxide [11-13], synthesis and secretion of various chemokines [14-17], losses in insulin secretion [18, 19] and diminished viability [20-22]. These cytokine-driven processes are thought to be a major cause of autoimmune-mediated (T1DM) diabetes mellitus [11, 23] and may also be involved with development of Type 2 diabetes mellitus (T2DM; ref [24, 25]).
Due to inflammatory processes underlying development of both T1DM and T2DM, IL-1 and TNF have been targeted individually as possible clinical treatments for treat diabetes in experimental rodent models, including non-obese diabetic (NOD) mice and Goto-Kakizaki (GK) non-obese type II diabetic rats [26-28]. In the NOD mouse, TNF-α can reduce the incidence of diabetes [29] or accelerate the development of diabetes [30, 31], depending on timing and modulatory strategy undertaken. TNF-α production directly from pancreatic islets of neonatal mice enhances diabetes development by promoting islet inflammation [31]. Moreover, therapies reducing IL-1 or IL-1 action in obese humans, or in individuals with overt diabetes, have revealed improved β-cell function with no parallel amelioration of peripheral insulin resistance [32-34].
Because of the prominent role of IL-1β in both major forms of diabetes as well as the apparently dichotomous role of TNF-α in modulating β-cell death and dysfunction leading to diabetes onset, we investigated the regulation of the TNF-α gene in pancreatic β-cells exposed to IL-1β. Several novel observations emerged: 1) Expression of the TNF-α gene is markedly upregulated in rat and human islets and β-cell lines in response to IL-1β exposure. 2) TNF-α induces Cd11b, but not Cd11c, expression in bone-marrow derived neutrophils. 3) The IκKβ protein is involved in mediating the intracellular response to IL-1β. 4) The p65 protein binds to κB response elements in the proximal TNF-α gene promoter. 5) The timing of TNF-α transcript accumulation is congruent with rapid signal-induced alterations in methylation status of histone H3 at K4 and K9 and recruitment of RNA polymerase II to the TNF-α promoter region.
2. Materials and Methods
2.1. Adenoviral vectors, cell culture, isolation of islets and reagents
Selection process and culture of 832/13 rat insulinoma cells has been described [35]. Isolation of rat islets has also been previously described [21]. Human islets were obtained commercially from Lonza (Clonetics™ Fresh Human Pancreatic Islets) on two separate occasions. Human islets used herein were from two different donors, with each shipment of islets handpicked into duplicates for each biological replicate used for cytokine exposure. The construction and use of adenoviruses encoding β-Galactosidase [36] and IκBαSR [37] have been previously documented. All reagents were from Fisher-Scientific unless otherwise noted.
2.2 Chromatin immunoprecipitation (ChIP) assays
832/13 cells were grown to confluence in 10cm dishes, treated as indicated in the figure legends, and harvested for ChIP as outlined in detail previously [12]. Antibodies used to immunoprecipitate anti-trimethyl-Histone H3 (Lysine 4 and 9) and RNA Polymerase II were from Millipore, while p65 was from Santa Cruz Biotechnology. Primers used to amplify regions of interest within promoter and coding regions are available upon request.
2.3 Isolation of total RNA, synthesis of cDNA, and detection of transcription by real-time PCR
832/13 cells, rat islets, and human islets were cultured in 12 well plates prior to and during exposure to cytokines. RNA isolation was carried out using Tri-Reagent for 832/13 cells and RNeasy kits (Qiagen) for rat and human islets. All procedures have been described [38]. Primers for detection of transcripts were designed using Primer3 Plus software and sequences are available upon request.
2.4 siRNA duplex transfection and immunoblot analysis
All siRNA duplexes were obtained via Ambion (Life Technologies) and transfected into 832/13 cells using Dharmafect I (Dharmacon) as recommended by the transfection reagent protocol and previous published methods [12, 16]. siRNA-mediated suppression of target genes was monitored using reverse transcription followed by real-time PCR analysis and immunoblotting using our previously described methodologies [12, 16].
2.5 Bone marrow neutrophil integrin expression following exposure to TNF-α
Four to six Balb/c mice were euthanized with isofluorane and femurs were flushed with PBS. After washing in PBS, erythrocytes were lysed with ammonium-chloride-potassium (ACK) lysis buffer and passed through a 70μm cell strainer (BD falcon). Cells were layered on top of a percoll gradient (58%) centrifuged for 30min at 1250 × g. The bone marrow neutrophil pellet was resuspended in PBS and counted. 106 neutrophils were placed into a snap cap tube and TNF-α was added to a final concentration of 1ng/ml followed by incubation at 37°C for 30min. After incubation, the cells were stained with anti-murine CD11b-PE and CD11c-APC (Biolegends) and analyzed on a BD LSRII. Data were analyzed using Flojo software (ver. 10.0.7r2).
2.5 ELISA and Statistical Analysis
832/13 cells were grown in 12-well plates, treated as indicated in the figures and associated legends. The cell culture media was supplemented with 0.3% BSA and collected at the indicated time points to analyze for secreted proteins. The cells were then lysed in M-PER (Thermo-Scientific) to analyze for intracellular TNF-α protein content. The TNF-α ELISA kit was from Life Technologies and was used exactly as recommended by the manufacturer to detect TNF-α protein in both supernatant samples and directly from lysed cell material. All ELISA data were normalized to total protein to account for any differences in cell number. All statistics were calculated using GraphPad Prism 6.0 software.
