Abstract
The nature of the oral cavity and host behaviors has mandated that the oral microbiota evolve mechanisms for coping with environmental fluctuations, especially changes in the type and availability of carbohydrates. In the case of human dental caries, the presence of excess carbohydrates is often responsible for altering the local environment to be more favorable for species associated with the initiation and progression of disease, including Streptococcus mutans. Some of the earliest endeavors to understand how cariogenic species respond to environmental perturbations were carried out using chemostat cultivation, which provides fine control over culture conditions and bacterial behaviors. The development of genome-scale methodologies has allowed for the combination of sophisticated cultivation technologies with genome-level analysis to more thoroughly probe how bacterial pathogens respond to environmental stimuli. Recent investigations in S. mutans and other closely related streptococci have begun to reveal that carbohydrate metabolism can drastically impact pathogenic potential and highlight the important influence that nutrient acquisition has on the success of pathogens; inside and outside of the oral cavity. Collectively, research into pathogenic streptococci, which have evolved in close association with the human host, has begun to unveil the essential nature of careful orchestration of carbohydrate acquisition and catabolism to allow the organisms to persist and, when conditions allow, initiate or worsen disease.
Keywords: carbohydrate transport, sugar phosphotransferase system, dental caries, biofilms, catabolite repression
The capability to sequence whole bacterial genomes and the knowledge gained in the ‘post-genomic’ era have significantly reshaped our understanding of the etiologies and pathogenic processes of many oral diseases, including dental caries (1). For example, new information from the fields of anthropology and evolutionary genetics has provided evidence that Streptococcus mutans appeared in human dental plaque and that an expansion of genes important for the success of dental caries pathogens occurred around the time humans transitioned from hunter-gatherers to stable agrarian communities (2, 3). This acquisition and diversification of caries pathogens thus occurred during the period when human civilizations became dependent on foods enriched in carbohydrates from domesticated grains, which was likely a major contributing factor in the appearance of dental caries beginning in ‘Post Agricultural’ humans and continuing to the present (4). Studies into the oral microbiome have broadened our understanding of the diversity of organisms present in the oral cavity of modern humans, with estimates of the number of microbial taxa inhabiting this environment ranging from 600 to over 1,200 (5, 6). Analysis of the oral microbiome in healthy and in carious sites provides support for the ‘ecological plaque hypothesis’, which posits that specific changes to the local environment allow for cariogenic species to outcompete health-associated flora and dominate as carious lesions are initiated and progress (7). This ‘ecological catastrophe’ (8) leads to demineralization of tooth enamel, which is largely driven by acid production by bacteria from sugary foodstuffs introduced via the diet of the host. Cariogenic species are particularly effective at metabolizing carbohydrates to produce strong organic acids and at surviving in acidic conditions as compared to species associated with healthy dentition (8, 9). It is now well accepted that physiological processes, including carbohydrate uptake, acid generation, and the tolerance of low pH, are the major characteristics of bacteria that contribute to the development of dental caries, rather than classical virulence factors, such as secreted toxins.
In the years since its discovery by Clarke in 1924, S. mutans has become the most intensively studied cariogenic organism and is the species that is most consistently associated with the initiation and progression of caries in humans (10). Studies conducted on the genetics and physiology of S. mutans have furthered our understanding of fundamental processes, including biofilm formation, quorum sensing, and stress responses, and how these physiological processes contribute to pathogenicity (11). Before the genomic era, major advances to our knowledge of bacterial physiology were achieved through the analysis of cell populations generated using chemostat culture, which allowed for tight control of environmental conditions and bacterial behaviors. In this review, we discuss how chemostat cultivation can be combined with genomic studies to more thoroughly assess bacterial responses to carbohydrate availability under finely controlled environmental conditions. Furthermore, as the oral cavity provides a diverse array of carbohydrate sources, we will also discuss some recent advances understanding how carbohydrate uptake and gene regulation in response to specific carbohydrates may influence persistence and virulence of S. mutans. We conclude with a brief discussion of how these advances are integrated with selected information about the influence of carbohydrate utilization on virulence of related pathogens.
Carbohydrate utilization by Streptococcus mutans
The expansion of genes related to carbohydrate uptake and metabolism appears to have been an essential evolutionary advancement contributing to the success of S. mutans as a caries pathogen in oral microbial biofilms (3, 12). The transport of oligosaccharides, including melibiose, raffinose, stachyose, and maltodextrans, is primarily conducted by the activity of ATP binding cassette (ABC) transporters encoded in the genome of S. mutans, which include the multiple sugar metabolism (msm) and malXFGK transport systems (13–15), The predominant route for uptake of mono- and disaccharides by S. mutans is the phosphoenolpyruvate: sugar phosphotransferase system (PTS). The characteristics, functions, and regulation of the genes for the PTS have been reviewed extensively elsewhere (16–20). The PTS consists minimally of Enzyme I (EI) and HPr, which participate in a phosphotransfer reaction to a variety of Enzyme II (EII) permeases that concomitantly phosphorylate and internalize a spectrum of mono- or disaccharides. The genomes of certain strains of S. mutans encode as many as 15 EII permeases, composed of A, B, C, and sometimes D domains. Most of these permeases are harbored by the majority of strains, but there are a few that are only present in particular isolates (3, 21, 22). Undoubtedly, the diversity in carbohydrate sources that can be internalized by S. mutans contributes to its pathogenic potential. Recent studies have suggested that additional accessory components may be required for full functionality of certain EII complexes. For example, an open reading frame (ORF), encoding a hypothetical protein, located within the cellobiose utilization operon of S. mutans and Streptococcus pneumoniae is necessary for the efficient internalization of cellobiose (23, 24). Similarly, the manO gene product of S. mutans appears to be required for the normal function of the EIIMan complex (25).
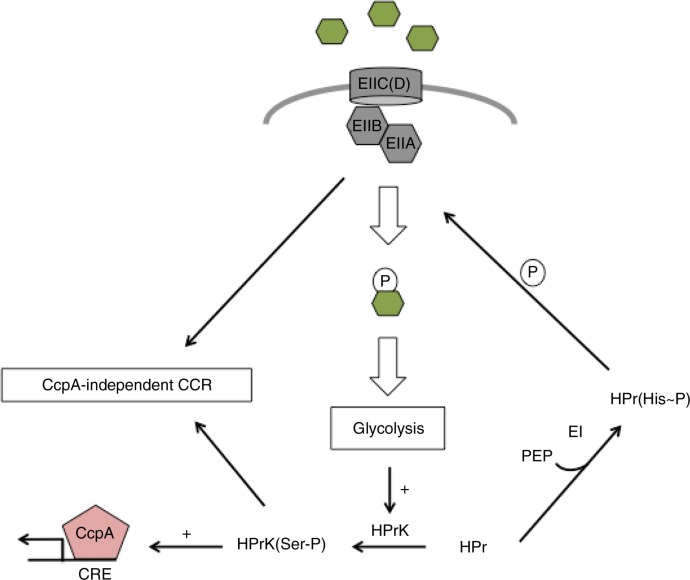
Carbohydrate catabolite repression (CCR) (16, 26, 27), the process by which bacteria delay the catabolism of non-preferred carbohydrates when preferred sources are simultaneously present, is a critical process that optimizes growth and competitive fitness. Perhaps not surprisingly – given its close co-evolution with humans, dietary changes in humans over centuries and the nature of the disease caused by this organism – S. mutans has evolved strategies for CCR that do not completely mirror those described for many other Gram-positive organisms. In general, CCR in Gram-positives can be controlled at multiple levels. When rapidly metabolizable carbon sources are internalized, glycolytic intermediates accumulate within the cell, some of which act as allosteric activators of a kinase (HPr kinase/phosphatase) that phosphorylates HPr at serine residue 46 (16). In S. mutans, serine-phosphorylated HPr (HPr(Ser-P)) plays a major role in the repression of genes for the uptake and metabolism of non-preferred carbohydrate sources (28). A primary route for CCR in many Gram-positive bacteria is through the activation of the transcriptional regulator CcpA, which when allosterically activated by HPr(Ser-P) can bind to the promoter regions of multiple CCR-sensitive genes at a conserved catabolite response element (CRE). However, inactivation of the ccpA gene in S. mutans almost never results in alleviation of CCR. On the other hand, microarray studies comparing ccpA and wild-type S. mutans clearly show a critical role for this regulator in direct and indirect control of transcription of genes that encode products involved in carbon flow, energy metabolism, and the fate of pyruvate (29). In contrast to HPr and CcpA, certain EII permeases exert a dominant effect on CCR-sensitive genes in S. mutans, particularly when cells are grown in the presence of the sugar(s) that are internalized by these permeases (21, 30, 31). There is also another layer of control of CCR in S. mutans. In particular, CcpA has the ability to indirectly influence non-preferred sugar uptake by regulating the expression of multiple EII permeases (31). Overall, then, CCR in S. mutans appears to have evolved to allow the organism to evaluate the source and availability of carbohydrates in the environment using EII permeases while monitoring carbohydrate flow through glycolysis and global energy demands using HPr and CcpA (Fig. 1). Organization of CCR in this manner would afford S. mutans the ability to adjust carbohydrate uptake and metabolism pathways rapidly and seamlessly in response to the ever-changing environment within the oral cavity.
Fig. 1.
S. mutans has evolved elegant strategies for coping with the diversity and availability of nutrients present within the oral cavity. When a preferred carbohydrate source, such as glucose, is present in abundant quantities, the elevated movement of metabolites through glycolysis profoundly alters many cellular properties. For instance, the kinase activity of the HPr kinase/phosphatase (HprK) is stimulated leading to an increase in the serine-phosphorylated species of HPr [HPr(Ser-P)]. HPr(Ser-P) acts as a co-factor of the major regulator CcpA to repress transcription of metabolic genes containing a catabolite response element (CRE) in their promoter regions, or in the cases of CcpA-independent CCR, directly affects transcription of genes associated with catabolism of non-preferred carbohydrate sources, a process that may also require certain PTS permeases. As the supply of available carbohydrate is diminished, the reduced quantity of readily metabolizable carbohydrate translates to a lower abundance of HPr(Ser-P). Without its co-factor, CcpA no longer functions as a repressor, and with lower levels of carbohydrate transport occurring, PTS porters remain predominately phosphorylated and play a less active role in CCR. Relief of repression by these factors allows for transcription of a variety of genes, including PTS porters, for sensing and accessing a diverse array of carbohydrate sources. Overall, PTS porters monitor the type and levels of carbohydrates present in the oral cavity, while the overall supply of carbohydrate in relation to cellular energy demands is monitored by the activity of HPr and CcpA.
