Abstract
Using quantitative PCR we differentiated asymptomatic and symptomatic Indian Leishmania donovani infection. qPCR on blood of 40 visceral leishmaniasis, 130 endemic and 40 nonendemic healthy controls showed 500 times less (p<0.0001) parasitemia in asymptomatic compared to the symptomatic ones, and threshold of 5 parasite genome/ml for the clinical disease.
Keywords: Visceral Leishmaniasis, Asymptomatic, patients, qPCR
Visceral leishamaniasis (VL, Kala-azar) is a serious public health problem in some states of India, with state of Bihar bearing 90% of the total VL burden (Trouiller et al., 2002). The symptomatic infection is characterized by prolonged fever, splenomegaly, weight loss, anemia, and hypergammaglobulinemia, and fatal if left unreated (Sundar and Rai, 2002). The diagnosis is confirmed by the demonstration of the parasites in splenic or bone marrow smears or indirectly through positive serology or nucleic acid demonstration with a typical clinical background (Adhya et al., 1995; Chulay and Bryceson, 1983; Githure et al., 1984; Lightner et al., 1983; Sundar and Rai, 2002; Sundar et al., 2002; Sundar et al., 2007; Zijlstra et al., 1998). However, healthy individuals from endemic region may also test positive either with serology or nucleic acid detection, and these are asymptomatic leishmania infection. Majority of these asymptomatically infected persons do not progress to VL (Bretagne et al., 2001; Srivastava et al., 2013). In a study from Bihar (India), seroepidemiological survey indicated prevalence of asymptomatic leishmania infection as 110 per 1,000 persons. The rate of progression to symptomatic illness was 17.85 per 1,000 person-months. The incidence rate ratio of progression to symptomatic case was 3.36 (Topno et al., 2010). In another study using DAT seroconversion as a marker of infection, incident asymptomatic infection was eight times more frequent than incident VL disease in India and Nepal, and about 1 in 50 of these latent infections lead to VL in the next 18 months (Ostyn et al., 2011). The conventional PCR shows a wide range of positivity in healthy subjects from endemic regions (Costa et al., 2002; Fakhar et al., 2008; le Fichoux et al., 1999). But an important drawback of the conventional PCR as well as that of serology is their inability to differentiate between active VL with those infected but asymptomatic. These drawbacks can be overcome by quantitative PCR (qPCR). Therefore the aim of this study was to validate qPCR for epidemiological purposes, by assessing infection levels in the endemic healthy population. In this study we primarily wanted to know whether in Indian VL quantification of parasite load could differentiate the asymptomatic with those with active VL. This study was carried out at the Department of Medicine, Banaras Hindu University (BHU), Varanasi and at its field site; Kala-Azar Medical Research Centre, Muzaffarpur, Bihar. The study was approved by the Ethics Committee of the Institute of Medical Sciences, BHU. Written informed consent was obtained from each participating individual.
For selection of the best suited primer in terms of sensitivity, six set of primers targeting Leishmania specific genes with different copy number were chosen to prepare the standard curve (Table1) (Weirather et al., 2011). For qPCR assay, kDNA specific primers were selected as these could detect up to 0.001 parasite genome/reaction (Figure.1).
Table 1.
Leishmania specific primers having different copy numbers.
| Leishmania specific Primer | Forward sequence | Reverse Sequence |
|---|---|---|
| DNA polymerase (single copy) | AGGAGGATGGCAAGCGGAAG | GCGACGGGTACAGGGAGTTG |
| Hsp 70 (~2 copy) | GAAGGTGCAGTCCCTCGTGT | CCTCCGTCTGCTTGCTCTTG |
| Maxicircle (~25–50 copy) | GCTTGGTTGGATTATTTTTGCTG | AACAACATTTTAACTCTTGTAGGATTCG |
| Miniexon (~200 copy) | CGAAACTTCCGGAACCTGTCTT | CACCACACGCACGCACAC |
| MAG region (≥18 copy) | AGAGCGTGCCTTGGATTGTG | CGCTGCGTTGATTGCGTTG |
| kDNA(minicircle) (~10,000 copy) | GGGTAGGGGCGTTCTGC | TACACCAACCCCCAGTTTGC |
Figure. 1.
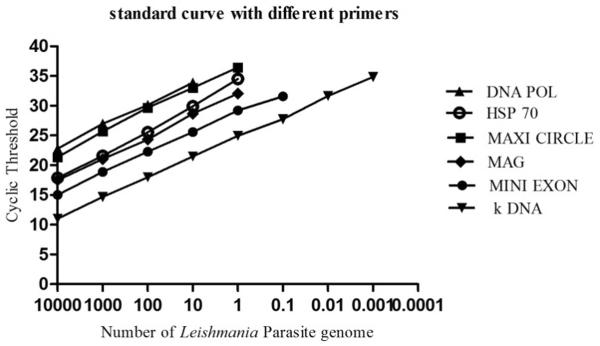
Standard curve generated by different primers.
