Abstract
Cardiovascular diseases (CVD) remain the leading cause of morbidity and mortality in modern societies, and advancing age is the major risk factor for CVD. Arterial dysfunction, characterized by large elastic artery stiffening and endothelial dysfunction, is the key event leading to age-associated CVD. Our work shows that regular aerobic exercise inhibits large elastic artery stiffening with aging (optimizes arterial compliance) and preserves endothelial function. Importantly, among previously sedentary late middle-aged and older adults, aerobic exercise improves arterial stiffness and enhances endothelial function in most groups and, therefore, also can be considered a treatment for age-associated arterial dysfunction. The mechanisms by which regular aerobic exercise destiffens large elastic arteries are incompletely understood, but existing evidence suggests that reductions in oxidative stress associated with decreases in both adventitial collagen (fibrosis) and advanced glycation end-products (structural protein cross-linking molecules), play a key role. Aerobic exercise preserves endothelial function with aging by maintaining nitric oxide bioavailability via suppression of excessive superoxide-associated oxidative stress, and by inhibiting the development of chronic low-grade vascular inflammation. Recent work from our laboratory supports the novel hypothesis that aerobic exercise may exert these beneficial effects by directly inducing protection to aging arteries against multiple adverse factors to which they are chronically exposed. Regular aerobic exercise should be viewed as a “first line” strategy for prevention and treatment of arterial aging and a vital component of a contemporary public health approach for reducing the projected increase in population CVD burden.
Keywords: arterial stiffness, endothelial dysfunction, oxidative stress, inflammation
despite reductions in prevalence over the last 3+ decades, cardiovascular diseases (CVD) remain the leading cause of morbidity and mortality in the US and other modern societies.1 The primary risk factor for CVD is advancing age. Indeed, the prevalence of CVD of all causes increases progressively with advancing age, such that the great majority of individuals with CVD are middle-aged and older (MA/O) adults (43). This fact, combined with the projected increases in numbers of older adults worldwide, has led to concern of a new epidemic of CVD in the near future. A recent policy statement by the American Heart Association suggests that such concerns are well founded (31). The report projects that, by 2030, 40% of US adults will have at least one form of CVD, medical costs of CVD will triple, and that these changes will be driven largely by the aging of the population. It concludes that effective prevention strategies will be needed to limit the projected increase in the burden of CVD.
As we seek to identify effective prevention strategies, it is helpful to consider how aging increases CVD risk. Undoubtedly, many factors contribute. However, it is now well accepted that the key intermediary event that drives the majority of the increase in CVD risk with aging is the development of arterial dysfunction (37). Several changes to arteries with aging likely contribute to the increase in susceptibility for clinical CVD as we grow older, but two events, in particular, appear to be important (37). One is the stiffening of the large elastic arteries, i.e., the aorta and carotid arteries. As the nomenclature suggests, these arteries are designed to expand as they accept the left ventricular stroke volume with each contraction of the heart, and then recoil to create the necessary kinetic energy to drive the blood distally to our tissues and cells. The stiffening of these arteries with aging leads to numerous pathophysiological effects that collectively increase our risk of CVD, including increases in arterial systolic and pulse pressures, left ventricular hypertrophy (caused by repeatedly ejecting blood out into stiff arteries), and tissue damage as a result of increases in pulsatile flow, especially in high-flow vital organs such as the brain and kidneys (37, 38, 45).
A second clinically important change with aging is the development of endothelial dysfunction (37, 64). The vascular endothelium is a single cell layer at the interface between the flow of blood in the lumen of the artery and the walls of the artery. Once believed to be primarily a physical barrier charged with filtering solutes moving between the blood and arterial wall, the vascular endothelium now is understood to synthesize and release a wide array of biologically active molecules that act in autocrine and/or paracrine fashion to influence the function and health (resistance to disease) of arteries and their surrounding tissues. The most important of these endothelial-derived molecules is nitric oxide (NO), which exerts a provasodilatory and anti-coagulative, -proliferative, and -inflammatory protective effect on arteries (9, 64, 79). Endothelial dysfunction can be defined as any alteration from the healthy endothelial phenotype and typically is associated with reduced NO bioavailability (9, 64, 79).
With this background, we can establish a model in which aging leads to arterial dysfunction, as reflected in part by the development of large elastic artery stiffness and endothelial dysfunction, and this, in turn, increases our risk of clinical CVD. Our laboratory is interested in lifestyle and pharmacological strategies that act to delay or prevent the development of large elastic artery stiffening and endothelial dysfunction with aging, and/or to improve stiffness and endothelial function in MA/O adults with already established (baseline) vascular dysfunction (Fig. 1). In this recap of the 2013 Adolph Lecture, I will summarize the key findings of our work over the last 15+ years related to one of the most potent available strategies for preserving vascular function with advancing age: regular aerobic exercise. The ensuing discussion of the effects of aerobic exercise on vascular function with aging will focus on evidence in human subjects, as there are sufficient data to make conclusions in these populations. Discussion of the mechanisms mediating the anti-aging effects of exercise on vascular function will draw from research performed in both humans and rodents, as animal models are required in many cases to provide the most in depth, mechanistic insight. Much of the work discussed has been highlighted in previous reviews of the topic (14, 63–65, 67).
Fig. 1.
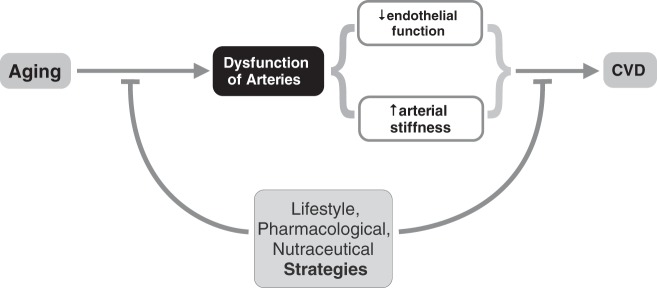
Aging, vascular dysfunction, and cardiovascular disease (CVD) risk. Aging causes increased risk of developing CVDs in large part through the development of arterial dysfunction, characterized in part by vascular endothelial dysfunction and stiffening of large elastic arteries (aorta and carotid arteries). As such, evidence-based lifestyle, pharmaceutical, and nutraceutical strategies that either delay/minimize/prevent arterial aging, or, in late middle-aged/older adults who already demonstrate features of vascular aging, improve arterial function and reduce the risk of CVD, are of high biomedical research priority.
LARGE ELASTIC ARTERY STIFFNESS, AGING, AND EXERCISE
Functional Effects
One important question is whether aerobic exercise can reduce or prevent increases in large elastic artery stiffness with aging, and/or reduce stiffness in middle-aged/older adults who already demonstrate large elastic artery stiffening.
Aortic pulse-wave velocity.
The gold standard clinical measurement of stiffness of the large elastic arteries is aortic pulse-wave velocity (PWV), typically measured as carotid-femoral PWV (3, 45, 46). As the term implies, aortic PWV is simply the velocity at which a pulse wave generated by left ventricular contraction travels down the aorta: the faster the velocity, the stiffer the artery. Aortic PWV increases progressively with adult aging, even in the absence of major CVD risk factors or clinical disease (3, 37, 75).
Using this technique, it was first shown that MA/O men who perform vigorous aerobic-endurance exercise (masters endurance athletes) demonstrated lower aortic PWV and systolic arterial blood pressure (SBP) compared with sedentary men of similar age, and closer to values observed in sedentary young controls (78). However, because this study did not report data on a young trained group, it was not possible to determine whether the lower aortic PWV in MA/O adults who perform aerobic exercise was the result of less stiffening with aging (i.e., a smaller age-associated difference) or the same change in stiffness from a lower (young trained group) baseline. Accordingly, we performed a cross-sectional comparison of premenopausal and postmenopausal healthy women who were either sedentary or engaged in endurance exercise training (75). Postmenopausal sedentary women had higher aortic PWV compared with premenopausal controls, but there were no significant differences between premenopausal and postmenopausal endurance exercise-trained women (Fig. 2), suggesting that regular aerobic exercise may prevent/reduce stiffening of the aorta with aging-menopause. The absence of a significant elevation in aortic PWV in the postmenopausal women who exercised was associated with a lack of increase in 24-h systolic arterial blood pressure and pulse pressure (66). Similar cross-sectional observations were later reported for healthy middle-aged men (39), and greater “light” dynamic physical activity was shown to be associated with lower aortic PWV in unfit older adults, suggesting that even a low-intensity aerobic exercise stimulus may exert destiffening effects (25). Importantly, moderate aerobic exercise interventions have been shown to reduce aortic PWV in healthy MA/O men and women (30, 87), indicating that aerobic exercise also can be viewed as a therapy for reducing aortic stiffness in this population.
Fig. 2.
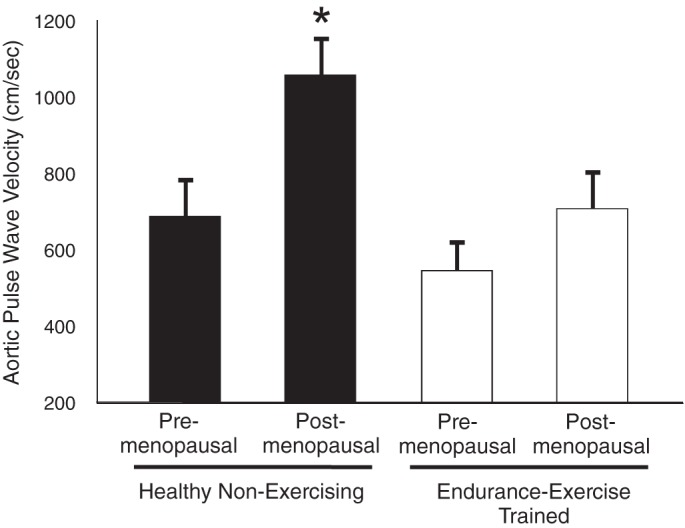
Aging, aortic pulse wave velocity, and regular aerobic exercise. Aortic (femoral to carotid artery) pulse wave velocity, a measure of aortic stiffness, is greater in healthy postmenopausal compared with premenopausal sedentary women, but not significantly different in premenopausal and postmenopausal endurance exercise-trained women. These and other findings support the concept that regular aerobic lessens/prevents aortic stiffing with aging in healthy adult humans. *P < 0.05 vs. other groups. Data are from Tanaka et al. (75).
