Abstract
The purpose of this investigation was to determine the effect of 4 wk of voluntary wheel running on cardiac performance in the 5/6 ablation-infarction (AI) rat model of chronic kidney disease (CKD). We hypothesized that voluntary wheel running would be effective in preserving cardiac function in AI. Male Sprague-Dawley rats were divided into three study groups: 1) sham, sedentary nondiseased control; 2) AI-SED, sedentary AI; and 3) AI-WR, wheel-running AI. Animals were maintained over a total period of 8 wk following AI and sham surgery. The 8-wk period included 4 wk of disease development followed by a 4-wk voluntary wheel-running intervention/sedentary control period. Cardiac performance was assessed using an isolated working heart preparation. Left ventricular (LV) tissue was used for biochemical tissue analysis. In addition, soleus muscle citrate synthase activity was measured. AI-WR rats performed a low volume of exercise, running an average of 13 ± 2 km, which resulted in citrate synthase activity not different from that in sham animals. Isolated AI-SED hearts demonstrated impaired cardiac performance at baseline and in response to preload/afterload manipulations. Conversely, cardiac function was preserved in AI-WR vs. sham hearts. LV nitrite + nitrate and expression of LV nitric oxide (NO) synthase isoforms 2 and 3 in AI-WR were not different from those of sham rats. In addition, LV H2O2 in AI-WR was similar to that of sham and associated with increased expression of LV superoxide-dismutase-2 and glutathione peroxidase-1/2. The findings of the current study suggest that a low-volume exercise intervention is sufficient to maintain cardiac performance in rats with CKD, potentially through a mechanism related to improved redox homeostasis and increased NO.
Keywords: cardiac function, kidney disease, exercise, nitric oxide, and oxidative stress
exercise training is an important adjunct therapy for the treatment and prevention of various cardiovascular diseases (9, 29, 43). Exercise provides sustainable protection against myocardial infarction and hypertension while improving cardiac function, cardiovascular outcomes, and quality of life (9, 29, 43). Yet few clinical and experimental studies have investigated the effect of exercise on cardiovascular function among those suffering with chronic kidney disease (CKD).
Cardiovascular disease (CVD) is the most significant cause of morbidity and mortality in patients with CKD (48a). At the heart, CVD manifests in part as cardiac dysfunction. Left ventricular (LV) systolic dysfunction and diastolic dysfunction are apparent early on in renal disease progression and are important risk factors for the development of heart failure and, ultimately, death (39, 41, 42, 49). Moreover, impaired cardiac function plays an important role in the risk of ischemic cardiac events as well as ensuing poor prognosis in patients with CKD (12, 38, 40). Previous animal studies from our laboratory and others have supported these findings by demonstrating impaired cardiac function in uremic rats that persists following short-duration ischemic insult (6, 27).
The purpose of this investigation was to determine the effect of a short, voluntary wheel-running intervention on cardiac function in the moderate-to-severe, 5/6 ablation-infarction (AI) rat model of CKD. In doing so, kidney disease and associated cardiac dysfunction were allowed to develop and progress over a period of 4 wk. A 4-wk voluntary wheel-running intervention was then initiated to test the hypothesis that wheel running would preserve cardiac function in AI after kidney disease had already been established. The overall cardiovascular benefits of exercise have been attributed to a multifactorial mechanism with effects observed at the heart and peripheral vasculature (9, 18). The underlying molecular mechanisms remain incompletely elucidated, but restoration of redox homeostasis and increased stable nitric oxide (NO) metabolites in the myocardium appear to play important roles (8, 9). Therefore, we also sought to determine whether preserved cardiac function with exercise was associated with alterations in markers of LV oxidative stress and NO.
MATERIALS AND METHODS
Animal care and use.
The following experimental protocol was approved by the University of Delaware Animal Care and Use Committee and followed the guidelines established by the National Institutes of Health Office of Laboratory Animal Welfare for the use of animals in research.
Male Sprague-Dawley rats were purchased from Harlan Laboratories (Frederick, MD) and housed in a climate-controlled environment with a 12:12-h light-dark cycle. Food and water were provided ad libitum. Animals were then randomly assigned at 10 wk of age to sham (sedentary nondiseased control) or AI study groups, including one AI subset to remain sedentary (AI-SED) and another to undergo a 4-wk voluntary wheel-running intervention (AI-WR). AI-WR rats were then allowed to acclimate to custom-made running wheels for 1 wk.
At 12 wk of age, sham and AI groups underwent surgery as previously described (27). In short, all animals were anesthetized using isofluorane (1.0–5.0%) and AI animals had 2/3 of the renal artery branches supplying the left kidney ligated followed by removal of the right kidney to induce CKD. The kidneys of sham animals were exposed and manipulated with no ligation or ablation being performed. After surgery, all animals were maintained over a total period of 8 wk until being killed at 20 wk of age to allow for moderate-to-severe CKD to develop in AI. The 8-wk period included 4 wk of disease development until AI-WR rats were placed in cages with free access to custom-made running wheels. Citrate synthase activity was measured in soleus muscles isolated from a subset of animals in each group following death using a colorimetric assay (Sigma-Aldrich, St. Louis, MO) (sham n = 5; AI-SED n = 5, AI-WR n = 5; replicates n = 3).
Blood and urine analysis.
Urine was collected overnight (16 h) in all groups using metabolic cages 1 wk prior to (baseline) and 4 and 7 wk following surgery. During this time, animals had free access to water but were restricted from food consumption. Urine volume was recorded for calculation of urine flow rate. Blood samples were collected from tail veins (baseline and 4 wk) or the inferior vena cava upon death (8 wk). The tail vein blood draw was carried out while rats were anesthetized using isofluorane (1.0–5.0%) and kept on a heating pad to control body temperature for a period of 10–15 min. Approximately 0.5 ml of whole blood was collected in a 1.5-ml microcentrifuge tube from each animal. The inferior vena cava blood draw was accomplished utilizing a Vacutainer Safety-Lok blood collection set (BD Medical, Franklin Lakes, NJ) to draw approximately 3–4 ml of blood. All blood was centrifuged at 3,000 g for 10 min at 4°C for isolation of serum within an hour of collection. Serum and urine samples were then stored at −80°C until later analysis.