Continuous culture as a valuable tool for analyzing bacterial physiology
Since its development (32, 33), chemostat cultivation has aided in the discovery and characterization of many processes related to microbial physiology, and additional opportunities for this technology remain in the ‘post-genomic’ era (34). Chemostat cultivation provides fine control of multiple variables that impact bacterial physiology and gene expression, including pH, temperature, growth rate, atmospheric composition, and the source and availability of specific nutrients. Continuous culture has proven to be extremely valuable to researchers seeking to unravel the impact of the availability of particular nutrients on the metabolic pathways of bacteria, including the mutans streptococci. Information on specific aspects of theory and design of continuous culture can be found elsewhere (34, 35).
The saccharolytic metabolism of lactic acid bacteria, including S. mutans and many other oral streptococci, makes these bacteria highly amenable to chemostat cultivation, as the organisms do not respire and fermentation of carbohydrates is the overwhelmingly dominant source of energy for growth and maintenance. In early studies of S. mutans, chemostat culture was exploited to determine how perturbations to the environment impacted the composition of the bacterial envelope, especially the lipoteichoic acids (LTAs) (36–41). As it was established that attachment to the tooth pellicle and the construction of extracellular polysaccharides by the activity of glucosyltranferases (GTFs) were critical for the success of S. mutans as a pathogen, a variety of chemostat studies were initiated to characterize these virulence factors, and they revealed that the production, activity, and localization of the GTFs were greatly influenced by growth rate, carbohydrate source, pH and other factors (42–45). Another major aspect contributing to the success of S. mutans in oral biofilms is its ability to uptake a variety of carbohydrate sources and alter its metabolism in response to the local microenvironment, and chemostat studies have greatly aided in our understanding of how environmental inputs impact PTS activity and the levels and phosphorylation state of various component of the PTS (46–53). In addition to the findings mentioned above, the chemostat model has been instrumental in shaping our understanding of the impact of various environmental stimuli and growth rate on fermentation end products (54), acidurance (55, 56), membrane fatty acid content (57) and the effects of trace metals on growth (58–60), among other critical processes. Collectively, these studies provided a firm foundation of how the basic physiological and virulence-related properties of S. mutans are influenced by environmental alterations.
Though batch culture is the overwhelming method for studying S. mutans genetics and physiology, the chemostat model is still utilized in low throughput studies of S. mutans where tight control of growth parameters is crucial. Some recent examples include explorations of the proteome of S. mutans in response to selected growth conditions, including an acidic environment (61, 62), of the GlnR-mediated response to low pH (63) and of the contribution of NADH oxidase to acid and oxidative stress (64). As genomic technologies continue to develop, prior knowledge generated can guide the design of new studies that merge continuous culture techniques with genome-scale methodologies to further broaden our understanding of the adaptive capabilities of this caries pathogen.
In order to integrate observations of physiological responses to carbohydrate availability with transcriptional methodologies, we conducted a continuous culture study of the reference strain S. mutans UA159 and a mutant lacking the EIIABMan permease, manL, grown in base medium formulated with either 10 mM glucose, which was determined to be glucose limiting, or 100 mM glucose, which supplied glucose in excess (65). Multiple parameters of the PTS were probed in the manL mutant and parental strains to demonstrate that UA159 responds similarly to previously characterized strains of S. mutans. Most notably, in pH drop experiments, cells of the parental strain grown in limiting carbohydrate lowered the pH faster and reached a lower terminal pH upon addition of exogenous glucose, compared with cells grown in excess glucose (65). In pH drop assays, cells halt metabolism due to the acidification of the cytoplasm and the acid-sensitive nature of the PTS enzymes, not because they have exhausted the supply of fermentable carbohydrate (55). Thus, cells able to achieve a lower terminal pH are generally regarded as more acid tolerant. Though there is evidence for the presence of a proton-glucose symporter, which is active in PTS-deficient cells (66), it is likely that under conditions of low pH the majority of carbohydrates are transported via residual PTS activity. A small number of sugars may also be transported by ABC transporters under these conditions.
To test the relative levels of intracellular glycogen-like energy stores, chemostat cultivated cells were also assayed for their ability to lower the pH without the addition of exogenous carbohydrates. It was found that cells grown in excess glucose produced a lower final pH than cells grown in limiting carbohydrate, indicative of the accumulation of higher levels of intracellular glycogen-like energy stores (65), closely resembling previous results (46). In the same study (65), the PEP-dependent transport of glucose, mannose and fructose was found to be lower in both the manL mutant and parental strains when cultured in excess versus limiting glucose, and in relation to the parental strain, the manL mutant displayed diminished transport of all sugars tested, but particularly of glucose and mannose (65). The protein levels of the general PTS proteins EI and HPr in the manL deletion and parental strains experienced very little change between limiting and excess carbohydrate conditions (65), and these observations are generally consistent with observations with batch-grown cells (67) and with chemostat cultivated S. mutans grown under various conditions (47–49). Finally, it was noted that under excess glucose conditions there was a higher abundance of both the HPr(Ser-P) and HPr(Ser-P)(His~P) forms of HPr in both the manL and parental strains, while under glucose limitation, the unphosphorylated and histidine-phosphorylated species predominated. These results were similar to what has been noted with batch-grown cells (68). Further, in continuous culture studies under increasing concentrations of glucose, availability of the HPr(Ser-P) and HPr(Ser-P)(His~P) forms of HPr was generally increased in S. mutans; with a corresponding decrease in the abundance of unphosphorylated and histidine-phosphorylated forms (69). Thus, growth in glucose replete conditions strongly favors serine phosphorylation of HPr, which can then serve as a repressor for the catabolism of more complex carbohydrates or non-preferred mono- and disaccharides (28). Collectively, studies of the regulation of PTS components in S. mutans have generally concluded that perturbations in the environment can strongly influence the availability and activity of EII enzymes. However, the absolute levels of the general PTS proteins do not fluctuate to nearly as great an extent in response to environmental inputs, albeit the phosphorylation status of HPr appears very sensitive to environmental conditions.
Physiologic analysis of the effects of carbohydrate availability on S. mutans UA159 is complex and attributes that are directly correlated with virulence potential are affected. Thus, a transcriptional study of S. mutans UA159 grown in limiting versus excess glucose was conducted using microarray techniques (65). Under conditions of glucose limitation, cells experienced a derepression of transporters associated with carbohydrate uptake, as evidenced by the elevation of several PTS EII enzymes and the genes for the msm operon. Genes involved in glycogen catabolism, energy metabolism, and pyruvate fate, including pyruvate dehydrogenase, were also elevated. Under glucose excess, where cells are likely limited for amino acids or a group of amino acids, we found that many transcripts for biosynthesis of amino acids, nucleotides, and cofactors were upregulated, as well as several ABC transporters, some of which were specific for the internalization of amino acids. Interestingly under glucose limitation, the ccpA transcript and many genes known to be part of the CcpA regulon were found to be upregulated, while the global transcriptional regulator CodY and several genes known to be under its control were upregulated when glucose was present in excess. From these observations, it was concluded that the coordinated activity of CcpA and CodY might be an important regulatory mechanism in cellular responses to alterations in carbohydrate availability and amino acid limitation.
Other methods for analyzing the impact of carbohydrates on bacterial physiology
Chemostat cultivation serves as an excellent method for obtaining reproducible data evaluating the impact of environmental perturbations on pure populations of planktonic bacteria. However, in the oral cavity, bacteria grow in multispecies biofilms where diffusion limitation and other factors lead to populations that are heterogeneous in terms of their microenvironments and their growth rate and growth phases. In addition, saliva and the rate of salivary flow contribute greatly to the microbial ecology of the oral cavity and the severity of dental caries, as well as other oral diseases. With these considerations in mind, we will briefly discuss several other models of oral microbial communities and how dietary carbohydrates contribute to the success of pathogens under these conditions.
Biofilm models of bacteria have been developed to monitor the formation of biofilm communities and their responses to environmental variables, such as nutrient type and availability. In addition to simple mono- and disaccharides, oral microbial biofilms likely encounter many structurally complex carbohydrate sources, including polysaccharides, such as starch, that are introduced via the diet or host-derived glycoproteins such as mucins, and these diverse carbohydrates can have a profound impact on bacterial physiology and virulence. In a recent study addressing the impact of complex carbohydrates on microbial biofilms, Klein et al. monitored the influences of starch and sucrose in the presence of host-derived amylase on the transcriptome and physiology of S. mutans in a biofilm setting (70). Importantly, bacteria were found to up-regulate genes associated with the transport and metabolism of maltose and maltotriose at early timepoints, indicating that the bacteria could access the sugars released via amylase (70). Further, an increased expression in genes related to glycogen metabolism and in the amount of glycogen detected in biofilms, as a result of addition of starch led the authors to propose that S. mutans could benefit from the slow release of nutrients from starch (70). Of particular interest, in the presence of starch and sucrose, the authors noted the upregulation of genes encoding proteins contributing to stress tolerance, including groEL and groES along with the osmo-responsive pacL and trkB genes (70). Similarly, when readily fermentable carbohydrates become limiting in the oral cavity, it is likely that microbial biofilms access host-derived glycoproteins for nutrients, including mucins. Though studies have shown that S. mutans cannot grow well with mucin as a sole carbon and energy source (71), when mucin was added to sucrose-starved biofilms, the bacteria displayed enhanced survival (72).