Carotid artery compliance.
The stiffness of the carotid arteries can be assessed using the combination of ultrasound imaging of changes in the internal diameter of one carotid artery throughout the cardiac cycle, with simultaneous measurement of changes in arterial pressure in the contralateral carotid artery using applanation tonometry (51, 76). Knowing the concurrent changes in diameter and pressure allows the calculation of carotid artery compliance (opposite in direction to stiffness) and the less arterial pressure-dependent measure of carotid β-stiffness (directly proportional to stiffness). Using this approach, we showed that carotid artery compliance decreased (β-stiffness increased) progressively with aging in healthy normotensive men and women, but that these age-associated changes were only ∼50% as great in subjects who engaged in regular aerobic exercise compared with their sedentary peers (51, 76) (Fig. 3A). These findings support the idea that regular aerobic exercise suppresses stiffening of the carotid arteries with advancing age in men and women free of clinical disease. Again, this effect of aerobic exercise appears to be specific for the large elastic arteries, as no differences are observed in the compliance of the peripheral large (femoral) artery between endurance exercise-trained and sedentary MA/O adults (51, 76).
Fig. 3.
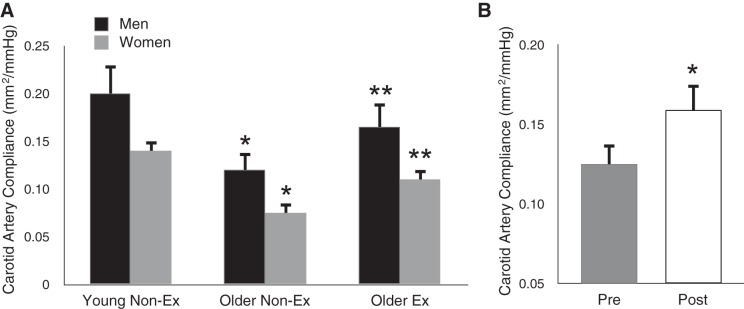
Aging, carotid artery compliance, and regular aerobic exercise. A: carotid artery compliance is lower in healthy older men and women compared with young controls. However, the age-associated difference in carotid compliance in aerobically exercising (Ex) adults is only ∼50% as great as that observed in their nonexercising (non-Ex) peers. *P < 0.05 vs. young. **P < 0.05 vs. older non-Ex. B: carotid artery compliance is increased by ∼30% after 12 wk of moderate-intensity aerobic exercise (brisk walking) in previously sedentary late middle-aged/older men and women. *P < 0.05 vs. preexercise training. Data are from Moreau et al. (51) and Tanaka et al. (76).
We (51, 76) and others (1) also have shown that moderate aerobic exercise interventions improve carotid artery compliance ∼30% in healthy MA/O men and women (Fig. 3B). These findings suggest that, as with the aorta, aerobic exercise can be used therapeutically to destiffen the large elastic arteries in this group.
For sake of completeness, it is important to emphasize that better maintenance of carotid artery compliance with aging in aerobically exercising adults also has important implications for integrative autonomic-cardiovascular function. Consistent with this notion, preserved carotid artery compliance in older men who exercise regularly is associated with corresponding maintenance carotid baroreflex sensitivity (49).
Types of aerobic exercise and resistance exercise.
Evidence suggests that several types of aerobic exercise may act to suppress the stiffening of the large elastic arteries with aging. MA/O adults engaged in running, cycling, swimming, or rowing all demonstrate enhanced carotid artery compliance compared with sedentary controls (1, 14, 51, 56, 76) (Fig. 4), and a swimming exercise intervention increases carotid compliance in this population (57). The fact that masters rowers, a group that engages in a combination of aerobic and resistance training activities, exhibit augmented carotid compliance (8), suggests that even an element of aerobic activity may be a sufficient stimulus to inhibit stiffening with aging. This is not an effect of exercise in general, because MA/O individuals who solely perform intensive resistance exercise have unchanged or even reduced carotid artery compliance compared with sedentary adults of the same age (47) (Fig. 4). Again, adding an aerobic component appears to offset the effects of vigorous resistance exercise on large elastic artery stiffness (8, 35). Finally, the influence of aerobic exercise appears to be specific for large elastic arteries, as no effect is observed in the more muscular femoral arteries (8, 56).
Fig. 4.
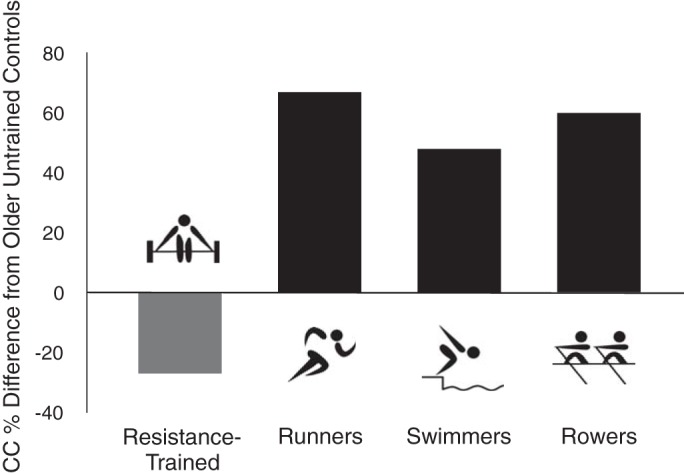
Aging, types of aerobic exercise, and large elastic artery stiffness. Differences in carotid artery compliance (CC) are compared with late middle-aged/older healthy untrained (non-Ex) controls in age-matched groups of (left to right) solely resistance Ex adults, runners, swimmers, and rowers. Groups performing some type of aerobic exercise all have greater carotid compliance than non-Ex controls, whereas solely resistance exercise-trained adults have lower compliance. Data are from DeVan and Seals (14), based on data from Refs. 8, 47, 56.
Summary.
In summary, several types of aerobic exercise act to inhibit the stiffening of the large elastic arteries with normal aging in adult humans. Importantly, regular aerobic exercise reduces large elastic artery stiffness in MA/O adults with baseline elevations in stiffness and thus can be viewed as a therapy for age-associated large elastic artery stiffening.
Mechanisms of Action
Stiffness and aging.
The biological mechanisms underlying the inhibitory effect of aerobic exercise on large elastic artery stiffness are largely unknown. Increases in stiffness with aging are believed to be mediated in part by structural changes in the arterial wall characterized by remodeling of the extracellular matrix, including increases in collagen (fibrosis), fragmentation of elastin filaments, and formation of advanced glycation end-products (AGEs), molecules derived from glycolysis that cross-link structural proteins, conferring additional stiffness to the artery (36, 37). So-called “functional” influences involving increases in smooth muscle tone, perhaps linked to abnormal smooth muscle signaling and endothelial dysfunction, also are thought to contribute (44, 69). These processes may be triggered and/or sustained by the development of vascular oxidative stress and inflammation (36, 37, 80, 84).
Age-associated stiffening and aerobic exercise in humans.
In healthy, estrogen-deficient postmenopausal women, acute systemic infusion of a supraphysiological concentration of ascorbic acid (vitamin C), which scavenges the reactive oxygen species, superoxide, selectively improves carotid artery compliance in sedentary subjects, increasing levels to those observed in endurance-trained women (52). This finding suggests the possibility that the greater carotid compliance in postmenopausal aerobically exercising women is associated with reduced superoxide bioavailability (reduced oxidative stress). However, no effect of vitamin C infusion or chronic oral supplementation on carotid compliance has been observed in sedentary MA/O healthy men (19). The influence of habitual aerobic exercise is not obviously attributable simply to favorable clinical characteristics and lower CVD risk factor burden in the trained state (51, 63, 76).
Age-associated stiffening and aerobic exercise in rodents.
The mechanisms contributing to the destiffening effects of aerobic exercise on large elastic arteries with aging are difficult to study in humans because of the central location of these vessels in the systemic circulation. This prohibits direct access to/sampling from these arteries. Moreover, administration of agents that might activate or inhibit potential signaling pathways involved in modulating the stiffness of large elastic arteries must not alter vascular resistance, or the measurements could be confounded by baroreceptor counterregulatory responses. Peripheral arteries cannot be used as surrogate vessels, because they do not demonstrate age-associated stiffening. As a result, animal models must be employed to provide mechanistic insight. Unfortunately, few data are available at present.
In rats, collagen content was shown to be greater and elastin lower in aorta of older compared with young adult sedentary animals (55). The age-related difference in aortic stiffness, as determined by the elastic modulus calculated from stress-strain responses, was found to be smaller in animals that underwent 4–5 mo of swim training. However, this effect of swimming was not associated with differences in collagen or elastin protein expression, or calcium or polar amino acid content of the elastin layer.
