Abstract
BACKGROUND
Bile acids (BAs) are nutrient-responsive hormones that modulate energy balance through cell surface and nuclear receptors. Postprandial plasma BAs have been found to be decreased in obesity.
OBJECTIVE
We aimed to determine whether meal-stimulated circulating BA levels are altered by Roux-en-Y gastric bypass (RYGB), an operation that modifies the neurohumoral determinants of food intake and energy expenditure to cause significant and durable weight loss.
DESIGN
Longitudinal study measuring fasting and postprandial plasma BAs before and after RYGB.
SUBJECTS
Five obese surgical patients and eight lean controls underwent frequent blood sampling after a standard liquid meal. Obese subjects were also tested at 1, 4 and 40 weeks after RYGB. Primary and secondary circulating BAs, as well as their glycine and taurine conjugates, were measured via reverse-phase high-performance liquid chromatography/mass spectroscopy.
RESULTS
We found that postprandial excursion of conjugated BAs was 52.4% lower in obese than in lean individuals by area-under-the-curve (AUC) analysis (378 vs 793 µmol min l−1, respectively, P < 0.05). By 40 weeks after RYGB, the meal-induced rise in conjugated BAs increased by 55.5% to the level of healthy lean controls (378 pre-op vs 850 µmol min l− post-op by AUC analyses, P < 0.05). In contrast, postprandial concentrations of unconjugated BAs were similar in lean and obese individuals and were not affected by surgery.
CONCLUSION
In light of the growing evidence that BAs have key roles in glucose, lipid and energy homeostasis, the observation that RYGB normalizes the blunted postprandial circulating BA response in obesity suggests that BAs may contribute to the improvement in meal-related physiology seen after RYGB. Further studies are warranted to examine this hypothesis and to determine the degree to which an augmented BA response to nutrient ingestion may mediate the increased incretin response, brown adipose tissue activation and thermic effect of feeding that has been observed after this operation.
Keywords: bariatric surgery, energy expenditure, metabolomics, postprandial bile acids, TGR5, FXR
INTRODUCTION
Obesity is a pathological state characterized by abnormalities in the neurohumoral regulation of energy balance.1 Previous studies have demonstrated that bile acids (BAs) have key roles in this regulation through the activation of specific nuclear and cell surface receptors such as FXR and TGR5, respectively.2–8 Roux-en-Y gastric bypass (RYGB) is a gastrointestinal weight loss procedure that alters the physiology of energy regulation to cause profound and durable weight loss. This operation, as well as other bariatric procedures including vertical sleeve gastrectomy and laparoscopic adjustable gastric banding, have been associated with elevations in circulating BAs in humans in the fasted state.9–11
Both the detergent and hormonal effects of BAs, however, are particularly relevant to postprandial physiology. Upon nutrient ingestion, BAs released into the intestine assist in the digestion of dietary fats and fat-soluble vitamins. Through an FXR-mediated pathway in the intestine, BAs have been found to stimulate postprandial release of FGF19, a protein that regulates glucose disposal and lipid homeostasis, and acts through FGFR4 to modulate BA synthesis in the liver.12–14 Through a TGR5-mediated pathway in enteroendocrine cells, BAs stimulate the release of glucagon-like peptide-1 (GLP-1), a regulatory peptide that promotes satiety and stimulates β-cell insulin release.15,16 The sites of action of BAs likely extend beyond the intestine since circulating BA concentrations triple after meal ingestion.17–20 Watanabe et al.21 have shown that BAs increase energy expenditure (EE) via a TGR5-cAMP-deiodinase 2-dependent pathway in skeletal muscle and brown adipose tissue (BAT). This finding suggests a direct physiologic function for the circulating component of the BA pool in the normal regulation of energy balance. Moreover, since BAT functions in the thermic effect of feeding, it also raises the possibility that these nutrient-responsive lipid hormones contribute to postprandial EE. Indeed, Ockenga et al.22 have recently found that postprandial levels of circulating BAs are strongly correlated with postprandial EE in lean individuals. In keeping with a role for the nutrient-stimulated BA fraction in the regulation of energy balance, Glicksman et al.23 have observed that postprandial circulating glycine-conjugated concentrations of BAs are preferentially decreased in obesity.
Several of the physiologic effects of RYGB are also selective for or more pronounced in the postprandial state. This operation is associated with significant elevations in postprandial GLP-1, which directly increases insulin release and promotes the weight loss-independent improvement in oral glucose tolerance observed after RYGB.24–28 We and others have observed a large increase in EE after RYGB in rodent models.29,30 Recent studies have also shown increased postprandial thermogenesis in humans after RYGB,31,32 extending earlier observations that RYGB in humans is associated with a blunting of the adaptive reduction in EE (metabolic adaption) normally associated with acute weight loss and the chronic weight-reduced state.33–35 The stimulation of EE in rodents after RYGB is dependent on feeding and is associated with an increased activation of BAT, suggesting that the surgery influences adaptive thermogenesis.36,37 BAT, the predominant mediator of feeding-related thermogenesis in small animals, is now known to be present in adult humans where it also functions in adaptive thermogenesis.38–41 Brown adipocytes have been shown to express the TGR5 cell surface BA receptor and respond to BA stimulation.21 Given the distribution and known physiologic effects of BAs on satiety, lipid, glucose and energy homeostasis, postprandial changes in circulating BA concentrations may well contribute to the enhanced thermic effect of feeding and to the ingestion-related metabolic changes observed after RYGB.