Serum creatinine, blood urea nitrogen (BUN), and protein excretion were used to assess renal function in a subgroup of all animals (sham n = 7; AI-SED n = 7, AI-WR n = 7; replicates n = 3). Serum creatinine (Cayman Chemical, Ann Arbor, MI) and BUN (Bioassay Systems, Hayward, CA) were measured using commercially available colorimetric assays after being filtered with 10 kDa Amicon-Ultra centrifugal filters (Millipore, Billerica, MA). Urine protein concentration was determined using the Bradford method, and excretion was calculated by adjusting for urine flow rate. In addition, urinary excretion of the stable NO metabolites, NO2− and NO3− (NO2− + NO3−:NOx), were measured using the Greiss reaction as an index of systemic NO production (Cayman Chemical).
Isolated perfused working heart.
Cardiac performance was assessed using an in vitro working heart preparation (Radnoti, Monrovia, CA) at baseline and in response to altering preload and afterload to construct LV function curves, as previously described (27). Animals (sham n = 10; AI-SED n = 11; AI-WR n = 10) were anesthetized using an ip injection of ketamine-xylazine (100 mg/kg). Hearts were excised rapidly and placed in an ice-cold saline solution, and mass was measured on an electronic scale (gross wet mass). The aorta was then cannulated and hearts were perfused in a retrograde fashion using a modified Krebs-Henseleit buffer solution (1.25 mM CaCl2, 130 mM NaCl, 5.4 mM KCl, 11 mM glucose, 0.5 mM MgCl2, 0.5 mM NaH2PO4, 25 mM NaHCO3, and 12 mU/ml insulin; pH 7.4–7.5). The buffer was kept at physiological temperature, ∼37°C, and aerated with 95% O2-5% CO2. During 5–15 min of retrograde perfusion, a second cannula was quickly secured in the left atrium, and the system was switched to working heart mode (i.e., anterograde flow). Experimental protocols for measures of baseline cardiac function as well as preload/afterload manipulation were then commenced. Cardiac output (CO) was determined as the sum of coronary and aortic flow rates measured via timed collection of coronary effluent and aortic column overflow, respectively. A Luer syringe fitting on the aortic cannula contained a pressure transducer attached to an instrument amplifier for measures of pressure and heart rate. Data were collected at 100 Hz with a BIOPAC-MP100 data acquisition system (Goleta, CA).
Measures of basal cardiac function were taken at 13.5 cmH2O of preload and 80 cmH2O of afterload. Preload and afterload were then independently altered to construct LV function curves by adjusting the height of a compliance chamber feeding the left atrium and an aortic overflow chamber, respectively. Afterload was maintained at 80 cmH2O; preload was set at 9.5, 13.5, 17.5, and 21.5 cmH2O in increments of 3–5 min to document the relationship between CO and preload (Starling curve). Preload was then held constant at 13.5 cmH2O, and afterload was set at 60, 70, 80, 90, and 100 cmH2O in increments of 3–5 min to establish the relationship between afterload and CO.
Tissue isolation and biochemical analysis.
Animal lungs were dissected following death, and gross wet mass was measured as an index of fluid accumulation. Hearts from a separate set of animals from each group (sham n = 7; AI-SED n = 7; AI-WR n = 5) that did not go through the cardiac function measurements were used to obtain LV tissue for biochemical tissue analysis including oxidative assays and Western blotting. Hearts were excised and mass was measured similarly to those used for in vitro cardiac function measurements. The LV was then dissected, sectioned, and snap-frozen in liquid nitrogen to be stored at −80°C until later use.
LV tissue samples were homogenized with a Next-Advance BulletBlender (Averill Park, NY) according to the manufacturer's instructions. Samples to be used for oxidative assays were homogenized in an ice-cold buffer containing phosphate buffered saline (PBS) + 0.1% Triton X-100, whereas the homogenization buffer for Western blot analysis consisted of PBS with 2% SDS, 10 mmol/liter Tris·HCL, 10% (vol/vol) glycerol, and 1 mM Protease Inhibitor Cocktail (Sigma-Aldrich). All tissue lysates were centrifuged at 10,000 g at 4°C for 10 min, and the supernatant was used for analysis. Protein concentration was determined using the Bradford method, and tissue lysates were either used immediately or stored at −80°C.
Cardiac NO substrate/production was assessed by measuring NOx (Cayman Chemical) in conjunction with nitrotyrosine (OxiSelect Nitrotyrosine ELISA assay; Cell Biolabs, San Diego, CA) in LV tissue using colorimetric assays. Finally, LV hydrogen peroxide (H2O2) was measured using the fluorescent Amplex Red enzyme (Life Technologies, Carlsbad, CA) assay according to the manufacturer's instructions.
Western blotting.
Protein abundance was determined using Western blotting in LV tissue samples resuspended in Laemmli sample buffer (Bio-Rad, Hercules, CA). Protein (10 μg) was loaded and separated using gel electrophoresis (1 h, 100 V) until being transferred to nitrocellulose membranes (1 h, 50 V) that were then blocked for 1 h using SuperBlock blocking buffer at room temperature (Thermo Scientific, Rockford, IL). The membranes were subsequently probed using primary antibodies for nitric oxide synthase-3 (NOS-3; BD Pharmingen, 1:1,000), nitric oxide synthase-2 (NOS-2; Santa Cruz Biotechnology, 1:200), NAPDH-oxidase-4 (Nox-4; Santa Cruz Biotechnology, 1:500), NADPH-oxidase-2 (Nox-2; Santa Cruz Biotechnology, 1:500), superoxide dismutase-1 (SOD-1; Santa Cruz Biotechnology, 1:500), superoxide dismutase-2 (SOD-2; Santa Cruz Biotechnology, 1:500), glutathione peroxidase-1/2 (GPx-1/2; Santa Cruz Biotechnology, 1:500), and catalase (Santa Cruz Biotechnology, 1:1,500) overnight followed by species-specific secondary antibodies at room temperature for 1 h. Membranes were then stained with enhanced chemiluminescence detection reagents (Thermo Scientific) for development. Image J software was used to analyze all digitized Western blots. Protein abundance is expressed as relative units normalized to β-actin (1:2,000; Santa Cruz Biotechnology) and a control sample loaded into each gel. Control analysis of β-actin indicated no differences between groups (P > 0.05), and exposure times were within the linear portion of the β-actin standard curves (band intensity vs. time).