More recently, we studied the effects of wheel running as a model of voluntary aerobic exercise on carotid artery stiffness in mice (22). Sedentary old animals demonstrated greater carotid stiffness than young controls. The age-related stiffening was associated with several changes in arterial wall composition, including increases in collagen I and III in the adventitial (outer) layer of the artery (Fig. 5), which were, in turn, associated with corresponding increases in the profibrotic cytokine, transforming growth factor-β1 (TGF-β1), and an increase in smooth muscle α-actin, a marker of a myofibroblast or collagen-synthesizing phenotype. Elastin was reduced in the medial (middle) layer of the carotid artery, and this was accompanied by decreases in lysyl oxidase, a prosynthetic elastin enzyme, and increases in metalloproteinase 2, an elastin-degrading enzyme. There also was a small increase in arterial calcification in the arteries of the old mice, although no differences were observed in fibronectin, a glycoprotein involved in extracellular matrix remodeling. Voluntary wheel running for 10–14 wk initiated late in life reversed the carotid stiffening in old animals. This was associated with reductions in collagen I (Fig. 5) and III, TGF-β1, smooth muscle α-actin, and calcification, whereas elastin and its modulating enzymes were unaffected. In vitro experiments linked increases in TGF-β1 to oxidative stress-associated stimulation of collagen in adventitial fibroblasts. Recent preliminary studies from our laboratory using this mouse model suggest that wheel running also reduces stiffness in the aorta (aortic PWV), which is associated with reduced superoxide-related oxidative stress and accumulation of AGEs.
Fig. 5.
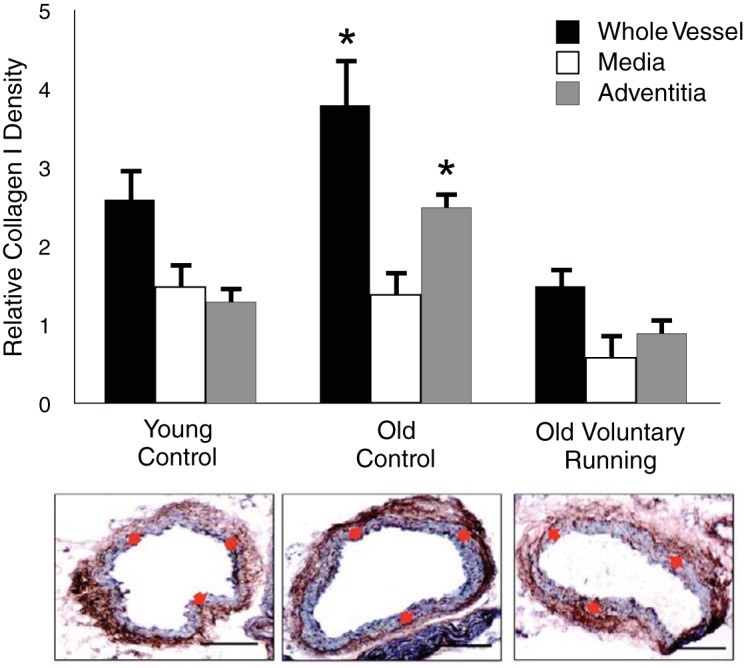
Aging, aerobic exercise, and large elastic artery collagen. Carotid artery collagen I expression is greater in old control (non-Ex) compared with young controls due to greater collagen in the adventitial layer. Voluntary wheel running (VR) for 10–14 wk in old mice reverses carotid artery collagen I to levels at or below those of young controls. *P < 0.05 vs. young. Data are from Fleenor et al. (22).
Summary.
In summary, the limited mechanistic data available suggest that voluntary aerobic exercise may destiffen large elastic arteries with aging, at least in part, by reducing oxidative stress and inducing a remodeling of the extracellular matrix. The latter appears to involve reductions in adventitial collagen I, the primary load-bearing structural protein in the arterial wall, associated with reduced TGF-β1, a reversal of the adventitial myofibroblast phenotype, a decrease in calcification, and a reduction in AGEs.
VASCULAR ENDOTHELIAL DYSFUNCTION, AGING, AND EXERCISE
Functional Effects
As with large elastic artery stiffness, the key questions here are if regular aerobic exercise can slow, reduce, or even prevent the development of vascular endothelial dysfunction with aging and/or improve endothelial function in MA/O adults who already demonstrate baseline endothelial dysfunction.
Assessment of endothelial function.
Vascular endothelial function is most commonly assessed in human subjects by measuring the dilation produced by a stimulus that evokes NO synthesis/release from the vascular endothelial cells (9, 64, 65, 79). This is termed an endothelium-dependent dilation (EDD): the greater the EDD, the greater the endothelial function (and vice versa). Two main types of stimuli are used to induce an EDD: a chemical stimulus (typically acetylcholine) or a mechanical stimulus (typically an increase in blood flow or shear rate). In either case, these stimuli activate the NO-producing enzyme in endothelial cells, endothelial NO synthase (eNOS), which synthesizes NO. The NO diffuses to the vascular smooth muscle cells in the medial layer of the artery, where it induces a guanylate cyclase-mediated increase in cyclic guanosine monophosphate, causing smooth muscle cell relaxation and dilation of the artery/increase in blood flow (EDD). The NO-dependent component of EDD can be assessed by chemically inhibiting NO production by eNOS using the competitive inhibitor NG-monomethyl-l-arginine or other agents.
Effects of aging and exercise on forearm blood flow responses to acetylcholine and NO bioavailability.
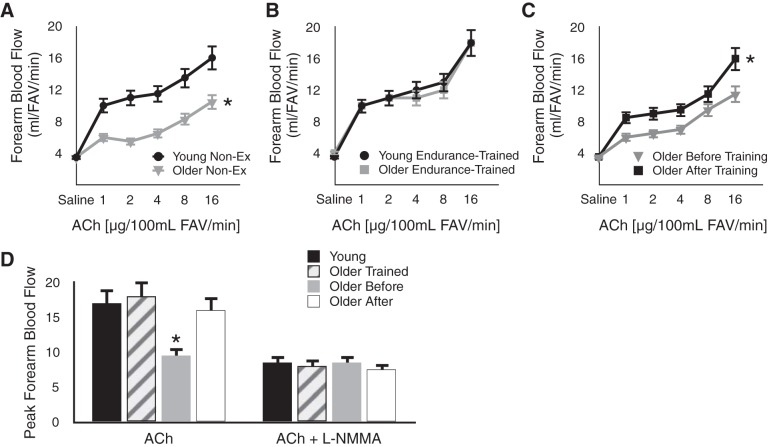
We (12, 63) and others (74) have used increases in forearm blood flow to brachial artery infusions of acetylcholine for assessing the influence of aerobic exercise on endothelial function in the microvasculature with aging. Among healthy men, sedentary older subjects demonstrate smaller increases in forearm blood flow in response to increasing concentrations of acetylcholine compared with young controls (12, 74) (Fig. 6A). In contrast, the forearm blood flow responses to acetylcholine do not differ in young and older men who regularly perform aerobic-endurance exercise (12, 74) (Fig. 6B), providing support for the concept that regular aerobic exercise may prevent age-associated microvascular endothelial dysfunction. Importantly, EDD in response to acetylcholine is improved by 12 wk of brisk walking in older previously sedentary men (12) (Fig. 6C). Indeed, such a walking program can restore EDD to levels observed in young sedentary and exercise-trained men and in older masters endurance athletes performing chronic, higher-intensity endurance exercise training (63) (Fig. 6D). This aerobic exercise program also improves other expressions of endothelial function in older men, such as endothelial fibrinolytic capacity (68). Taken together, these findings provide evidence that aerobic exercise also can be viewed as a treatment for endothelial dysfunction of resistance arteries in MA/O men.
Fig. 6.
Aging, aerobic exercise, and microvascular endothelial function. A: forearm blood flow (FBF) responses to acetylcholine (ACh) are impaired in older vs. young Non-Ex healthy men. B: FBF to ACh does not differ in older vs. young endurance exercise-trained men. C: FBF to ACh is improved by 12 wk of aerobic exercise in older previously Non-Ex men. D: mean peak FBF to ACh is lower in healthy Non-Ex older men (Older Before) vs. combined young Non-Ex and trained men (Young), older trained men (Older Trained), and older Non-Ex men after aerobic exercise training (Older After) (left). Group differences in mean peak FBF to ACh are abolished under conditions of nitric oxide inhibition using NG monomethyl-l-arginine (l-NMMA) (right). *P < 0.05 vs. other group(s) or condition(s). FAV, forearm tissue volume. Data are from DeSouza et al. (12) and Taddei et al. (74).
The greater EDD in young men and in older exercising men appears to be mediated by greater NO bioavailability because group differences in EDD are abolished with inhibition of eNOS (74) (Fig. 6D). Moreover, compared with young controls, basal NO production is reduced in sedentary, but not in endurance exercise-trained, healthy older men, and regular moderate-intensity aerobic exercise restores basal NO production to young adult levels in previously sedentary older men (63). It is not known if aerobic exercise has similar effects in postmenopausal women because, to our knowledge, there are no published data on this group.
Effects of aging and exercise on brachial artery flow-mediated dilation in MA/O men and women.
The interaction between aging, aerobic exercise, and macrovascular endothelial function has been investigated using mechanical (flow-mediated) stimulation of EDD. We have shown that brachial artery flow-mediated dilation (FMD) is only ∼50% of young control levels in healthy late MA/O sedentary men, but is well preserved in male masters endurance athletes of the same age (20, 21, 59) (Fig. 7A). In general, higher levels of brachial artery FMD in older aerobically exercising compared with sedentary men have been reported in the literature (23, 24, 60, 61). Recent work by our laboratory extended these cross-sectional observations by establishing that 8 wk of moderate-intensity regular aerobic exercise (i.e., brisk walking) improves brachial artery FMD by ∼50% in previously sedentary healthy older men (59) (Fig. 7B). This exercise program restored brachial artery FMD to levels close to those observed in young men and in older endurance exercise-trained men (Fig. 7). Other laboratories have reported improvements in brachial artery FMD in response to aerobic exercise training in middle-aged healthy and/or mildly hypertensive men (62, 85). The results of studies performed in rodent models assessing EDD, generally in the carotid arteries and skeletal muscle arterioles, support these observations in human subjects (17, 70, 71).