We therefore sought to evaluate the impact of RYGB and associated weight loss on circulating BAs in humans in the postprandial state. We observed that the meal-induced BA response normalizes after RYGB with increased and accelerated postprandial excursion of circulating BAs, an effect that is selective for conjugated BA moieties. These observations likely have implications for the regulation of BA metabolism and the contribution of BAs to the regulation of energy balance and metabolic function.
MATERIALS AND METHODS
Subjects
We undertook a prospective pilot study of five subjects with obesity (BMI≥35 kgm−2) undergoing RYGB and conducted a detailed analysis of the fasting and postprandial circulating BA responses before and at several time points after surgery. We compared this group to 8 healthy lean controls (BMI 18–25 kgm−2). RYGB subjects were recruited from the surgical management program at our obesity medicine and bariatric surgery center. Lean controls were recruited from internet-based advertisements and locally posted flyers. We excluded subjects with evidence of liver disease, with a disrupted enterohepatic circulation from previous bowel resection, diarrheal or malabsorptive syndromes, on oral contraceptives or hormone replacement therapy, on treatments expected to alter the gut microbiota, such as probiotics or antibiotics, or on treatments with BA sequestrants. Written informed consent was obtained from each subject. The study was approved by the Massachusetts General Hospital Institutional Review Board.
Study procedures
Subjects with obesity undergoing RYGB were evaluated longitudinally 4 weeks before surgery and 1, 4 and 40 weeks after surgery. To assess the potential effects of diet and activity on BA levels, subjects completed a 4-day food diary and a 3-day Bouchard physical activity diary prior to each study visit.42,43 Subjects were admitted to the Clinical Research Center at 0900 hours after an 8-h overnight fast. Upon admission, height and weight were measured in light clothing without shoes using a calibrated stadiometer and scale. Subjects were asked to drink a standard 8-ounce liquid meal (TwoCalHN, 475 calories, 40% carbohydrate, 40% fat, 20% protein) slowly over a 20-min period. The 20-min period was allotted to standardize intake across groups and was based on our clinical experience with the tolerability of liquid meal ingestion early after RYGB. Blood samples were drawn through an indwelling intravenous catheter 15 min before meal ingestion (time −15), at the completion of meal ingestion (time 0), and at 15, 30, 60, 90, 120, 150 and 180 min after meal completion (sampling time points for lean controls were −15, 0, 30, 60, 120 and 180 min). Samples were collected into EDTA-containing tubes and processed within 15 min of collection. Plasma aliquots were stored at −80 °C for later analysis.
BA measurements
Concentrations of individual BAs were determined using reverse-phase high-performance liquid chromatography/mass spectroscopy (TNO Laboratories, Zeist, The Netherlands), as previously described.44 We measured the primary BAs (cholic acid and chenodeoxycholic acid) and the secondary BAs (deoxycholic acid, ursodeoxycholic acid and lithocholic acid) as well as the taurine and glycine conjugates of each of these five BAs. Pooled aliquots from each of the study samples were used as quality control samples that were run in every tenth position. To more stringently control for measurement error, we included quality control samples in random positions within and between batches to which the testing laboratory was blinded so as to obtain an unbiased estimate of the within-batch and between-batch variation for individual BAs. Assays were run in multiple batches. Intra-assay and inter-assay coefficients of variation for individual BAs ranged from 2.3% to 7.8% and 2.7% to 13.3%, respectively, with the exception of UDCA and LCA, both of which were present in low concentrations and had higher coefficients of variation. The inclusion or exclusion of these two BAs did not alter the results of the analysis of specific BA subsets that include them.
Statistical analysis
BA concentrations were compared in obese and lean groups using independent sample t-tests, and before and after RYGB using paired t-tests. We conducted analyses of each fasting and postprandial time point, as well as area-under-the-curve (AUC) analysis of postprandial BA excursion. AUC was calculated using the trapezoidal method.45 Since adequate circulating concentrations need to be achieved for ligands, such as BAs, to reach and stimulate their cognate receptors on muscle and BAT, we also assessed the maximum postprandial rise in circulating BA concentration, defined as the maximum increase in plasma concentration detected during a 3-h postprandial period. To quantitate changes in acceleration of postprandial BA levels before and after RYGB, we calculated the %AUC in the first 90 min after meal ingestion (90-min AUC×100/total AUC). Statistical analyses were performed using SPSS, version 18 (IBM, Armonk, NY, USA). P < 0.05 was considered significant. Data are plotted as mean ± standard error of the mean.
RESULTS
Subject characteristics
Subject characteristics are outlined in Table 1. The mean BMI was 47.7 ± 7.4 kgm−2 in obese subjects and 21.7 ± 1.6 kgm−2 in lean subjects. The mean age was 44.8 ± 12.9 and 41.0 ± 11.3 years in the obese and lean groups, respectively. Total dietary intake measured by weight or energy content (kcal) did not differ between the obese and lean groups. Subjects with obesity had a significantly greater percentage of their calories derived from protein (19.2 ± 1.1% (obese) vs 14.8 ± 3.3% (lean); P = 0.02) and a significantly lower percentage derived from carbohydrates (38.8 ± 4.4% (obese) vs 48.1 ± 8.3% (lean); P = 0.04). They also tended to have a greater percentage of their calories derived from fat (41.8 ± 5.6% (obese) vs 35.1 ± 6.3% (lean); P = 0.08). There was no significant difference in physical activity between lean and obese subjects.
