Abstract
Vβ5+ regulatory T cells (Tregs), which are specific for a mouse endogenous retroviral superantigen, become activated and proliferate in response to Friend retrovirus (FV) infection. We previously reported that FV-induced expansion of this Treg subset was dependent on CD8+ T cells and TNFα, but independent of IL-2. We now show that the inflammatory milieu associated with FV infection is not necessary for induction of Vβ5+ Treg expansion. Rather, it is the presence of activated CD8+ T cells that is critical for their expansion. The data indicate that the mechanism involves signaling between the membrane-bound form of TNFα (memTNFα) on activated CD8+ T cells and TNF receptor 2 (TNFR2) on Tregs. CD8+ T cells expressing memTNFα but no soluble TNFα (solTNFα) remained competent to induce strong Vβ5+ Treg expansion in vivo. In addition, Vβ5+ Tregs expressing only TNFR2 but no TNFR1 were still responsive to expansion. Finally, treatment of naïve mice with solTNFα did not induce Vβ5+ Treg expansion, but treatment with a TNFR2-specific agonist did. These results reveal a new mechanism of intercellular communication between activated CD8+ T cell effectors and Tregs that results in the activation and expansion of a Treg subset that subsequently suppresses CD8+ T cell functions.
Introduction
CD4+Foxp3+ regulatory T cells (Tregs) constitute the major subset of immunosuppressive T cells (1). Their function is essential for both the prevention of autoimmune diseases in healthy individuals (2–4) and protection from immunopathological tissue damage during immune responses to viral infections and other infectious agents (5, 6). While generally beneficial, the suppressive effects of Tregs can result in delayed or incomplete clearance of pathogens leading to the establishment of chronic infections (5, 7, 8). The immunosuppressive role of Tregs in antiviral immune responses was first described in Friend Virus (FV) -infected mice (9), but has since been reported for viruses causing human infections such as herpes simplex virus (HSV), hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) (reviewed in (6, 7, 10)). FV is a retroviral complex that causes chronic infections in leukemia-resistant C57BL/6 (B6) mice. During acute FV infection, cytotoxic CD8+ T cells are critical for the elimination of virus-infected cells (11), although with time an expanding population of Tregs suppresses the anti-viral function of virus-specific CD8+ T cells (12–15). This Treg-mediated suppression results in CD8+ T cell exhaustion allowing the establishment of a chronic FV infection (8, 12, 16).
Recently we demonstrated that FV infection induced disproportionately greater expansion of Tregs expressing the T cell receptor (TCR) Vβ5 chain compared to Tregs expressing other Vβ chains (17). Investigation revealed that the proliferation of this subpopulation was driven by a mechanism distinct from other Tregs. IL-2 is well known to be an important growth and survival factor for Tregs (1, 18–20), and although IL-2 neutralization during FV infection significantly impaired the expansion of all Tregs not expressing the Vβ5 TCR, the Vβ5+ subset was not significantly affected. In contrast, depletion of CD8+ T cells or neutralization of TNFα uniquely affected the Vβ5+ subset (17). This was likely due to the known specificity of Vβ5+ TCRs for superantigens (Sags) encoded by endogenous mouse mammary tumor viruses (mtv) (21, 22). Superantigens are known to deliver unusually potent TCR signals distinct from those delivered by cognate antigens (23–25) and have been shown to stimulate conventional CD4+ T cells in an IL-2 independent manner (26). In the current study we examine the unique dependence of the Vβ5+ Treg subset on CD8+ T cells, TNFα, and the connection between the two.
TNFα is a pleiotropic cytokine that occurs in both a soluble (solTNFα) and a membrane-bound (memTNFα) form. There are two receptors for TNFα, TNFR1 and TNFR2. TNFR1 is efficiently activated by both solTNFα and memTNFα, but TNFR2 signaling is mainly triggered by memTNFα (27). TNFR1 belongs to the death receptor subgroup of the TNF receptor family and mediates the majority of the many pro-inflammatory effects of TNFα, but can also trigger cell death under certain circumstances (27). TNFR2 does not possess a death domain and signals with the help of adapter proteins of the TRAF family. TNFR2 modulates TNFR1 signaling but also elicits various anti-inflammatory activities (27, 28). Interestingly, both human and mouse Tregs constitutively express TNFR2 (29–31) and mouse Tregs can be expanded in a TNFR2-dependent pathway (32–35). We now demonstrate that Vβ5+ Treg expansion is induced specifically through TNFR2-signaling by interactions with memTNFα expressed by activated CD8+ T cells. In addition, we show that treatment of mice with a TNFR2-specific agonist can substitute for CD8+ T cells to induce expansion of Vβ5+ Tregs.
Materials and Methods
Mice and virus
Unless otherwise noted, 8 to 24 weeks old female C57BL/6 mice (abbreviated B6; Harlan Laboratories, Germany or bred at the Rocky Mountain Laboratories (RML), Hamilton, MT) or (C57BL/10 x A.BY)F1 mice (abbreviated Y10; bred at RML, Hamilton, MT) were used for the experiments. All transgenic mice used in this study were on B6 background. Mice were maintained at the central animal facility at the Institute for Virology, University Hospital Essen or at RML, Hamilton, MT. CD4+ adoptive transfer experiments were done using either congenic CD45.1+ B6 mice as recipients and B6, TNFR1-KO (36) (kind gift from Karl Lang) or TNFR1+2-KO (kind gift from Percy Knolle, Institute for Molecular Medicine and Experimental Immunology, University of Bonn, Bonn, Germany) mice as donors or B6 and B6.MHC II−/− (37) (Jackson Labs, B6.129S2-H2dlAb1-Ea/J) as recipients and Thy1.1+ Foxp3 eGFP (hereafter called Foxp3GFP) reporter mice (38) as donors (B6.MHC II−/− and Foxp3GFP bred at RML). CD8+ T cell adoptive transfer experiments were done using either CD8-KO (39) mice as recipients and iRhom2-WT or iRhom2-KO (40) (kind gift from Tak Mak) mice as donors; or Y10 mice as recipients and TCR transgenic mice specific for the DbGagL FV epitope expressing a Thy1.1+ congenic marker (hereafter called CD8.TCR transgenic (Tg)) or OT-1 mice that express a transgenic TCR specific for SIINFEKL peptide and the CD45.1+ congenic marker. TNFR2-KO mice (41) used for TNFα treatment experiments were a kind gift from Daniela Männel, Institute for Immunology, University Regensburg, Regensburg, Germany.
The FV stock used in these experiments was FV complex containing replication competent B-tropic Friend murine leukemia helper retrovirus (F-MuLV), replication defective polycythemia-inducing spleen focus-forming retrovirus (SFFV), and was free of lactate dehydrogenase-elevating virus (LDV) (42). B6 mice were infected with FV by intravenous (i.v.) injection of 0.3 ml PBS containing 20,000 spleen focus-forming units (SFFU) of FV complex and Y10 mice were given i.v. injection of 6000 SFFU. Animal experiments in Germany were performed in strict accordance with the German regulations of the Society for Laboratory Animal Science (GV-SOLAS) and the European Health Law of the Federation of Laboratory Animal Science Associations (FELASA). Animal experiments in the USA were approved by the NIAID RML IACUC. MHC Class II knockout mice (B6.129S2-H2dlAb1-Ea/J) were from Jackson Labs stock # 003584.