Fig. 7.
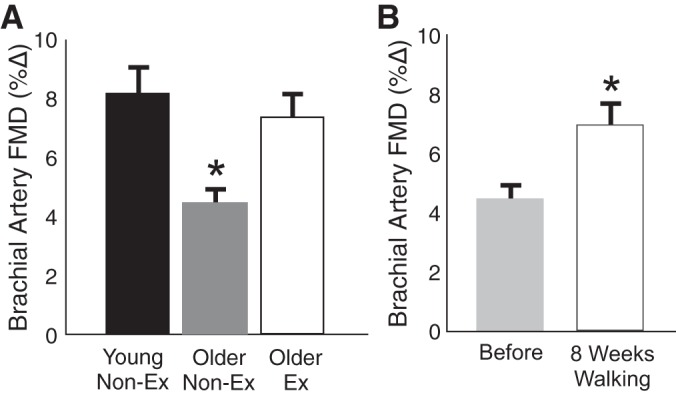
Aging, aerobic exercise, and macrovascular endothelial function. A: brachial artery flow-mediated dilation (FMD) is impaired in older vs. young Non-Ex healthy men, but is preserved in older aerobically Ex men. B: brachial artery FMD is improved by 8 wk of aerobic exercise in older previously Non-Ex men. *P < 0.05 vs. other group(s) or condition. Δ, Change. Data are from Eskurza et al. (20) and Pierce et al. (59).
The influence of regular aerobic exercise on macrovascular endothelial function with aging in women is far less clear. In the largest cross-sectional analysis of sedentary and endurance exercise-trained healthy MA/O men and women performed to date (n = 167), we found that brachial artery FMD was ∼50% greater in the exercising compared with sedentary men (59). In contrast, there was no difference between the exercising and sedentary postmenopausal women, all of whom were estrogen deficient. No factor was identified during extensive post hoc analysis of the results that could explain this lack of difference in brachial artery FMD between the two groups. Comparisons of smaller samples of sedentary and aerobically exercising postmenopausal women have reported either similar observations to ours, i.e., no group differences (7, 53), or higher values in exercising women (5, 28). Using an exercise intervention design, we found that the same 8-wk program of brisk walking that produced a 50% increase in brachial artery FMD in MA/O men failed to increase FMD in estrogen-deficient postmenopausal women (59). Other aerobic exercise trials in postmenopausal women have reported either no improvements (7, 73) or significant increases (2, 72, 86) in brachial artery FMD, and a single bout of aerobic exercise has been reported to increase brachial artery FMD in postmenopausal, estrogen-deficient women (29).
In our initial trial (59), absence of increases in FMD in response to aerobic exercise in our postmenopausal subjects may have been linked to their estrogen-deficient hormone status. To address this issue, we recently completed a follow-up intervention trial in which healthy, but sedentary estrogen-deficient postmenopausal women underwent 12-wk of aerobic exercise training with a preceding 12-wk period of either placebo treatment or estrogen replacement (53). Brachial artery FMD was significantly improved in response to aerobic exercise training in estrogen-replaced, but not estrogen-deficient (placebo treatment) women (Fig. 8). These findings support the possibility that circulating estrogen concentration plays a permissive role for improvements in endothelial function with aerobic exercise in postmenopausal women. It has been suggested that sedentary estrogen-deficient postmenopausal women with lower baseline endothelial function may respond more consistently to aerobic exercise intervention than women with higher baseline levels (73); however, the evidence supporting this possibility is not uniform (59), and the question will require further investigation.
Fig. 8.
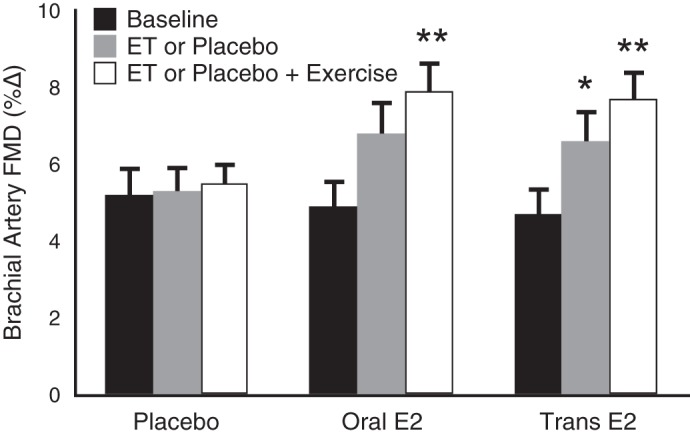
Aging, aerobic exercise, estrogen status, and endothelial function. In estrogen-deficient postmenopausal women, 12 wk of aerobic exercise did not improve brachial artery FMD, unless preceded by estrogen replacement treatment (ET) using oral or transdermal (Trans) estradiol (E2). These results suggest that estrogen sufficiency may be required to induce improvements in endothelial function in at least some postmenopausal women. *P < 0.05 vs. baseline. **P < 0.05 vs. estrogen or placebo. Data are from Moreau et al. (53).
In a broader sense, the question of whether or not regular aerobic exercise induces consistent benefits on endothelial function in estrogen-deficient postmenopausal women is a cautionary tale about making assumptions that an intervention shown to have efficacy in some groups will have similar effects on all groups.
Types of aerobic exercise and resistance exercise.
Relatively little systematic information is available concerning the type, frequency, intensity, and duration of aerobic exercise training required to preserve/improve endothelial function with aging. With regard to type of exercise, MA/O masters runners were reported to have higher brachial artery FMD than either swimmers or sedentary adults, with the latter two groups not differing in brachial artery FMD (56). However, an intervention trial in which healthy men and women underwent swim training found a significant improvement in brachial artery FMD (57). In men, both walk/jog/run- and cycling-based aerobic exercise programs have been associated with enhanced endothelial function in MA/O subjects (12, 59, 62), although lack of improvements also has been reported with cycle training (77). The intervention studies in women showing improvements in brachial artery FMD with aerobic exercise have utilized walking and/or cycling training (2, 72, 86). The limited available data do not support a beneficial effect of resistance exercise training on endothelial function in MA/O adults (postmenopausal women) (7), and the addition of resistance training to aerobic exercise has been reported to suppress improvements in brachial artery FMD induced by aerobic exercise training alone in middle-aged men (62). Several studies in MA/O adults have shown improvements in endothelial function with aerobic exercise frequencies of ≥3 days/wk (2, 12, 57, 59, 62, 72, 86). Moderate-vigorous intensity aerobic exercise programs based on a target range of 65–80% of maximal heart rate appears sufficient to improve endothelial function in healthy MA/O subjects, and even lower intensities of aerobic training may increase function in certain groups, including those with CVD risk factors at baseline (2, 12, 57, 59, 86). On the other hand, in male and female patients with essential hypertension, high-intensity interval aerobic exercise training has been reported to improve brachial artery FMD, whereas continuous, moderate-intensity training was found to have no effect (48). Improvements in endothelial function have been observed in MA/O adults with durations of 30–60 min/day of aerobic exercise (2, 12, 57, 59, 62, 72, 86). Training periods of 8 wk up to 6 mo of aerobic training have been found to improve endothelial function in this group (2, 12, 48, 57, 59, 62, 72, 86), whereas a single bout of aerobic exercise also has been reported to increase brachial artery FMD in postmenopausal women (29). In general, much more insight is needed to establish the necessary aerobic exercise stimulus for preserving/improving endothelial function in older adults.
Summary.
In summary, evidence obtained from both cross-sectional and intervention studies support the concept that regular aerobic exercise is associated with enhanced NO-mediated endothelial function in MA/O men. Moreover, experimental findings suggest that aerobic exercise also increases basal NO production and other endothelial functions in this group. Based on either an absence of data (forearm blood flow responses to acetylcholine) and/or inconsistent results (brachial artery FMD), the effect of aerobic exercise on endothelial function in estrogen-deficient postmenopausal women is uncertain at present. It is possible that circulating estrogen concentrations modulate endothelial responsiveness to aerobic exercise in postmenopausal women, and that low baseline function may influence improvements with training more in this group than in older men. Finally, multiple types of moderate-intensity aerobic exercise that can be performed by most MA/O adults appear to be sufficient for improving endothelial function. However, much more information is needed on the minimal and optimal overall aerobic exercise stimulus for enhancing endothelial function in this population.
Mechanisms of Action
In comparison to large elastic artery stiffness, much more is known about the mechanisms underlying the beneficial effects of aerobic exercise on vascular endothelial function with aging. This is because more studies have been performed using preclinical models, as well as the fact that the vascular endothelium is more accessible experimentally in peripheral vasculature of human subjects than is the case with the large elastic arteries. In general, oxidative stress and inflammation are the primary “macro-mechanistic” processes thought to be involved in mediating endothelial dysfunction with aging, and regular aerobic exercise is believed to preserve/restore endothelial function with aging, largely by reversing these mechanisms (14, 63–65, 67) (Fig. 9). Changes in expression and activation of eNOS as a possible contributor to altered NO signaling with aging and exercise training and the bioavailability of other vasodilatory molecules also have been investigated to a limited extent (63–65, 67).
Fig. 9.