Statistical analyses.
One-way ANOVAs were used to compare animal characteristics and cardiac biochemical measures, and Dunnet's post hoc tests were conducted to determine whether either AI group differed from sham animals. Similarly, a two-way repeated measures mixed model ANOVA was used with a Dunnet's post hoc tests to compare cardiac function between groups and with alterations in pressure level. If a significant interaction existed, two separate one-way ANOVAs were conducted. All statistical tests were performed using SPPS statistical software 22 (IBM) and GraphPad Prism (La Jolla, CA) with two-tailed probability values reported. Alpha was set at 0.05, and data are presented as means ± SE. An a priori power analysis was performed to determine the number of animals needed (n = 10 per group, 80% power) to examine differences in cardiac function in AI-SED and AI-WR groups compared with the sham group.
RESULTS
Animal characteristics.
Animal characteristics are presented in Table 1. As shown, both groups of AI animals had decreased body mass and increased heart mass relative to sham animals at 8 wk postsurgery (P < 0.05). In contrast, no significant differences were observed in lung mass. By design, renal function (Table 2) was not different at baseline but became significantly impaired in AI-SED and AI-WR animals at 4 and 8 wk following surgery as indicated by elevated protein excretion and increased BUN. Serum creatinine was also elevated at 8 wk in AI animals, but was not different in AI-SED or AI-WR compared with sham animals at 4 wk (P > 0.05). In conjunction with impaired renal function, urinary NOx excretion was reduced at 4 and 8 wk following surgery in both AI groups, whereas all animals demonstrated significant reductions in NOx over the course of the study from baseline to 4 and 8 wk postsurgery (Table 2; all P < 0.05). Urine flow rate was not different between AI groups and sham animals at baseline (sham 4.9 ± 0.4; AI-SED 4.0 ± 0.5; AI-WR 5.5 ± 1.0 ml·24 h−1·100 g of body mass−1) but became significantly elevated in AI animals relative to sham at 4 wk (sham 4.1 ± 0.8; AI-SED 8.8 ± 0.5; AI-WR 7.2 ± 0.7 ml·24 h−1·100 g of body mass−1) and 8 wk (sham 2.9 ± 0.6; AI-SED 10.0 ± 1.2; AI-WR 8.5 ± 1.3 ml·24 h−1·100 g of body mass−1). In addition, urine flow rate was increased relative to baseline at 4 wk in AI-SED animals and both AI groups at 8 wk (all P < 0.05).
Table 1.
Animal characteristics and baseline cardiac function
| Sham | AI-SED | AI-WR | |
|---|---|---|---|
| Animal characteristics* | |||
| Body mass, g | 441 ± 6 | 347 ± 19‡ | 385 ± 9‡ |
| Heart mass/tibia length, mg/mm | 39 ± 1 | 45 ± 2‡ | 45 ± 2‡ |
| Lung mass/tibia length, mg/mm | 46 ± 1 | 49 ± 3 | 48 ± 2 |
| Baseline cardiac function† | |||
| Heart rate, bpm | 295 ± 13 | 271 ± 21 | 272 ± 14 |
| Coronary flow, ml/min | 17 ± 1 | 12 ± 1‡ | 13 ± 1 |
| Cardiac output, ml/min | 43 ± 2 | 28 ± 3‡ | 35 ± 3 |
| Stroke volume, μl/beat | 147 ± 7 | 101 ± 10‡ | 129 ± 9 |
| Systolic pressure, mmHg | 84 ± 2 | 77 ± 2‡ | 80 ± 2 |
| Diastolic pressure, mmHg | 39 ± 1 | 44 ± 1‡ | 42 ± 1 |
| Cardiac work, SP/CO | 3,629 ± 237 | 2,125 ± 278‡ | 2,787 ± 239 |
| Stroke work, SP/SV | 12,411 ± 790 | 7,827 ± 918‡ | 10,356 ± 920 |
| RPP, HR/SP | 24,622 ± 117 | 20,476 ± 1,357§ | 21,514 ± 1,099 |
| Maximum rate of ΔP (+dP/dt), mmHg/s | 1,230 ± 50 | 902 ± 53‡ | 1,057 ± 55 |
| Minimum rate of ΔP (−dP/dt), mmHg/s | −955 ± 55 | −746 ± 46‡ | −810 ± 46 |
AI-SED, 5/6 ablation-infarction sedentary rats; AI-WR, 5/6 ablation-infarction wheel-running rats; CO, cardiac output; ΔP, change in pressure; dP/dt, change in pressure over time; HR, heart rate; RPP, rate pressure product; SP, systolic pressure; SV, stroke volume. Values are means ± SE.
Sham n = 20, AI-SED n = 17, AI-WR n = 17.
Sham n = 10, AI-SED n = 11, AI-WR n = 10.
P < 0.05 vs. sham;
P = 0.05 for ANOVA.
Table 2.