Surface and intracellular staining antibodies and flow cytometry
The antibodies used for cell staining were purchased from BD Biosciences, BioLegend or eBioscience, unless otherwise noted. The following antibodies were used for cell surface staining: anti-CD4 (GK1.5); anti-CD8 (53–6.7); anti-CD43 (1B11); anti-Thy1.1 (OX-7); anti-CD45.1 (A20); anti-Vβ5 (MR9-4); anti-TNFα (MP6-XT22); anti-TNFR1 (55R-286); anti-TNFR2 (TR7554) (R&D Systems). Intracellular Foxp3 staining was performed according to the manufacturer’s recommendation using anti-Foxp3 (FJK-16s) and the Foxp3 staining kit from eBioscience. For intracellular staining of Ki67, cells were surfaced stained before fixation and permeabilized using reagents from the Foxp3 kit (eBioscience) and then stained with anti-Ki67 (SolA15). For intracellular GzmB staining the Cytofix/Cytoperm intracellular staining kit from BD was used according to the manufacturer’s recommendations. Dead cells were excluded by singlet gating strategy and Fixable Viability Dye (eBioscience). The flow cytometric data was collected with an LSRII (BD Biosciences) and analyzed using FlowJo (Tree Star) software.
CD8+ T cell stimulation for TNFα-surface staining
Bead-isolated CD8+ T cells from naïve FV-specific CD8.TCR Tg mice were incubated for 6 or 24 hours with bone marrow-derived dendritic cells (DCs), loaded with the F-MuLV peptide (AbuAbuLAbuLTVFL (43)), in complete RPMI at a ratio of 5:1. After incubation the cells were stained for surface expression of TNFα and subsequently fixed with 4% formaldehyde. For surface TNFα staining on OT-1 cells, whole splenocytes from naïve OT-1 transgenic mice were cultured for 6 hours in vitro at 37°C and 5% CO2 in media alone or with 5 µg/ml SIINFEKL peptide, as indicated. Cells were stained for surface expression of TNFα and then fixed overnight in 0.5% paraformaldehyde.
Depletion of cell populations, cell purifications and adoptive transfers
For the depletion of Vβ5+ T cells 300 µg of anti-Vβ5 monoclonal antibody (MR9-4) was administered intraperitoneal (i.p.) on day 4 post infection. The treatment depleted more than 99% of the Vβ5+ Tregs at 12 dpi in the spleen.
In the CD8 depletion and add-back experiments, naïve Y10 mice were CD8 depleted by three 0.5 ml i.p. injections of approximately 300 µg anti-CD8 tissue culture supernatant (clone 169.4) given every other day, then 7 days later were given either 1×105 bead purified CD8+ T cells i.v. from the spleens of naïve CD8.TCR Tg mice or 1×106 bead purified CD8+ T cells from the spleens of naïve OT-1 mice. The following day mice were FV-infected as described, and the mice receiving the OT-1 CD8+ T cells also received 100 µg SIINFEKL peptide i.p. in a balanced salt solution and were boosted 7 days later with an additional 100 µg SIINFEKL peptide.
For CD4+ T cell transfer experiments, splenocytes and lymph node cells from naïve B6 (WT, wild type), TNFR1-KO, TNFR1+2-KO, or Thy1.1+ Foxp3GFP mice were enriched using magnetic bead mouse CD4+ T cell isolation kit II (Miltenyi) and the Miltenyi MACS system following the manufacturer’s recommendations. 3–5×106 CD4+ T cells from either B6, TNFR1-KO or TNFR1+2-KO (all CD45.1−) were transferred i.v. into 6 days FV-infected CD45.1+ B6 recipients and the spleen samples were analyzed 6 days post transfer (12 dpi). The Thy1.1+ Foxp3GFP were CellTrace™ Violet labeled (Invitrogen) following the manufacturer’s recommendations and 5×106 cells were transferred into B6 or B6.MHC II−/− recipients by i.v. injection in 0.25 ml PBBS and the recipient mice were FV-infected at the time of cell transfer,
For CD8+ T cell transfer experiments, splenocytes and lymph node cells from naïve iRhom2-WT or iRhom2-KO mice were enriched using the magnetic bead mouse CD8+ T cell isolation kit (Miltenyi). Only cell populations reaching purity of >90% were used for transfers into CD8-deficient mice. 5×106 CD8+ T cells were transferred i.v. into FV-infected CD8-KO recipients at 5 days post infection and the spleens were analyzed 5 days later (10 dpi).
TNFα treatment and TNFα blockade of naïve mice
Two different TNFα constructs were used to treat naïve B6 or TNFR2-KO mice. Recombinant human soluble TNFα (solTNFα) (Peprotech) was used as a murine TNFR1-specific agonist. To selectively stimulate murine TNFR2, a nonameric variant of murine TNFα has been used that corresponds in structure and function to a recently published nonameric TNFR2-specific mutant of human TNFα (TNFR2-spec. nona-muTNFα) (64). Due to a nonameric structure of the molecule it overcomes the limited activity of soluble trimeric TNFα on TNFR2. The specificity of the construct for murine TNFR2 was achieved by homologous to known mutations in human TNFα that confer selectivity for human TNFR2 (44). A detailed characterization of the nonameric TNFR2-specific variant of murine TNFα is part of a manuscript submitted elsewhere. For TNFα treatment naïve mice were injected twice on day 0 and day 2 with either 8.33 µg solTNFα (Peprotech) or 25 µg of TNFR2-spec. nona-muTNFα i.p for equal molecule availability. On day 4 after the first treatment spleen samples were analyzed via flow cytometry. For TNFα and IL-2 blocking studies, (A.BY x B10)F1 mice were adoptively transferred with 2 × 106 OT-1 CD8+ T cells on day zero. The following day (day 1) the OT-1 cells were stimulated in vivo by i.p. injection with cognate peptide (100 µg SIINFEKL peptide diluted in 200 µl of sterile pharmaceutical grade saline) or negative control peptide. The mice were boosted on day 7 in the same manner. On day 1 the mice were injected i.p. with either control non-specific antibody, anti-TNFα treatment antibody (200 µg of functional grade XT3.11 (BioXCell)) or anti-IL-2 antibodies (50 µg each of functional grade JES6-5H4 and JES6-1A12 (BioXCell)). The antibody treatments were administered every other day for a total of 7 treatments. Unstimulated control mice were given 5 × 106 OT-1 CD8+ T cells on day zero and were given the anti-TNFα treatment as described above. The mice were euthanized for analysis on day 14.