Table 1.
Subject characteristics
| Lean | Obese (pre-op) |
P-valuea | 1 week post-op |
P-valueb | 4 weeks post-op |
P-valueb | 40 weeks post-op |
P-valueb | |
|---|---|---|---|---|---|---|---|---|---|
| N (male) | 8 (4) | 5 (4) | 5 (4) | 5 (4) | 5 (4) | ||||
| Age, years, mean (s.d.) | 41.0 (11.3) | 44.8 (12.9) | |||||||
| Weight, kg, mean (s.d.) | 63.9 (8.7) | 158 (26.8) | 150 (24.4) | 141 (23.3) | 105 (21.2) | ||||
| BMI, kg m−2, mean (s.d.) | 21.7 (1.6) | 47.7 (7.4) | 45.2 (6.7) | 42.7 (6.9) | 31.5 (5.0) | ||||
| EBWL, %, mean (s.d.) | NA | NA | 11.5 (4.8) | 23.3 (6.9) | 73.8 (19.7) | ||||
| TBWL, %, mean (s.d.) | NA | NA | 5.3 (2.0) | 10.6 (2.3) | 33.7 (7.2) | ||||
| Activity, kcal kg−1 per day, mean (s.d.) | 45.9 (7.4) | 41.7 (6.2) | 0.31 | 33.7 (2.7) | 0.009 | 38.4 (2.6) | 0.25 | 46.8 (7.8) | 0.09 |
| Total food intake | |||||||||
| g per day, mean (s.d.) | 3027 (731) | 2951 (570) | 0.85 | 1715 (602) | 0.03 | 2241 (362) | 0.04 | 2299 (1093) | 0.09 |
| kcal per day, mean (s.d.) | 2350 (510) | 1978 (184) | 0.15 | 423 (158) | <0.001 | 679 (205) | 0.004 | 1367 (315) | 0.02 |
| Fat intake | |||||||||
| g per day, mean (s.d.) | 94.0 (26.9) | 91.8 (17.6) | 0.88 | 6.6 (5.0) | <0.001 | 28.0 (9.5) | 0.02 | 63.1 (29.1) | 0.19 |
| % of total calories, mean (s.d.) | 35.1 (6.3) | 41.8 (5.6) | 0.08 | 13.1 (8.5) | <0.001 | 36.2 (4.9) | 0.26 | 38.5 (12.4) | 0.68 |
| Protein intake | |||||||||
| g per day, mean (s.d.) | 88.0 (30.4) | 94.8 (10.7) | 0.64 | 25.5 (6.9) | <0.001 | 45.5 (21.5) | 0.03 | 69.5 (19.7) | 0.07 |
| % of total calories, mean (s.d.) | 14.8 (3.3) | 19.2 (1.1) | 0.02 | 26.8 (7.4) | 0.07 | 26.9 (5.2) | 0.03 | 21.1 (7.9) | 0.61 |
| Carbohydrate intake | |||||||||
| g per day, mean (s.d.) | 286 (75.0) | 204 (21.6) | 0.04 | 66.8 (27.1) | <0.001 | 63.6 (14.1) | 0.001 | 140 (34.5) | 0.02 |
| % of total calories, mean (s.d.) | 48.1 (8.3) | 38.8 (4.4) | 0.04 | 60.1 (5.6) | 0.001 | 36.8 (8.5) | 0.85 | 40.1 (13.2) | 0.88 |
Abbreviations: EBWL, excess body weight loss; TBWL, total body weight loss; n/a, not applicable.
Lean vs obese.
Compared to preoperative values.
All subjects with obesity successfully underwent RYGB and had uncomplicated perioperative and postoperative courses. The average percent excess body weight loss was 11.5 ± 4.8% at postoperative week 1, 23.3 ± 6.9% at postoperative week 4 and 73.8 ± 19.7% at postoperative week 40, which corresponded to an average percent total body weight loss of 5.3 ± 2.0%, 10.6 ± 2.3% and 33.7 ± 7.2% at these time points, respectively. The percent excess body weight loss and percent total body weight loss exhibited by these subjects were consistent with the distribution of weight loss clinically observed and previously reported at our center.46 By 40 weeks after surgery, weight loss had either ceased or dramatically slowed for all subjects.
In the early postoperative period (1 week after surgery), subjects had dramatic reductions in absolute food intake (423 ± 158 kcal (post-op) vs 1978 ± 184 kcal (pre-op); P < 0.001) accompanied by a reduction in the percentage of calories derived from fat (13.1 ± 8.5% (post-op) vs 41.8 ± 5.6% (pre-op); P < 0.001) and increases in the percentage of calories derived from carbohydrate and protein (60.1 ± 5.6 (post-op) vs 38.8 ± 4.4% (pre-op); P = 0.001, and 26.8 ± 7.4% (post-op) vs 19.2 ± 1.1% (pre-op); P = 0.07, respectively). By postoperative week 40, absolute intake had increased but remained significantly lower than the preoperative baseline (1367 ± 315 kcal (post-op) vs 1978 ± 184 kcal (pre-op); P = 0.02). Early postoperative changes in dietary composition did not persist, and by 40 weeks after surgery, the percent intakes of fat, carbohydrate and protein were similar to the preoperative baseline. Subjects reported a significant decrease in physical activity at postoperative week 1, reflecting the early postsurgical recovery phase. Physical activity subsequently returned to baseline with no significant difference in activity between baseline and 40 weeks after surgery.