Renal function and urinary NOx excretion
| Baseline | 4 Weeks | 8 Weeks | |
|---|---|---|---|
| Serum creatinine, mg/dl | |||
| Sham | 1.01 ± 0.11 | 1.23 ± 0.13 | 0.72 ± 0.05 |
| AI-SED | 1.01 ± 0.07 | 1.80 ± 0.28 | 2.39 ± 0.36*† |
| AI-WR | 0.95 ± 0.07 | 1.76 ± 0.18# | 2.09 ± 0.21*† |
| Blood urea nitrogen (mg/dl) | |||
| Sham | 24 ± 1 | 29 ± 3 | 22 ± 2 |
| AI-SED | 26 ± 3 | 50 ± 5*† | 56 ± 6*† |
| AI-WR | 25 ± 1 | 57 ± 3*† | 62 ± 5*† |
| Protein excretion, mg·24 h−1·100 g of body mass−1 | |||
| Sham | 5.5 ± 0.8 | 4.9 ± 0.9 | 5.9 ± 1.0 |
| AI-SED | 5.9 ± 0.6 | 41.3 ± 4.9*† | 54.7 ± 6.6*† |
| AI-WR | 6.0 ± 0.8 | 35.9 ± 5.2*† | 54.9 ± 7.6*† |
| Urinary NOx excretion, nmol·24 h−1·100 g of body mass−1 | |||
| Sham | 1,595 ± 260 | 1,112 ± 222† | 1,002 ± 154† |
| AI-SED | 1,496 ± 242 | 303 ± 62*† | 400 ± 74*† |
| AI-WR | 1,588 ± 298 | 368 ± 87*† | 239 ± 43*† |
Different measures of renal function and urinary NOx excretion in sham (n = 7) and AI groups (AI-SED: n = 7, AI-WR: n = 7) at baseline and 4 and 8 wk following surgery. NOx, nitrite + nitrate. Values are means ± SE.
P < 0.05 vs. sham;
P < 0.05 vs. baseline.
Wheel running and baseline cardiac function.
AI-WR animals ran an average of 13 ± 2 km total (range 4–22 km) over the 4-wk intervention period, indicating that the volume of activity performed was low. Running behavior was consistent across the duration of the intervention as indicated by weekly running totals recorded in a subset of animals (n = 6; week 1, 2.7 ± 0.7 km; week 2, 2.7 ± 0.6 km; week 3, 2.1 ± 0.5 km; week 4, 1.8 ± 0.7 km; all P > 0.05 vs. week 1). Soleus muscle citrate synthase activity was significantly attenuated in AI-SED animals (sham 580 ± 46 vs. AI-SED 455 ± 9 nmol·ml−1·min−1; P < 0.05 vs. sham) and was not different from sham animals with voluntary wheel running (AI-WR 530 ± 29 nmol·ml−1·min−1; P > 0.05 vs. sham). Wheel running coincided with preserved baseline CO as well as other measures of in vitro cardiac function (Table 1). Stroke volume in isolated perfused hearts from AI-WR and sham animals were not different, suggesting inotropic-mediated effects of wheeling running. In addition, systolic dysfunction and diastolic dysfunction were not evident in isolated AI-WR hearts as shown by preserved systolic and diastolic pressure and rates of pressure development. In contrast, AI-SED animals demonstrated impaired in vitro CO and systolic and diastolic function. Coronary flow was also significantly decreased in AI-SED but not AI-WR hearts, indicating preserved myocardial perfusion in these animals.
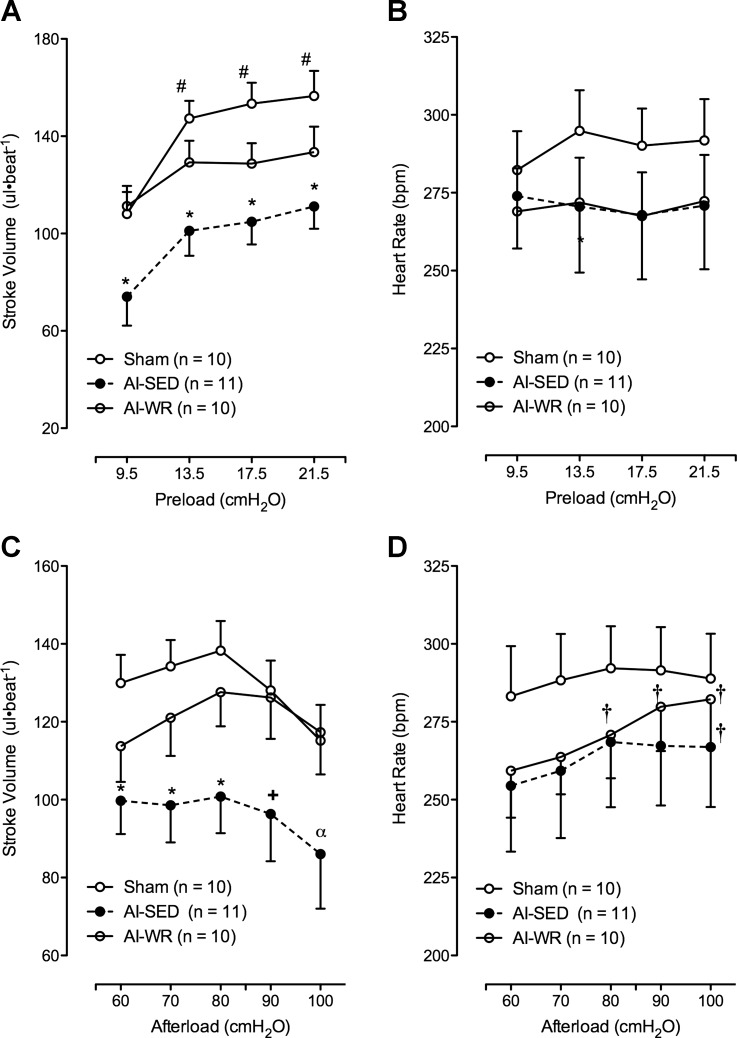
CO was impaired in AI-SED hearts in response to both alterations in preload (Fig. 1, A and C) and afterload (Fig. 1, B and D), an effect not observed in AI-WR hearts with the exception of one preload level. Preloads of 9.5, 13.5, and 21.5 cmH2O elicited impaired CO in AI-SED (P < 0.05) but not AI-WR (P > 0.05) hearts while an afterload was maintained at 80 cmH2O, whereas at a preload of 17.5 cmH2O, CO was impaired in both groups (P < 0.05). Afterloads of 60, 70, and 80 cmH2O (all P < 0.05 AI-SED vs. sham), 90 cmH2O (P = 0.05 AI-SED vs. sham), and 100 cmH2O (P = 0.12 AI-SED vs. sham) also elicited impaired CO in AI-SED but not AI-WR hearts with preload set at 13.5 cmH2O (all P > 0.05 for AI-WR vs. sham). Furthermore, isolated hearts from two AI-SED animals were unable to achieve aortic overflow during the 100 cmH2O pressure level. Area under the curve, as an index of the overall response of CO to afterload (Fig. 1D) and preload (Fig. 1C) manipulation, also indicated significantly impaired functional responses in AI-SED but preserved function in AI-WR. Differences in CO, or lack thereof, in AI groups during afterload and preload manipulation may be predominately attributed to changes in stroke volume (Fig. 2, A and C); however, heart rate (Fig. 2D) did increase in both AI groups with increasing afterload, suggesting some chronotropic-induced increases in CO during the afterload manipulation.