Results
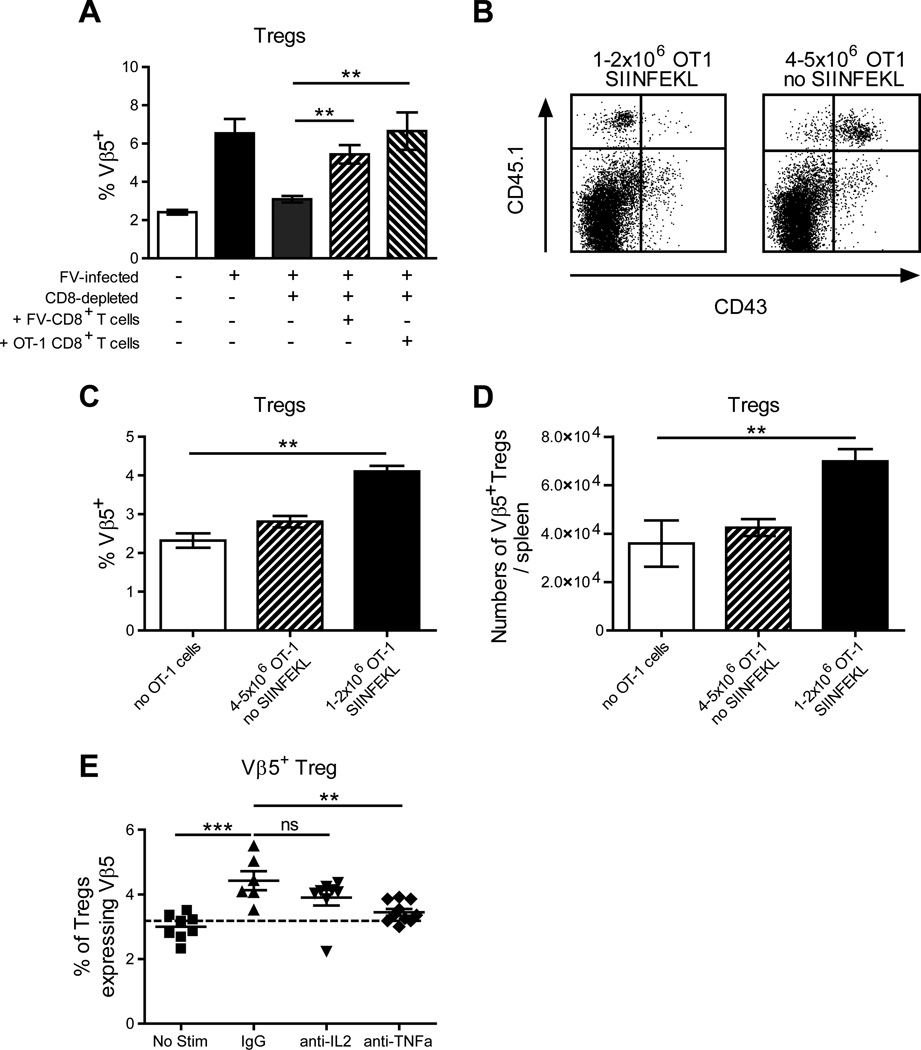
Previous results demonstrated that FV-induced expansion of Vβ5+ Tregs was dependent on CD8+ T cells and did not occur in CD8+ T cell-depleted mice (16). To determine if Vβ5+ Treg expansion could be restored in depleted mice by adoptive transfer of CD8+ T cells, the experiment was repeated with and without adoptive transfer of TCR transgenic CD8+ T cells. Mice were CD8+ T cell-depleted for one week, allowed to rest for one week to reduce anti-CD8 antibody titers, and then adoptively transferred with TCR transgenic CD8+ T cells the day before FV infection. To determine whether virus specificity was important, both virus-specific TCR transgenic CD8+ T cells (45) and ovalbumin (OVA)-specific TCR transgenic OT-1 CD8+ T cells (46) were adoptively transferred. Since the virus-specific CD8+ T cells would be activated by FV infection, the mice transferred with OT-1 cells were administered OVA peptide (SIINFEKL) to provide antigen-specific stimulation. All mice were analyzed at two weeks post-infection. As found previously, depletion of CD8+ T cells ablated the FV-induced expansion of Vβ5+ Tregs (Fig. 1A). Interestingly, the expansion of Vβ5+ Tregs was restored as well by adoptive transfer of non-specific (OT-1) CD8+ T cells as by virus-specific CD8+ T cells (Figure 1A).
Figure 1. Activated FV-specific or non-specific CD8+ T cells can induce Vβ5+ Tregs with or without FV infection.
(A) CD8-depleted mice were adoptively transferred with 1×105 bead purified CD8+ T cells from spleens of naïve CD8.TCR Tg mice or 1×106 bead purified CD8+ T cells from spleens of naïve OT-1 mice, with control mice as indicated. At 24 hrs post-transfer, mice were FV-infected and mice receiving OT-1 cells were also given 100 µg of SIINFEKL peptide and boosted at 1 weeks post infection (wpi). At 2 wpi the spleens of all mice were analyzed for the proportion of CD4+ Foxp3+ Tregs expressing Vβ5. Data are from 3 independent experiments, n=6–11 mice, using Bonferroni multi-comparisons (** < 0.005). (B-D) Bead purified CD8+ T cells from the spleens of naïve OT-1 mice were adoptively transferred into Y10 recipients; either 4–5×106 cells and left unstimulated, or 1–2×106 cells and given 100 µg SIINFEKL the next day followed by a 1 week post treatment (wpt) boost. At 2 wpt the spleens of mice were analyzed for (B) the expression profile of activation marker CD43 on CD45.1+ donor cells (representative plots shown) and (C) the proportion and (D) the absolute number of CD4+ Foxp3+ Vβ5+ Tregs. Data are from 2 independent experiments, n=4–9 mice, using a Student’s t-test (** < 0.005). (E) Mice were given an adoptive transfer of 2×106 OT-1 cells with SIINFEKL stimulation as described above with a group receiving control IgG, anti-IL-2, or anti-TNFα blocking antibodies every other day starting at the time of SIINFEKL stimulation. The no OT-1 stimulation (No Stim) control mice were given 5 × 106 OT-1 CD8+ T cells and anti-TNFα treatment. At 2 wpt the spleens of mice were analyzed for the proportion of CD4+ Foxp3+ Vβ5+ Tregs. Data are from 2 independent experiments, n=6–10 mice, using One Way ANOVA with Tukey’s multiple comparison test. The dashed line indicates the levels of the indicated cell type in naïve mice.
The finding that non-FV specific CD8+ T cells could drive Vβ5+ Treg expansion led us to question whether this could happen outside the context of virus infection and whether activation of the CD8+ T cells was important. To answer this we again used adoptive transfer of OT-1 TCR transgenic CD8+ T cells, but into naïve rather than FV-infected mice. The mice were adoptively transferred with one to two million cells and then primed with SIINFEKL peptide. To investigate the role of activation a control group received OT-1 cells but no SIINFEKL peptide. This group was transferred with two to five times as many OT-1 cells to compensate for their lack of antigen-induced proliferation. One week later the primed mice were boosted with peptide and analysis was done at two weeks post-adoptive transfer. In control mice that received 4–5 million CD8+ T cells but no SIINFEKL stimulus, the donor (CD45.1+) T cells remained negative for expression of the activation-induced isoform of CD43 (Figure 1B). These non-activated OT-1 cells did not significantly stimulate the expansion of Vβ5+ Tregs (Figure 1C, D). In contrast, the OT-1 donor cells from SIINFEKL-primed and boosted mice were predominantly activated (CD43+) (Figure 1B) and induced significant expansion of Vβ5+ Tregs in terms of both proportion (Figure 1C) and absolute numbers (Figure 1D). These results indicated that it was the activation status of the CD8+ T cells rather than their specificity or the inflammatory context of the viral infection that was important in driving the expansion of Vβ5+ Tregs.
Previous experiments indicated that CD8+ T cells and TNFα, but not IL-2, were required for Vβ5+ Treg expansion during FV infection (17). To determine if this was also true outside the context of FV infection, OT-1 adoptive transfer experiments were done in combination with blockade of either TNFα or IL-2. The induction of Vβ5+ Treg expansion by activated OT-1 cells was not significantly diminished by IL-2 blockade, but was dependent on TNFα (Fig. 1E).