Human saliva serves a primary role in maintaining the health of oral sites, and the unique proteins and finely tuned buffering capacity of these fluids greatly impact the composition of the oral microbiota, in many cases preferentially selecting for the survival of non-cariogenic organisms (73). The utilization of human saliva in models of dental caries is often limited due to the difficulty of obtaining sufficient volumes of material for experiments. In some instances, these challenges have been overcome by the creation of artificial saliva, and though not as complex as saliva obtained from human subjects, the results obtained with this medium are in some cases comparable to those of human saliva (74, 75). As described by van der Hoeven and co-workers, S. mutans cannot survive with mucin as a sole source of carbohydrates (71) and fares much better in artificial and human saliva supplemented with carbohydrates (75). This requirement for supplemental carbohydrates in saliva is further illustrated in classical studies of S. mutans in continuous chemostat cultivation with artificial and human saliva, which noted an overall lower recovery of the organism in the absence of supplemented fermentable carbohydrates (76). Further, in studies of human subjects, a decrease in the presence of S. mutans has been observed when carbohydrates were removed from the diet with a reemergence of the organism when the consumption of carbohydrates was resumed (77). The nutrient content and ionic strength of saliva is quite different from the components of complex media sources typically utilized in the laboratory. It is however sometimes necessary to utilize rich and defined media during experimentation in order to ensure the vigorous growth of the organism under study while assessing the characteristics of interest.
When approaching the topic of carious lesions from the perspective of preventing or assessing damage to odontological tissues, experiments utilizing animal models and studies of populations of humans have been particularly useful for advancing our understanding of the role that carbohydrates play in the initiation and progression of disease. A most helpful review discussing the utility of the rat caries model and its importance in the study of dental caries was recently written by Bowen (78). In this review, Bowen describes several instances where the rat caries model has shaped our understanding of critical concepts related to dental caries. For example, studies of germ free and antibiotic-treated rats were instrumental in demonstrating that the presence of microbes within the oral cavity is critical for the development of carious lesions. In addition, studies using rats have illustrated that it is not as much the consumption of particular sugars but the frequency of consumption that strongly contributes to caries, and further, many anti-caries treatments succeed mostly due to their impact on the frequency of meals (78). Similar results have been observed in human studies, and as presented in a review by Bradshaw and Lynch, the frequency and amount of sugar, particularly sucrose, consumed by human subjects is strongly linked to the development of dental caries (79). The results generated from early human studies precipitated the original, perhaps oversimplified, implication of sucrose as ‘the arch criminal of dental caries’ (80) and S. mutans as the causal organism. Further studies of the physiology and cariogenic potential of oral microbial pathogens have altered our understanding of how carious lesions originate and modified the strategy for preventing carious lesions from directly eliminating carious bacteria from the oral cavity to reestablishing a balance in the ecology between health and caries associated flora (8). Similarly, though sucrose does hold a strong causal link to caries, research conducted on the association of other carbohydrates sources with caries formation continues to broaden our view (81). Additional evidence suggests that other dietary carbohydrates, including particularly high concentrations of starch, can also result in caries due notably to the retention of these foodstuffs in the oral cavity (79). These observations lead us to the conclusion that though sucrose is critical to our understanding of caries etiology, it must also be considered that other sugars may still play important roles (81).
Recent progress on effects of carbohydrate source
Oral microbial biofilms are exposed to a vast array of carbohydrate sources that are provided via the diet or host secretions, or that are generated by the oral flora. As the nutrient source can significantly influence the composition and pathogenic potential of oral biofilms, it is important to determine how various carbohydrate sources are internalized and metabolized. Further, the diverse repertoire of PTS permeases encoded in the genome of S. mutans significantly contributes to its survival and cariogenic potential. The advent of genome-scale technologies has greatly enhanced our ability to study the influence of various nutrient sources on the transcriptome of organisms, and a major leap in our understanding of the impact that carbohydrate sources have on gene expression in S. mutans was achieved in a systematic analysis of transcriptional changes that occur in response to a variety of mono- and oligosaccharides using microarray methodology (82). This study clearly illustrates how discrete alterations to the transcriptome can occur as a result of changes in nutrient type. In the following section, we will focus our discussion on recent studies that delve into mechanistic aspects of the control of the metabolism by a select group of carbohydrate sources and how these processes may be integrated with the virulence of S. mutans. Rather than present an exhaustive discussion of all carbohydrate sources, we have selected certain carbohydrates that are regularly encountered by S. mutans in the oral cavity where significant progress in understanding the mechanistic aspects of metabolism has recently been made.
Sucrose
Sucrose, a β2,1-linked disaccharide of fructose and glucose, remains a major constituent of the human diet and is strongly linked to the initiation and progression of dental cares. S. mutans produces multiple exoenzymes capable of acting on sucrose. These include the glucosyltransferase enzyme GtfB (sometimes referred to as GTF-I), which acts on sucrose to produce water-insoluble glucans composed predominantly of α1,3-linkages, and GtfD (sometime called GTF-S) that converts sucrose to mostly soluble α1,6-linked glucans. An additional enzyme GtfC (sometimes GTF-SI) makes a glucan with mixed α1,3 and α1,6 linkages (83–85). The high molecular weight polysaccharides produced by the GTF enzymes have been implicated in attachment and biofilm formation by S. mutans on the smooth surfaces of the tooth (86). In addition, a fructosyltransferase (FTF) enzyme acts on sucrose to produce a fructose homopolymer that is primarily composed of β2,1-linked fructose (inulin). Unlike S. mutans-derived glucans, fructans serve mainly as an extracellular storage polymer (87). To access fructose polymers, S. mutans produces a secreted exo-β-D-fructosidase, FruA, that releases fructose from β2,6– and β2,1-linked fructan polymers (88, 89). FruA can also act as an invertase, cleaving extracellular sucrose into fructose and glucose. At the surface of the cell, the PTS enzyme EIIScr, encoded by the single gene scrA, phosphorylates and internalizes sucrose. In fact, the majority of sucrose encountered by S. mutans is internalized and metabolized to produce organic acids, with a relatively minor portion being converted to extracellular polymers (90), at least in in vitro studies.
Though carbohydrate transporters other than ScrA have been implicated in the internalization of sucrose, it has been challenging to determine the exact contribution of the PTS and/or ABC transporters to sucrose uptake because of the presence of the many sucrolytic exoenzymes produced by S. mutans. To address this problem, a variety of strains containing mutations in some or all of the genes for sucrolytic enzymes, including gtfA, gtfBC, gtfD, ftf, and fruA, were created in the S. mutans UA159 genetic background (30). Through growth studies conducted using these mutants, it was demonstrated that metabolism of sucrose in planktonic S. mutans cultures is primarily attributable to the activity of the exoenzymes GtfBC that release free fructose as they generate glucans, and to the PTS enzyme EIIScr, It was also observed that the exoenzyme GtfD played a minor role in the metabolism of sucrose, and little contribution to extracellular sucrose hydrolysis was noted for FruA, although this is probably because the fruA gene was poorly expressed under the conditions tested. Another question addressed by this study was what role major carbohydrate uptake systems had on the transport of sucrose in the absence of sucrolytic enzymes. It was demonstrated that the primary PTS permease (EIITre) for trehalose (α-D-glucopyranosyl-1,1-α-D-glucopyranoside) and the msm ABC transporter contributed modestly to growth on sucrose, so these systems appear to function in lower-affinity sucrose uptake, as suggested previously (14, 91). Further, it was also possible to assess the PTS-dependent transport of sucrose in this study without the confounding influence of the sucrolytic exoenzymes, and the results clearly showed that EIIScr was the major route for transport of sucrose, whereas EIITre was capable of only modest levels of transport (30). Also of note, the msm system was shown by microarray analysis to display lower expression when S. mutans was growing in sucrose as compared to glucose (82). Conversely, expression of scrA was shown to be constitutively high under all carbohydrate conditions tested (82), perhaps indicative of evolutionary pressure for S. mutans to be capable of assimilating sucrose quickly when it appears in the diet.
Additional evidence presented in the report by Zeng et al. demonstrated that EIIScr could also exert effects on sucrose metabolism by influencing the expression of levD (the EIIA component of a fructose PTS), fruA, and other genes (30). First, loss of scrA was shown to result in elevated expression of fruA and improved growth on the fructooligosaccharide, FOS. This phenotype could implicate EIIScr in substrate-dependent CCR, perhaps acting through HPr and bypassing the CcpA circuit. Second, loss of scrA caused loss of sucrose-dependent activation of the LevQRST pathway. The LevQRST four-component system activates fruA and levDEFG expression in response to extracellular signals, including the presence of fructose and mannose, and is sensitive to CcpA-independent catabolite repression (31, 92). Using mutants lacking sucrolytic exoenzymes, it was demonstrated that activation of the levD promoter could be achieved by pulsing the strain with increasing concentrations of sucrose. However, deletion of EIIScr in strains lacking multiple sucrolytic enzymes resulted in loss of levD promoter activation, as did the deletion of major components of the LevQRST system (30). These results could be explained as EIIScr directly participating in LevQRST signaling, with the potential to fine-tune gene regulation in response to the sucrose concentration in the environment. Alternatively, it was suggested that fructose-expulsion resulted from sucrose metabolism by EIIScr and ScrB, a sucrose-6-phosphate hydrolase that cleaves the internalized product of EIIScr. This hypothesis was corroborated when significant concentrations of free fructose (~100 µM) were found to be released by cells lacking GtfABCD, FTF, and FruA, but not ScrA, following pulsing with 10 mM sucrose (Zeng and Burne, unpublished data). Thus, the use of a suite of mutants has helped reveal important contributions of various sucrolytic systems to regulation of S. mutans gene expression and ScrA is clearly an important contributor to CCR when sucrose is present.
More recently, a new PTS transport system relevant to sucrose utilization was identified. In particular, it was determined that previously unannotated genes SMU.100–105 in S. mutans UA159 showed enhanced expression when cells were cultivated in biofilms in the presence of sucrose, but not when glucose or fructose were provided for growth in biofilms (93). Encoded in this gene cluster was a permease designated as PTSBio. Interestingly, it was noted that optimal expression of these genes was dependent upon the presence of functional GtfB and GtfC and that PTSBio was capable of internalizing the α1,3-linked disaccharide nigerose. Thus, PTSBio appears to transport oligosaccharides that could arise from the action of the GTFs (93), while other genes in the cluster are required for catabolism of the sugars. Clearly, then, great progress has been realized recently on understanding the complexities of the biochemistry and genetics of sucrose dissimilation, although additional work will be required to fully understand the influence that sucrose has on S. mutans physiology and virulence potential.