Fig. 1.
Left ventricular (LV) function curves and mean area under the curve (AUC) data depicting the response of cardiac output (CO) to preload and afterload manipulations. As shown, in vitro CO was significantly impaired in 5/6 ablation-infarction sedentary (AI-SED) animals but not in ablation-infarction wheel-running (AI-WR) animals vs. sham animals during preload (A and C) and afterload manipulations (B and D). Values are means ± SE. *P < 0.05 vs. sham; +P = 0.05 vs. sham; αP = 0.12 vs. sham; #P < 0.05 vs. 9.5 cmH20 value.
Fig. 2.
Stroke volume and heart rate responses to preload and afterload manipulations. Stroke volume was impaired similarly to CO in AI-SED hearts in response to altering preload (A) and afterload (C) while not different from sham in the AI-WR hearts. In contrast, heart rate was not different between groups during both manipulations (B and D). Moreover, heart rate did not change with increasing preload (B) but may have contributed to changes in CO with increasing afterload in both AI groups (D). *P < 0.05 vs. sham; +P = 0.07 vs. sham; αP = 0.12 vs. sham; #P < 0.05 vs. 9.5 cmH20 value; †P < 0.05 vs. 60 cmH2O value.
Biochemical markers of oxidative stress.
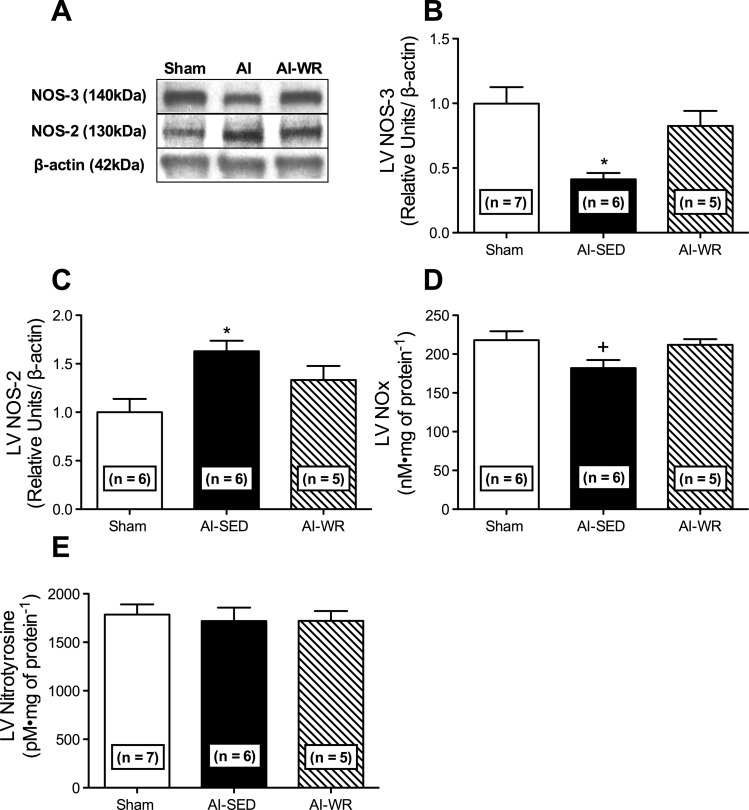
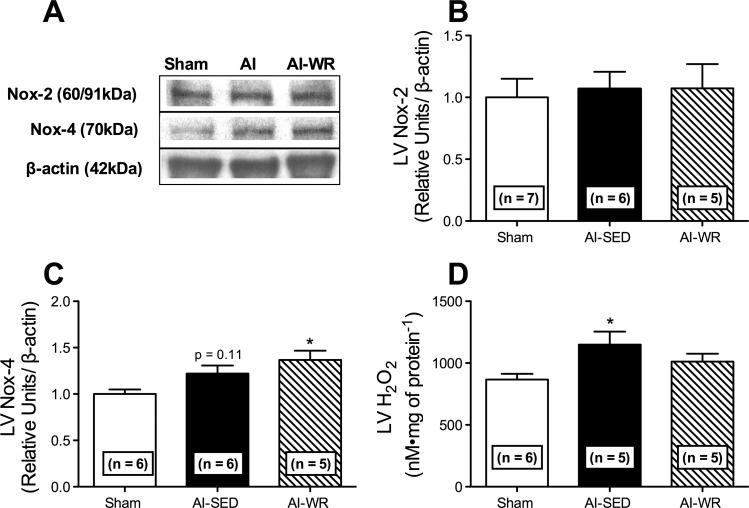
Differential LV NO production and nitric oxide synthase expression were observed in AI-SED and AI-WR animals. LV NOx (P = 0.05; Fig. 3D) as well as NOS-3 (P < 0.05; Fig. 3, A and B) was reduced in AI-SED animals, whereas NOS-2 was increased (P < 0.05; Fig. 3, A and C). Conversely, LV NOx, NOS-3, and NOS-2 (Fig. 3, A–D) in AI-WR animals were not different from those of sham animals (P > 0.05). LV nitrotyrosine was not significantly different between AI groups and sham animals (Fig. 3E). Similar decreases in LV SOD-1 (P < 0.001; Fig. 4A) and increases in LV GPx-1/2 (P < 0.05; Fig. 4C) were observed in AI-SED and AI-WR animals. In contrast, LV catalase expression was similar between sham and AI-WR animals (Fig. 4D), and greater differences were observed in LV SOD-2 in AI-WR relative to sham animals (P < 0.01), although SOD-2 was also elevated in the LV of AI-SED animals (P < 0.05; Fig. 4B). LV NOX-2 was not significantly different between AI groups and sham animals (Fig. 5B), whereas LV Nox-4 was elevated in AI-WR (P < 0.05) and AI-SED (Fig. 5C) groups, approaching statistical significance (P = 0.12). LV H2O2 was increased in AI-SED but not AI-WR animals, suggesting decreased production in the myocardium with wheel running (Fig. 5D).