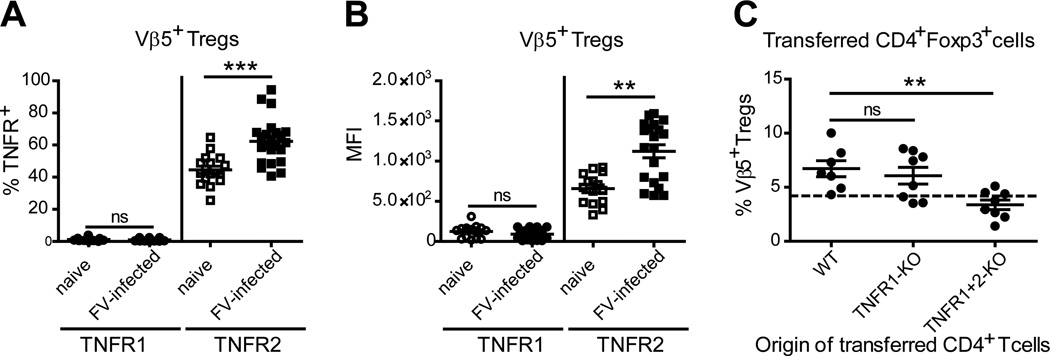
TNFα is expressed in two different forms, a membrane-bound and a soluble form that have different receptor stimulating abilities and distinct biological activities. A direct effect of TNFα on Vβ5+ Tregs would require expression of one of these receptors. Flow cytometric analysis revealed that TNFR1 was expressed on very few Tregs of the Vβ5+ Treg subpopulation or the total Treg population (CD4+Foxp3+) from naive mice (less than 5%), and neither the percentage of TNFR1-expressing Tregs, nor TNFR1 expression levels on Vβ5+ Tregs were induced following FV infection (Fig. 2A and B; Suppl. Fig. 1A). In contrast, TNFR2 was expressed on almost half of the Vβ5+ Treg population or the total Treg population (CD4+Foxp3+) in naïve mice and this percentage significantly increased after FV infection with the highest levels for Vβ5+ Tregs (Fig. 2A; Suppl. Fig. 1A–B). In addition, the mean fluorescence intensity (MFI) of TNFR2 staining on Vβ5+ Tregs was also significantly increased after FV infection (Fig. 2B).
Figure 2. Expression of TNFR1 and TNFR2 on Vβ5+ Tregs and the role of these receptors in Treg expansion.
B6 mice were FV-infected or left un-infected and the expression of TNFR1 and TNFR2 was investigated on Vβ5+ Tregs (CD4+ Foxp3+) using flow cytometry at 12 dpi. (A) The frequency and (B) the MFI of TNFR1 and TNFR2 on Vβ5+ Tregs in naïve and FV-infected mice are shown. Six independent experiments were analyzed with each dot representing a single mouse. Bars indicate the mean and SEM. For statistical analysis a Student’s t-test was used (ns = not significant, ** < 0.005 and *** < 0.0005). (C) CD45.1+ B6 mice were FV-infected (n=7–8) and on day 6 post infection 3–5×106 magnetic bead isolated CD4+ T cells of either wild type B6 (WT), TNFR1-KO or TNFR1+2-KO mice (all CD45.1 mice) were transferred i.v. and left to proliferate in the recipients for another six days. On day 12 post infection the spleens were investigated for Vβ5+ Treg expansion using flow cytometry. The percentage of Vβ5+ Tregs of transferred (CD45.1) CD4+ Foxp3+ Tregs was evaluated. The origin of transferred CD4+ T cells is shown on the x-axis. The dotted line indicates the level of Vβ5+ Tregs in the spleens of naïve mice. For statistical analysis a one-way ANOVA (Dunnett’s multiple comparison test) was performed (ns = not significant, ** < 0.005).
The increased expression of TNFR2 was consistent with positive feedback from memTNFα binding on Tregs as previously reported (47) and implicated TNFR2 as a mediator of Treg expansion during FV infection. To test this experimentally we took advantage of transgenic mice lacking either TNFR1 or both TNF receptors. Genetically-labeled (CD45.1−) Tregs from such animals were isolated and adoptively transferred into FV-infected wild type (CD45.1+ WT) mice. As a positive control WT Tregs were transferred. Both the WT Vβ5+ Tregs and TNFR1-deficient Vβ5+ Tregs expanded to similar extents (Fig. 2C) indicating that TNFR1-signaling was not required. In contrast, Vβ5+ Tregs lacking expression of TNFR2 in addition to TNFR1 failed to expand in FV-infected mice (Fig. 2C). Importantly, the frequency of Vβ5+ Tregs did not differ between naïve WT and TNFR1+2-KO donor mice and similar numbers of Tregs were transferred (data not shown), indicating that the inability of TNFR1+2 deficient Tregs to expand during FV infection was only restricted to the Vβ5+ subset and not a general impairment of all TNFR1+2 deficient Tregs to proliferate. Thus, TNFR2 appeared to be the critical receptor for the expansion of the Vβ5+ Tregs during FV infection. Furthermore, these results suggested that the dependence of Vβ5+ Treg expansion on both activated CD8+ T cells and TNFα might be explained by activation-induced expression of memTNFα on CD8+ T cells.
To ascertain whether memTNFα expression was induced by the activation of CD8+ T cells, FV-specific TCR transgenic CD8+ T cells were incubated in vitro with bone marrow-derived DC loaded with cognate FV peptide (48) and then analyzed for cell surface expression of TNFα. Indeed, after 6 hours of stimulation the CD8+ T cells significantly up-regulated cell surface expression of TNFα (Fig. 3A). Similar results were obtained from OT-1 cells stimulated with SIINFEKL peptide (Suppl. Fig. 2). Cell surface expression of TNFα was transient and down-regulated after 18 hours likely due to enzymatic cleavage to the soluble form of TNFα by TNFα convertase (TACE) (data not shown). These results indicated dynamic control of memTNFα expression on activated CD8+ T cells.
Figure 3. Expression of memTNFα on activated CD8+ T cells correlates with Vβ5+ Treg expansion during FV infection.
(A) For the evaluation of memTNFα, CD8+ T cells of CD8.TCR Tg mice were stimulated with bone-marrow derived DCs loaded or not with the cognate antigen. After six hours the CD8+ T cells were subject to TNFα surface analysis by flow cytometry. The mean MFI of the isotype control stain on CD8+ T cells for non-activated (shaded gray) was 318 and for activated (dashed gray) was 343. The memTNFα stain on CD8+ T cells for non-activated (dotted gray) was 221 and for activated (solid black) was 1317, with one representative histogram given. (B-D) CD8+ T cell deficient mice were FV-infected (n=4–6) and on day 5 post infection 7–8×106 magnetic bead isolated CD8+ T cells of either naïve iRhom2-WT or iRhom2-KO mice were transferred via i.v. injection into the infected CD8+ T cell deficient mice. 10 dpi the spleens were analyzed using flow cytometry. (B) The numbers of cells positive for memTNFα per million live cells were determined 5 days after the transfer (10 dpi). (C) The number of Vβ5+ Ki67+ Tregs and (D) the number of all Vβ5+ Tregs per million live cells is shown for mice that received either iRhom2-WT or iRhom2-KO CD8+ T cells. The results of two independent experiments are shown with the bars indicating the mean and the SEM. The dotted line indicates the level of Vβ5+ Tregs in the spleen of naïve iRhom2-KO/WT mice. For statistical analysis a Student’s t-test was used (* < 0.05 and ** < 0.005).