3. Results
3.1 Interleukin-1β rapidly increases TNF-α production in pancreatic β-cells
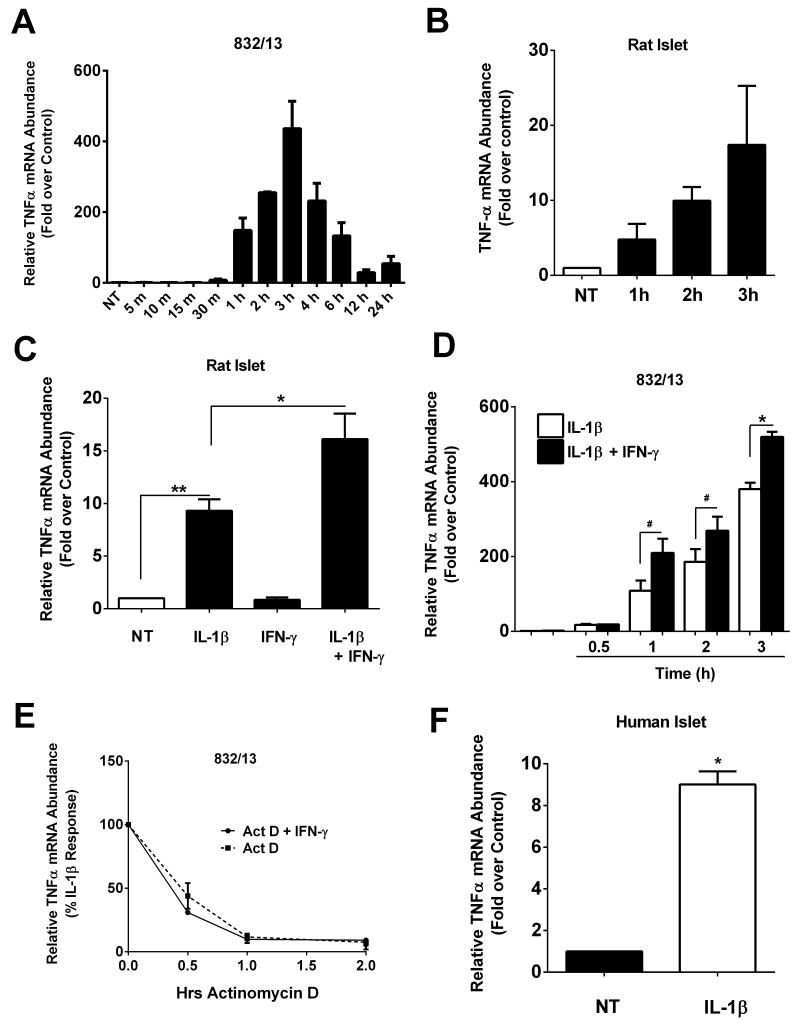
Alterations in TNF-α levels in serum of humans and rodents correlate with insulitis and development of T1DM [39-41]. Therefore, we examined the regulation of the gene encoding TNF-α using rat and human islets and β-cell lines. In 832/13 rat insulinoma cells, we discovered that TNF-α transcript levels were increased as early as one hour and peaked at three hours in response to 1ng/mL IL-1β (Figure 1A). A similar pattern was observed in isolated rat islets (Figure 1B). In addition, the presence of IFN-γ potentiated the response to IL-1β by 42% in rat islets (Figure 1C) and by 93%, 45%, and 38% at one, two, and three hours, respectively, in 832/13 cells (Figure 1D). Comparable results were also obtained using clonal INS-1E cells (not shown).
Figure 1. Cytokine-mediated induction of TNFα mRNA in rat and human islets and a β-cell line.
A. 832/13 cells were untreated (NT) or treated with 1 ng/mL IL-1β for the indicated times. B. Isolated rat islets were untreated (NT) or treated with 10 ng/mL IL-1β for the 1, 2 or 3h. C. Isolated rat islets were treated for 6 h with 10 ng/mL IL-1β, 100 U/mL IFN-γ or both cytokines in combination. **, P < 0.01, *, P < 0.05. D. 832/13 cells were treated with either 1 ng/mL IL-1β or IL-1β + 100 U/mL IFN-γ for 0.5, 1, 2 or 3 h. *, P < 0.05, #, P < 0.1. E. Following a 3 h stimulation with 1 ng/mL IL-1β (pre-exposure response induce by IL-1β is set at 100%), 832/13 cells were exposed to Actinomycin D (to block transcription) in the presence or absence of 100 U/mL IFN-γ. Total RNA was isolated at 0, 0.5, 1 and 2 h. F. Human islets were untreated (NT) or stimulated with IL-1β for 3 h.*, P < 0.05. A-F. TNFα transcript abundance was normalized to the housekeeping gene Ribosomal S9 (RS9). Data are shown as means ± SEM from three individual experiments.
To investigate whether the IFN-γ-mediated potentiation of the IL-1β response was due to stabilization of the mRNA, we monitored transcript abundance in the presence and absence of IFN-γ following a 3h pre-exposure to IL-1β. We found that IFN-γ was unable to induce a significant increase in stability of the TNF-α mRNA after removal of the IL-1β stimulus (Figure 1E). The transcript encoding TNF-α degraded rapidly after removal of the IL-1β stimulus (Figure 1E), indicating that regulation of the TNF-α gene is most likely at the transcriptional level. We note that results similar to those shown in Figure 1E were also obtained following a 6h pre-exposure to IL-1β (not shown). Finally, we found that IL-1β also induced a 9-fold increase in TNF-α mRNA in human islets (Figure 1F).