N-Acetylglucosamine/glucosamine
N-Acetylglucosamine (GlcNAc) and the deacetylated version of the sugar glucosamine (GlcN) are among the most abundant carbohydrates on the planet. Found in biological structures as diverse as the shells of arthropods to the cell walls of fungi and bacteria, these amino sugars could serve as attractive nutrients for S. mutans, as they provide a source of both carbon and nitrogen. Researchers in the field of oral biology have long known that S. mutans is capable of utilizing both GlcN and GlcNAc, and GlcNAc has classically been utilized as a method for distinguishing S. mutans from the related cariogenic species Streptococcus sobrinus (94, 95). Early studies using the sugar analog streptozotocin led to the conclusion that GlcNAc enters S. mutans through a relatively high-affinity porter as well as through a lower-affinity permease (96), though the identity of these systems was not determined. Recently, it was found that import of GlcNAc in S. mutans occurs predominantly through the glucose/mannose EII enzyme complex (EIIMan) as N-acetylglucosamine-6-phosphate (25), which is converted to glucosamine-6-phosphate by the activity of the enzyme N-acetylglucosamine-6-phosphate deacetylase (NagA). Subsequently, glucosamine-6-phosphate can be used directly for cell wall biosynthesis or acted on by the enzyme glucosamine-6-phosphate deaminase (NagB) to produce fructose-6-phosphate, followed by entry into the Embden–Meyerhof–Parnas pathway. The EIIMan complex also serves as the primary mechanism for internalizing GlcN, which generates glucosamine-6-phosphate, although it appears that residual transport by other permeases, including the cellobiose and fructose permeases, contributes to internalizing GlcN (25). When cells require glucosamine-6-phosphate for cell wall biosynthesis and exogenous sources are unavailable, glucosamine-6-phosphate can be synthesized de novo using free fructose-6-phosphate and glutamine by glucosamine-6-phosphate synthase (GlmS).
Early investigations into the metabolism of GlcNAc by oral streptococci demonstrated that S. mutans predominated when grown in a mixed culture with S. sobrinus in medium containing a combination of glucose and GlcNAc as the primary carbohydrate sources, and this was attributed in part to the fact that S. mutans produced higher activity of the NagA and NagB enzymes than S. sobrinus (97). Recent findings regarding the utilization of GlcNAc by S. mutans UA159 have demonstrated that the levels of nagB and glmS mRNA, as well as the levels of the enzymes in the cell, are inversely related (98). NagB levels in the cell were elevated as the concentration of GlcNAc in the media was increased, whereas GlmS levels decreased under the same conditions. Further, a mutation of nagB resulted in an inability to grow in the presence of GlcNAc, a decrease in biofilm formation and the diminished levels of virulence-associated proteins, including GtfBC and PAc. Loss of glmS resulted in cells being unable to grow in the absence of GlcNAc and displaying an increase in the expression of GtfBC and biofilm formation (98). Control of production of these enzymes in this manner would prevent metabolism of GlcNAc when needed for anabolic processes.
The regulation of amino sugar catabolism has been well characterized in several notable organisms (99–102). In the case of S. mutans, transcription of nagA and nagB, which are located within separate operons, is under the control of a GntR/HutC-type transcriptional regulator, NagR, which binds near the nagA and nagB promoter (25). Initial functional studies conducted in vitro showed that the NagR protein of S. mutans could be allosterically regulated by glucosamine-6-phosphate (25). Surprisingly, it was also found that NagR is capable of binding to the promoter region upstream of glmS and has a direct role in regulating its transcription. Past reports have demonstrated that regulation of glmS is controlled by small RNAs (sRNAs) in E. coli and other members of Enterobacteriaceae (103, 104), or by a metabolite-binding ribozyme in many Gram-positive organisms (105, 106). Analysis of the genomes of multiple Gram-positive species has revealed that the canonical residues for the formation of the ribozyme structure are notably absent in species of streptococci and most species in the genus Lactobacillus (98, 105). Our working hypothesis is that differential regulation of nagA, nagB and glmS is mediated through NagR in response to various pools of metabolites via allosteric regulation; however, the possibility remains that additional currently unknown regulators exist to ensure proper balance of NagAB and GlmS levels and/or activity.
Galactose
Galactose is an epimer of glucose and is common in dairy products and on the surface of many microbial cells and host proteins. The import of galactose by S. mutans strain UA159 is conducted primarily through low-affinity transport via the activity of the glucose/mannose (EIIMan) and lactose PTS permeases (107). Within the cell, galactose-6-phosphate enters either the Leloir or tagatose pathways for further catabolism. Early co-culture studies of S. mutans UA159 and the oral commensal Streptococcus gordonii DL1 demonstrated that S. gordonii can effectively outcompete S. mutans due to the presence of a high-affinity galactose PTS, a trait that may help to promote dental health (108, 109). However, a recent analysis of the genome sequences of 57 isolates of S. mutans from around the world (3) revealed that a predicted galactose-specific PTS system is present in the genomes of 35 of the 57 isolates (21). It was then shown that these strains did in fact produce a high-affinity PTS transporter of galactose that was inactivated when the genes were mutated. Also of note, a strain harboring the galactose PTS system was more effective at competing with S. gordonii in a mixed co-culture experiment and lowering the pH than an otherwise-isogenic mutant lacking this system (21). As galactose is generally considered a non-preferred carbohydrate, the presence of this high-affinity galactose PTS system could serve to enhance the cariogenic potential of S. mutans by adding to its repertoire of preferred carbohydrates. However, another interpretation of these results is that there is a divergence among isolates of S. mutans, with one group of bacteria that underwent a niche adaptation that favored the acquisition or retention of high-affinity galactose transport and one that did not. One could postulate that perhaps more-cariogenic species lost the galactose transporter, whereas those that retained the system are better adapted to co-existence with commensals in less-cariogenic biofilms or in sites in the mouth that may be enriched for galactose, e.g. sites near mucus-secreting salivary glands.
One abundant source of galactose in the oral cavity is mucin glycoproteins. A recent study on the long-term survival of S. mutans UA159 demonstrated that the addition of mucin to a medium containing sufficient quantities of amino acids significantly enhanced the survival of S. mutans, and this benefit required the presence of an intact tagatose pathway (110), which is a primary route for galactose catabolism in S. mutans. This study provides an interesting example of how S. mutans may exploit mucin as a supplementary nutrient source during fasting periods by the host, though not as the sole carbon and energy source (71). As the highly cariogenic reference strain UA159 possesses only the glucose/mannose and lactose PTS for galactose transport, it remains to be seen what effect the presence of a high-affinity galactose permease in S. mutans might have on the long-term survival of this organism in the presence of galactose-containing glycoproteins, such as mucin. Another issue that remains unresolved is whether S. mutans strains possess glycosidases that can effectively access galactose in glycoconjugates or if other members of the oral microbiota provide these activities in trans.
Cellobiose
As discussed above, the regulation of CCR in S. mutans is not dominated by CcpA, and this is particularly notable for catabolism of the β-glucoside, cellobiose, which is composed of two glucose moieties joined by a β1,4 linkage (4-β-D-glucopyranosyl-D-glucopyranose). Genetic analysis of the cel operon has revealed that cellobiose is internalized by the EIICCel permease (celD) and phosphorylated by the activity of EIIACel (celC) and EIIBCel (celB) (111). The internalized disaccharide can then be acted on by the activity of a phospho-β-glucosidase enzyme encoded by celA. The deletion of celA or celD in S. mutans UA159 resulted in a strain that was unable to grow in the presence of cellobiose, which indicated that the EIICel complex is the exclusive system for the transport of cellobiose and that there are no exoenzymes expressed under the growth conditions tested that can cleave cellobiose to release free glucose (111). The genes for cellobiose utilization are regulated by CelR, which contains two DNA-binding domains at the N-terminus, two PTS regulatory domains (PRD) (112) in the center of the protein and one EIIA-like domain at the C-terminus. Five of the histidine residues in CelR are predicted to undergo phosphorylation, two located in each PRD (H226 and H284; H332 and H391) and one (H576) located in the EIIA-like domain. The primary structure of CelR is similar to the mannitol transcriptional regulator MtlR of Geobacillus stearothermophilus (24, 113–115). Microarray studies comparing a strain lacking the manL gene for the glucose/mannose EIIAB protein revealed that celA, celC, and celD were highly expressed in the manL mutant when compared to the wild-type strain (116). Also of note, a ccpA mutant did not display a similar upregulation of the cel operon when assayed by microarray (29), though a subsequent RNA-Seq study did show modest elevation in cel operon expression upon deletion of ccpA (117).
In order to determine the contribution of ManL, certain other PTS components and CcpA to cellobiose utilization, a study characterizing the genetic regulation of the cel operon was initiated (24). It was demonstrated that CelR is required to activate the cel operon through a mechanism dependent on the PRD domains located within CelR. Mutation of PRD histidine residues 284 or 391 to alanine resulted in constitutive activation of the cel operon, whereas similar mutations of histidine residues 226, 332 or 576 resulted in a loss of cel operon expression. Further testing showed that with regard to CelR activity, the latter three histidine residues were dominant to the former two, and it was interpreted that histidine residues 226, 332, and 576 are targets for the general PTS proteins EI and HPr, and that phosphorylation of these residues is required for activation of CelR (24). The loss of EIIACel led to constitutive expression of cel genes and an EIIBCel deletion drastically, although not completely, reduced the ability for cellobiose to activate cel genes. Initially, the former result was interpreted as a requirement of phosphorylation at histidine residues 284 and 391 by EIIACel to deactivate CelR, which was removed with the loss of EIIACel. Likewise, deletion of EIIBCel was thought to cause constitutive phosphorylation of EIIACel, leading to inactivation of CelR (24). Notably, lack of EIICCel, which should also lead to constitutive phosphorylation of EIIACel, resulted in complete loss of cel gene activation in the presence of cellobiose. In a recent study of the homologous PRD-containing regulator MtlR of Bacillus subtilis (118), it was reported that membrane sequestration of MtlR by a dephosphorylated EIIBMtl is essential for full functionality of MtlR and the subsequent activation of downstream catabolic genes. This observation with MtlR could provide a possible explanation for the behavior of S. mutans UA159 lacking EIIBCel, whereby phosphorylation by EIIBCel serves to inactivate CelR, and dephosphorylated EIIBCel serves as a tether to keep CelR associated with the membrane and facilitates cel operon activation. However, it remains to be experimentally proven if CelR of S. mutans is regulated in this fashion.