Circulating BAs in obesity and after RYGB
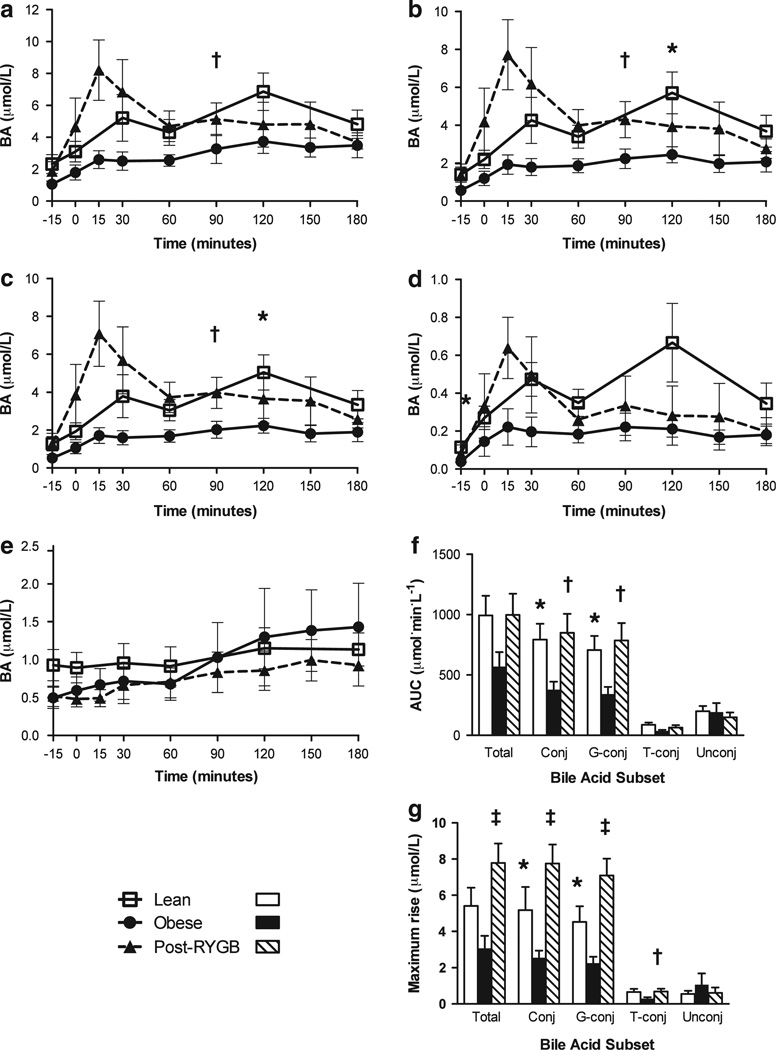
The fasting circulating BA concentrations were not significantly different between lean and obese subjects with the exception of the taurine-conjugated subset, which was lower in obesity (Table 2). In contrast, despite the small number of subjects studied, we detected significant differences in the postprandial circulating BA response (Figure 1). Subjects with obesity had a blunted conjugated BA excursion after a meal compared to lean controls. The AUC of the conjugated BA subset was 52.4% lower in subjects with obesity (378 ± 70 (obese) vs 793 ± 131 (lean); P < 0.05). The maximum increase in circulating conjugated BAs during the first 180 min after a meal was also diminished in subjects with obesity (2.2 ± 0.44 (obese) vs 5.2 ± 1.0 µmol l−1 (lean); P < 0.05), as was the plasma-conjugated BA concentration at 120 min post ingestion (2.4 ± 0.47 (obese) vs 5.7 ± 1.1 µmol l−1 (lean); P < 0.05). The majority of these differences in postprandial conjugated BAs were attributed to the glycine-conjugated BA subset, for which subjects with obesity had a lower postprandial AUC (342 ± 63 (obese) vs 706 ± 118 (lean); P < 0.05), a lower maximum postprandial increase (2.0 ± 0.38 (obese) vs 4.5 ± 0.86 µmol l−1 (lean); P < 0.05), and a lower concentration at 120 min after meal ingestion (2.2 ± 0.43 (obese) vs 5.0 ± 0.93 µmol l−1 (lean); P < 0.05). The taurine-conjugated subset showed a trend in the same direction, with obese subjects having a lower postprandial AUC (35.8 ± 9.1 (obese) vs 87.1 ± 19.0 (lean); P = 0.07). There was no difference between groups in the unconjugated BA subset as determined by AUC (190 ± 76.2 (obese) vs 200 ± 43.8 (lean); P = 0.90), maximum postprandial increase (1.0 ± 0.63 (obese) vs 0.54 ± 0.18 µmol l−1 (lean); P = 0.38) or any single postprandial time point (Figure 1).
Table 2.