To better define the role of memTNFα in Vβ5+ Treg expansion we took advantage of iRhom2-KO mice that have defective TACE activity and therefore fail to cleave memTNFα into the soluble form. Compared to wild type, iRhom2-KO mice have slightly higher constitutive levels of memTNFα and display significantly higher inducible levels of the molecule (49). CD8+ T cells from iRhom2-KO mice were adoptively transferred into FV-infected CD8+ knockout mice, which do not show disproportionate expansion of Vβ5+ Tregs after FV infection because of the lack of CD8+ T cells (data not shown). The adoptive transfer of CD8+ T cells from either iRhom2-WT or iRhom2-KO origin into FV-infected mice resulted in activation of the transferred cells as a result of the ongoing viral infection (data not shown). Significantly more CD8+ T cells from iRhom2-KO mice expressed mbTNFα in FV-infected mice compared to CD8+ T cells of iRhom2-WT origin following transfer (Fig. 3B). Compared to mice that received wild type CD8+ T cells, the ones that received CD8+ T cells from iRhom2-KO mice had significantly more Vβ5+ Tregs expressing the Ki67 proliferation marker (Fig. 3C). Furthermore, there was also significantly greater expansion of Vβ5+ Tregs in those mice (Fig. 3D). These results suggested that increased memTNFα expression on CD8+ T cells provided a stronger signal for the expansion of Vβ5+ Tregs.
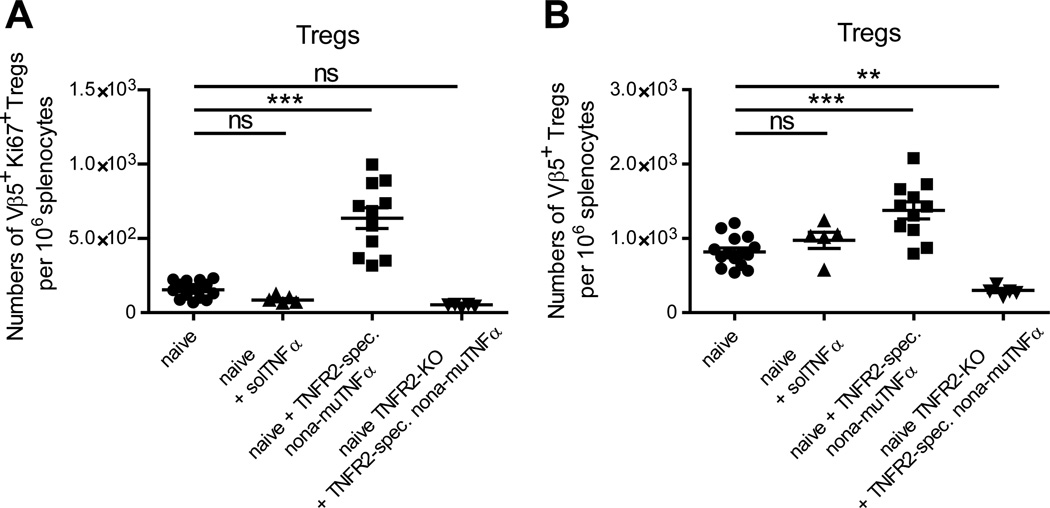
To determine if the dependence of Vβ5+ Treg expansion on CD8+ T cells could be circumvented by direct stimulation of the Tregs via TNFR2, we treated naïve mice with a nonameric variant of murine TNFα with mutations conferring specificity for TNFR2 (TNFR2-spec. nona-muTNFα). This murine TNFR2 variant is similar in structure and activity to a corresponding human TNFR2-specific TNFα variant that was recently for use with human cells (50) and acts as highly potent agonist of murine TNFR2. As a control, mice were treated with recombinant human solTNFα, which binds to mouse TNFR1 but not TNFR2 (51). solTNFα failed to expand Vβ5+ Tregs in naïve mice whereas treatment with nonameric TNFR2-specific murine TNFα significantly increased the number of Vβ5+ Ki67+ Tregs (Fig. 4A) and the number of all Vβ5+ Tregs (Fig. 4B). Thus, stimulation of TNFR2 was sufficient to trigger the proliferation of Vβ5+ Tregs. Taken together, the results suggest that in the context of FV infection, activation-induced up-regulation of memTNFα on CD8+ T cells triggers TNFR2 on Tregs to drive their expansion.
Figure 4. Expansion of Vβ5+ Tregs by complexed mouse TNFα.
Naïve B6 were treated twice (days 0 and 2) i.p. with 8.33 µg recombinant human soluble TNFα (solTNFα) or 25 µg of a nonameric variant of TNFR2-specific murine TNF mutant (TNFR2-spec. nona-muTNFα). As a control, the latter was also used to treat TNFR2-KO mice. At day 4 after the start of the treatment the spleens were subject to flow cytometric analysis. (A) The numbers of Vβ5+ Ki67+ Tregs and (B) of all Vβ5+ Tregs per million live cells were determined. One to two independent experiments were carried out, with each dot representing a single mouse. Bars indicate the mean and the SEM. For statistical analysis a one-way ANOVA (Dunnett´s multiple comparison test) was performed (ns not significant, ** < 0.005).
Like conventional CD4+ T cells, the activation of Tregs to proliferate generally requires two signals: the first is recognition of antigen through the TCR and the second is a co-stimulatory signal (reviewed in (52)). The first signal for Vβ5 TCR is recognition of mtv-9-encoded Sag in the context of MHC class II molecules (17, 22), while the second signal appeared to be TNFR2 signaling via memTNFα. Although previous results indicated that mtv-9 Sag is constitutively expressed in antigen presenting cells at low levels (17), likely providing relatively constant signal 1 to Vβ5+ Tregs, it was important to evaluate the possibility that TNFR2-mediated signal 2 was sufficient to induce the proliferation of Vβ5+ Tregs in the absence of TCR-mediated signal 1. To this end we tested for Vβ5+ Treg expansion in FV-infected MHC II knockout mice, which cannot present Sag (signal 1) to CD4+ T cells. CD4+ T cells from naïve WT mice were labeled with CellTrace™ Violet fluorescent proliferation tracer, adoptively transferred into either B6.MHC II−/− or WT B6 mice, and the mice were infected with FV the same day. At six days post-infection the spleen cells were analyzed for proliferation of the adoptively transferred Vβ5+ Tregs by dilution of the fluorescent label. Whereas a large number of Vβ5+ Tregs transferred into FV-infected WT mice underwent multiple rounds of cell division, almost none of the cells transferred into B6.MHC II−/− mice proliferated (Fig. 5A, B). Lack of proliferation was not due to lack of CD8+ T cell activation as the FV-infected B6.MHC II−/− mice had even higher levels of activated CD8+ T cells than WT mice (Fig. 5C). These results indicated that Vβ5+ Treg proliferation was dependent on both signal 1 (Vβ5 TCR stimulation by MHC class II presented Sag) and signal 2 (TNFR2 signaling from memTNFα on activated CD8+ T cells).
Figure 5. FV induced Vβ5+ Treg proliferation is dependent on MHC II expression.
5×106 cell tracer-labeled, bead purified CD4+ T cells from the spleens of naïve Foxp3GFP mice were adoptively transferred into WT (B6) and MHC II−/− (B6.MHC II−/−) mice and infected with FV the same day. At 6 days post-transfer the spleens of recipients were analyzed for the proliferation profiles of donor (Thy1.1+) CD4+ GFP+ Vβ5+ Tregs. (A) A representative proliferation histogram overlay of B6 (black line) and MHC II−/− (solid gray) donor Vβ5+ Tregs and (B) the percentage of Vβ5+ Tregs within the proliferation gate (mean and SEM) from each group, as defined in panel A is shown. (C) The percent of CD8+ T cells expressing the activation marker CD43 in recipient mice is depicted. Data are from 2 independent experiments, n=4, with a Student’s t-test (*** p=0.0007).