Males and females in the age group between 6–55 years were enrolled in the study. Three groups of the subjects were studied, i) Randomly selected healthy individuals(n=130) living in the VL endemic region of Muzaffarpur (EHC). ii) Parasitologically confirmed patients with VL(AVL, n=40). The clinical and laboratory characteristics of VL patients are in Table 2, and iii) Non endemic Controls(n=40) were subjects coming to the BHU hospital from non-endemic regions for VL. DNA extraction was done by QIAamp DNA mini kit (Qiagen, Hidden Germany) as per the manufacturer's instructions from 30μl of buffy coat(total 200 μl) was isolated from 2 ml of fresh blood. Standard curve based absolute quantification method for quantification of parasite load was used according to a previously described technique (Sudarshan et al., 2011). Laboratory personnel were blinded to the nature of samples being used in the study.
Table 2.
Clinical and laboratory features of patients with Visceral leishmaniasis (n=40)
| Parameters | Mean±SD |
|---|---|
| Gender (Male) | 23 (57.5%) |
| Age(year) | 28.84±17.29 |
| Weight (kg.) | 37.7±13.6 |
| Duration of fever (days) | 58.6±42 |
| Maximum Temp. | 101.7±2.1 |
| Spleen size(cm) | 4.2±3.1 |
| Splenic scoreƪ | 2.33±1.22 |
| RBC (×106/μL) | 3.37±0.78 |
| Haemoglobin(g/dl) | 7.86±0.05 |
| WBC (×103/μL) | 3.7±0.6 |
| Platelet count (×106/μL) | 1.5±0.2 |
WBC-White Blood Cells, RBC-Red Blood Cells
Scoring of parasite load is logarithmic scale from 1 to 6, where 0 means no parasites/1,000 microscopic field (1,000×), 1 is 1–10 parasites/field, and 6 is ≥ 100 parasites/field
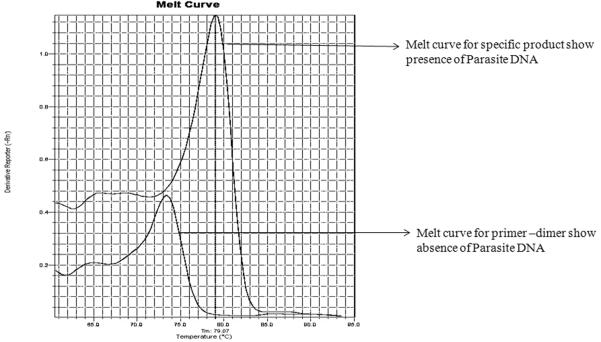
Data analysis was done using Prism (Graph pad software, La Jalla, CA, USA). Non parametric Mann-Whitney test was applied to assess statistical significance at 95% confidence interval. Specific product had melt peak at approx 79°C. No subject from the NEHC group showed specific melt peak and therefore were negative for Leishmania DNA (Figure 2.). Amongst EHC, 36.9% (48 in 130) showed Leishmania specific DNA in their peripheral blood. The median value of parasitemia in EHC was 0.17 (range 0.009 to 5.07) while in AVL it was in the range 8 to 54,610 (Median = 574.5) parasite genome/mL of blood.
Figure. 2.
Melt curve analysis for presence or absence of Leishmania parasite.