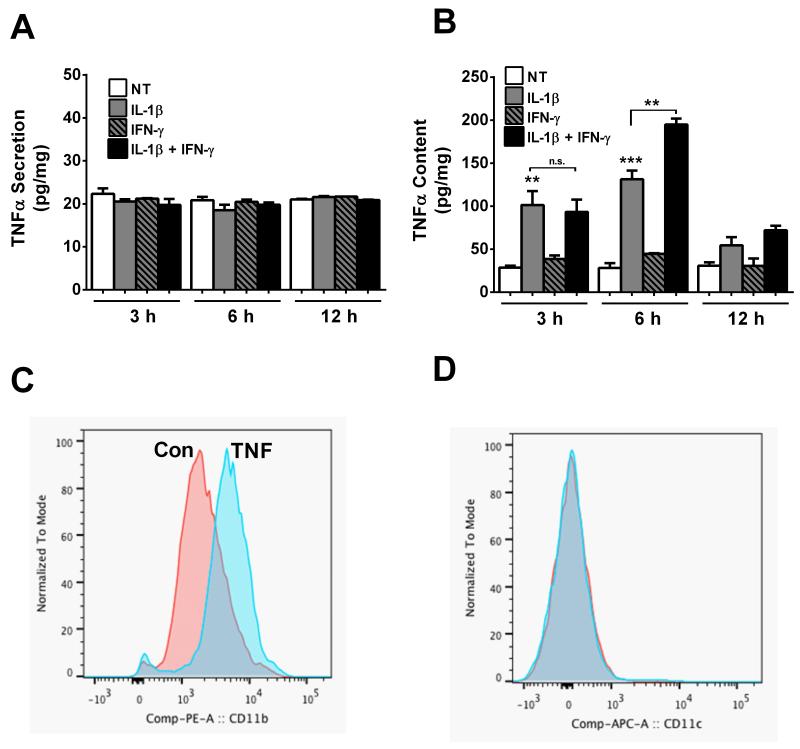
Because TNF-α transcript levels accumulate rapidly after IL-1β exposure, we next measured TNF-α content and secretion. To our surprise, TNF-α was not released from the cells under any condition examined (Figure 2A). We then measured TNF-α content within the cell and found that TNF-α protein accumulated within three hours after cells were exposed to IL-1β with no early potentiation by IFN-γ (Figure 2B). By contrast, the increase in TNF-α content was potentiated by IFN-γ at the 6 hour time point (Figure 2B). The decrease in TNF-α transcript by 12 hours (Figure 1A) is consistent with the detectable decline in protein by 12 hours (Figure 2B).
Figure 2. Cytokines induce production of TNFα in 832/13 cells and TNF-α enhances surface expression of Cd11b, but not Cd11c, in bone-marrow derived neutrophils.
A, B. 832/13 cells were treated with IL-1β (1 ng/mL), IFN-γ (100 U/mL) or the combination for 3, 6 or 12 h. TNFα secretion into the media (A) and cellular TNFα content (B) were quantified by ELISA and normalized to total protein. ***, P < 0.001 vs. NT, **, P < 0.01 (grey bars vs. NT), n.s. = not significant. ELISA assays were performed on three separate occasions. Data are expressed as means ± SEM. C,D. Isolated murine bone marrow neutrophils were exposed to 1ng/ml of TNFα for 30min (TNF) or media alone (Con). Cells were stained with antibodies to CD11c APC or CD11b PE and analyzed by flowcytometry. Results shown are representative of three biological replicates.
If TNF-α is released from the dying β-cells, the first response would ostensibly be circulating neutrophils. These neutrophils would serve to amplify the inflammatory response, contributing to T1DM. Using the mouse model, we examined the effect of TNF-α on bone marrow-derived (BM) neutrophils. Up regulation of integrin expression on neutrophils can increase the tight attachment of the neutrophil to vascular endothelial cells and subsequent extravasation to a site of inflammation. To examine whether TNF-α alters integrin expression, BM neutrophils were exposed to TNF-α. This resulted in a marked shift in expression of the integrin, CD11b (Mac-1) [mean fluorescence intensity (MFI): TNF (6244) vs CON: (2554)] (see Fig. 2C), while there was little to no change in CD11c expression (Fig. 2D). This observation demonstrates that upregulation of integrins in response to TNF-α is specific and not a result of generalized augmentation of all integrins.
3.2 Interference with IκKβ decreases IL-1β-mediated induction of TNF-α mRNA abundance
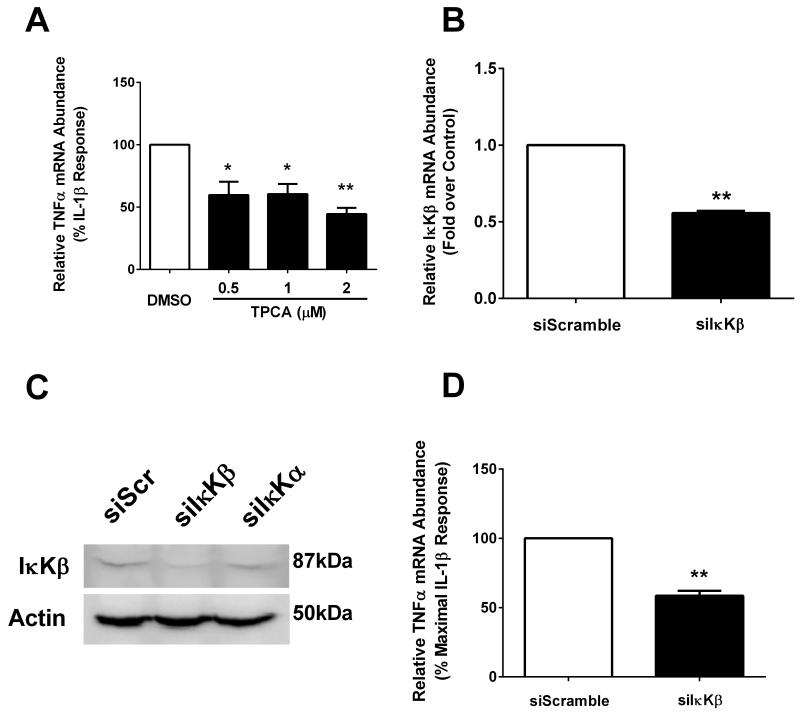
We have reported previously that the chemokine CCL2 requires p38 MAPK and IκKβ for synthesis and secretion in response to IL-1β [17]. Since TNF-α is also a soluble secreted mediator of inflammation, we tested whether the expression of the TNF-α gene also involves similar signaling pathway activation. First, we used 2-[(Aminocarbonyl)amino]-5-(4-fluor-ophenyl)-3-thiophenecarboxamide (TPCA) at doses that protect β-cells against cytokine-mediated cell death [20] and inhibit expression of the CCL2 gene [17]. We discovered that TPCA blunted the ability of IL-1β to increase TNF-α mRNA accumulation (Figure 3A). However, inhibiting p38 MAPK with a variety of pyridinyl imidazole based compounds, which effectively inhibit expression of the CCL2 gene [17] did not block the increase in TNF-α mRNA by IL-1β (not shown). Furthermore, siRNA-mediated suppression of the IκKβ mRNA (Figure 3B) and protein (Figure 3C) confirmed the results seen with TPCA, i.e., diminished IκKβ abundance is associated with a reduction in the IL-1β-driven accumulation of TNF-α transcripts (Figure 3D).