Fig. 3.
Markers of LV nitric oxide production and bioavailability. Representative Western blots (A) and quantification of the LV nitric oxide synthase-3 (NOS-3) (B), and NOS-2 (C) isoforms as well as total LV nitrite + nitrate (NOx) (D) and nitrotyrosine (E) in sham and AI animals. LV NOS-3, NOS-2, and NOx were no longer significantly different from sham in AI-WR. No differences were observed in LV nitrotyrosine levels between AI groups and sham animals. Values are means ± SE. *P < 0.05 vs. sham; +P = 0.05 for ANOVA.
Fig. 4.
Representative blots and quantification of LV antioxidant enzymes. Western blot analysis of LV superoxide dismutase-1 (SOD-1) (A), SOD-2 (B), glutathione peroxidase-1/2 (GPx1/2) (C), and catalase (D) is shown. Antioxidant enzyme expression was similar in the LV of AI-SED and AI-WR animals. However, LV SOD-2 was elevated in AI-WR more so than AI-SED relative to shams, and LV catalase was not significantly different from that of sham animals in AI-WR animals. Values are means ± SE. *P < 0.05 vs. sham, **P < 0.01 vs. sham, ***P < 0.001 vs. sham.
Fig. 5.
LV NADPH-oxidase expression and hydrogen peroxide (H2O2) production. Quantification and representative blots of LV NADPH-oxidase-2 (Nox-2) (A), Nox-4 (B), and H2O2 production (D). LV H2O2 was not different from that of sham animals with wheel running; however, LV NADPH-oxidase-4 expression was elevated in AI-WR animals and no difference was observed in LV NOX-2 expression between AI groups and sham. Values are means ± SE. *P < 0.05 vs. sham.
DISCUSSION
The present investigation is the first to demonstrate preserved cardiac function following a low-volume, 4-wk, voluntary wheel-running intervention in the AI model of moderate-to-severe CKD. Isolated perfused hearts from sedentary AI animals demonstrated significantly impaired cardiac performance at baseline and in response to alterations in preload and afterload. Conversely, measures of cardiac performance were not different from those of sham animals in AI-WR rats that ran an average of only 13 ± 2 km total (∼0.48 km/day) over the 4-wk intervention period. Taken together, a short-term, low-volume exercise intervention is sufficient to maintain cardiac function in kidney-diseased rats.
Previous studies investigating cardiac dysfunction in experimental CKD have yielded mixed results, potentially related to the severity of the model used (27, 44). Nevertheless, systolic dysfunction and diastolic dysfunction are apparent in uremic cardiomyocytes, and isolated perfused hearts from CKD rats demonstrate depressed CO at baseline and with manipulation of preload and afterload (25, 27, 33). Other studies have demonstrated in vitro and in vivo functional impairments in animals with CKD that persist following ischemia-reperfusion injury (6, 27). Here, we support previous findings and demonstrate significantly impaired cardiac function in AI-SED animals. The AI model of CKD is an accelerated moderate-to-severe uremic model characterized by decreased systemic NO production and endothelial dysfunction (2, 31, 45). AI rats in the current study had elevated levels of BUN, significant cardiac hypertrophy, and decreased urinary NOx excretion in the absence of changes in lung mass. Therefore, uremia and concomitant hemodynamic effects may be contributing to the cardiac dysfunction observed in the absence of significant fluid accumulation in the lungs.
Experimental evidence is limited regarding the effect of increased physical activity or exercise training on cardiac function in CKD. Exercise studies conducted in CKD have predominately focused on its ability to limit kidney disease progression. Kidney function is not altered or improved with aerobic exercise in humans and animal models (3, 13, 22, 24, 26, 35, 36), an effect that may be dependent upon the modality of exercise utilized (30, 36). However, diastolic function has been shown to improve following 12 mo of a lifestyle and exercise intervention in patients with CKD (23). Furthermore, in a study conducted by da Silva Luiz et al., a high-intensity aerobic swimming intervention maintained myocardial function in nephrectomized rats while improving papillary muscle contractility (13). In the current study, several measures of cardiac function were preserved in AI animals that underwent a short, 4-wk period of low-volume voluntary wheel running. AI-WR animals only ran an average of 13 ± 2 km total but had preserved soleus muscle citrate synthase activity levels in response to this level of stimulus. The average running distance of AI-WR animals is comparable to that observed in rodent models of aging, but less than other models of CKD, and despite this, cardiac performance was preserved (1, 16). Furthermore, unpublished data from our laboratory suggests that cardiac function is impaired at 4 wk in AI rats (AI-SED CO at 4-wk 31 ± 5 ml/min, n = 4; P < 0.05 vs. sham at 8 wk 43 ± 2 ml/min, n = 10). Therefore, cardiac function may be improved with the 4-wk voluntary wheel-running intervention despite further progression of kidney disease.
CO was preserved in AI-WR animals and persisted during preload and afterload manipulations, with all isolated hearts from AI-WR animals able to withstand these perturbations. In contrast, 2 out of 11 isolated AI-SED hearts were unable to achieve aortic overflow at the highest level of afterload. Increased CO in AI-WR animals is potentially the result of inotropic effects because stroke volume was preserved in the absence of changes in heart mass. In addition, systolic dysfunction and diastolic dysfunction were not apparent in the AI-WR group, and wheel running did not alter renal function in these animals.
Enhanced redox homeostasis and increased NO production, NO bioavailability, or both may be partly responsible for the preserved cardiac function observed with wheel running in AI. In the absence of adequate sequestration by antioxidant enzymes, excessive reactive oxygen species (ROS) production has various harmful effects on the heart. ROS can disrupt the cardiac Ca2+ transport system, elicit mitochondrial injury, and cause cell death that are implicated in cardiac dysfunction (20). In addition, ROS diminish the bioavailability of NO, which is essential for normal cardiac function in humans and animal models (4, 48).