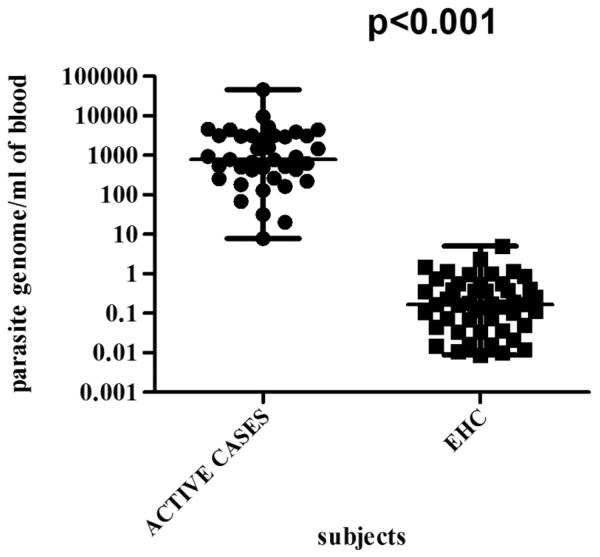
For epidemiological surveys to be fully informative, it is necessary to include such a test which can be considered as a marker of infection and can clearly distinguish symptomatic from asymptomatic. This study demonstrated the use of qPCR to delineate the status of Leishmania infection. Large proportion of positive EHC indicate widespread leishmanial infection. Similar findings has been reported from Mediterranean region where qPCR showed more than 50% population harboring Leishmania specific DNA in their blood (Mary et al., 2006). In the current study, we confirmed that levels of circulating kDNA detected in asymptomatic individuals are nearly 500 times lower than those found in AVL (p<0.0001) (Figure 3.). In asymptomatics maximum limit of parasitemia was 5 parasite genome/ml of blood while in symptomatics it remained above this level. Therefore it can be assumed that the asymptomatic individuals, having parasite level of more than 5, are at the risk of the development of clinical VL. Thus, qPCR assay can be use as a tool to follow up asymptomatics who have parasitemia at the threshold level and monitor whether they convert to symptomatic infection by increasing parasite number in their blood or clear the infection. Usually asymptomatic infection does not persist for more than one year, but rarely asymptomatic infection may last for decades (Badaro et al., 1986; Buchetont et al., 2003; Guevara et al., 1993). In asymptomatics, inspite of low parasitemia, their huge number would constitute a formidable reservoir of infection especially in case of the anthroponotic transmission and may be playing a significant role in driving it (Costa et al., 2002). The ability of these parasite positive asymptomatic individuals to infect the sand flies (through xenodiagnosis) should be the next logical step. Though qPCR appears to be a clearly superior technique, its application in the endemic areas of developing world is not simple. Initial investment for the equipment and other infrastructure is quite significant, further running cost for such laboratories are going to be quite high and skilled personnel will be required to run the facility. Whether it is possible to have one central qPCR laboratory catering to each endemic district, remains to be seen. Serology, particularly rK39 rapid diagnostic tests, seems to do well in the Indian Subcontinent, however, up to 32% endemic healthy subjects may be positive. Therefore for diagnosis of VL, a test specific for symptomatic VL will provide a significant advance, and qPCR appears to be an improvement over serology notwithstanding the limitations discussed above.
Figure. 3.
Parasitemia level in active cases and EHC(asymptomatics).
Acknowledgments
We acknowledge the subjects and staff members of KAMRC, Muzaffarpur for participating in the study.
Financial support The study was partially funded by National Institute of Allergy and Infectious disease (NIAID), DMID funding mechanism, Tropical Medicine Research Center Grant number, P50AI074321. Author Medhavi Sudarshan got financial support from Council of Scientific and Industrial Research (CSIR), New Delhi, India.
Footnotes
Conflict of interest No declared
Transparency declarations None declared
References
- Adhya S, Chatterjee M, Hassan MQ, Mukherjee S, Sen S. Detection of Leishmania in the blood of early kala-azar patients with the aid of the polymerase chain reaction. Trans R Soc Trop Med Hyg. 1995;89:622–4. doi: 10.1016/0035-9203(95)90416-6. [DOI] [PubMed] [Google Scholar]
- Badaro R, Jones TC, Carvalho EM, Sampaio D, Reed SG, Barral A, et al. New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–11. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- Bretagne S, Durand R, Olivi M, Garin JF, Sulahian A, Rivollet D, et al. Real-time PCR as a new tool for quantifying Leishmania infantum in liver in infected mice. Clin Diagn Lab Immunol. 2001;8:828–31. doi: 10.1128/CDLI.8.4.828-831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchetont B, El-Safi SH, Hammad A, Kheir MM, Eudes N, Mirgani A, et al. Antileishmanial antibodies in an outbreak of visceral leishmaniasis in eastern Sudan: high antibody responses occur in resistant subjects and are not predictive of disease. Trans R Soc Trop Med Hyg. 2003;97:463–8. doi: 10.1016/s0035-9203(03)90092-7. [DOI] [PubMed] [Google Scholar]