Another level of regulation seen for cellobiose utilization involves the EII permeases enzymes ManL, FruCD, and LevD. Deletion of manL results in upregulation of the cel operon, and the most profound derepression occurs in the presence of glucose, as it serves as a secondary substrate of EIICel. Enhanced expression of the cel operon in the presence of the repressing sugars fructose or mannose required deletion of multiple fructose and mannose porters, i.e. manL, fruCD, fruI, and levD, to become apparent. The authors concluded that ManL, in the presence of glucose, siphons PEP-derived phosphate groups from CelR, which results in decreased expression from the operon (24). In this manner, the PRD-containing transcription regulator CelR adds another dimension to the picture of CcpA-independent CCR in S. mutans and provides further examples of the profound influence that EII permeases can have on metabolism of preferred and non-preferred carbohydrates.
Impact of carbohydrate metabolism in other pathogenic streptococci
When considering bacterial pathogens of humans, a great deal of attention has been placed on the production of virulence factors that mediate the destruction of host cells, such as secreted exotoxins. However, a critical area of study, often neglected, is the requirement by invading pathogens to acquire nutrients both during asymptomatic carriage and while eliciting pathologies. Not surprisingly, carbohydrate utilization pathways are intimately tied to colonization and the production of virulence compounds in many pathogens, including many bacteria that are related to S. mutans. As we conclude this discussion of carbohydrate metabolism and how it may influence pathogenesis, we will highlight certain recent advances in carbohydrate utilization and catabolite repression in the human pathogens Streptococcus pyogenes and S. pneumoniae and how these studies provided interesting perspectives on concepts discussed above.
S. pyogenes is capable of eliciting diseases in a diverse range of sites across the human body, including the skin and deeper tissues, the respiratory tract and in extreme cases, the bloodstream. Recent studies have begun to illustrate the critical importance of nutrient acquisition to the success of this pathogen. For example, the latest study of the general PTS proteins, EI and HPr, of S. pyogenes has implicated EI (ptsI) in the regulation of the sag operon, which produces the streptolysin S (SLS) toxin (119), while HPr is considered absolutely required for growth; as has been demonstrated in S. mutans as well as S. pneumoniae (28, 119, 120). In addition to the role played by the general PTS proteins, three carbohydrate responsive regulators in S. pyogenes have recently been shown to be critically important to the pathogen's success. Firstly, CcpA plays an important role in coordinating virulence gene expression, and deletion of ccpA has in some cases been linked to a loss of virulence in murine models of infection (121, 122). Other researchers have observed an enhancement of virulence in the ccpA deletion strain similarly to that exhibited by the ptsI mutant though less severe (123).
A second nutrient responsive regulator of S. pyogenes is LacD.1, a tagatose-1,6-bisphosphate aldolase, which is encoded in one of two lac operons located in the S. pyogenes genomes (124, 125). Interestingly, regulation by LacD.1 is independent of the enzyme's catalytic activity though likely dependent on substrate binding. Instead, LacD.1 is primarily believed to serve as a metabolite-sensing co-regulator of enzymes including the DNA-binding regulator Rgg (also called RopB) (124, 125). S. mutans UA159 has also been noted to contain a functional lacD gene (126–128) and an uncharacterized, orthologous gene lacD2 (Abranches et al., unpublished results). Investigations into the role of nutrient-sensing regulators in S. pyogenes, including CcpA and LacD.1, during the course of a murine infection generally concluded that CcpA is critical to virulence gene regulation inside mature lesions, whereas LacD.1 appears more important at early and late timepoints. These differences were perhaps mediated by the regulators sensing and responding to different environmental signals, such as glucose, during the course of infection, and may also be influenced by the site of infection (129). Finally, the major ‘stand-alone’ regulator of S. pyogenes Mga structurally resembles the transcriptional regulator MtlR of G. stearothermophilus, yet S. pyogenes possesses a CcpA-binding site upstream of the promoter for mga (130, 131). Studies of the PRD domain of Mga in a mouse model indicated that maintenance of its phosphorylation level is critical to virulence expression, further illustrating the importance of nutrient source and availability during the course of an active infection (131). Collectively, these studies demonstrate the complexity of metabolite-sensing regulatory circuits during in vivo infections and raise important questions of how metabolite sensitive regulators are coordinated during various phases of infection and disease.
S. pneumoniae is another major human pathogen that colonizes the pharynx and is often cleared asymptomatically, but can cause more severe disease in some cases. As S. pneumoniae moves away from the oral cavity and ventures into the airways, it is likely that this pathogen must liberate and metabolize nutrients from host glycoconjugates, including host defense molecules (132). In addition to a complement of glycosidases, a recent comprehensive study across several strains of S. pneumoniae has identified as many as 32 carbon sources directly metabolizable by this bacterium, with the vast majority being transported by PTS permeases (133). Of interest, this study demonstrated the importance of a mannose-type PTS transporter in the internalization of glucose, galactose, mannose, GlcNAc, and GlcN (133), similar to what has been seen in S. mutans (25, 107, 134); though, it was notable that the operon in S. pneumoniae lacks the accessory gene manO (133). As demonstrated in other streptococcal species, the success of S. pneumoniae within an in vivo infection model is greatly diminished in the absence of the major catabolite regulator CcpA (135, 136). The prominent influence of CcpA as a master regulator within S. pneumoniae was recently demonstrated in a study using transposon sequencing (Tn-seq), which implicated CcpA in 64 genetic interactions (137), and another study combining transcriptomic and metabolic analyses (138). Collectively, these recent advancements in our understanding of the human pathogens S. pyogenes and S. pneumoniae are beginning to reveal the critical role of carbohydrate uptake and related gene regulation during the course of infections.
Conclusions and future direction
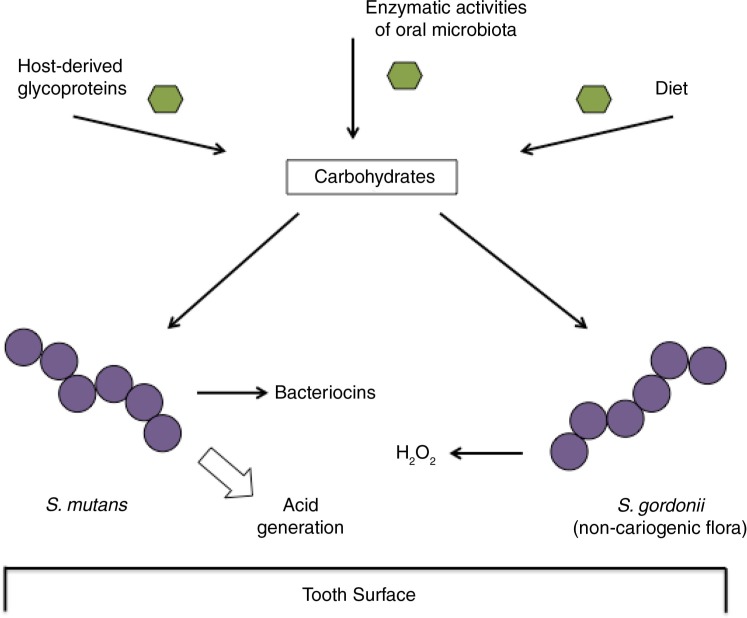
For pathogenic streptococci, acquisition of nutrients is a key event in the survival within and exploitation of the host. It is readily apparent that oral cariogenic bacteria are a product of the dynamic environment that they inhabit and differences in the levels and types of carbohydrates presented to cariogenic species have a great influence on the caries process. The ability of S. mutans to coordinate carbohydrate metabolism in response to changing environmental factors is critical to its ability to outcompete other members of the oral microbiome when conditions are conducive for caries development (Fig. 2). Further, carbohydrate sources have been demonstrated to impact the ability of the pathogen to antagonize commensals via the production of bacteriocins (139) and conversely, the capacity of commensals to respond through the production of defensive compounds, such as hydrogen peroxide (140, 141). While the numerous and often complex interactions of S. mutans with other members of the oral microbiome undoubtedly impact carbohydrate utilization and its regulation, the many studies of the impact of carbohydrate availability on S. mutans in pure cultures have greatly enhanced our understanding of how phenotypic and genotypic events converge and provided a model for more complex, in vivo studies of this major pathogen. As tools evolve to study whole populations at the transcriptomic and metabolomic level, it will be a fascinating journey to understand how S. mutans and other abundant plaque streptococci thrive in the human host.
Fig. 2.
Illustration of the response of S. mutans and non-cariogenic flora to carbohydrates introduced to the oral cavity.