Discussion
Previous studies demonstrated that Tregs potently suppress CD8+ T cell responses during FV infection (8, 12, 13). The current study provides strong evidence that it is the CD8+ T cell response itself that drives the response of the Vβ5+ Tregs, a subset that expands disproportionately more than other Treg subsets not only during FV infection (17), but other infections as well (22). Interestingly, Vβ5+ Tregs fail to proliferate in response to FV infection when no CD8+ T cells are present (Fig. 1A) or when TNFα is neutralized (17). We now show that FV infection is not necessary to induce the expansion of Vβ5+ Tregs and that activated, but not naive CD8+ T cells are sufficient (Fig. 1). Activated CD8+ T cells transiently up-regulate the expression of the membrane-bound form of TNFα, which in contrast to soluble TNFα is a potent activator of TNFR1 and TNFR2 (Fig. 3). We further found that TNFR2 signaling by itself is both sufficient (Fig. 4) and required (Fig. 2) to induce Vβ5+ Treg expansion. Thus the results indicate that the requirement for activated CD8+ T cells for Vβ5+ Treg expansion is related to their expression of memTNFα. Indeed, CD8+ T cells that express high levels of memTNFα due to a defect in the cleavage of memTNFα, induced even higher levels of Vβ5+ Tregs (Fig. 3D).
The role of TNFα signaling in Tregs remains a controversial area of research due to some seemingly conflicting reports. On one hand, two studies have indicated that TNFR signaling inhibits the suppressive function of human Tregs in patients with active rheumatoid arthritis (30, 53) and a similar observation was made in a human TNFα transgenic mouse model of arthritis (54). When TNFα was blocked by antibodies, the suppressive activity of Tregs was restored (30, 53) and this effect was shown to be TNFR2 dependent (31). Similar findings have been made in children with type 1 diabetes (55). Another study in patients infected with HBV reported that solTNFα partially abrogated Treg-mediated suppression of HBV-specific effector T cell responses (56). On the other hand, Tregs with high TNFR2 expression are associated with suppression of effector responses in acute myeloid leukemia patients and in malaria patients (57–59), more similar to what has been observed in mouse models, which indicates that TNFR2 expression marks a highly suppressive subset of Tregs (60). Recent studies demonstrated that TNFα can be involved in Treg activation, expansion and Foxp3 up-regulation / stabilization, but the source of TNFα was not directly linked to T effector cells (32, 61, 62). However, it has been demonstrated that TNFα from CD4+ T effectors boosts Treg expansion and function in a diabetes autoimmunity model (35). The complexity of the regulation of these immune responses makes it is very important to understand precisely what is happening in individual cases before attempting therapies such as neutralization of TNFα.
Not surprisingly, it is becoming increasingly clear that there are distinct subsets of Tregs with different modes of activation and proliferation. The differences in the outcomes of TNFR2-signaling described above may be explained by the distinct subtypes of Tregs being studied. Housley et al. showed that suppression mediated by natural Tregs (nTregs) was dependent on TNFα, whereas the function of TGFβ-induced iTregs was not (63). Consistent with this finding the expanding Vβ5+ Treg population in our model is a unique subset exclusively derived from nTregs (17). These Vβ5+ Tregs have immunosuppressive functions in vivo as antibody-mediated depletion of this subset results in enhanced expression of cytotoxic effector molecules by CD8+ T cells (Fig. 6). Disease-specific parameters such as the inflammatory milieu (64) might also explain some of the disparate results on mechanisms of Treg activation in the literature. However, in our experiments using adoptive transfer of OT-1 cells into non-infected mice, we eliminated the innate immune responses and inflammatory effects induced by FV infection as variables and showed that activated CD8+ T cells alone are sufficient to provide a proliferation signal to Vβ5+ Tregs.
Figure 6. Depletion of Vβ5+ T cells during acute FV infection.
B6 mice were FV-infected and one group was treated with 300 µg of α-Vβ5 depletion MAb i.p. once at day 4 post infection. The CD8+ T cell response was investigated in the spleen at 12 dpi for the frequencies of GzmB producing cells of activated virus-specific CD8+ T cells using flow cytometry. Four independent experiments were analyzed with each dot representing a single mouse. Bars indicate the mean and the SEM. For statistical analysis a Student’s t-test was used (ns not significant and * < 0.05).
There is evidence that TNFR2-signaling through memTNFα is important for the homeostatic stability of Foxp3 expression and Treg function (65). In this respect memTNFα appears to function as a growth and survival factor similar to IL-2 (1, 18–20). It has been shown that TNFR2 stimulation in concert with IL-2 can up-regulate in Tregs not only the expression of the co-stimulatory molecules TNFR2, 4-1BB, and OX40 but also results in induction of proliferation (47). Thus, one way that CD8+ T cells cross-communicate with Tregs is by production of IL-2 (66, 67). Activated CD4+ T cells can also be a source of IL-2 (68, 69). However, we have shown that the expansion of the Vβ5+ Treg subset is independent of IL-2 (17), a unique property that is likely related to the very strong signal 1 being provided by Sag, which can overcome the requirement for IL-2 (26). Thus, whereas a second signal delivered by memTNFα on CD8+ T cells is sufficient to fully activate proliferation of the Sag-stimulated Vβ5+ Tregs, the remaining Tregs retain dependence on IL-2 for proliferation (17) and we showed that the IL-2 driving the expansion of the non-Vβ5+ Tregs during FV infection was mainly produced by virus-specific effector CD4+ T cells (70).
Although it remains to be determined whether humans have infection-induced, Sag-specific Treg subsets, circumstantial evidence points in that direction. It is known that, like mice, humans encode endogenous retroviral superantigens that can induce the proliferation of conventional CD4+ T cells (71–73). Furthermore, Sag transcription has been shown to be induced by infectious agents (50, 72, 74, 75). As discussed above, it is also known that TNFR2 signaling plays an important role in human Treg function (57–59). If Sag-specific Tregs are involved in the immunological control of human diseases they make good targets for intervention since they could be regulated independently of other Treg subsets. They could be activated by TNFR2-specific agonists (50) to increase immunosuppression and dampen immunopathogenic responses or they could be deactivated by anti-TNFα to increase effector responses and help to clear infections. Furthermore, broader regulatory responses might be induced by combination therapies with IL-2 and a TNFR2 agonist, which should have more generalized effects on proliferation and function of different Treg subsets.
Supplementary Material
Acknowledgements
We thank Daniela Männel (Institute for Immunology, University Regensburg, Germany) for kindly providing TNFR2-KO mice and Percy Knolle (Institute for Molecular Medicine and Experimental Immunology, University of Bonn, Germany) for kindly providing TNFR1+2-KO mice.