Figure 3. IL-1β-dependent stimulation of TNFα requires IκKβ.
A. 832/13 cells were pre-treated for 1 h with either DMSO (vehicle control) or the indicated concentrations of the IκKβ inhibitor TPCA. Cells were subsequently treated for 3 h with 1 ng/mL IL-1β. Relative TNFα mRNA abundance was normalized to RS9. **, P < 0.01 vs. DMSO, *, P < 0.01 vs. DMSO. B. 832/13 cells were transfected with siRNA duplexes targeting either a scrambled control sequence (siScramble) or IκKβ (siIκKβ). After 48 h exposure to siRNA, cells were harvested and mRNA levels of IκKβ were quantified. **, P <0 .01. C. 832/13 cells were transfected with siScramble (siScr), siIκKβ or siIκKβ or siIκKα. After 48 h culture with the indicated siRNA duplexes, cells were harvested and an immunoblot performed to determine the cellular abundance of IκKβ; Actin was used as the loading control. The immunoblot shown is representative of two independent experiments. D. 832/13 cells were transfected with siScramble and siIκKβ duplexes. After 48 h, cells were stimulated with 1 ng/mL IL-1β for 3 h. TNFα mRNA level was quantified and normalized to RS9. **, P < 0.01. For mRNA experiments, three individual replicates were generated and expressed as means ± SEM.
3.3 NF-κB subunit RelA/p65 is required for the IL-1β-mediated induction of the TNF-α gene
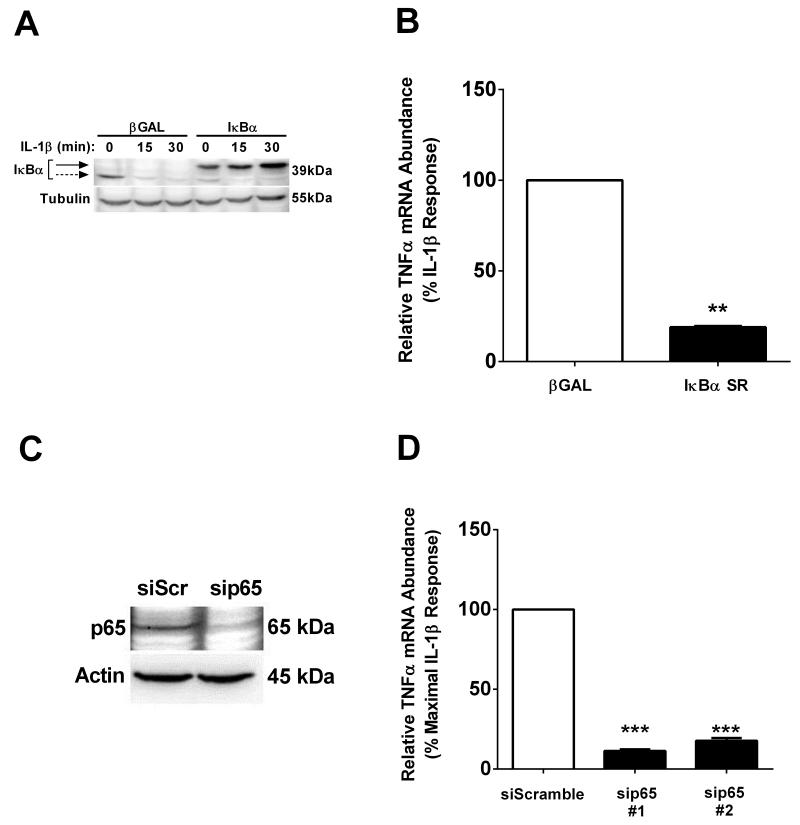
IL-1β activates the NF-κB pathway and promotes the expression of a variety of pro-inflammatory genes within pancreatic β-cells [10, 12, 16, 38, 42, 43]. Thus, we next examined whether or not the prototypical heterodimer of NF-κB, consisting of p65/p50, was involved in the IL-1β-mediated induction of the TNF-α gene. Using the IκBα super-repressor (IκBαSR), a non-degradable form of the inhibitor of NF-κB transcription factor proteins [37], we found that the abundance of this ectopically-expressed regulatory protein is maintained in the presence of IL-1β, while the endogenous protein is degraded (Figure 4A). TNF-α mRNA accumulation was decreased by 82% in the presence of IκBαSR relative to the β-Gal control after exposure to IL-1β (Figure 4B).
Figure 4. NF-κB subunit p65, but not p50, is required for IL-1β-dependent activation of the TNFα gene.