Acknowledgements
The authors’ research in this area was supported by DE12236 from the National Institute of Dental and Craniofacial Research. A University of Florida Alumni Fellowship supported ZDM.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
References
- 1.Burne RA, Zeng L, Ahn SJ, Palmer SR, Liu Y, Lefebure T, et al. Progress dissecting the oral microbiome in caries and health. Adv Dent Res. 2012;24:77–80. doi: 10.1177/0022034512449462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamond J. Evolution, consequences and future of plant and animal domestication. Nature. 2002;418:700–7. doi: 10.1038/nature01019. [DOI] [PubMed] [Google Scholar]
- 3.Cornejo OE, Lefébure T, Pavinski Bitar PD, Lang P, Richards VP, Eilertson K, et al. Evolutionary and population penomics of the cavity causing bacteria Streptococcus mutans . Mol Biol Evol. 2013;30:881–93. doi: 10.1093/molbev/mss278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukacs JR. Dental paleopathology and agricultural intensification in south Asia: new evidence from Bronze Age Harappa. Am J Phys Anthropol. 1992;87:133–50. doi: 10.1002/ajpa.1330870202. [DOI] [PubMed] [Google Scholar]
- 5.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkinson HF. Beyond the oral microbiome. Environ Microbiol. 2011;13:3077–87. doi: 10.1111/j.1462-2920.2011.02573.x. [DOI] [PubMed] [Google Scholar]
- 7.Marsh PD. Sugar, fluoride, pH and microbial homeostasis in dental plaque. Proc Finn Dent Soc. 1991;87:515–25. [PubMed] [Google Scholar]
- 8.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–94. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 9.Burne RA. Oral streptococci … products of their environment. J Dent Res. 1998;77:445–52. doi: 10.1177/00220345980770030301. [DOI] [PubMed] [Google Scholar]
- 10.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–80. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemos JA, Quivey RG, Jr, Koo H, Abranches J. Streptococcus mutans: a new Gram-positive paradigm? Microbiology. 2013;159:436–45. doi: 10.1099/mic.0.066134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler CJ, Dobney K, Weyrich LS, Kaidonis J, Walker AW, Haak W, et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat Genet. 2013;45:450–5. doi: 10.1038/ng.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell RR, Aduse-Opoku J, Sutcliffe IC, Tao L, Ferretti JJ. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J Biol Chem. 1992;267:4631–7. [PubMed] [Google Scholar]
- 14.Tao L, Sutcliffe IC, Russell RR, Ferretti JJ. Transport of sugars, including sucrose, by the msm transport system of Streptococcus mutans . J Dent Res. 1993;72:1386–90. doi: 10.1177/00220345930720100701. [DOI] [PubMed] [Google Scholar]
- 15.Webb AJ, Homer KA, Hosie AH. Two closely related ABC transporters in Streptococcus mutans are involved in disaccharide and/or oligosaccharide uptake. J Bacteriol. 2008;190:168–78. doi: 10.1128/JB.01509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vadeboncoeur C, Pelletier M. The phosphoenolpyruvate: sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol Rev. 1997;19:187–207. doi: 10.1111/j.1574-6976.1997.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 18.Postma PW, Lengeler JW, Jacobson GR. Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–94. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robillard GT, Broos J. Structure/function studies on the bacterial carbohydrate transporters, enzymes II, of the phosphoenolpyruvate-dependent phosphotransferase system. Biochim Biophys Acta. 1999;1422:73–104. doi: 10.1016/s0304-4157(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 20.Postma PW, Lengeler JW. Phosphoenolpyruvate: carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985;49:232–69. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng L, Xue P, Stanhope MJ, Burne RA. A galactose-specific sugar: phosphotransferase permease is prevalent in the non-core genome of Streptococcus mutans . Mol Oral Microbiol. 2013;28:292–301. doi: 10.1111/omi.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajdić D, McShan WM, McLaughlin RE, Savić G, Chang J, Carson MB, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A. 2002;99:14434–9. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKessar SJ, Hakenbeck R. The two-component regulatory system TCS08 is involved in cellobiose metabolism of Streptococcus pneumoniae R6. J Bacteriol. 2007;189:1342–50. doi: 10.1128/JB.01170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng L, Burne RA. Transcriptional regulation of the cellobiose operon of Streptococcus mutans . J Bacteriol. 2009;191:2153–62. doi: 10.1128/JB.01641-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moye ZD, Burne RA, Zeng L. Uptake and metabolism of N-Acetylglucosamine and glucosamine by Streptococcus mutans . Appl Environ Microbiol. 2014;80:5053–67. doi: 10.1128/AEM.00820-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr Opin Microbiol. 2008;11:87–93. doi: 10.1016/j.mib.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Titgemeyer F, Hillen W. Global control of sugar metabolism: a gram-positive solution. Antonie Van Leeuwenhoek. 2002;82:59–71. [PubMed] [Google Scholar]
- 28.Zeng L, Burne RA. Seryl-phosphorylated HPr regulates CcpA-independent carbon catabolite repression in conjunction with PTS permeases in Streptococcus mutans . Mol Microbiol. 2010;75:1145–58. doi: 10.1111/j.1365-2958.2009.07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abranches J, Nascimento MM, Zeng L, Browngardt CM, Wen ZT, Rivera MF, et al. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans . J Bacteriol. 2008;190:2340–9. doi: 10.1128/JB.01237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng L, Burne RA. Comprehensive mutational analysis of sucrose-metabolizing pathways in Streptococcus mutans reveals novel roles for the sucrose phosphotransferase system permease. J Bacteriol. 2013;195:833–43. doi: 10.1128/JB.02042-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng L, Burne RA. Multiple sugar: phosphotransferase system permeases participate in catabolite modification of gene expression in Streptococcus mutans . Mol Microbiol. 2008;70:197–208. doi: 10.1111/j.1365-2958.2008.06403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monod J. La technique de culture continue: théorie et applications. Ann Inst Pasteur (Paris) 1950;79:390–410. [Google Scholar]
- 33.Novick A, Szilard L. Description of the chemostat. Science. 1950;112:715–6. doi: 10.1126/science.112.2920.715. [DOI] [PubMed] [Google Scholar]
- 34.Hoskisson PA, Hobbs G. Continuous culture – making a comeback? Microbiology. 2005;151:3153–9. doi: 10.1099/mic.0.27924-0. [DOI] [PubMed] [Google Scholar]
- 35.Burne RA, Chen YM. The use of continuous flow bioreactors to explore gene expression and physiology of suspended and adherent populations of oral streptococci. Methods Cell Sci. 1998;20:181–90. [Google Scholar]
- 36.Knox KW, Jacques NA, Campbell LK, Wicken AJ, Hurst SF, Bleiweis AS. Phenotypic stability of the cell wall of Streptococcus mutans Ingbritt grown under various conditions. Infect Immun. 1979;26:1071–8. doi: 10.1128/iai.26.3.1071-1078.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacques NA, Hardy L, Campbell LK, Knox KW, Evans JD, Wicken AJ. Effect of carbohydrate source and growth conditions on the production of lipoteichoic acid by Streptococcus mutans Ingbritt. Infect Immun. 1979;26:1079–87. doi: 10.1128/iai.26.3.1079-1087.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacques NA, Hardy L, Knox KW, Wicken AJ. Effect of growth conditions on the formation of extracellular lipoteichoic acid by Streptococcus mutans BHT. Infect Immun. 1979;25:75–84. doi: 10.1128/iai.25.1.75-84.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardy L, Jacques NA, Forester H, Campbell LK, Knox KW, Wicken AJ. Effect of fructose and other carbohydrates on the surface properties, lipoteichoic acid production, and extracellular proteins of Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1981;31:78–87. doi: 10.1128/iai.31.1.78-87.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linzer R, Campbell LK, Knox KW. Characterization of polysaccharide antigens of Streptococcus mutans B13 grown under various conditions. Infect Immun. 1984;44:76–81. doi: 10.1128/iai.44.1.76-81.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grossi S, Prakobphol A, Linzer R, Campbell LK, Knox KW. Characterization of serological cross-reactivity between polysaccharide antigens of Streptococcus mutans serotypes c and d . Infect Immun. 1983;39:1473–6. doi: 10.1128/iai.39.3.1473-1476.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wexler DL, Hudson MC, Burne RA. Streptococcus mutans fructosyltransferase (ftf) and glucosyltransferase (gtfBC) operon fusion strains in continuous culture. Infect Immun. 1993;61:1259–67. doi: 10.1128/iai.61.4.1259-1267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wenham DG, Hennessey TD, Cole JA. Regulation of glucosyl- and fructosyltransferase synthesis by continuous cultures of Streptococcus mutans . J Gen Microbiol. 1979;114:117–24. doi: 10.1099/00221287-114-1-117. [DOI] [PubMed] [Google Scholar]
- 44.Walker GJ, Brown RA, Taylor C. Activity of Streptococcus mutans α-D-glucosyltransferases released under various growth conditions. J Dent Res. 1984;63:397–400. doi: 10.1177/00220345840630030801. [DOI] [PubMed] [Google Scholar]
- 45.Walker G, Morrey-Jones J, Svensson S, Taylor C. Effect of variation in growth conditions on the activity of D-glucosyltransferases and the synthesis of α-D-glucans by Streptococcus mutans OMZ176. In: Doyle RJ, Ciardi JE, editors. Glucosyltransferases, glucans, sucrose and dental caries (Sp. Suppl. Chemical Senses) Washington, DC: Information Retrieval Limited; 1983. pp. 179–200. [Google Scholar]