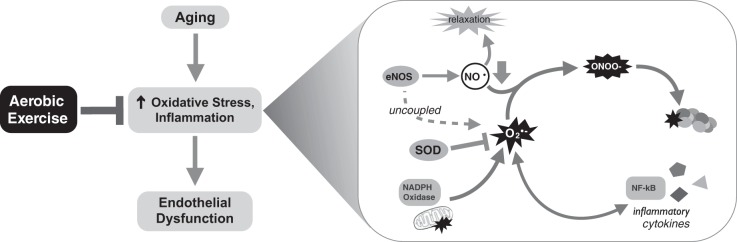
Mechanisms of aging and aerobic exercise on endothelial function. Aging induces endothelial dysfunction via vascular oxidative stress and inflammation involving excessive superoxide (O2·−) production as modulated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, superoxide dismutase (SOD), uncoupling of endothelial nitric oxide synthase (eNOS), and other mechanisms (e.g., dysfunctional mitochondria). Excessive superoxide causes peroxynitrite formation (ONOO−) and protein oxidation (nitrotyrosine), as well as activation of nuclear factor-κB (NF-κB) and induction of inflammatory cytokines.
Oxidative stress.
Endothelial dysfunction with aging in preclinical models and adult humans is associated with arterial oxidative stress as a result of excessive vascular superoxide production and either unchanged or reduced antioxidant defenses (64, 65). Excessive vascular superoxide production with aging, in turn, is mediated primarily by a combination of increased expression and activity of the oxidant enzyme, nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), eNOS uncoupling, and/or increased synthesis during mitochondrial oxidative phosphorylation (64, 65). Reduced antioxidant defenses with vascular aging, when observed, may be linked in part to downregulation of endogenous antioxidant enzymes, such as the key vascular superoxide neutralizing enzyme, superoxide dismutase (64, 65). In some cases, no changes have been observed in antioxidant enzymes with aging, but the lack of consistent increases in antioxidant defenses in response to the stimulus of cellular oxidative stress represents an abnormal compensatory adaptation in the aging vasculature (64, 65). The excessive availability of vascular superoxide with aging impairs NO-dependent EDD by reacting with NO to form peroxynitrite, thus directly reducing the bioavailability of NO. In addition, peroxynitrite, a potent reactive oxygen species in its own right, readily oxidizes tetrahydrobiopterin (BH4), a critical cofactor for NO production by eNOS, to its inactive form (BH2), causing eNOS to become “uncoupled”, a state in which the enzyme produces more superoxide and less NO in a vicious cycle. Several recent reviews of these and other cellular and molecular mechanisms of endothelial dysfunction with aging are available elsewhere (4, 18, 32).
We have investigated the possibility that regular aerobic exercise prevents or reverses the development of oxidative stress-dependent endothelial dysfunction with aging by influencing one or more of the mechanisms described above. To do so, we have employed an integrative translational research approach combining observations made in preclinical mouse models and clinical studies in human subjects varying in age and aerobic exercise status.
NITROTYROSINE ABUNDANCE.
In our investigations, we have assessed oxidative stress using the cellular marker, nitrotyrosine, which reflects oxidant modification of proteins by depicting the extent of nitration of tyrosine residues. In our initial preclinical study in mice, we found that 10–14 wk of daily wheel running, a model of voluntary aerobic exercise training in humans, started late in life (∼26–28 mo), restored NO-mediated EDD in old mice to that of young controls (17). Aortic nitrotyrosine was 200% greater in old compared with young cage control (sedentary) animals, but wheel running reduced levels in old mice to those observed in the young controls (Fig. 10A). We then showed that, in endothelial cells biopsied from the brachial artery of human subjects, nitrotyrosine was markedly greater in cells from older compared with young sedentary men (58). However, endothelial cells obtained from older men who regularly performed aerobic exercise demonstrated levels of nitrotyrosine as low or lower than those observed in young controls (58) (Fig. 10A). Taken together, these results support the view that regular aerobic exercise prevents/reverses the development of oxidative stress with aging in whole arteries of mice, as well as in vascular endothelial cells of healthy adult humans.
Fig. 10.
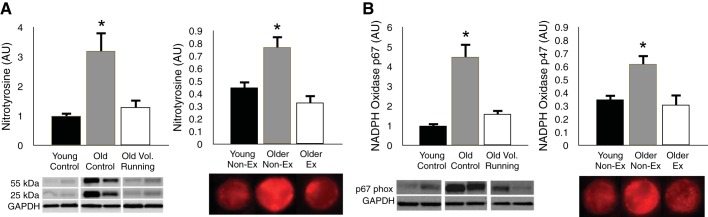
Aging, aerobic exercise, and arterial oxidative stress. A: nitrotyrosine, a cellular marker of oxidative stress, is markedly increased with sedentary aging in whole aorta of mice (left) and endothelial cells sampled from the brachial artery of healthy men (right); this age-associated increase in nitrotyrosine is reversed by 10–14 wk of voluntary wheel (Vol) running in old mice and is not observed in older men who regularly perform aerobic Ex. B: similarly, expression of the oxidant enzyme NADPH oxidase is increased with sedentary aging, but reversed/prevented by voluntary aerobic exercise in both mice (left) and humans (right). *P < 0.05 vs. the other groups. AU, arbitrary units. Data are from Durrant et al. (17) and Pierce et al. (58).
SUPEROXIDE.
To determine the potential role of superoxide in these age- and aerobic exercise-related differences in endothelial function, our laboratory and others have performed functional studies in which superoxide bioavailability was experimentally manipulated, and its effects on function measured. In our original study in mice (17), ex vivo administration of the superoxide scavenging compound, TEMPOL, restored EDD in old cage control mice, but had no effect on young cage controls or on old mice given access to voluntary running wheels late in life. Similarly, infusion of ascorbic acid (vitamin C), a potent antioxidant when administered at doses/rates that create supraphysiological circulating concentrations, selectively restored EDD in older sedentary men, while having no effect on young controls or on older men who performed regular vigorous aerobic exercise (19, 74). Recently, we found that ascorbic acid infusion improved brachial artery FMD in both sedentary and aerobic exercise-trained estrogen-deficient postmenopausal women, but not in estrogen-replaced women who had undergone aerobic exercise training (53). Collectively, these findings indicate that excessive superoxide bioavailability contributes to endothelial dysfunction with aging, and that the enhanced endothelial function in aerobically exercising older men and estrogen-sufficient postmenopausal women is the result of reduced superoxide suppression of endothelial function.
NADPH OXIDASE.
A reasonable next question may be the source(s) of the age- and aerobic exercise-associated differences in vascular superoxide production. In mice, we found that NADPH oxidase expression (p67phox subunit) and activity were increased in the aorta with sedentary aging, but that these changes were reversed in old animals that ran voluntarily on wheels for 10–14 wk late in life (17) (Fig. 10B). Moreover, we showed that ex vivo inhibition of this enzyme system with apocynin restored EDD in old cage control mice to levels observed in young animals, but had no effect on old mice given access to running wheels (17). Consistent with these preclinical findings, we found that the expression of NADPH oxidase (p47phox subunit) in endothelial cells obtained from the brachial arteries of older sedentary men was much greater than in young controls, but that expression in cells of older aerobically exercising men was as low or lower than in young men (58) (Fig. 10B). These observations support the idea that aerobic exercise enhances endothelial function with aging, at least in part, by inhibiting superoxide production/signaling by the oxidant enzyme, NADPH oxidase.
eNOS UNCOUPLING AND MITOCHONDRIA SUPEROXIDE PRODUCTION.
Regular aerobic exercise may inhibit excessive superoxide-associated suppression of NO-mediated EDD through other mechanisms as well. For example, we (21) and others (33) have shown that administration of BH4 to reduce eNOS uncoupling restores EDD in MA/O sedentary adults to levels observed in young controls, and this effect appears to be mediated by restoration of NO bioavailability (33). Similar observations have been reported in skeletal muscle arterioles with aging in rodents (10). In contrast to sedentary aging, we find that BH4 administration has no effect on brachial artery FMD in MA/O men who regularly perform aerobic exercise (21), indicating that habitual exercise preserves BH4 bioactivity with aging, presumably by reducing vascular oxidative stress and consequent oxidation-inactivation of this molecule. Moreover, preliminary data from our laboratory suggest that aortic mitochondrial superoxide production increases with aging in cage control mice, but that this is reversed in old mice engaged in voluntary wheel running. Our preliminary work also suggests that ex vivo administration of mitochondrial-specific antioxidants improves EDD in old sedentary mice, but has no effect on young animals or on old wheel-running mice (27). Collectively, these observations support a role for eNOS “recoupling” and reduced mitochondrial superoxide production in the ameliorative effects of voluntary aerobic exercise on age-associated endothelial dysfunction.
SUPEROXIDE DISMUTASE.
We have shown that old mice given access to running wheels late in life demonstrate increases in aortic total, manganese (mitochondrial), and copper-zinc (cytosolic) + extracellular superoxide dismutase activity compared with old cage controls (17) (Fig. 11A). Consistent with these preclinical results, we find that expression of manganese superoxide dismutase in brachial artery endothelial cells obtained from MA/O men who perform regular aerobic exercise is 50% greater than in cells from older healthy sedentary men and equivalent to levels in young controls (58) (Fig. 11B). In addition, we have shown that circulating endothelium-derived extracellular superoxide dismutase activity is reduced with aging in sedentary men, but preserved at young adult levels in MA/O men engaged in habitual aerobic exercise (58) (Fig. 11C). These observations indicate that preserved/enhanced superoxide dismutase antioxidant defenses may represent still another mechanism by which regular aerobic exercise inhibits vascular oxidative stress and maintains healthy endothelial function with aging.