Fasting bile acid concentrations, µmol l−1, mean (s.d.)
| Lean | Obese pre-RYGB | P-valuea | 40 weeks post-RYGB | P-valueb | |
|---|---|---|---|---|---|
| Total bile acids | 2.3 (1.7) | 1.1 (0.66) | 0.14 | 1.9 (1.5) | 0.31 |
| Conjugated bile acids | 1.4 (1.1) | 0.56 (0.35) | 0.14 | 1.4 (1.5) | 0.33 |
| Glycine-conjugated bile acids | 1.3 (1.1) | 0.52 (0.34) | 0.16 | 1.3 (1.4) | 0.32 |
| Taurine-conjugated bile acids | 0.11 (0.07) | 0.04 (0.02) | <0.05 | 0.08 (0.11) | 0.48 |
| Unconjugated bile acids | 0.93 (0.59) | 0.50 (0.34) | 0.17 | 0.52 (0.33) | 0.75 |
Lean vs obese.
Pre-RYGB vs post-RYGB.
Figure 1.
Circulating BA response to nutrient ingestion is decreased in obesity and normalized by RYGB. Time course of (a) total BAs, (b) conjugated BAs, (c) glycine-conjugated BAs, (d) taurine-conjugated BAs and (e) unconjugated BAs in plasma before and after ingestion of a standard liquid meal. (f) Area under the postprandial BA excursion curves. (g) Maximum postprandial rise, defined as the maximum increase in plasma BA level from baseline after meal ingestion. *P < 0.05 lean vs pre-RYGB obese, †P < 0.05 pre-RYGB vs post-RYGB, ‡P < 0.01 pre-RYGB vs post-RYGB.
After RYGB, postprandial BA excursion in subjects with obesity was normalized such that the AUC and the maximum postprandial rise of total BAs and conjugated and unconjugated BA subsets in post-RYGB subjects were not significantly different from lean controls (all P-values > 0.3; Figure 1). In addition, there was no significant difference between post-RYGB and lean subjects at any individual fasting or postprandial time point for total, conjugated or unconjugated BAs (Figure 1). By 40 weeks after surgery, the postprandial AUC of the conjugated BA subset was significantly greater than the preoperative baseline (850 ± 157 (post-op) vs 378 ± 65.8 (pre-op); P < 0.05), as was the maximum postprandial rise of conjugated BAs (7.8 ± 1.0 (post-op) vs 2.5 ± 0.41 µmol l−1 (pre-op); P < 0.01) and the conjugated BA concentration at 90 min after a meal (4.3 ± 1.0 (post-op) vs 2.2 ± 0.56 µmol l−1 (pre-op); P < 0.05; Figure 1). These changes were reflected in the maximum rise of total BAs (7.8 ± 1.1 (post-op) vs 3.1 ± 0.69 µmol l−1 (pre-op); P < 0.01) and the 90-min postprandial total BA concentration (5.1 ± 1.1 (post-op) vs 3.3 ± 0.99 µmol l−1 (pre-op); P < 0.05), both of which were significantly greater 40 weeks after surgery than before surgery. The changes in the postprandial response of total and conjugated BAs were mainly attributable to the glycine-conjugated subset, which showed postsurgical normalization in AUC (786 ± 145 (post-op) vs 342 ± 58.3 (pre-op); P < 0.05), in the maximum postprandial rise (7.1 ± 0.94 (post-op) vs 2.3 ± 0.35 µmol l−1 (pre-op); P < 0.01) and in the 90-min postprandial time point (4.0 ± 0.90 (post-op) vs 2.0 ± 0.49 µmol l−1 (pre-op); P < 0.05). The taurine-conjugated subset also increased after RYGB, as detected by a significant rise in the maximum increase after a meal (0.69 ± 0.15 (post-op) vs 0.29 ± 0.08 µmol l−1 (pre-op); P <0.05). In contrast, the unconjugated BA subset was not altered after RYGB (AUC=149 ± 40.3 (post-op) vs 192 ± 75.5 (pre-op); P = 0.62; maximum postprandial rise = 0.61 ± 0.30 (post-op) vs 1.1 ± 0.62 µmol l−1 (pre-op); P = 0.58; Figure 1).