ROS are elevated systemically and in the myocardium in experimental kidney failure, and elicit cardiac pathogenesis likely via NO-dependent and -independent mechanisms (11, 27, 34, 45). Nonphagocytic NADPH-oxidases (Noxs) that are responsible for enzymatic production of superoxide (O2−) and hydrogen peroxide (H2O2) are increased in the uremic heart and contribute to LV hypertrophy and cardiac dysfunction (27, 34). ROS have also been linked to cardiac fibrosis in uremia and impaired endothelial function in the peripheral vasculature (14, 21). Furthermore, a deficiency in NO is apparent in CKD and related to hypertension and systolic dysfunction (2, 4). ROS are believed to contribute to decreased NO in kidney disease by oxidizing critical cofactors essential for its synthesis, increasing endogenous nitric oxide synthase (NOS) inhibitors, and reacting with NO to form the damaging radical, peroxynitrite (ONOO−) (2).
AI-SED animals in the current study presented with elevated levels of LV H2O2 and nonsignificant increases in Nox-4, whereas no difference was observed in expression of Nox-2. This supports previous findings and potentially reflects the source of ROS production and related AI cardiac disease (27). Nox-2 represents a membrane-associated multisubunit protein complex that is analogous to phagocytic Nox and produces O2−, whereas Nox-4 may be predominately localized in the mitochondria in cardiomyocytes and is a major source of O2−, H2O2, or both, in response to pressure overload-induced cardiomyopathy (28). Consistent with this finding, the superoxide dismutase-2 (SOD-2; MnSOD) isoform, which is responsible for sequestering O2− to produce H2O2 in the mitochondria, was increased in the LV of AI-SED animals; conversely, cytosolic SOD-1 (CuZn SOD) was reduced, suggesting a mitochondrial source of ROS production (15). Other antioxidant enzymes including LV glutathione peroxidase-1/2 (GPx) and catalase were also increased in the AI-SED group but were apparently unable to adequately decrease overall myocardial H2O2 levels.
Contrary to AI-SED rats, AI-WR animals had LV H2O2 levels not different from those of sham animals, despite reduced SOD-1 and significantly elevated Nox-4. The unchanged levels of LV catalase, sustained increase in GPx, and increased SOD-2 with wheel running may explain this finding. Also, Nox-4 is considered a unique Nox isoform that has been shown to be vascular protective by inducing angiogenesis and preventing apoptosis (46). In this regard, Nox-4 may also represent a novel mediator of the low-volume exercise-induced cardioprotective effects observed. Overall, ROS production was decreased in the heart with wheel running as associated with elevated antioxidant defense demonstrating an attenuation of the prooxidant environment in CKD.
Consistent with enhanced redox homeostasis; LV NO production was increased in the AI-WR group with differential expression of NOS isoforms. NO signaling in the myocardium is complex, with exogenous and endogenous sources of NO (32). However, in general, NO is produced enzymatically in the myocardium from three NOS isoforms (NOS-1 to NOS-3) and nonenzymatically from the stable NO metabolites, NO2− and NO3− (NOx) (8, 32). AI-SED animals demonstrated similar NOS expression profiles to that of heart failure; overall LV NOx levels were reduced with decreased constitutive NOS-3 and increased inducible NOS-2 (7, 32). NOS-2 has been associated with impaired function and mortality in the failing myocardium and may be an important component of the cardiac dysfunction observed in AI-SED animals (7). Decreases in LV NOx observed in AI-SED animals may be due to increased ROS production through inhibition of NOS-3 as well as increased ONOO−. Specifically, LV nitrotyrosine, as a marker of ONOO−, was not different between AI-SED and sham animals. However, decreased LV NOx in the AI-SED group suggests that O2− is likely the dominant substrate for ONOO− in these animals and NO bioavailability is decreased. NOS-2 may be the primary isoform responsible for this observation, because its overexpression has been shown to induce ONOO−, cardiomyopathy, and sudden cardiac death (37).
AI-WR rats had LV NOx, NOS-3, and NOS-2 levels maintained at the same level as sham animals. The slightly improved cardiac NO production observed could be related to the improved redox homeostasis in this group. Increased NO in the heart was not accompanied by increased systemic NO production as indicated by urinary NOx excretion. The validity of urinary NOx as a marker of systemic NO production as opposed to primarily kidney NO production may explain this discrepancy. Nevertheless, increased LV NO observed in AI-WR could elicit a number of different effects related to improved cardiac function. Specifically, coronary flow rate was preserved with wheel running, suggesting improved myocardial perfusion. In addition, cardiac hypertrophy was not further augmented in the AI-WR group, a finding that contrasts previous studies conducted in other models of CVD (29). LV NO may have conferred these effects because it is suggested to prevent cardiac hypertrophy and mediate coronary vasodilation via guanosine 3′,5′-cyclic monophosphate (cGMP)-dependent mechanisms (17). Finally, NO is involved in regulation of various Ca2+ handling proteins through S-nitrosylation and cGMP, and could contribute to increased stroke volume (contractility) in AI-WR animals (17, 19). Interestingly, in the absence of sympathetic-induced β-adrenergic stimulation, endogenous inhibition of NO prevents the positive inotropic effects of increased preload in isolated perfused hearts (47).
Clinical perspectives.
CKD affects ∼11% of the United States adult population (∼26 million Americans), and thus represents a major public health concern (10). Patients with CKD are at increased risk of developing CVD and dying from it, and are more likely to die of CVD than progress to end-stage renal disease (48a). CVD manifests at the heart as cardiac dysfunction related to congestive heart failure, ischemic heart disease, and death (12, 38–42, 49). In this study, we demonstrated preserved cardiac function in a rat model of CKD following a 4-wk period of low-volume exercise. Therefore, habitual physical activity, and chronic exercise, are likely ideal adjunct treatment strategies for cardiac dysfunction and associated mortality in renal disease. Indeed, exercise has already been shown to improve quality of life and reduce hospitalization in a number of other cardiovascular diseases (9, 18, 29, 43). Consequentially, further clinical study is needed to confirm our findings and demonstrate the beneficial effects of exercise in patients with CKD.