- Chulay JD, Bryceson AD. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am J Trop Med Hyg. 1983;32:475–9. doi: 10.4269/ajtmh.1983.32.475. [DOI] [PubMed] [Google Scholar]
- Costa CH, Stewart JM, Gomes RB, Garcez LM, Ramos PK, Bozza M, et al. Asymptomatic human carriers of Leishmania chagasi. Am J Trop Med Hyg. 2002;66:334–7. doi: 10.4269/ajtmh.2002.66.334. [DOI] [PubMed] [Google Scholar]
- Fakhar M, Motazedian MH, Hatam GR, Asgari Q, Kalantari M, Mohebali M. Asymptomatic human carriers of Leishmania infantum: possible reservoirs for Mediterranean visceral leishmaniasis in southern Iran. Ann Trop Med Parasitol. 2008;102:577–83. doi: 10.1179/136485908X337526. [DOI] [PubMed] [Google Scholar]
- Githure JI, Oster CN, Chulay JD. Comparison of three culture media for isolating Leishmania donovani from splenic aspirates in Kenyan visceral leishmaniasis. East Afr Med J. 1984;61:539–43. [PubMed] [Google Scholar]
- Guevara P, Ramirez JL, Rojas E, Scorza JV, Gonzalez N, Anez N. Leishmania braziliensis in blood 30 years after cure. Lancet. 1993;341:1341. doi: 10.1016/0140-6736(93)90845-8. [DOI] [PubMed] [Google Scholar]
- le Fichoux Y, Quaranta JF, Aufeuvre JP, Lelievre A, Marty P, Suffia I, et al. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J Clin Microbiol. 1999;37:1953–7. doi: 10.1128/jcm.37.6.1953-1957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner LK, Chulay JD, Bryceson AD. Comparison of microscopy and culture in the detection of Leishmania donovani from splenic aspirates. Am J Trop Med Hyg. 1983;32:296–9. doi: 10.4269/ajtmh.1983.32.296. [DOI] [PubMed] [Google Scholar]
- Mary C, Faraut F, Drogoul MP, Xeridat B, Schleinitz N, Cuisenier B, et al. Reference values for Leishmania infantum parasitemia in different clinical presentations: quantitative polymerase chain reaction for therapeutic monitoring and patient follow-up. Am J Trop Med Hyg. 2006;75:858–63. [PubMed] [Google Scholar]
- Ostyn B, Gidwani K, Khanal B, Picado A, Chappuis F, Singh SP, et al. Incidence of symptomatic and asymptomatic Leishmania donovani infections in high-endemic foci in India and Nepal: a prospective study. PLoS Negl Trop Dis. 2011;5:e1284. doi: 10.1371/journal.pntd.0001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P, Gidwani K, Picado A, Van der Auwera G, Tiwary P, Ostyn B, et al. Molecular and serological markers of Leishmania donovani infection in healthy individuals from endemic areas of Bihar, India. Trop Med Int Health. 2013;18:548–54. doi: 10.1111/tmi.12085. [DOI] [PubMed] [Google Scholar]
- Sudarshan M, Weirather JL, Wilson ME, Sundar S. Study of parasite kinetics with antileishmanial drugs using real-time quantitative PCR in Indian visceral leishmaniasis. J Antimicrob Chemother. 2011;66:1751–5. doi: 10.1093/jac/dkr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9:951–8. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Sahu M, Mehta H, Gupta A, Kohli U, Rai M, et al. Noninvasive management of Indian visceral leishmaniasis: clinical application of diagnosis by K39 antigen strip testing at a kala-azar referral unit. Clin Infect Dis. 2002;35:581–6. doi: 10.1086/342057. [DOI] [PubMed] [Google Scholar]
- Sundar S, Singh RK, Bimal SK, Gidwani K, Mishra A, Maurya R, et al. Comparative evaluation of parasitology and serological tests in the diagnosis of visceral leishmaniasis in India: a phase III diagnostic accuracy study. Trop Med Int Health. 2007;12:284–9. doi: 10.1111/j.1365-3156.2006.01775.x. [DOI] [PubMed] [Google Scholar]
- Topno RK, Das VN, Ranjan A, Pandey K, Singh D, Kumar N, et al. Asymptomatic infection with visceral leishmaniasis in a disease-endemic area in bihar, India. Am J Trop Med Hyg. 2010;83:502–6. doi: 10.4269/ajtmh.2010.09-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N. Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet. 2002;359:2188–94. doi: 10.1016/S0140-6736(02)09096-7. [DOI] [PubMed] [Google Scholar]
- Weirather JL, Jeronimo SM, Gautam S, Sundar S, Kang M, Kurtz MA, et al. Serial quantitative PCR assay for detection, species discrimination, and quantification of Leishmania spp. in human samples. J Clin Microbiol. 2011;49:3892–904. doi: 10.1128/JCM.r00764-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra EE, Daifalla NS, Kager PA, Khalil EA, El-Hassan AM, Reed SG, et al. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin Diagn Lab Immunol. 1998;5:717–20. doi: 10.1128/cdli.5.5.717-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]