Glossary
- compTNFα
complexed tumor necrosis factor α
- dpi
days post infection
- FV
Friend Virus
- memTNFα
membrane-bound tumor necrosis factor α
- MFI
mean fluorescence intensity
- Mtv
mammary tumor virus
- Sag
superantigen
- solTNFα
soluble tumor necrosis factor α
- TACE
tumor necrosis factor α convertase
- Tg
transgenic
- TNFR2
tumor necrosis factor receptor
- Tregs
regulatory T cells
Footnotes
# This research was partially funded by the Division of Intramural Research of the NIAID, NIH and by the German Research Association (DFG) Transregio 60 project B4 and the graduate school GK1045.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 2.Groux H, Powrie F. Regulatory T cells and inflammatory bowel disease. Immunol Today. 1999;20:442–445. doi: 10.1016/s0167-5699(99)01510-8. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 4.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 5.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 6.Veiga-Parga T, Sehrawat S, Rouse BT. Role of regulatory T cells during virus infection. Immunol Rev. 2013;255:182–196. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson SJ, Hasenkrug KJ. The role of virus-induced regulatory T cells in immunopathology. Springer Semin Immunopathol. 2006;28:51–62. doi: 10.1007/s00281-006-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietze KK, Zelinskyy G, Gibbert K, Schimmer S, Francois S, Myers L, Sparwasser T, Hasenkrug KJ, Dittmer U. Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proc Natl Acad Sci U S A. 2011;108:2420–2425. doi: 10.1073/pnas.1015148108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc Natl Acad Sci U S A. 2001;98:9226–9230. doi: 10.1073/pnas.151174198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural regulatory T cells and persistent viral infection. J Virol. 2008;82:21–30. doi: 10.1128/JVI.01768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelinskyy G, Myers L, Dietze KK, Gibbert K, Roggendorf M, Liu J, Lu M, Kraft AR, Teichgraber V, Hasenkrug KJ, Dittmer U. Virus-specific CD8+ T cells upregulate programmed death-1 expression during acute friend retrovirus infection but are highly cytotoxic and control virus replication. Journal of immunology. 2011;187:3730–3737. doi: 10.4049/jimmunol.1101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittmer U, Hasenkrug KJ. Functional impairment of CD8+ T cells by regulatory T cells during persistent retroviral infection (vol 20, pg 293, 2004) Immunity. 2004;20:653–653. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 13.Zelinskyy G, Dietze K, Sparwasser T, Dittmer U. Regulatory T cells suppress antiviral immune responses and increase viral loads during acute infection with a lymphotropic retrovirus. PLoS Pathog. 2009;5:e1000406. doi: 10.1371/journal.ppat.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelinskyy G, Dietze KK, Husecken YP, Schimmer S, Nair S, Werner T, Gibbert K, Kershaw O, Gruber AD, Sparwasser T, Dittmer U. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood. 2009;114:3199–3207. doi: 10.1182/blood-2009-03-208736. [DOI] [PubMed] [Google Scholar]
- 15.Zelinskyy G, Kraft AR, Schimmer S, Arndt T, Dittmer U. Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during Friend retrovirus infection. Eur J Immunol. 2006;36:2658–2670. doi: 10.1002/eji.200636059. [DOI] [PubMed] [Google Scholar]
- 16.Dietze KK, Zelinskyy G, Liu J, Kretzmer F, Schimmer S, Dittmer U. Combining regulatory T cell depletion and inhibitory receptor blockade improves reactivation of exhausted virus-specific CD8+ T cells and efficiently reduces chronic retroviral loads. PLoS Pathog. 2013;9:e1003798. doi: 10.1371/journal.ppat.1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers L, Joedicke JJ, Carmody AB, Messer RJ, Kassiotis G, Dudley JP, Dittmer U, Hasenkrug KJ. IL-2-independent and TNF-alpha-dependent expansion of Vbeta5+ natural regulatory T cells during retrovirus infection. J Immunol. 2013;190:5485–5495. doi: 10.4049/jimmunol.1202951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouse M, Nagarkatti M, Nagarkatti PS. The role of IL-2 in the activation and expansion of regulatory T-cells and the development of experimental autoimmune encephalomyelitis. Immunobiology. 2013;218:674–682. doi: 10.1016/j.imbio.2012.08.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 21.Blish CA, Gallay BJ, Turk GL, Kline KM, Wheat W, Fink PJ. Chronic modulation of the TCR repertoire in the lymphoid periphery. J Immunol. 1999;162:3131–3140. [PubMed] [Google Scholar]
- 22.Punkosdy GA, Blain M, Glass DD, Lozano MM, O’Mara L, Dudley JP, Ahmed R, Shevach EM. Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proc Natl Acad Sci U S A. 2011;108:3677–3682. doi: 10.1073/pnas.1100213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, Supper E, Shpilka T, Minis A, Kaempfer R. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 2011;9:e1001149. doi: 10.1371/journal.pbio.1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bueno C, Lemke CD, Criado G, Baroja ML, Ferguson SS, Rahman AK, Tsoukas CD, McCormick JK, Madrenas J. Bacterial superantigens bypass Lck-dependent T cell receptor signaling by activating a Galpha11-dependent, PLC-beta-mediated pathway. Immunity. 2006;25:67–78. doi: 10.1016/j.immuni.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Zamoyska R. Superantigens: supersignalers? Sci STKE. 2006;2006:pe45. doi: 10.1126/stke.3582006pe45. [DOI] [PubMed] [Google Scholar]
- 26.Jin H, Gong D, Adeegbe D, Bayer AL, Rolle C, Yu A, Malek TR. Quantitative assessment concerning the contribution of IL-2Rbeta for superantigen-mediated T cell responses in vivo. Int Immunol. 2006;18:565–572. doi: 10.1093/intimm/dxh398. [DOI] [PubMed] [Google Scholar]
- 27.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 28.Faustman DL, Davis M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front Immunol. 2013;4:478. doi: 10.3389/fimmu.2013.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol. 2010;40:1099–1106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagar M, Jacob-Hirsch J, Vernitsky H, Berkun Y, Ben-Horin S, Amariglio N, Bank I, Kloog Y, Rechavi G, Goldstein I. TNF activates a NF-kappaB-regulated cellular program in human CD45RA- regulatory T cells that modulates their suppressive function. J Immunol. 2010;184:3570–3581. doi: 10.4049/jimmunol.0902070. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 33.Kleijwegt FS, Laban S, Duinkerken G, Joosten AM, Zaldumbide A, Nikolic T, Roep BO. Critical role for TNF in the induction of human antigen-specific regulatory T cells by tolerogenic dendritic cells. J Immunol. 2010;185:1412–1418. doi: 10.4049/jimmunol.1000560. [DOI] [PubMed] [Google Scholar]
- 34.Chopra M, Riedel SS, Biehl M, Krieger S, Krosigk Vvon, Bauerlein CA, Brede C, Garrote ALJordan, Kraus S, Schafer V, Ritz M, Mattenheimer K, Degla A, Mottok A, Einsele H, Wajant H, Beilhack A. Tumor necrosis factor receptor 2-dependent homeostasis of regulatory T cells as a player in TNF-induced experimental metastasis. Carcinogenesis. 2013;34:1296–1303. doi: 10.1093/carcin/bgt038. [DOI] [PubMed] [Google Scholar]