A. 832/13 cells were transduced with adenoviruses overexpressing βGalactosidase (βGAL) or IκBα Superrepressor (SR). Following a 24 h transduction with the indicated adenoviruses, cells were stimulated for 15 or 30 min with 1 ng/mL IL-1β. An immunoblot was performed using whole cell lysates and antibodies against IκBα using actin as the loading control. B. 832/13 cells were transduced with adenoviruses overexpressing βGAL or IκBα SR. Following a 24 h exposure to adenoviruses, cells were stimulated for 3 h with 1 ng/mL IL-1β. **, P < 0.01. C, D. . 832/13 cells were transfected with siRNA targeted to p65 and incubated for 48 h. C. 48 h post-transfection, whole cell lysates were blotted to determine abundance of p65. Actin served as the loading control. D. After 48 h exposure to siRNA cells were stimulated for 3 h with 1 ng/mL IL-1β. ***, P < 0.001, n.s. = not significant. B-D. mRNA levels of TNFα were measured and normalized to RS9. Immunoblots were performed on two separate occasions and a representative image is shown. Data are shown as means ± SEM from three independent experiments.
To confirm the IκBαSR results, we next used siRNA duplexes to reduce either p65 or p50 abundance. Transfection of siRNA duplexes that each target a distinct exon with the p65 mRNA sequence decrease p65 protein levels (duplex #1; Figure 4C) and blocked the IL-1β-mediated increase in TNF-α transcript accumulation (by 89% and 83%, respectively; Figure 4D). By contrast, targeting the p50 subunit of NF-κB, also using siRNA transfection, did not interfere with IL-1β stimulated increases in TNF-α transcript levels (data not shown). Thus, the p65 subunit appears to be the major factor controlling expression of the TNF-α gene in response to IL-1β.
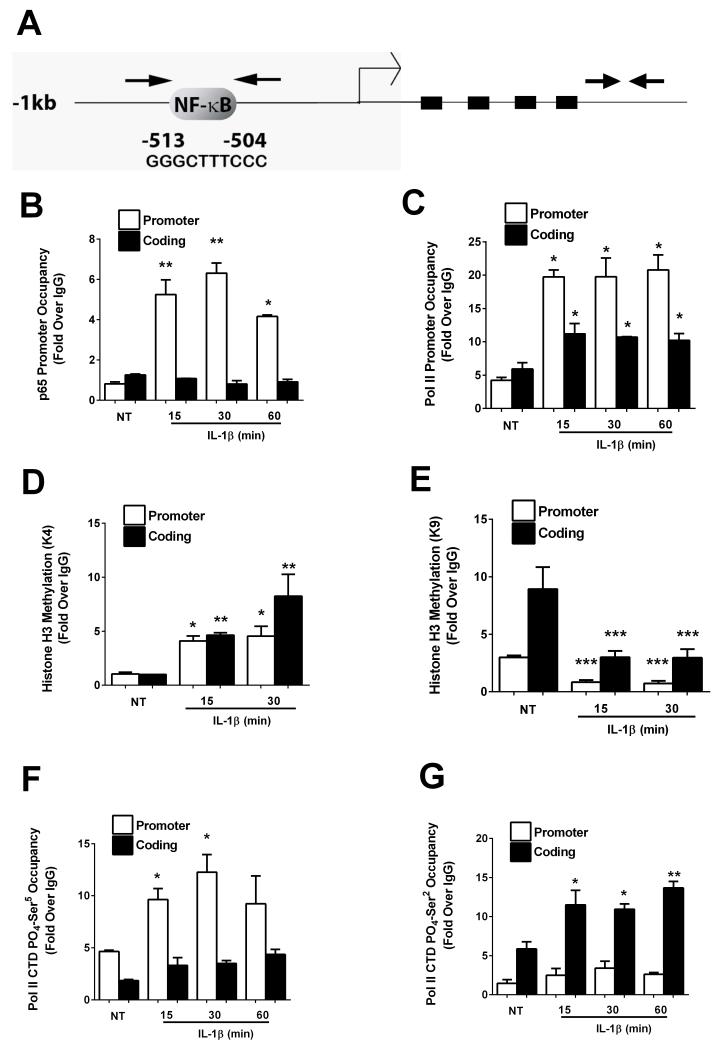
3.4 p65 binding, histone chemical modifications, and RNA polymerase II recruitment to the TNF-α gene promoter are IL-1β stimulated events
Because the p65 subunit of NF-κB is required for expression of the TNF-α gene in response to IL-1β (Figure 4), we examined its occupancy on the proximal gene promoter. In silico analysis of 1.2kb of the TNF-α proximal gene promoter revealed a κB response element approximately 500bp upstream of the transcriptional start site (Figure 5A). Using primers spanning this region of the promoter to amplify DNA recovered after chromatin immunoprecipitation, we detected 6.43−, 7.74− , and 5.10− fold increases in p65 occupancy after a 15, 30, and 60min exposure to IL-1β, respectively (Figure 5B). No binding over baseline was observed in the absence of IL-1β, indicating a stimulus-specific occupancy of p65 within the proximal TNF-α gene promoter. In addition, there was no detectable binding of p65 within the coding region (shown as arrow-indicated amplicon on the right hand side of Figure 5A), indicating specific occupancy at genomic regions containing κB sites.
Figure 5. IL-1β recruits p65 to the TNFα promoter and promotes changes in histone methylation and RNA Pol II phosphorylation.
A. Schematic representation of the TNFα promoter and coding regions. Arrows indicate regions amplified by PCR using recovered DNA as a template. B-G. 832/13 cells were treated with 1 ng/mL IL-1β for the indicated times. ChIP assays were performed using antisera to, methylated histone H3 (lysine 4; B and lysine 9; C), p65 (D) ,total Pol II (E) and Pol II CTD-phospho Serine 5 (F) and Serine 2 (G) on both the TNFα promoter and coding regions. ***, P < 0.001 vs. NT, **, P < 0.01 vs. NT, *, P < 0.05 vs. NT. ChIP signal is shown relative to IgG control as the means ± SEM from 3-4 individual experiments.