Fig. 11.
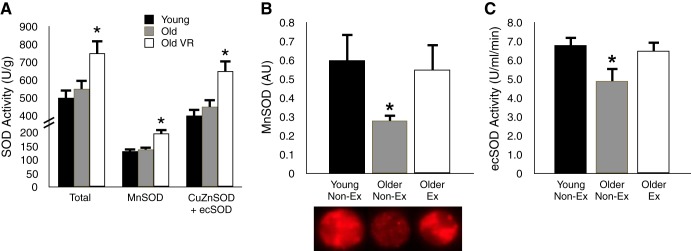
Aging, aerobic exercise, and arterial SOD enzyme defenses. A: total activity of the antioxidant enzyme SOD and the activities of the manganese (Mn; mitochondrial) and copper-zinc + extracellular (CuZn/ec) SOD isoforms are increased in old mice that engage in VR for 10–14 wk. B and C: MnSOD expression in endothelial cells obtained from the brachial artery and the activity of circulating ecSOD of healthy men are decreased in older sedentary (Non-Ex), but not older Ex subjects. *P < 0.05 vs. the other groups. Data are from Durrant et al. (17) and Pierce et al. (58).
eNOS.
Finally, upregulation of eNOS also may contribute to preserved NO bioavailability in the setting of regular aerobic exercise and aging. We (17) and others (70) have reported that expression of eNOS is greater in arteries from old mice who regularly exercise in running wheels or on motorized treadmills. In addition, we have shown that activation of eNOS, as indicated by increased phosphorylation at Ser1177, is increased in aorta of old mice given late-life access to running wheels compared with cage control old animals (17). Greater eNOS expression and activation could result in greater constitutive endothelial NO production and, therefore, contribute to enhanced vascular NO bioavailability in the aerobic exercise-trained state with aging.
Inflammation.
Inflammation and oxidative stress are mutually reinforcing processes, so it is not surprising that the development of chronic low-grade inflammation is a hallmark of primary vascular aging, i.e., aging in the absence of other major risk factors or clinical disease. This is supported by multiple lines of evidence, including age-associated increases in proinflammatory proteins in the whole arteries of experimental animals and autopsied adult humans, as well as in vascular endothelial cells obtained from peripheral arteries and veins of living human subjects (40, 41, 64, 65, 83, 84). This chronic low-grade inflammatory state is associated with upregulation of the major proinflammatory transcription factor nuclear factor-κB (NF-κB), and the transcription and expression of several of the inflammatory cytokines it regulates, as well as oxidative stress (40, 41, 64, 65). Consistent with these observations, we have shown that inhibiting NF-κB with sodium salicylate in old mice reduces the expression of proinflammatory cytokines in the aorta and restores EDD to levels not different from young mice by reducing vascular oxidative stress (40). These observations demonstrate that activation of NF-κB stimulates vascular inflammation with aging and induces chronic oxidative stress-related suppression of endothelial function.
INITIAL PRECLINICAL WORK WITH EXERCISE.
Regular aerobic exercise has long been suggested to have anti-inflammatory effects based in part on selective observations of lower circulating markers of systemic inflammation, such as C-reactive protein, in aerobically exercising compared with sedentary MA/O adults (34, 54). Until recently, however, there was little or no direct evidence on the potential anti-inflammatory effects of aerobic exercise on vascular aging per se. In our initial (preclinical) work on this issue (41), we found that, compared with young (6 mo) animals, old (31 mo) cage control mice demonstrated increased aortic activation of NF-κB, as indicated by greater phosphorylated-to-total ratios of both the inhibitor of NF-κB kinase and the p65 subunit of NF-κB. These changes in NF-κB activation with aging were associated with increased aortic expression of the proinflammatory cytokines interleukin-1β (IL-1β), IL-6, interferon-γ, and tumor necrosis factor-α (TNF-α) (Fig. 12), as well as impaired NO-dependent EDD. Most importantly, 10–14 wk of voluntary wheel running, begun late in life (27–28 mo), normalized the age-related increases in aortic inhibitor of NF-κB kinase, NF-κB activation and proinflammatory cytokine expression (Fig. 12), while restoring endothelial function and NO bioavailability to levels of young animals. This was the first direct evidence to our knowledge of the anti-inflammatory effects of regular aerobic exercise on vascular inflammation with aging.
Fig. 12.

Aging, aerobic exercise, and arterial inflammation. Aortic expression of the inflammatory cytokines interleukin-6 (IL-6), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) increases with sedentary aging in mice, but this effect is reversed by 10–14 wk of voluntary (Vol) running in old animals. *P < 0.05 vs. the other groups. Data are from Lesniewski et al. (41).
TRANSLATIONAL STUDIES WITH EXERCISE IN HUMANS.
In our translational work on this topic, we first showed increased expression of the proinflammatory markers total and nuclear NF-κB, IL-6, TNF-α and monocyte chemoattractant protein-1 (MCP-1) in vascular endothelial cells obtained from healthy MA/O men and women compared with young controls (15). The MA/O subjects also had greater plasma C-reactive protein and IL-6 concentrations and exhibited impaired brachial artery FMD compared with the young subjects. These findings suggested that, consistent with our findings in mice, primary vascular aging in humans is associated with development of a proinflammatory phenotype in the vascular endothelium featuring upregulation of NF-κB. In a follow-up investigation, we were able to show that the age-related increase in NF-κB expression in vascular endothelial cells is not observed in MA/O men who regularly perform aerobic exercise, and that this was associated with preserved brachial artery FMD (58). Preliminary results from our laboratory also suggest that the absence of elevations in NF-κB in endothelial cells of MA/O exercising adults is associated with a lack of increase in endothelial cell expression of IL-6.
Recently, we have attempted to gain insight into the possible role of reduced endothelial NF-κB activation in the enhanced vascular endothelial function associated with regular aerobic exercise in MA/O humans. To do so, we treated groups of young controls and MA/O sedentary and aerobically exercising subjects (∼70% male) for 4 days with salsalate, a potent NF-κB-inhibiting agent, or placebo (82). Salsalate reduced NF-κB expression in endothelial cells of MA/O sedentary subjects in the absence of changes in the cells of the young controls or MA/O exercising group. Brachial artery FMD was impaired in the MA/O sedentary subjects compared with the other two groups under placebo conditions, as expected. Salsalate restored FMD in the MA/O sedentary group to levels not different from those of the young controls or MA/O exercising subjects, while having no effects in the latter two groups (Fig. 13). Experiments in which vitamin C was infused at supraphysiological concentrations to acutely reduce superoxide bioavailability revealed that the effects of regular aerobic exercise in inhibiting inflammation-associated suppression of brachial artery FMD with aging are mediated by reduced oxidative stress. Together, these observations suggest that upregulation of NF-κB is associated with oxidative stress-related impairment of EDD with aging in sedentary adults, but that regular aerobic exercise prevents these events to preserve endothelial function.
Fig. 13.
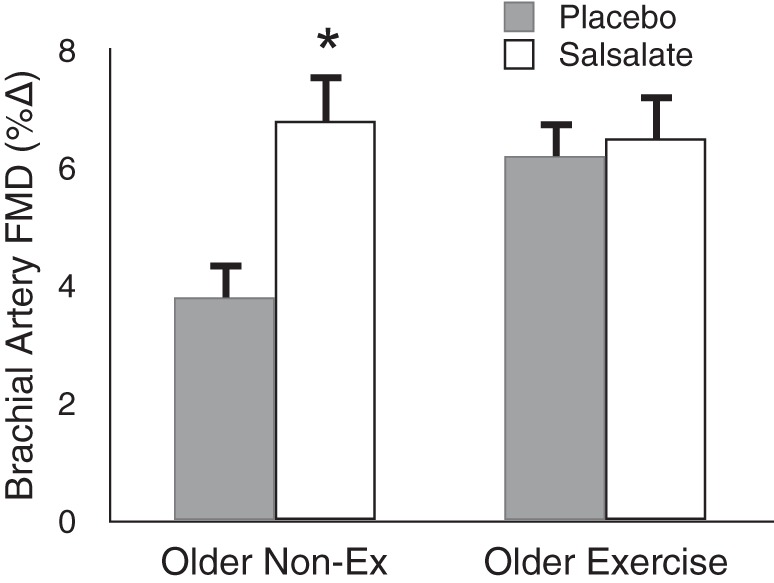
Aging, aerobic exercise, and inflammatory suppression of endothelial function. Brachial artery FMD is improved in sedentary, but not aerobically Ex, older healthy adults in response to short-term inhibition of NF-κB activity with oral salsalate treatment. This suggests that the enhanced baseline brachial artery FMD of older Ex adults is associated with an absence of the tonic NF-kB-related inhibition of endothelial function seen with sedentary aging. *P < 0.05 vs. placebo. Data are from Walker et al. (82).
UPSTREAM OR REINFORCING MECHANISMS OF EXERCISE.
We also have sought to obtain initial insight into the upstream or, perhaps, reinforcing events associated with these anti-inflammatory effects of aerobic exercise on aging arteries. It is well established that circulating immune cells, including monocytes, adhere to the vascular endothelium, attracted by chemoattractant proteins such as MCP-1 (11). Some percentage of the immune cells infiltrate the endothelial layer, with the infiltrating monocytes differentiating into macrophages. The macrophages and other immune cells lodging in the arterial wall can release superoxide and proinflammatory cytokines, stimulating inflammation and possibly contributing to a chronic low-grade inflammatory state.