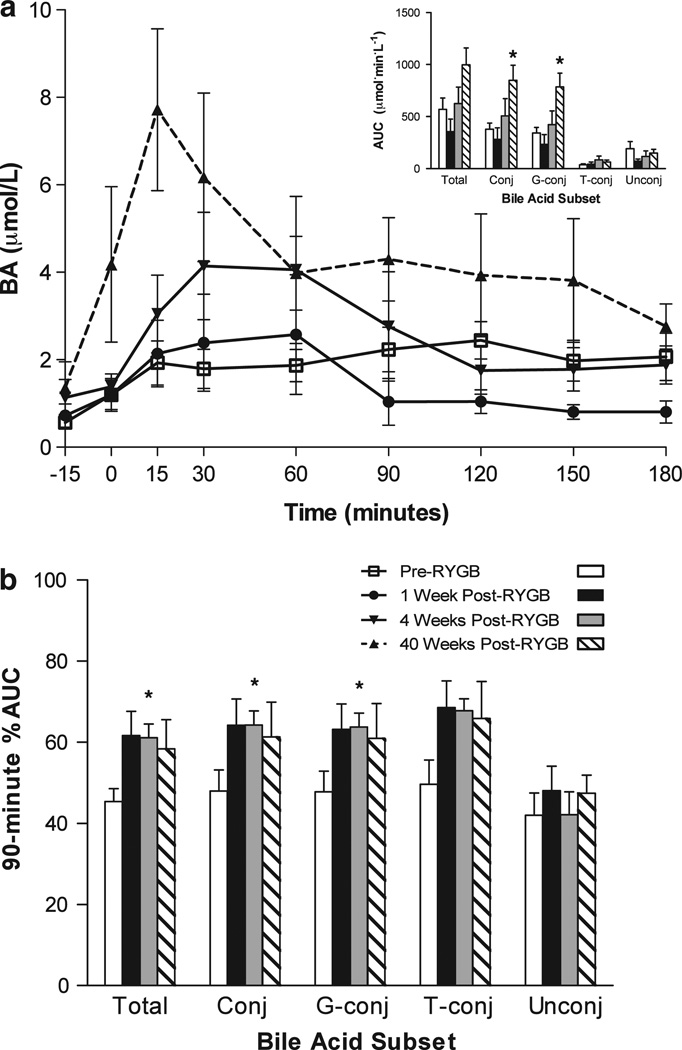
These RYGB-related changes were not detected 1 week or 4 weeks after surgery. However, at these earlier postoperative time points, there was a visibly earlier rise and fall of the circulating BA concentration after a meal (Figure 2). We quantified this difference in pattern by calculating the percentage of the excursion occurring within the first 90 min after the meal (90-min %AUC). Compared to the preoperative baseline, the 90-min %AUC at 4 weeks after surgery was significantly higher for total BAs (61.1 ± 3.4% (post-op) vs 45.4 ± 3.2% (pre-op); P <0.05), conjugated BAs (64.2 ± 3.5% (post-op) vs 48.0 ± 5.2% (pre-op); P < 0.05) and the glycine-conjugated subset (63.7 ± 3.5% vs 47.8 ± 5.1% (pre-op); P < 0.05). The taurine-conjugated subset showed a similar trend (67.8 ± 2.9% (post-op) vs 49.6 ± 6.0% (pre-op); P = 0.06), while the unconjugated BAs were unchanged.
Figure 2.
RYGB accelerates the postprandial excursion of conjugated BAs. (a) Time course of plasma-conjugated BAs before and after ingestion of a standard liquid meal in individuals before RYGB, 1 week after RYGB, 4 weeks after RYGB and 40 weeks after RYGB. The inset displays the 180-min AUC at each of these time points for total BAs (Total), conjugated BAs (Conj), glycine-conjugated BAs (G-conj), taurine-conjugated BAs (T-conj) and unconjugated BAs (Unconj). (b) Proportion of the area under the BA excursion curve occurring during the first 90 min after ingestion of a standard liquid meal for individuals before RYGB,1 week after RYGB, 4 weeks after RYGB and 40 weeks after RYGB. *P < 0.05 compared to pre-op.
DISCUSSION
In this longitudinal, prospective pilot study of individuals undergoing RYGB, we observed that obesity is associated with a blunted postprandial rise in circulating conjugated BAs despite fasting BA levels similar to those in lean individuals. Although several studies have shown that fasting BAs increase after RYGB, the postprandial responses of BAs are particularly important to their hormonal effects, including stimulation of FGF19, GLP-1 and BAT activity. Accordingly, we evaluated the effect of RYGB on postprandial BA excursion and found that RYGB treatment normalizes the blunted BA response associated with obesity, leading to a significant increase in postprandial circulating glycine- and taurine-conjugated BAs. This effect was evident in the total 3-h postprandial BA excursion, as represented by the AUC, and in the maximum rise in BA concentration, which reflects the highest concentration achieved within 3 h of a meal stimulus. We also found that RYGB accelerates the postprandial BA response, leading to an earlier rise and fall in glycine- and taurine-conjugated BAs after meal ingestion. While significant increases in meal-stimulated total BA excursion were limited to the late postoperative period (40 weeks after surgery), acceleration of the postprandial curve was seen as early as 4 weeks after surgery. Notably, this small pilot study is likely underpowered to detect the more limited changes in circulating BAs seen early after surgery.