Moreover, we found that occupancy of the RNA polymerase II was increased on the promoter as well as on the coding region (Figure 5C) of the TNF-α gene, congruent with active transcription. In addition, methylation of histone H3 lysine 4 (H3K4), a well-known marker of gene activation [44], was enhanced in response to IL-1β (Figure 5D). By contrast, methylation of histone H3 at lysine 9 (H3K9), a modification associated with gene repression, was decreased after exposure to IL-1β (Figure 5E). No corresponding changes were detected at genes unresponsive to IL-1β (not shown). We also observed an increase in phosphorylation of the RNA Pol II carboxy terminal domain (CTD) at sites associated with promoter clearance (Ser5; Figure 5F) and movement along the coding region (Ser2; Figure 5G). The time frame of p65 occupancy (Figure 5A) and histone chemical modifications induced by IL-1β coincided with an increase in total and phosphorylated RNA polymerase II recruitment to the promoter and coding regions of the TNF-α gene. These results are consistent with robust appearance of transcript within 1 hour after IL-1β exposure (Figure 1A).
4. Discussion
Immune cell infiltration and islet inflammation are key features of both T1DM and T2DM [11, 23, 45, 46]. A major component of the inflammatory process leading to β-cell destruction is exposure to pro-inflammatory cytokines, such as TNF-α and IL-1β. Cytokine trap strategies blocking IL-1β action improve islet transplantation in rodents [47] while IL-1 receptor antagonism improves β-cell function in humans [33, 34]. In addition, TNF-α is markedly elevated in islets from humans with T2DM and correlates with poor islet function [48].
TNF-α can either prevent or enhance T1DM, depending on the model system used. For example, systemic administration of recombinant TNF-α decreases insulitis [49] and diabetes [28] in NOD mice. In addition, transgenic production of TNF-α directly from islet β-cells does not prevent insulitis, but does prevent autoimmune-mediated diabetes [29]. This contrasts with the toxic effects on TNF-α on islets in culture [50]. Thus, it is critical to understand the signal-driven production of TNF-α because of its unmistakably complex role in immune-mediated β-cell destruction.
We found that TNF-α is regulated transcriptionally by IL-1β in rat and human islets and the 832/13 rat β-cell line (Figure 1). These results are consistent with a previous report using mouse β-cells [51] and also extend those earlier findings by identifying key signaling events associated with increases in TNF-α transcription. For example, the IκKβ protein, a critical signaling node in cellular inflammatory responses, is involved in both systemic and organ-specific inflammatory diseases, making it a target for drug discovery [52]. Herein, we identify IκKβ as a critical component of the signal-specific induction of TNF-α gene transcription (Figure 3).
In addition, the p65 subunit of NF-κB, a strong transcriptional activator downstream of IκKβ, is required for TNF-α expression after β-cell exposure to IL-1β (Figure 4). p65 controls the expression of a number of genes involved in islet inflammation, including COX2, iNOS, CXCL1, CXCL2, CXCL10, and CCL2 [12, 14, 16, 17, 38]. Similar to the complex role TNF-α plays in islet inflammation [39], CCL2 can also promote either insulitis or diabetes, or both, on genetic backgrounds that are not associated with autoimmunity [53, 54]. Alternatively, CCL2 produced directly from β-cells prevents diabetes in autoimmune-predisposed settings, such as the NOD mouse [55]. Thus, specific genes directly regulated by NF-κB proteins, such as CCL2 and TNF-α, can have diametrically opposing effects on disease development, which may depend on timing and quantity of expression as well as genetic context. This explanation fits with the promising results of anti-TNF-α therapy in diabetic children [56] and adults with metabolic syndrome [57] and also with the negative outcomes observed during other situations of TNF-α modulation [58].
Remarkably, the IL-1β-mediated production of TNF-α transcript and protein were not coupled to release of TNF-α from the cell (Figure 2). Although we and others have shown that several chemokine proteins are synthesized and secreted upon synthesis in the pancreatic β-cell (e.g., CXCL1 and CXCL2 [14, 15], CXCL10 [16, 59], and CCL2 [17]), we speculate that TNF-α either requires signals in addition to IL-1β (and γ-IFN) for release from the cell or may only be released upon β-cell necrosis. For the latter possibility, a similar situation occurs where the immunological adjuvant protein HMGB1 is released during cytokine-mediated necrotic β-cell death, but not during bona fide apoptosis [22].
Since TNF-α specifically modulates surface expression of the CD11b integrin on neutrophils (Figure 2C), it is possible that a spillage of TNF-α from dying β-cells alter neutrophil function and migration, initiating the development or progression to diabetes. In addition, macrophage-derived TNF-α could also modulate neutrophil function, consistent with the immune cell crosstalk known to occur during T1DM [23]. Finally, TNF-α also contributes to diabetes development via islet antigen-specific Th17 cells [60], further increasing the complexity associated with leukocyte-mediated β-cell destruction. Conceptually, these possibilities fit with islet inflammation being associated with altered immune cell responses leading to β-cell destruction during progression to diabetes [11, 23, 45].
Thus, many challenges remain before the efficacy of single or combination immuno-modulatory strategies are proven completely effective for treatment of inflammation-associated diabetes in humans. Part of the remaining puzzle is the lack of comprehensive mechanistic information regarding expression of subsets of genes and associated gene networks regulated by cytokines and other signaling molecules. Understanding the signal-specific, tissue-specific, and gene-specific regulatory mechanisms will enable the development of more selective therapeutic strategies to target immune-cell/β-cell crosstalk.
Highlights.
TNF-α mRNA and protein are increased by IL-1β in pancreatic β-cells
Induction of TNF-α requires IκKβ, a component of the NF-κB signaling pathway
p65, a NF-κB transcriptional subunit, is recruited to the TNF-α gene promoter
Methylation status of histone H3 at the TNF-α gene is altered in response to IL-1β
RNA polymerase II is recruited to the TNF-α gene promoter in response to IL-1β
Acknowledgements
This study used the PBRC Genomics core facilities that are supported in part by COBRE (NIH8 P20-GM103528) and NORC (NIH 1P30-DK072476) center grants from the National Institutes of Health. This study was also partially supported by NIH grant R01 AI071042-01A2 (to T.E.S.).