In our initial investigation in mice, we found that the abundance of macrophages and T lymphocytes was increased in the adventitial and perivascular regions of the aorta of old compared with young cage control mice (41). There was no increase in the number of immune cells in the intimal and medial layers of the aorta in the old animals, suggesting either an “outside-in” infiltration pattern of the arterial wall or infiltration from the lumen to the outer regions of the wall with sedentary aging (6). Late-life voluntary wheel running for 10–14 wk reversed the age-related increases in aortic adventitial and perivascular macrophage infiltration and tended to have the same influence on T lymphocyte abundance. These observations suggest that regular aerobic exercise may exert its anti-inflammatory actions on arteries with aging, at least in part, by suppressing immune cell infiltration into the outer layer and surrounding (perivascular) region of the vascular wall. Fewer immune cells would result in less proinflammatory signaling and cytokine release into the arterial wall, reduced vascular inflammation, and enhanced function.
To provide translational insight into the findings in mice, we isolated peripheral blood mononuclear cells (PBMC) from groups of young and MA/O healthy sedentary men and women, as well as in a subset of the MA/O subjects before and after 8 wk of moderate-intensity aerobic exercise (26). Compared with the young controls, PBMC from the MA/O sedentary subjects had higher mRNA expression (PCR) of several proinflammatory/prooxidant factors, including NF-κB, the receptor for AGEs, TNF-α, MCP-1, NADPH oxidase, and the inducible form of NOS (Fig. 14). Regular aerobic exercise improved fitness in the MA/O subjects, and this was accompanied by reductions or a trend for reductions in all of these PBMC proinflammatory markers (Fig. 14). Preliminary findings from our laboratory also suggest that MCP-1 expression is higher in endothelial cells obtained from MA/O healthy sedentary adults compared with young controls, but that MA/O subjects who regularly perform aerobic exercise express MCP-1 at levels at or below those of young adults.
Fig. 14.
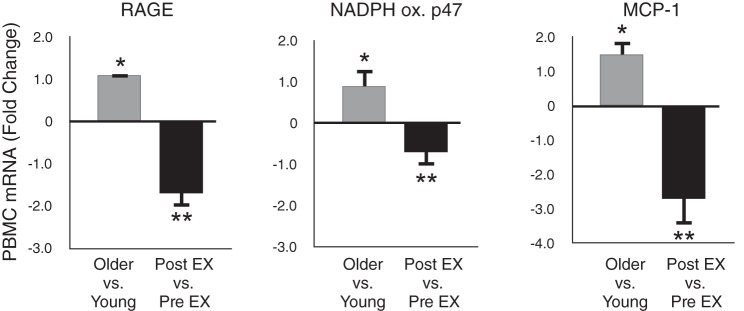
Aging, aerobic exercise, and oxidative/inflammatory gene expression. Expression of genes associated with oxidative stress and inflammation, including receptor for advanced glycation end-products (RAGE), NADPH oxidase (p47 subunit) and monocyte chemoattractant protein-1 (MCP-1), are increased with sedentary aging in peripheral blood mononuclear cells (PBMCs) of healthy adults; these increases are reversed in older subjects by ∼8 wk of regular aerobic exercise (EX). *P < 0.05 vs. young. **P < 0.05 vs. before exercise training. Data are from Gano et al. (26).
Collectively, these translational observations indicate that circulating immune cells develop a proinflammatory phenotype with sedentary human aging. Increased endothelial expression of chemoattractant proteins could increase infiltration of these cells into the arterial wall, eventually locating in the adventitial and perivascular regions of the arterial wall. Alternatively, greater macrophage accumulation in the outer regions of the wall may originate from the “outside-in” (adventitial-perivascular regions). In either case, increased abundance of these cells in the arterial wall may contribute to a proinflammatory process featuring increased superoxide and proinflammatory cytokine production, ultimately developing into a state of chronic-low grade inflammation. Regular aerobic exercise appears to inhibit the development of a prooxidant/-inflammatory phenotype in the circulating immune cells with aging and prevents the consequent downstream events leading to vascular inflammation.
Summary.
In summary, regular aerobic exercise may act to preserve vascular endothelial function with aging via several mechanisms. Translational observations in mice and humans support the concept that aerobic exercise inhibits the development of excessive superoxide-dependent vascular oxidative stress with aging via some combination of suppressing the upregulation of the NADPH oxidant enzyme system, preventing the uncoupling of eNOS, constraining mitochondrial superoxide production, and preserving endogenous antioxidant enzyme defenses. This restraint of superoxide-associated oxidative stress, along with induction of eNOS expression and activation, contribute to greater NO bioavailability and enhanced EDD. Habitual aerobic exercise also exerts a potent anti-inflammatory effect on aging arteries by potentially inhibiting several steps in the process of developing chronic low-grade inflammation and its suppression of endothelial function.
VASCULAR PROTECTION FROM “ADVERSE” FACTORS
Historically, much of the influence of regular aerobic exercise for reducing the risk of CVD with aging has been attributed to the secondary effects of exercise on traditional risk factors for CVD. That is, it has been assumed that habitual aerobic exercise favorably modifies risk factors such as blood pressure, plasma lipids, blood glucose control, and body weight/fatness, and that this lower risk factor burden is responsible for the maintenance of vascular health and associated reductions in CVD observed in exercising adults. However, epidemiological data indicate that, at most, only about one-half of the CVD risk-lowering effects of regular aerobic exercise can be attributed to such an influence (50). So clearly some other mechanism(s) are involved. Several years ago, we advanced the complementary hypothesis that regular aerobic exercise may be exerting at least part of its CVD-protective effects not by lowering traditional risk factors, but rather by inducing an increased “resistance” against the potentially harmful effects of existing levels of risk factors or, even more broadly, against a wide array of harmful factors to which arteries are commonly and chronically exposed (67, 81).
In our initial study in humans seeking support for this hypothesis (81), we used plasma low-density lipoprotein cholesterol (LDL-C) as the potential “harmful factor” and brachial artery FMD as the measure of vascular function. We found that having even a borderline elevation in plasma LDL-C was associated with greater impairment of brachial artery FMD in older sedentary adults compared with their peers who had normal/optimal plasma LDL-C. In contrast, among sedentary young adults, borderline elevations in plasma LDL-C had no influence on brachial artery FMD, as if young age exerted a protective effect against the potentially adverse influence of elevated plasma LDL-C on endothelial function. Most importantly, older adults who performed regular aerobic exercise demonstrated the same “resistance” to elevated plasma LDL-C as observed in young adults. A follow-up study in which impaired fasting blood glucose was used as a potential adverse factor (13) confirmed the results of the initial investigation, i.e., that regular aerobic exercise protects aging arteries from the possible harmful effects of circulating stressors (Fig. 15).
Fig. 15.
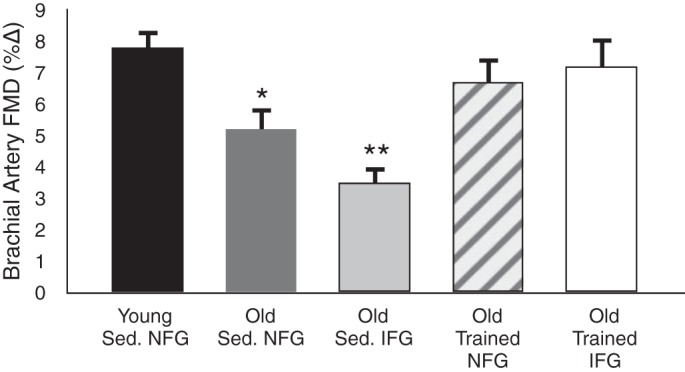
Aging, aerobic exercise, and “resistance” to cardiovascular risk factors. Brachial artery FMD is reduced with sedentary (Sed) aging in healthy adults with normal fasting blood glucose (NFG; <100 mg/dl), and FMD is decreased further in sedentary older subjects with impaired fasting blood glucose (IFG; 100–126 mg/dl). Older adults with NFG who regularly perform aerobic exercise demonstrate preserved brachial artery FMD. Most importantly, older subjects with IFG who exercise also have values for brachial artery FMD similar to those of healthy young adults, suggesting that regular aerobic exercise protects against aging and additional risk factors like IFG. *P < 0.05 vs. young. **P < 0.05 vs. old NFG. Data are from DeVan et al. (13).
More recently, we have used a preclinical mouse model to gain insight into the mechanisms by which regular aerobic exercise confers this apparent increased resistance to stress in aging arteries. In our initial investigation, we found that ingestion of a “Western” diet (40% fat, 19% sucrose) for 10–14 wk started late in life exacerbated age-associated endothelial dysfunction (reduction in ex vivo carotid EDD) in old cage control mice (42). However, old mice given access to running wheels were protected against the adverse effects of the Western diet on endothelial function. The primary mechanism underlying the harmful influence of Western diet on vascular function in the old cage control mice was excessive superoxide-associated oxidative stress and resulting reduction in NO bioavailability. Voluntary wheel running in old mice preserved endothelial function during consumption of the Western diet by preventing superoxide-related reductions in NO bioavailability. More recent preclinical work by our laboratory suggests that the increased resistance to Western diet and other forms of arterial stress conferred by voluntary aerobic exercise is linked to reductions in mitochondrial-derived oxidative stress and improved mitochondrial homeostasis (27).
Summary
Taken together, these translational findings support the novel concept that regular aerobic exercise protects aging arteries, not only by lowering the risk factor burden to which they are chronically exposed, but also by improving the intrinsic resistance of the arteries to existing levels of a variety of potentially harmful factors. Our work also suggests that aerobic exercise confers this protection, at least in part, by preventing exacerbation of vascular oxidative stress in response to these adverse factors, which allows the preservation of NO bioavailability and arterial function.