- 46.Hamilton IR, Phipps PJ, Ellwood DC. Effect of growth rate and glucose concentration on the biochemical properties of Streptococcus mutans Ingbritt in continuous culture. Infect Immun. 1979;26:861–9. doi: 10.1128/iai.26.3.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton IR, Gauthier L, Desjardins B, Vadeboncoeur C. Concentration-dependent repression of the soluble and membrane components of the Streptococcus mutans phosphoenolpyruvate: sugar phosphotransferase system by glucose. J Bacteriol. 1989;171:2942–8. doi: 10.1128/jb.171.6.2942-2948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodrigue L, Lacoste L, Trahan L, Vadeboncoeur C. Effect of nutritional constraints on the biosynthesis of the components of the phosphoenolpyruvate: sugar phosphotransferase system in a fresh isolate of Streptococcus mutans . Infect Immun. 1988;56:518–22. doi: 10.1128/iai.56.2.518-522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vadeboncoeur C, Thibault L, Neron S, Halvorson H, Hamilton IR. Effect of growth conditions on levels of components of the phosphoenolpyruvate: sugar phosphotransferase system in Streptococcus mutans and Streptococcus sobrinus grown in continuous culture. J Bacteriol. 1987;169:5686–91. doi: 10.1128/jb.169.12.5686-5691.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellwood DC, Hamilton IR. Properties of Streptococcus mutans Ingbritt growing on limiting sucrose in a chemostat: repression of the phosphoenolpyruvate phosphotransferase transport system. Infect Immun. 1982;36:576–81. doi: 10.1128/iai.36.2.576-581.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellwood DC, Phipps PJ, Hamilton IR. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979;23:224–31. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamilton IR, Bowden GH. Response of freshly isolated strains of Streptococcus mutans and Streptococcus mitior to change in pH in the presence and absence of fluoride during growth in continuous culture. Infect Immun. 1982;36:255–62. doi: 10.1128/iai.36.1.255-262.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton IR, Ellwood DC. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978;19:434–42. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlsson J, Griffith CJ. Fermentation products and bacterial yields in glucose-limited and nitrogen-limited cultures of streptococci. Arch Oral Biol. 1974;19:1105–9. doi: 10.1016/0003-9969(74)90238-6. [DOI] [PubMed] [Google Scholar]
- 55.Belli WA, Marquis RE. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl Environ Microbiol. 1991;57:1134–8. doi: 10.1128/aem.57.4.1134-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamilton IR, Buckley ND. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol Immunol. 1991;6:65–71. doi: 10.1111/j.1399-302x.1991.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 57.Quivey RG, Jr, Faustoferri R, Monahan K, Marquis R. Shifts in membrane fatty acid profiles associated with acid adaptation of Streptococcus mutans . FEMS Microbiol Lett. 2000;189:89–92. doi: 10.1111/j.1574-6968.2000.tb09211.x. [DOI] [PubMed] [Google Scholar]
- 58.Martin ME, Strachan RC, Aranha H, Evans SL, Salin ML, Welch B, et al. Oxygen toxicity in Streptococcus mutans: manganese, iron, and superoxide dismutase. J Bacteriol. 1984;159:745–9. doi: 10.1128/jb.159.2.745-749.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aranha H, Strachan RC, Arceneaux JE, Byers BR. Effect of trace metals on growth of Streptococcus mutans in a teflon chemostat. Infect Immun. 1982;35:456–60. doi: 10.1128/iai.35.2.456-460.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strachan RC, Aranha H, Lodge JS, Arceneaux JE, Byers BR. Teflon chemostat for studies of trace metal metabolism in Streptococcus mutans and other bacteria. Appl Environ Microbiol. 1982;43:257–60. doi: 10.1128/aem.43.1.257-260.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Len AC, Cordwell SJ, Harty DW, Jacques NA. Cellular and extracellular proteome analysis of Streptococcus mutans grown in a chemostat. Proteomics. 2003;3:627–46. doi: 10.1002/pmic.200300391. [DOI] [PubMed] [Google Scholar]
- 62.Len AC, Harty DW, Jacques NA. Proteome analysis of Streptococcus mutans metabolic phenotype during acid tolerance. Microbiology. 2004;150:1353–66. doi: 10.1099/mic.0.26888-0. [DOI] [PubMed] [Google Scholar]
- 63.Chen PM, Chen YY, Yu SL, Sher S, Lai CH, Chia JS. Role of GlnR in acid-mediated repression of genes encoding proteins involved in glutamine and glutamate metabolism in Streptococcus mutans . Appl Environ Microbiol. 2010;76:2478–86. doi: 10.1128/AEM.02622-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Derr AM, Faustoferri RC, Betzenhauser MJ, Gonzalez K, Marquis RE, Quivey RG., Jr Mutation of the NADH oxidase gene (nox) reveals an overlap of the oxygen- and acid-mediated stress responses in Streptococcus mutans . Appl Environ Microbiol. 2012;78:1215–27. doi: 10.1128/AEM.06890-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moye ZD, Zeng L, Burne RA. Modification of gene expression and virulence traits in Streptococcus mutans in response to carbohydrate availability. Appl Environ Microbiol. 2014;80:972–85. doi: 10.1128/AEM.03579-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cvitkovitch DG, Boyd DA, Thevenot T, Hamilton IR. Glucose transport by a mutant of Streptococcus mutans unable to accumulate sugars via the phosphoenolpyruvate phosphotransferase system. J Bacteriol. 1995;177:2251–8. doi: 10.1128/jb.177.9.2251-2258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thibault L, Vadeboncoeur C. Phosphoenolpyruvate-sugar phosphotransferase transport system of Streptococcus mutans: purification of HPr and enzyme I and determination of their intracellular concentrations by rocket immunoelectrophoresis. Infect Immun. 1985;50:817–25. doi: 10.1128/iai.50.3.817-825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vadeboncoeur C, Brochu D, Reizer J. Quantitative determination of the intracellular concentration of the various forms of HPr, a phosphocarrier protein of the phosphoenolpyruvate: sugar phosphotransferase system in growing cells of oral streptococci. Anal Biochem. 1991;196:24–30. doi: 10.1016/0003-2697(91)90112-7. [DOI] [PubMed] [Google Scholar]
- 69.Thevenot T, Brochu D, Vadeboncoeur C, Hamilton IR. Regulation of ATP-dependent P-(Ser)-HPr formation in Streptococcus mutans and Streptococcus salivarius . J Bacteriol. 1995;177:2751–9. doi: 10.1128/jb.177.10.2751-2759.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klein MI, DeBaz L, Agidi S, Lee H, Xie G, Lin AH, et al. Dynamics of Streptococcus mutans transcriptome in response to starch and sucrose during biofilm development. PLoS One. 2010;5:e13478. doi: 10.1371/journal.pone.0013478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van der Hoeven JS, van den Kieboom CW, Camp PJ. Utilization of mucin by oral Streptococcus species. Antonie Van Leeuwenhoek. 1990;57:165–72. doi: 10.1007/BF00403951. [DOI] [PubMed] [Google Scholar]
- 72.Renye JA, Jr, Piggot PJ, Daneo-Moore L, Buttaro BA. Persistence of Streptococcus mutans in stationary-phase batch cultures and biofilms. Appl Environ Microbiol. 2004;70:6181–7. doi: 10.1128/AEM.70.10.6181-6187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–9. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 74.Shellis RP. A synthetic saliva for cultural studies of dental plaque. Arch Oral Biol. 1978;23:485–9. doi: 10.1016/0003-9969(78)90081-x. [DOI] [PubMed] [Google Scholar]
- 75.Björklund M, Ouwehand AC, Forssten SD. Improved artificial saliva for studying the cariogenic effect of carbohydrates. Curr Microbiol. 2011;63:46–9. doi: 10.1007/s00284-011-9937-x. [DOI] [PubMed] [Google Scholar]
- 76.Glenister D, Salamon KE, Smith K, Beighton D, Keevil C. Enhanced growth of complex communities of dental plaque bacteria in mucin-limited continuous culture. Microb Ecol Health Dis. 1988;1:31–8. [Google Scholar]
- 77.De Stoppelaar JD, Van Houte J, Backer Dirks O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4:114–23. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- 78.Bowen WH. Rodent model in caries research. Odontology. 2013;101:9–14. doi: 10.1007/s10266-012-0091-0. [DOI] [PubMed] [Google Scholar]
- 79.Bradshaw DJ, Lynch RJ. Diet and the microbial aetiology of dental caries: new paradigms. Int Dent J. 2013;63(Suppl 2):64–72. doi: 10.1111/idj.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Newbrun E. Sucrose, the arch criminal of dental caries. ASDC J Dent Child. 1969;36:239–48. [PubMed] [Google Scholar]
- 81.Zero DT. Sugars – the arch criminal? Caries Res. 2004;38:277–85. doi: 10.1159/000077767. [DOI] [PubMed] [Google Scholar]
- 82.Ajdić D, Pham VT. Global transcriptional analysis of Streptococcus mutans sugar transporters using microarrays. J Bacteriol. 2007;189:5049–59. doi: 10.1128/JB.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aoki H, Shiroza T, Hayakawa M, Sato S, Kuramitsu HK. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986;53:587–94. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanada N, Kuramitsu HK. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989;57:2079–85. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hanada N, Kuramitsu HK. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988;56:1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shiroza T, Kuramitsu HK. Sequence analysis of the Streptococcus mutans fructosyltransferase gene and flanking regions. J Bacteriol. 1988;170:810–6. doi: 10.1128/jb.170.2.810-816.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burne RA, Schilling K, Bowen WH, Yasbin RE. Expression, purification, and characterization of an exo-β-D-fructosidase of Streptococcus mutans . J Bacteriol. 1987;169:4507–17. doi: 10.1128/jb.169.10.4507-4517.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burne RA, Penders JE. Characterization of the Streptococcus mutans GS-5 fruA gene encoding exo-β-D-fructosidase. Infect Immun. 1992;60:4621–32. doi: 10.1128/iai.60.11.4621-4632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tanzer JM, Chassy BM, Krichevsky MI. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim Biophys Acta. 1971;261:379–87. doi: 10.1016/0304-4165(72)90062-1. [DOI] [PubMed] [Google Scholar]
- 91.Poy F, Jacobson GR. Evidence that a low-affinity sucrose phosphotransferase activity in Streptococcus mutans GS-5 is a high-affinity trehalose uptake system. Infect Immun. 1990;58:1479–80. doi: 10.1128/iai.58.5.1479-1480.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeng L, Wen ZT, Burne RA. A novel signal transduction system and feedback loop regulate fructan hydrolase gene expression in Streptococcus mutans . Mol Microbiol. 2006;62:187–200. doi: 10.1111/j.1365-2958.2006.05359.x. [DOI] [PubMed] [Google Scholar]
- 93.Ajdić D, Chen Z. A novel phosphotransferase system of Streptococcus mutans is responsible for transport of carbohydrates with α-1,3 linkage. Mol Oral Microbiol. 2013;28:114–28. doi: 10.1111/omi.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beighton D, Russell RR, Whiley RA. A simple biochemical scheme for the differentiation of Streptococcus mutans and Streptococcus sobrinus . Caries Res. 1991;25:174–8. doi: 10.1159/000261363. [DOI] [PubMed] [Google Scholar]
- 95.Kral TA, Daneo-Moore L. Biochemical differentiation of certain oral streptococci. J Dent Res. 1981;60:1713–8. doi: 10.1177/00220345810600091301. [DOI] [PubMed] [Google Scholar]