We thank Tiantain Jiang and Matthew Goff for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12:147–168. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- [4].Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- [5].Oral H, Kapadia S, Nakano M, Torre-Amione G, Lee J, Lee-Jackson D, Young JB, Mann DL. Tumor necrosis factor-alpha and the failing human heart. Clinical cardiology. 1995;18:IV20–27. doi: 10.1002/clc.4960181605. [DOI] [PubMed] [Google Scholar]
- [6].Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- [7].Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends in cell biology. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- [9].Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- [10].Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci. 2013 doi: 10.1111/j.1749-6632.2012.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burke SJ, Updegraff BL, Bellich RM, Goff MR, Lu D, Minkin SC, Jr., Karlstad MD, Collier JJ. Regulation of iNOS Gene Transcription by IL-1beta and IFN-gamma Requires a Coactivator Exchange Mechanism. Mol Endocrinol. 2013;27:1724–1742. doi: 10.1210/me.2013-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Corbett JA, McDaniel ML. Does nitric oxide mediate autoimmune destruction of beta-cells? Possible therapeutic interventions in IDDM. Diabetes. 1992;41:897–903. doi: 10.2337/diab.41.8.897. [DOI] [PubMed] [Google Scholar]
- [14].Burke SJ, Lu D, Sparer TE, Masi T, Goff MR, Karlstad MD, Collier JJ. NF-kappaB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Endocrinol Metab. 2014;306:E131–149. doi: 10.1152/ajpendo.00347.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cowley MJ, Weinberg A, Zammit NW, Walters SN, Hawthorne WJ, Loudovaris T, Thomas H, Kay T, Gunton JE, Alexander SI, Kaplan W, Chapman J, O’Connell PJ, Grey ST. Human islets express a marked proinflammatory molecular signature prior to transplantation. Cell Transplant. 2012;21:2063–2078. doi: 10.3727/096368911X627372. [DOI] [PubMed] [Google Scholar]
- [16].Burke SJ, Goff MR, Lu D, Proud D, Karlstad MD, Collier JJ. Synergistic Expression of the CXCL10 Gene in Response to IL-1beta and IFN-gamma Involves NF-kappaB, Phosphorylation of STAT1 at Tyr701, and Acetylation of Histones H3 and H4. J Immunol. 2013;191:323–336. doi: 10.4049/jimmunol.1300344. [DOI] [PubMed] [Google Scholar]
- [17].Burke SJ, Goff MR, Updegraff BL, Lu D, Brown PL, Minkin SC, Jr., Biggerstaff JP, Zhao L, Karlstad MD, Collier JJ. Regulation of the CCL2 Gene in Pancreatic beta-Cells by IL-1beta and Glucocorticoids: Role of MKP-1. PLoS One. 2012;7:e46986. doi: 10.1371/journal.pone.0046986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rehman KK, Bertera S, Bottino R, Balamurugan AN, Mai JC, Mi Z, Trucco M, Robbins PD. Protection of islets by in situ peptide-mediated transduction of the Ikappa B kinase inhibitor Nemo-binding domain peptide. J Biol Chem. 2003;278:9862–9868. doi: 10.1074/jbc.M207700200. [DOI] [PubMed] [Google Scholar]
- [19].Giannoukakis N, Rudert WA, Trucco M, Robbins PD. Protection of human islets from the effects of interleukin-1beta by adenoviral gene transfer of an Ikappa B repressor. J Biol Chem. 2000;275:36509–36513. doi: 10.1074/jbc.M005943200. [DOI] [PubMed] [Google Scholar]
- [20].Collier JJ, Burke SJ, Eisenhauer ME, Lu D, Sapp RC, Frydman CJ, Campagna SR. Pancreatic beta-Cell Death in Response to Pro-Inflammatory Cytokines Is Distinct from Genuine Apoptosis. PLoS One. 2011;6:e22485. doi: 10.1371/journal.pone.0022485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Collier JJ, Fueger PT, Hohmeier HE, Newgard CB. Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes. 2006;55:1398–1406. doi: 10.2337/db05-1000. [DOI] [PubMed] [Google Scholar]
- [22].Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. 2006;3:e17. doi: 10.1371/journal.pmed.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- [24].Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- [26].Wilson CA, Jacobs C, Baker P, Baskin DG, Dower S, Lernmark A, Toivola B, Vertrees S, Wilson D. IL-1 beta modulation of spontaneous autoimmune diabetes and thyroiditis in the BB rat. J Immunol. 1990;144:3784–3788. [PubMed] [Google Scholar]
- [27].Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, Kergoat M, Portha B, Homo-Delarche F, Donath MY. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci U S A. 2009;106:13998–14003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jacob CO, Aiso S, Michie SA, McDevitt HO, Acha-Orbea H. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): similarities between TNF-alpha and interleukin 1. Proc Natl Acad Sci U S A. 1990;87:968–972. doi: 10.1073/pnas.87.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Grewal IS, Grewal KD, Wong FS, Picarella DE, Janeway CA, Jr., Flavell RA. Local expression of transgene encoded TNF alpha in islets prevents autoimmune diabetes in nonobese diabetic (NOD) mice by preventing the development of auto-reactive islet-specific T cells. J Exp Med. 1996;184:1963–1974. doi: 10.1084/jem.184.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang XD, Tisch R, Singer SM, Cao ZA, Liblau RS, Schreiber RD, McDevitt HO. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Green EA, Eynon EE, Flavell RA. Local expression of TNFalpha in neonatal NOD mice promotes diabetes by enhancing presentation of islet antigens. Immunity. 1998;9:733–743. doi: 10.1016/s1074-7613(00)80670-6. [DOI] [PubMed] [Google Scholar]