OVERALL SUMMARY AND CONCLUSIONS
“Aging” of arteries is the major risk factor for CVD, and this will become a progressively greater challenge in the future with projected worldwide increases in the number of older adults. Regular aerobic exercise should be viewed as a “first-line” strategy for the prevention and treatment of arterial aging and, therefore, reducing population CVD risk. Aerobic exercise enhances the compliance of the large elastic arteries (inhibits arterial stiffening) and preserves endothelial function during adult aging. These benefits of aerobic exercise are attributable largely to the suppression of vascular oxidative stress and inflammation. Habitual aerobic exercise preserves arterial health by not only exerting favorable effects on selective CVD risk factors, but also by inducing a direct protective effect to aging arteries against multiple potentially “adverse” factors to which they are chronically exposed. Given the public health implications for prevention of CVD and related age-associated disorders, more information is needed regarding the minimal and optimal types, durations, intensities, and frequencies of aerobic exercise for preserving arterial function with aging.
GRANTS
This work was supported by National Institutes of Health awards AG-013038 (MERIT), HL-107120, AG-031141, AG-006537, AG-000279, AG-022241, AG-020683, AG-016071, RR-000051/TR-001082, HL-007822, AG-031617, AG-033196, AG-000847, and HL-003840, and American Heart Association Grant 0715735Z.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.R.S. conception and design of research; D.R.S. interpreted results of experiments; D.R.S. drafted manuscript; D.R.S. edited and revised manuscript; D.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The author thanks Dr. Thomas LaRocca for the figure illustrations.
Footnotes
This article is the topic of an Invited Editorial by Michael J. Joyner (33a).
REFERENCES
- 1.Akazawa N, Choi Y, Miyachi M, Tanabe Y, Sugawara J, Ajisaka R, Maeda S. Effects of curcumin intake and aerobic exercise training on arterial compliance in postmenopausal women. Artery Res 7: 67–72, 2013 [Google Scholar]
- 2.Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R, Maeda S. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res 32: 795–799, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O'Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation 71: 202–210, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Bachschmid MM, Schildknecht S, Matsui R, Zee R, Haeussler D, Cohen RA, Pimental D, Loo B. Vascular aging: chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann Med 45: 17–36, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell KA, Lipinski MJ, Doran AC, Skaflen MD, Fuster V, McNamara CA. Lymphocytes and the adventitial immune response in atherosclerosis. Circ Res 110: 889–900, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey DP, Pierce GL, Howe KS, Mering MC, Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol 100: 403–408, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cook JN, DeVan AE, Schleifer JL, Anton MM, Cortez-Cooper MY, Tanaka H. Arterial compliance of rowers: implications for combined aerobic and strength training on arterial elasticity. Am J Physiol Heart Circ Physiol 290: H1596–H1600, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 115: 1285–1295, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29: 313–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 13.DeVan AE, Eskurza I, Pierce GL, Walker AE, Jablonski KL, Kaplon RE, Seals DR. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin Sci (Lond) 124: 325–331, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeVan AE, Seals DR. Vascular health in the ageing athlete. Exp Physiol 97: 305–310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Assar M, Angulo J, Vallejo S, Peiro C, Sanchez-Ferrer CF, Rodriguez-Manas L. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol 3: 132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol 286: H1528–H1534, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588: 3971–3982, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens 18: 510–516, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Galetta F, Franzoni F, Plantinga Y, Ghiadoni L, Rossi M, Prattichizzo F, Carpi A, Taddei S, Santoro G. Ambulatory blood pressure monitoring and endothelium-dependent vasodilation in the elderly athletes. Biomed Pharmacother 60: 443–447, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Gando Y, Yamamoto K, Murakami H, Ohmori Y, Kawakami R, Sanada K, Higuchi M, Tabata I, Miyachi M. Longer time spent in light physical activity is associated with reduced arterial stiffness in older adults. Hypertension 56: 540–546, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Gano LB, Donato AJ, Pierce GL, Pasha HM, Magerko KA, Roeca C, Seals DR. Increased proinflammatory and oxidant gene expression in circulating mononuclear cells in older adults: amelioration by habitual exercise. Physiol Genomics 43: 895–902, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gioscia-Ryan RA, Battson ML, Sindler AL, Cuevas LM, Zigler MC, Seals DR. Aerobic exercise increases stress resistance in arteries of old mice. FASEB J 28: 1106.–9., 2014 [Google Scholar]
- 28.Hagmar M, Eriksson MJ, Lindholm C, Schenck-Gustafsson K, Hirschberg AL. Endothelial function in post-menopausal former elite athletes. Clin J Sport Med 16: 247–252, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Harvey PJ, Morris BL, Kubo T, Picton PE, Su WS, Notarius CF, Floras JS. Hemodynamic after-effects of acute dynamic exercise in sedentary normotensive postmenopausal women. J Hypertens 23: 285–292, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol 55: 235–239, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Herrera MD, Mingorance C, Rodriguez-Rodriguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev 9: 142–152, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 186: 390–395, 2006 [DOI] [PubMed] [Google Scholar]
- 33a.Joyner MJ. Buying into healthy blood vessels: exercise and aging. J Appl Physiol; 10.1152/japplphysiol.00474.2014 [DOI] [PubMed] [Google Scholar]
- 34.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 45: 1563–1569, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Kawano H, Tanaka H, Miyachi M. Resistance training and arterial compliance: keeping the benefits while minimizing the stiffening. J Hypertens 24: 1753–1759, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. III. Cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. I. Aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. II. The aging heart in health: links to heart disease. Circulation 107: 346–354, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Laurent P, Marenco P, Castagna O, Smulyan H, Blacher J, Safar ME. Differences in central systolic blood pressure and aortic stiffness between aerobically trained and sedentary individuals. J Am Soc Hypertens 5: 85–93, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci 66: 409–418, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301: H1025–H1032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lesniewski LA, Zigler ML, Durrant JR, Nowlan MJ, Folian BJ, Donato AJ, Seals DR. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol 48: 1218–1225, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee, and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 44.McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin Newby DE, Cockcroft JR, Wilkinson IB. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 48: 602–608, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43: 1239–1245, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Miyachi M, Donato AJ, Yamamoto K, Takahashi K, Gates PE, Moreau KL, Tanaka H. Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension 41: 130–135, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Molmen-Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, Wisloff U, Ingul CB, Stoylen A. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol 19: 151–160, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation 104: 1627–1632, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation 116: 2110–2118, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res 57: 861–868, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Moreau KL, Gavin KM, Plum AE, Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause 13: 951–958, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc 56: 2045–2052, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nosaka T, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol 28: 204–212, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Nualnim N, Barnes JN, Tarumi T, Renzi CP, Tanaka H. Comparison of central artery elasticity in swimmers, runners, and the sedentary. Am J Cardiol 107: 783–787, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Nualnim N, Parkhurst K, Dhindsa M, Tarumi T, Vavrek J, Tanaka H. Effects of swimming training on blood pressure and vascular function in adults >50 years of age. Am J Cardiol 109: 1005–1010, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 10: 1032–1037, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120: 13–23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol 88: 761–766, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Rywik TM, Blackman MR, Yataco AR, Vaitkevicius PV, Zink RC, Cottrell EH, Wright JG, Katzel LI, Fleg JL. Enhanced endothelial vasoreactivity in endurance-trained older men. J Appl Physiol 87: 2136–2142, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Schaun MI, Dipp T, Rossato Jda S, Wilhelm EN, Pinto R, Rech A, Plentz RD, Homem de Bittencourt PI, Reischak-Oliveira A. The effects of periodized concurrent and aerobic training on oxidative stress parameters, endothelial function and immune response in sedentary male individuals of middle age. Cell Biochem Funct 29: 534–542, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol 105: 1323–1332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You're only as old as your arteries: Translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 29: 250–264, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seals DR, Stevenson ET, Jones PP, DeSouza CA, Tanaka H. Lack of age-associated elevations in 24-h systolic and pulse pressures in women who exercise regularly. Am J Physiol Heart Circ Physiol 277: H947–H955, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol 587: 5541–5549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith DT, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Effects of ageing and regular aerobic exercise on endothelial fibrinolytic capacity in humans. J Physiol 546: 289–298, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol 101: 1751–1759, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spier SA, Delp MD, Stallone JN, Dominguez JM, 2nd, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol 292: H3119–H3127, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Swift DL, Earnest CP, Blair SN, Church TS. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. Br J Sports Med 46: 753–758, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swift DL, Weltman JY, Patrie JT, Saliba SA, Gaesser GA, Barrett EJ, Weltman A. Predictors of improvement in endothelial function after exercise training in a diverse sample of postmenopausal women. J Womens Health (Larchmt) 23: 260–266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000 [DOI] [PubMed] [Google Scholar]
- 75.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 18: 127–132, 1998 [DOI] [PubMed] [Google Scholar]
- 76.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 77.Thijssen DH, Rongen GA, van Dijk A, Smits P, Hopman MT. Enhanced endothelin-1-mediated leg vascular tone in healthy older subjects. J Appl Physiol 103: 852–857, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 79.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation 112: 2193–2200, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens 22: 250–256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals DR. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor-κB. Clin Sci (Lond) 127: 645–654, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension 41: 1308–1316, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50: 219–227, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 116: 433–441, 2009 [DOI] [PubMed] [Google Scholar]
- 86.Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am J Hypertens 23: 368–372, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Yoshizawa M, Maeda S, Miyaki A, Misono M, Saito Y, Tanabe K, Kuno S, Ajisaka R. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: a randomised controlled trial in women aged 32–59 years. Br J Sports Med 43: 615–618, 2009 [DOI] [PubMed] [Google Scholar]