- 96.Jacobson GR, Poy F, Lengeler JW. Inhibition of Streptococcus mutans by the antibiotic streptozotocin: mechanisms of uptake and the selection of carbohydrate-negative mutants. Infect Immun. 1990;58:543–9. doi: 10.1128/iai.58.2.543-549.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Homer KA, Patel R, Beighton D. Effects of N-acetylglucosamine on carbohydrate fermentation by Streptococcus mutans NCTC 10449 and Streptococcus sobrinus SL-1. Infect Immun. 1993;61:295–302. doi: 10.1128/iai.61.1.295-302.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawada-Matsuo M, Mazda Y, Oogai Y, Kajiya M, Kawai T, Yamada S, et al. GlmS and NagB regulate amino sugar metabolism in opposing directions and affect Streptococcus mutans virulence. PLoS One. 2012;7:e33382. doi: 10.1371/journal.pone.0033382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bertram R, Rigali S, Wood N, Lulko AT, Kuipers OP, Titgemeyer F. Regulon of the N-acetylglucosamine utilization regulator NagR in Bacillus subtilis . J Bacteriol. 2011;193:3525–36. doi: 10.1128/JB.00264-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vogler AP, Lengeler JW. Analysis of the nag regulon from Escherichia coli K12 and Klebsiella pneumoniae and of its regulation. Mol Gen Genet. 1989;219:97–105. doi: 10.1007/BF00261163. [DOI] [PubMed] [Google Scholar]
- 101.Rigali S, Nothaft H, Noens EE, Schlicht M, Colson S, Müller M, et al. The sugar phosphotransferase system of Streptomyces coelicolor is regulated by the GntR-family regulator DasR and links N-acetylglucosamine metabolism to the control of development. Mol Microbiol. 2006;61:1237–51. doi: 10.1111/j.1365-2958.2006.05319.x. [DOI] [PubMed] [Google Scholar]
- 102.Światek MA, Tenconi E, Rigali S, van Wezel GP. Functional analysis of the N-acetylglucosamine metabolic genes of Streptomyces coelicolor and role in control of development and antibiotic production. J Bacteriol. 2012;194:1136–44. doi: 10.1128/JB.06370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Görke B, Vogel J. Noncoding RNA control of the making and breaking of sugars. Genes Dev. 2008;22:2914–25. doi: 10.1101/gad.1717808. [DOI] [PubMed] [Google Scholar]
- 104.Göpel Y, Lüttmann D, Heroven AK, Reichenbach B, Dersch P, Görke B. Common and divergent features in transcriptional control of the homologous small RNAs GlmY and GlmZ in Enterobacteriaceae . Nucleic Acids Res. 2011;39:1294–309. doi: 10.1093/nar/gkq986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McCown PJ, Roth A, Breaker RR. An expanded collection and refined consensus model of glmS ribozymes. RNA. 2011;17:728–36. doi: 10.1261/rna.2590811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–6. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- 107.Zeng L, Das S, Burne RA. Utilization of lactose and galactose by Streptococcus mutans: transport, toxicity, and carbon catabolite repression. J Bacteriol. 2010;192:2434–44. doi: 10.1128/JB.01624-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeng L, Martino NC, Burne RA. Two gene clusters coordinate galactose and lactose metabolism in Streptococcus gordonii. Appl Environ Microbiol. 2012;78:5597–605. doi: 10.1128/AEM.01393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu J, Wu C, Huang IH, Merritt J, Qi F. Differential response of Streptococcus mutans towards friend and foe in mixed-species cultures. Microbiology. 2011;157:2433–44. doi: 10.1099/mic.0.048314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mothey D, Buttaro BA, Piggot PJ. Mucin can enhance growth, biofilm formation, and survival of Streptococcus mutans . FEMS Microbiol Lett. 2014;350:161–7. doi: 10.1111/1574-6968.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Old LA, Lowes S, Russell RR. Genomic variation in Streptococcus mutans: deletions affecting the multiple pathways of β-glucoside metabolism. Oral Microbiol Immunol. 2006;21:21–7. doi: 10.1111/j.1399-302X.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 112.Stülke J, Arnaud M, Rapoport G, Martin-Verstraete I. PRD — a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol Microbiol. 1998;28:865–74. doi: 10.1046/j.1365-2958.1998.00839.x. [DOI] [PubMed] [Google Scholar]
- 113.Henstra SA, Tolner B, ten Hoeve Duurkens RH, Konings WN, Robillard GT. Cloning, expression, and isolation of the mannitol transport protein from the thermophilic bacterium Bacillus stearothermophilus . J Bacteriol. 1996;178:5586–91. doi: 10.1128/jb.178.19.5586-5591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henstra SA, Tuinhof M, Duurkens RH, Robillard GT. The Bacillus stearothermophilus mannitol regulator, MtlR, of the phosphotransferase system. A DNA-binding protein, regulated by HPr and IICBmtl-dependent phosphorylation. J Biol Chem. 1999;274:4754–63. doi: 10.1074/jbc.274.8.4754. [DOI] [PubMed] [Google Scholar]
- 115.Henstra SA, Duurkens RH, Robillard GT. Multiple phosphorylation events regulate the activity of the mannitol transcriptional regulator MtlR of the Bacillus stearothermophilus phosphoenolpyruvate-dependent mannitol phosphotransferase system. J Biol Chem. 2000;275:7037–44. doi: 10.1074/jbc.275.10.7037. [DOI] [PubMed] [Google Scholar]
- 116.Abranches J, Candella MM, Wen ZT, Baker HV, Burne RA. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans . J Bacteriol. 2006;188:3748–56. doi: 10.1128/JB.00169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeng L, Choi SC, Danko CG, Siepel A, Stanhope MJ, Burne RA. Gene regulation by CcpA and catabolite repression explored by RNA-Seq in Streptococcus mutans . PLoS One. 2013;8:e60465. doi: 10.1371/journal.pone.0060465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bouraoui H, Ventroux M, Noirot-Gros MF, Deutscher J, Joyet P. Membrane sequestration by the EIIB domain of the mannitol permease MtlA activates the Bacillus subtilis mtl operon regulator MtlR. Mol Microbiol. 2013;87:789–801. doi: 10.1111/mmi.12131. [DOI] [PubMed] [Google Scholar]
- 119.Gera K, Le T, Jamin R, Eichenbaum Z, McIver KS. The phosphoenolpyruvate phosphotransferase system in group A Streptococcus acts to reduce streptolysin S activity and lesion severity during soft tissue infection. Infect Immun. 2014;82:1192–204. doi: 10.1128/IAI.01271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song JH, Ko KS, Lee JY, Baek JY, Oh WS, Yoon HS, et al. Identification of essential genes in Streptococcus pneumoniae by allelic replacement mutagenesis. Mol Cells. 2005;19:365–74. [PubMed] [Google Scholar]
- 121.Kietzman CC, Caparon MG. CcpA and LacD.1 affect temporal regulation of Streptococcus pyogenes virulence genes. Infect Immun. 2010;78:241–52. doi: 10.1128/IAI.00746-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shelburne SA, 3rd, Keith D, Horstmann N, Sumby P, Davenport MT, Graviss EA, et al. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus . Proc Natl Acad Sci U S A. 2008;105:1698–703. doi: 10.1073/pnas.0711767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kinkel TL, McIver KS. CcpA-mediated repression of streptolysin S expression and virulence in the group A streptococcus. Infect Immun. 2008;76:3451–63. doi: 10.1128/IAI.00343-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Loughman JA, Caparon MG. Comparative functional analysis of the lac operons in Streptococcus pyogenes . Mol Microbiol. 2007;64:269–80. doi: 10.1111/j.1365-2958.2007.05663.x. [DOI] [PubMed] [Google Scholar]
- 125.Loughman JA, Caparon MG. A novel adaptation of aldolase regulates virulence in Streptococcus pyogenes . EMBO J. 2006;25:5414–22. doi: 10.1038/sj.emboj.7601393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smorawinska M, Hsu JC, Hansen JB, Jagusztyn-Krynicka EK, Abiko Y, Curtiss R., 3rd Clustered genes for galactose metabolism from Streptococcus mutans cloned in Escherichia coli . J Bacteriol. 1983;153:1095–7. doi: 10.1128/jb.153.2.1095-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosey EL, Stewart GC. Nucleotide and deduced amino acid sequences of the lacR, lacABCD, and lacFE genes encoding the repressor, tagatose 6-phosphate gene cluster, and sugar-specific phosphotransferase system components of the lactose operon of Streptococcus mutans . J Bacteriol. 1992;174:6159–70. doi: 10.1128/jb.174.19.6159-6170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jagusztyn-Krynicka EK, Hansen JB, Crow VL, Thomas TD, Honeyman AL, Curtiss R., 3rd Streptococcus mutans serotype c tagatose 6-phosphate pathway gene cluster. J Bacteriol. 1992;174:6152–8. doi: 10.1128/jb.174.19.6152-6158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kietzman CC, Caparon MG. Distinct time-resolved roles for two catabolite-sensing pathways during Streptococcus pyogenes infection. Infect Immun. 2011;79:812–21. doi: 10.1128/IAI.01026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Almengor AC, Kinkel TL, Day SJ, McIver KS. The catabolite control protein CcpA binds to Pmga and influences expression of the virulence regulator Mga in the Group A streptococcus. J Bacteriol. 2007;189:8405–16. doi: 10.1128/JB.01038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hondorp ER, Hou SC, Hause LL, Gera K, Lee CE, McIver KS. PTS phosphorylation of Mga modulates regulon expression and virulence in the group A streptococcus. Mol Microbiol. 2013;88:1176–93. doi: 10.1111/mmi.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buckwalter CM, King SJ. Pneumococcal carbohydrate transport: food for thought. Trends Microbiol. 2012;20:517–22. doi: 10.1016/j.tim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bidossi A, Mulas L, Decorosi F, Colomba L, Ricci S, Pozzi G, et al. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae . PLoS One. 2012;7:e33320. doi: 10.1371/journal.pone.0033320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abranches J, Chen YY, Burne RA. Characterization of Streptococcus mutans strains deficient in EIIABMan of the sugar phosphotransferase system. Appl Environ Microbiol. 2003;69:4760–9. doi: 10.1128/AEM.69.8.4760-4769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iyer R, Baliga NS, Camilli A. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae . J Bacteriol. 2005;187:8340–9. doi: 10.1128/JB.187.24.8340-8349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Giammarinaro P, Paton JC. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect Immun. 2002;70:5454–61. doi: 10.1128/IAI.70.10.5454-5461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Opijnen T, Bodi KL, Camilli A. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods. 2009;6:767–72. doi: 10.1038/nmeth.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carvalho SM, Kloosterman TG, Kuipers OP, Neves AR. CcpA ensures optimal metabolic fitness of Streptococcus pneumoniae. PLoS One. 2011;6:e26707. doi: 10.1371/journal.pone.0026707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Merritt J, Qi F. The mutacins of Streptococcus mutans: regulation and ecology. Mol Oral Microbiol. 2012;27:57–69. doi: 10.1111/j.2041-1014.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zheng L, Itzek A, Chen Z, Kreth J. Environmental influences on competitive hydrogen peroxide production in Streptococcus gordonii. Appl Environ Microbiol. 2011;77:4318–28. doi: 10.1128/AEM.00309-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kreth J, Zhang Y, Herzberg MC. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol. 2008;190:4632–40. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]