The comparison of lean and obese individuals is consistent with the findings of Glicksman et al.,23 who observed that people with obesity exhibited a decreased circulating glycine-conjugated BA response to meal ingestion. In the current study, these findings are also extended to total conjugated BAs with a similar trend in the taurine-conjugated subset. Taurine-conjugated BAs have been shown to have a greater affinity for TGR5 than the glycine-conjugated moieties and may thus be particularly relevant for the effect of BAs on postprandial GLP-1 release and postprandial EE.47
In prior studies evaluating fasting BAs after gastrointestinal weight loss procedures, accelerated delivery of BAs to the distal ileum (the dominant BA-absorptive component of the intestine) has been suggested as a potential mechanism to explain the increases in circulating BA levels.48–50 As RYGB shortens the distance from the stomach to the ileum by bypassing the proximal intestine and creating a Roux limb, this ‘accelerated delivery hypothesis’ could explain the acceleration of the postprandial BA excursion curve that we detected as early as 4 weeks after surgery. However, the anatomic changes associated with RYGB, despite having immediate effects on baseline GI motility and transit time, are unlikely explanations for the greater total rise in circulating, postprandial BA concentrations, which did not become apparent until several months after surgery. The late effects on circulating BA levels suggest that they reflect time-dependent mechanisms such as developmental or adaptive changes that alter GI response to nutrient or meal ingestion. RYGB may also alter bile acid synthesis, secretion, modification and transport through direct physiological effects of the surgery. After RYGB in mice, we have observed a selective increase in intestinal expression of ABCC3 (MRP3) and SLC51B (OSTB), genes that encode for multidrug-resistant protein 3 and organic solute transporter beta, respectively, two BA transporters found in the basolateral membranes of intestinal epithelial cells (IJ Hatoum and LM Kaplan, unpublished).51,52 RYGB is also associated with alterations in the intestinal microbiota, a key regulator of BA conjugation and secondary BA formation.53–55
The normalization of the postprandial plasma conjugated BA response by RYGB may contribute to some of the metabolic effects of this operation. With the recent identification of BAT as a regulator of EE in adult humans, and the observation that BAs activate the TGR5 receptor on brown adipocytes to induce thermogenesis, the increase in circulating BAs after RYGB may contribute to the blunting of metabolic adaptation to weight loss and the increased thermic effect of feeding observed after this operation in humans.31–34 To the extent that TGR5 is located on the basolateral surface of enteroendocrine cells in humans, the circulating component of the BA pool may also stimulate secretion of GLP-1 to promote the decreased food intake and improved glucose homeostasis seen after RYGB.15,16 Notably, the rise in circulating GLP-1 in rodents and human patients after RYGB is predominantly postprandial.24–28 In rodent models, the elevated EE after RYGB is dependent on the fed state,36,37 and at least two recent studies have found that TEF is enhanced after RYGB in human patients.31,32 In this study, the increase in circulating BAs after RYGB is most pronounced in response to meal ingestion, providing further support for the hypothesis that circulating BAs may contribute to the neurohumoral cascade that alters the energy regulatory response to feeding after this operation.
With the exception of the taurine-conjugated BA subset, we did not detect the increases in fasting BA levels after RYGB reported previously. The absence of detectable changes in these levels is likely due to the small number of subjects studied. However, the significant differences in postprandial BA response detected in even this small cohort suggest that the effect of RYGB on postprandial BAs is more pronounced and perhaps more physiologically relevant. As the rise in postprandial BAs occurs late after RYGB, both adaptive changes and progressive weight loss during the first postoperative year may be important contributors. Similar to previous reports, we observed that the changes in dietary composition seen immediately after surgery do not persist late after the procedure, making changes in diet composition a less likely contributor to the observed surgically induced alterations in circulating BAs.56 Total dietary intake, which was similar in the lean and obese groups, is also unlikely to explain the observed differences in BA excursion between these groups.
In conclusion, we have observed that RYGB normalizes the disrupted BA response to meal ingestion associated with obesity by substantially increasing and accelerating the conjugated BA excursion. The observed meal-related changes support the hypothesis that altered concentrations of these hormonally active lipids may contribute to the metabolic effects of RYGB. They also raise the possibility that modulation of postprandial BA levels may provide new opportunities for the treatment of obesity and related metabolic disorders. Studies that directly examine the effect of circulating BAs on satiety and meal-related EE will help further define the role of BAs in the regulation of energy balance and the response to gastrointestinal weight loss surgery.
ACKNOWLEDGEMENTS
This study was supported by grants DK083230, KL2 RR025757, DK08866, DK090956 and UL1 RR025758 from the National Institutes of Health and a research grant from Ethicon Endo-Surgery.
Footnotes
This work was presented in part at the annual meeting of the American Gastroenterological Association in May 2010.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J. Lipid Res. 2009;50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of BAs and BA receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 4.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for BAs. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 5.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. BAs: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 8.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to BAs. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 9.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 13.Wu A, Coulter S, Liddle C, Wong A, Eastham-Anderson J, French DM, et al. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. Plos One. 2011;6:e17868. doi: 10.1371/journal.pone.0017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, et al. FGF19 as a postprandial insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;6024:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 16.Thomas C, Gioiella A, Noriega L, Strehle A, Oury J, Rizzo G. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaRusso NF, Korman MG, Hoffman NE, Hofmann AF. Dynamics of the enterohepatic circulation of bile acids: postprandial serum concentrations of conjugates of cholic acid in health, cholecystectomized patients, and patients with bile acid malabsorption. N Engl J Med. 1974;291:689–692. doi: 10.1056/NEJM197410032911401. [DOI] [PubMed] [Google Scholar]