- [32].Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32:1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].van Asseldonk EJ, Stienstra R, Koenen TB, Joosten LA, Netea MG, Tack CJ. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2119–2126. doi: 10.1210/jc.2010-2992. [DOI] [PubMed] [Google Scholar]
- [34].Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- [35].Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- [36].Herz J, Gerard RD. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993;90:2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner DA, Sartor RB. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- [38].Burke SJ, Collier JJ. The gene encoding cyclooxygenase-2 is regulated by IL-1beta and prostaglandins in 832/13 rat insulinoma cells. Cell Immunol. 2011;271:379–384. doi: 10.1016/j.cellimm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- [39].Green EA, Flavell RA. Tumor necrosis factor-alpha and the progression of diabetes in non-obese diabetic mice. Immunol Rev. 1999;169:11–22. doi: 10.1111/j.1600-065x.1999.tb01302.x. [DOI] [PubMed] [Google Scholar]
- [40].el-Nawawy A, Soliman T, el-Azzouni O, Abbassy AA, Massoud MN, Marzouk S, Ibrahim F, Helal L. Interleukin-1-beta, tumor necrosis factor-alpha, insulin secretion and oral glucose tolerance in non-diabetic siblings of children with IDDM. Indian J Pediatr. 1998;65:455–460. doi: 10.1007/BF02761143. [DOI] [PubMed] [Google Scholar]
- [41].Lorini R, De Amici M, d’Annunzio G, Vitali L, Scaramuzza A. Low serum levels of tumor necrosis factor-alpha in insulin-dependent diabetic children. Horm Res. 1995;43:206–209. doi: 10.1159/000184279. [DOI] [PubMed] [Google Scholar]
- [42].Mokhtari D, Barbu A, Mehmeti I, Vercamer C, Welsh N. Overexpression of the Nuclear Factor-{kappa}B subunit c-Rel protects against human islet cell death in vitro. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00212.2009. [DOI] [PubMed] [Google Scholar]
- [43].Kwon G, Corbett JA, Rodi CP, Sullivan P, McDaniel ML. Interleukin-1 beta-induced nitric oxide synthase expression by rat pancreatic beta-cells: evidence for the involvement of nuclear factor kappa B in the signaling mechanism. Endocrinology. 1995;136:4790–4795. doi: 10.1210/endo.136.11.7588208. [DOI] [PubMed] [Google Scholar]
- [44].Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- [45].Donath MY, Boni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- [46].Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- [47].Rydgren T, Oster E, Sandberg M, Sandler S. Administration of IL-1 trap prolongs survival of transplanted pancreatic islets to type 1 diabetic NOD mice. Cytokine. 2013;63:123–129. doi: 10.1016/j.cyto.2013.04.020. [DOI] [PubMed] [Google Scholar]
- [48].Butcher MJ, Hallinger D, Garcia E, Machida Y, Chakrabarti S, Nadler J, Galkina EV, Imai Y. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014 doi: 10.1007/s00125-013-3116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Campbell IL, Oxbrow L, Harrison LC. Reduction in insulitis following administration of IFN-gamma and TNF-alpha in the NOD mouse. J Autoimmun. 1991;4:249–262. doi: 10.1016/0896-8411(91)90022-5. [DOI] [PubMed] [Google Scholar]
- [50].Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab. 1990;71:152–156. doi: 10.1210/jcem-71-1-152. [DOI] [PubMed] [Google Scholar]
- [51].Yamada K, Takane N, Otabe S, Inada C, Inoue M, Nonaka K. Pancreatic beta-cell-selective production of tumor necrosis factor-alpha induced by interleukin-1. Diabetes. 1993;42:1026–1031. doi: 10.2337/diab.42.7.1026. [DOI] [PubMed] [Google Scholar]
- [52].Bamborough P, Callahan JF, Christopher JA, Kerns JK, Liddle J, Miller DD, Morse MA, Rumsey WL, Williamson R. Progress towards the development of anti-inflammatory inhibitors of IKKbeta. Curr Top Med Chem. 2009;9:623–639. doi: 10.2174/156802609789007336. [DOI] [PubMed] [Google Scholar]
- [53].Grewal IS, Rutledge BJ, Fiorillo JA, Gu L, Gladue RP, Flavell RA, Rollins BJ. Transgenic monocyte chemoattractant protein-1 (MCP-1) in pancreatic islets produces monocyte-rich insulitis without diabetes: abrogation by a second transgene expressing systemic MCP-1. J Immunol. 1997;159:401–408. [PubMed] [Google Scholar]
- [54].Martin AP, Rankin S, Pitchford S, Charo IF, Furtado GC, Lira SA. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes. 2008;57:3025–3033. doi: 10.2337/db08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kriegel MA, Rathinam C, Flavell RA. Pancreatic islet expression of chemokine CCL2 suppresses autoimmune diabetes via tolerogenic CD11c+ CD11b+ dendritic cells. Proc Natl Acad Sci U S A. 2012;109:3457–3462. doi: 10.1073/pnas.1115308109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244–1249. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, Khor VK, Ahima RS, Grinspoon SK. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E146–150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tack CJ, Kleijwegt FS, Van Riel PL, Roep BO. Development of type 1 diabetes in a patient treated with anti-TNF-alpha therapy for active rheumatoid arthritis. Diabetologia. 2009;52:1442–1444. doi: 10.1007/s00125-009-1381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schulthess FT, Paroni F, Sauter NS, Shu L, Ribaux P, Haataja L, Strieter RM, Oberholzer J, King CC, Maedler K. CXCL10 impairs beta cell function and viability in diabetes through TLR4 signaling. Cell Metab. 2009;9:125–139. doi: 10.1016/j.cmet.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [60].Li CR, Mueller EE, Bradley LM. Islet antigen-specific Th17 cells can induce TNF-alpha-dependent autoimmune diabetes. J Immunol. 2014;192:1425–1432. doi: 10.4049/jimmunol.1301742. [DOI] [PMC free article] [PubMed] [Google Scholar]