- 35.Grinberg-Bleyer Y, Saadoun D, Baeyens A, Billiard F, Goldstein JD, Gregoire S, Martin GH, Elhage R, Derian N, Carpentier W, Marodon G, Klatzmann D, Piaggio E, Salomon BL. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J Clin Invest. 2010;120:4558–4568. doi: 10.1172/JCI42945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 37.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 39.Fung-Leung WP, Schilham MW, Rahemtulla A, Kundig TM, Vollenweider M, Potter J, Ewijk Wvan, Mak TW. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 40.McIlwain DR, Lang PA, Maretzky T, Hamada K, Ohishi K, Maney SK, Berger T, Murthy A, Duncan G, Xu HC, Lang KS, Haussinger D, Wakeham A, Itie-Youten A, Khokha R, Ohashi PS, Blobel CP, Mak TW. iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science. 2012;335:229–232. doi: 10.1126/science.1214448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erickson SL, Sauvage FJde, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan KC, Schreiber RD, Goeddel DV, Moore MW. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- 42.Robertson SJ, Ammann CG, Messer RJ, Carmody AB, Myers L, Dittmer U, Nair S, Gerlach N, Evans LH, Cafruny WA, Hasenkrug KJ. Suppression of acute anti-friend virus CD8+ T-cell responses by coinfection with lactate dehydrogenase-elevating virus. J Virol. 2008;82:408–418. doi: 10.1128/JVI.01413-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schepers K, Toebes M, Sotthewes G, Vyth-Dreese FA, Dellemijn TA, Melief CJ, Ossendorp F, Schumacher TN. Differential kinetics of antigen-specific CD4+ and CD8+ T cell responses in the regression of retrovirus-induced sarcomas. J Immunol. 2002;169:3191–3199. doi: 10.4049/jimmunol.169.6.3191. [DOI] [PubMed] [Google Scholar]
- 44.Loetscher H, Stueber D, Banner D, Mackay F, Lesslauer W. Human tumor necrosis factor alpha (TNF alpha) mutants with exclusive specificity for the 55-kDa or 75-kDa TNF receptors. J Biol Chem. 1993;268:26350–26357. [PubMed] [Google Scholar]
- 45.Ohlen C, Kalos M, Cheng LE, Shur AC, Hong DJ, Carson BD, Kokot NC, Lerner CG, Sather BD, Huseby ES, Greenberg PD. CD8+ T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J. Exp. Med. 2002;195:1407–1418. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 47.Hamano R, Huang J, Yoshimura T, Oppenheim JJ, Chen X. TNF optimally activatives regulatory T cells by inducing TNF receptor superfamily members TNFR2, 4-1BB and OX40. Eur J Immunol. 2011;41:2010–2020. doi: 10.1002/eji.201041205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Qin H, Chesebro B, Cheever MA. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J. Virol. 1996;70:7773–7782. doi: 10.1128/jvi.70.11.7773-7782.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adrain C, Zettl M, Christova Y, Taylor N, Freeman M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science. 2012;335:225–228. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rauert H, Wicovsky A, Muller N, Siegmund D, Spindler V, Waschke J, Kneitz C, Wajant H. Membrane tumor necrosis factor (TNF) induces p100 processing via TNF receptor-2 (TNFR2) J Biol Chem. 2010;285:7394–7404. doi: 10.1074/jbc.M109.037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, Chen EY, Goeddel DV. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci U S A. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, Liu X, Xiao L, Chen X, Wan B, Chin YE, Zhang JZ. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19:322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 54.Biton J, Semerano L, Delavallee L, Lemeiter D, Laborie M, Grouard-Vogel G, Boissier MC, Bessis N. Interplay between TNF and regulatory T cells in a TNF-driven murine model of arthritis. J Immunol. 2011;186:3899–3910. doi: 10.4049/jimmunol.1003372. [DOI] [PubMed] [Google Scholar]
- 55.Ryba M, Marek N, Hak L, Rybarczyk-Kapturska K, Mysliwiec M, Trzonkowski P, Mysliwska J. Anti-TNF rescue CD4+Foxp3+ regulatory T cells in patients with type 1 diabetes from effects mediated by TNF. Cytokine. 2011;55:353–361. doi: 10.1016/j.cyto.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Stoop JN, Woltman AM, Biesta PJ, Kusters JG, Kuipers EJ, Janssen HL, van der Molen RG. Tumor necrosis factor alpha inhibits the suppressive effect of regulatory T cells on the hepatitis B virus-specific immune response. Hepatology. 2007;46:699–705. doi: 10.1002/hep.21761. [DOI] [PubMed] [Google Scholar]
- 57.Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, Price RN, Engwerda CR, Anstey NM, Plebanski M. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, Lawrence E, Ngwa-Amambua A, Jayasooriya S, Cheeseman IH, Gomez-Escobar N, Okebe J, Conway DJ, Riley EM. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009;5:e1000364. doi: 10.1371/journal.ppat.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wammes LJ, Wiria AE, Toenhake CG, Hamid F, Liu KY, Suryani H, Kaisar MM, Verweij JJ, Sartono E, Supali T, Smits HH, Luty AJ, Yazdanbakhsh M. Asymptomatic plasmodial infection is associated with increased tumor necrosis factor receptor II-expressing regulatory T cells and suppressed type 2 immune responses. J Infect Dis. 2013;207:1590–1599. doi: 10.1093/infdis/jit058. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X, Oppenheim JJ. TNF-alpha: an activator of CD4+FoxP3+TNFR2+ regulatory T cells. Curr Dir Autoimmun. 2010;11:119–134. doi: 10.1159/000289201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X, Oppenheim JJ. Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 2011;585:3611–3618. doi: 10.1016/j.febslet.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Housley WJ, Adams CO, Nichols FC, Puddington L, Lingenheld EG, Zhu L, Rajan TV, Clark RB. Natural but not inducible regulatory T cells require TNF-alpha signaling for in vivo function. J Immunol. 2011;186:6779–6787. doi: 10.4049/jimmunol.1003868. [DOI] [PubMed] [Google Scholar]
- 64.Tanriver Y, Martin-Fontecha A, Ratnasothy K, Lombardi G, Lechler R. Superantigen-activated regulatory T cells inhibit the migration of innate immune cells and the differentiation of naive T cells. J Immunol. 2009;183:2946–2956. doi: 10.4049/jimmunol.0803953. [DOI] [PubMed] [Google Scholar]
- 65.Chen X, Wu X, Zhou Q, Howard OM, Netea MG, Oppenheim JJ. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J Immunol. 2013;190:1076–1084. doi: 10.4049/jimmunol.1202659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kish DD, Gorbachev AV, Fairchild RL. CD8+ T cells produce IL-2, which is required for CD(4+)CD25+ T cell regulation of effector CD8+ T cell development for contact hypersensitivity responses. J Leukoc Biol. 2005;78:725–735. doi: 10.1189/jlb.0205069. [DOI] [PubMed] [Google Scholar]
- 67.McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci U S A. 2011;108:7529–7534. doi: 10.1073/pnas.1103782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Gorman WE, Dooms H, Thorne SH, Kuswanto WF, Simonds EF, Krutzik PO, Nolan GP, Abbas AK. The initial phase of an immune response functions to activate regulatory T cells. J Immunol. 2009;183:332–339. doi: 10.4049/jimmunol.0900691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 70.Joedicke JJ, Dietze KK, Zelinskyy G, Dittmer U. The phenotype and activation status of regulatory T cells during Friend retrovirus infection. Virol Sin. 2014 doi: 10.1007/s12250-014-3396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conrad B, Weissmahr RN, Boni J, Arcari R, Schupbach J, Mach B. A human endogenous retroviral superantigen as candidate autoimmune gene in type I diabetes. Cell. 1997;90:303–313. doi: 10.1016/s0092-8674(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 72.Stauffer Y, Marguerat S, Meylan F, Ucla C, Sutkowski N, Huber B, Pelet T, Conrad B. Interferon-alpha-induced endogenous superantigen. a model linking environment and autoimmunity. Immunity. 2001;15:591–601. doi: 10.1016/s1074-7613(01)00212-6. [DOI] [PubMed] [Google Scholar]
- 73.Sutkowski N, Conrad B, Thorley-Lawson DA, Huber BT. Epstein-Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity. 2001;15:579–589. doi: 10.1016/s1074-7613(01)00210-2. [DOI] [PubMed] [Google Scholar]
- 74.Turcanova VL, Bundgaard B, Hollsberg P. Human herpesvirus-6B induces expression of the human endogenous retrovirus K18-encoded superantigen. J Clin Virol. 2009;46:15–19. doi: 10.1016/j.jcv.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 75.Hsiao FC, Lin M, Tai A, Chen G, Huber BT. Cutting edge: Epstein-Barr virus transactivates the HERV-K18 superantigen by docking to the human complement receptor 2 (CD21) on primary B cells. J Immunol. 2006;177:2056–2060. doi: 10.4049/jimmunol.177.4.2056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.