- 18.Van Berge-Henegouwen GP, Hofmann AF. Systemic spill-over of bile acids. Euro J Clin Invest. 1983;13:433–437. doi: 10.1111/j.1365-2362.1983.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 19.Angelin B, Bjorkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man: fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724–731. doi: 10.1172/JCI110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenfield SM, Soloway RD, Carithers RL, Soper K, Silva de Barros SG, Balistreri WF. Evaluation of postprandial serum bile acid response as a test of hepatic function. Dig Dis Sci. 1986;31:785–791. doi: 10.1007/BF01296044. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 22.Ockenga J, Valentini L, Schuetz T, Wohlgemuth F, Glaesar S, Omar A, et al. Plasma bile acids are associated with energy expenditure and thyroid function in humans. J Clin Endocrinol Metab. 2012;97:535–542. doi: 10.1210/jc.2011-2329. [DOI] [PubMed] [Google Scholar]
- 23.Glicksman C, Pournaras DJ, Wright M, Roberts R, Mahon D, Welbourn R, et al. Postprandial plasma bile acid responses in normal weight and obese subjects. Ann Clin Biochem. 2010;47:482–484. doi: 10.1258/acb.2010.010040. [DOI] [PubMed] [Google Scholar]
- 24.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, Conget I, et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab. 2009;94:884–891. doi: 10.1210/jc.2008-1620. [DOI] [PubMed] [Google Scholar]
- 26.Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 28.Plum L, Ahmed L, Febres G, Bessler M, Inabnet W, Kunreuther E, et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity. 2011;19:2149–2157. doi: 10.1038/oby.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity. 2009;17:1839–1847. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bueter M, Lowenstein C, Olbers T, Wang M, Cluny NL, Bloom SR, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845–1853. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Faria SL, Faria OP, de Almeida Cardeal M, de Gouvea HR, Buffington C. Dietinduced thermogenesis and respiratory quotient after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2012;8:797–802. doi: 10.1016/j.soard.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Wilms B, Ernst B, Thurnheer M, Schultes B. Increased thermic effect of food after gastric bypass surgery. Obes Rev. 2012;13(Suppl 2):125. [Google Scholar]
- 33.Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78:22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 34.Thivel D, Brakonieki K, Duche P, Beatrice M, B Yves, Laferrere B. Surgical weight loss: impact on energy expenditure. Obes Surg. 2013;23:255–266. doi: 10.1007/s11695-012-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenbaum M, Hirsch J, Gallagher DA, Leibel R. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88:906–912. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- 36.Stylopoulos N, Zhang XB, Brownell AL, Kaplan LM. Roux-en-Y gastric bypass activates brown adipose tissue and increases energy expenditure in obese mice. Gastroenterology. 2010;138(Suppl 1):S754. (abstract W1854). [Google Scholar]
- 37.Nestoridi E, Kvas S, Kucharczyk J, Stylopoulos N. Resting energy expenditure and energetic cost of feeding are augmented after Roux en Y gastric bypass in obese mice. Endocrinology. 2012;153:2234–2244. doi: 10.1210/en.2011-2041. [DOI] [PubMed] [Google Scholar]
- 38.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected brown adipose tissue in humans. J Clin Endocrinol Metab. 2011;96:192–199. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- 39.Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–1793. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts J, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 41.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson FE, Byers T. Dietary assessment resource manual. J Nutr. 1994;124:2245S–2317S. doi: 10.1093/jn/124.suppl_11.2245s. [DOI] [PubMed] [Google Scholar]
- 43.Bouchard C, Tremblay A, Leblanc C, Lortie G, Savard R, Theriault G. A method to assess energy expenditure in children and adults. Am J Clin Nutr. 1983;37:461–467. doi: 10.1093/ajcn/37.3.461. [DOI] [PubMed] [Google Scholar]
- 44.Bobeldijk I, Hekman M, de Vries-van der Weij J, Coulier L, Ramaker R, Kleemann R, et al. Quantitative profiling of bile acids in biofluids and tissues based on accurate mass high resolution LC-FT-MS: compound class targeting in a metabolomics workflow. J Chromatogr B Analyt Technol Biomed Life Sc. 2008;871:306–313. doi: 10.1016/j.jchromb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diab Care. 1994;17:152–154. doi: 10.2337/diacare.17.2.152. [DOI] [PubMed] [Google Scholar]
- 46.Hatoum IJ, Greenawalt DM, Cotsapas C, Reitman ML, Daly MJ, Kaplan LM. Heritability of the weight loss response to gastric bypass surgery. J Clin Endocrinol Metab. 2011;96:E1630–E1633. doi: 10.1210/jc.2011-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato H, Macchairulo A, Thomas C, Gioiello A, Une M, Hofmann AF, et al. Novel potent and selective BA derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51:1831–1841. doi: 10.1021/jm7015864. [DOI] [PubMed] [Google Scholar]
- 48.Kohli R, Kirby M, Setchell KDR, Jha P, Klustaitis K, Woollett LA, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299:652–660. doi: 10.1152/ajpgi.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patriti A, Facchiano E, Annetti C, Aisa M, Galli F, Fanelli C, et al. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg. 2005;15:1258–1264. doi: 10.1381/096089205774512573. [DOI] [PubMed] [Google Scholar]
- 50.Boza C, Munoz R, Yung E, Milone L, Gagner M. Sleeve gastrectomy with ileal transposition induces a significant weight loss and diabetes improvement without exclusion of the proximal intestine. J Gastrointest Surg. 2011;15:928–934. doi: 10.1007/s11605-010-1369-6. [DOI] [PubMed] [Google Scholar]
- 51.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, et al. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:923–932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- 52.Vanwijngaerden Y, Wauters J, Langouche L, Perre SV, Liddle C, Coulter S, et al. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology. 2011;54:1741–1752. doi: 10.1002/hep.24582. [DOI] [PubMed] [Google Scholar]
- 53.De La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge C, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:440–448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furet J, Kong L, Tap J, Poitou C, Basdevant A, Bouillot J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liou AP, Paziuk M, Luevano JM, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota caused by Roux-en-Y gastric bypass contribute to reduced host weight and adiposity. Sci Transl Med. 2013;5:178ra41, 1–11. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novais PF, Rasera I, Jr, Leite CV, Marin FA, de Oliveira MR. Food intake in women two years or more after bariatric surgery meets adequate intake requirements. Nutr Res. 2012;32:335–341. doi: 10.1016/j.nutres.2012.03.016. [DOI] [PubMed] [Google Scholar]