Conclusion.
In conclusion, cardiac function is preserved in AI following a low-volume exercise intervention. Preserved cardiac performance following 4 wk of voluntary wheel running was associated with increased expression of LV NOx and the constitutive NOS-3 isoform as well as reduced inducible NOS-2. In addition, LV H2O2 was lower in AI-WR animals and was associated with increased expression of SOD-2 and GPx1/2. Therefore, preserved cardiac function in AI with wheel running may be mediated in part by improved redox homeostasis and increased NO. Future investigation is needed to determine the effectiveness of pharmaceuticals or nutraceuticals that elicit similar effects as exercise in treating impaired cardiac function in CKD. Recently, a paper published by Correa et al. demonstrated that curcumin, a natural pigment with antioxidant capacity, maintained both cardiac and mitochondrial function in AI animals (11). Additional study could focus on the efficacy of similar nutraceuticals and more novel, targeted antioxidants to treat and prevent oxidative-related cardiac dysfunction in CKD.
GRANTS
This work was supported by National Institutes of Health Grants P20 RR-016472-12 and P20 GM-103446-12, and by an ACSM Foundation grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.K., C.R.M., S.L.L.-E., and D.G.E. conception and design of research; J.M.K., C.R.M., and J.K. performed experiments; J.M.K., C.R.M., and J.K. analyzed data; J.M.K., C.R.M., J.K., S.L.L.-E., and D.G.E. interpreted results of experiments; J.M.K. prepared figures; J.M.K. drafted manuscript; J.M.K., C.R.M., J.K., S.L.L.-E., and D.G.E. edited and revised manuscript; J.M.K., C.R.M., J.K., S.L.L.-E., and D.G.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. Guers for assistance with data collection.
REFERENCES
- 1.Adams GR, Zhan CD, Haddad F, Vaziri ND. Voluntary exercise during chronic renal failure in rats. Med Sci Sports Exerc 37: 557–562, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol 294: F1–F9, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bergamaschi CT, Boim MA, Moura LA, Picarro IC, Schor N. Effects of long-term training on the progression of chronic renal failure in rats. Med Sci Sports Exerc 29: 169–174, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Bongartz LG, Braam B, Verhaar MC, Cramer MJ, Goldschmeding R, Gaillard CA, Doevendans PA, Joles JA. Transient nitric oxide reduction induces permanent cardiac systolic dysfunction and worsens kidney damage in rats with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 298: R815–R823, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Bongartz LG, Joles JA, Verhaar MC, Cramer MJ, Goldschmeding R, Tilburgs C, Gaillard CA, Doevendans PA, Braam B. Subtotal nephrectomy plus coronary ligation leads to more pronounced damage in both organs than either nephrectomy or coronary ligation. Am J Physiol Heart Circ Physiol 302: H845–H854, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Bronzwaer JG, Zeitz C, Visser CA, Paulus WJ. Endomyocardial nitric oxide synthase and the hemodynamic phenotypes of human dilated cardiomyopathy and of athlete's heart. Cardiovasc Res 55: 270–278, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Calvert JW. Cardioprotective effects of nitrite during exercise. Cardiovasc Res 89: 499–506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campos JC, Gomes KM, Ferreira JC. Impact of exercise training on redox signaling in cardiovascular diseases. Food Chem Toxicol 62C: 107–119, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Correa F, Buelna-Chontal M, Hernández-Reséndiz S, R García-Niño W, J Roldán F, Soto V, Silva-Palacios A, Amador A, Pedraza-Chaverrí J, Tapia E, Zazueta C. Curcumin maintains cardiac and mitochondrial function in chronic kidney disease. Free Radic Biol Med 61C: 119–129, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Curtis BM, Parfrey PS. Congestive heart failure in chronic kidney disease: disease-specific mechanisms of systolic and diastolic heart failure and management. Cardiol Clin 23: 275–284, 2005 [DOI] [PubMed] [Google Scholar]
- 13.da Silva Luiz R, Silva KA, Rampaso RR, Antonio EL, Montemor J, Bocalini DS, Dos Santos L, Moura L, Tucci PJ, de Abreu NP, Schor N. Exercise attenuates renal dysfunction with preservation of myocardial function in chronic kidney disease. PLoS One 8: e55363, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkareh J, Periyasamy SM, Shidyak A, Vetteth S, Schroeder J, Raju V, Hariri IM, El-Okdi N, Gupta S, Fedorova L, Liu J, Fedorova OV, Kahaleh MB, Xie Z, Malhotra D, Watson DK, Bagrov AY, Shapiro JI. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: implications for uremic cardiomyopathy. Am J Physiol Renal Physiol 296: F1219–F1226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588: 3971–3982, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis SH. The role of cGMP-dependent protein kinase in controlling cardiomyocyte cGMP. Circ Res 107: 1164–1166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation 122: 1221–1238, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez DR, Treuer A, Sun QA, Stamler JS, Hare JM. S-Nitrosylation of cardiac ion channels. J Cardiovasc Pharmacol 54: 188–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hafstad AD, Nabeebaccus AA, Shah AM. Novel aspects of ROS signalling in heart failure. Basic Res Cardiol 108: 359, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Hasdan G, Benchetrit S, Rashid G, Green J, Bernheim J, Rathaus M. Endothelial dysfunction and hypertension in 5/6 nephrectomized rats are mediated by vascular superoxide. Kidney Int 61: 586–590, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Howden EJ, Fassett RG, Isbel NM, Coombes JS. Exercise training in chronic kidney disease patients. Sports Med 42: 473–488, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol 8: 1494–1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanazawa M, Kawamura T, Li L, Sasaki Y, Matsumoto K, Kataoka H, Ito O, Minami N, Sato T, Ootaka T, Kohzuki M. Combination of exercise and enalapril enhances renoprotective and peripheral effects in rats with renal ablation. Am J Hypertens 19: 80–86, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kennedy D, Omran E, Periyasamy SM, Nadoor J, Priyadarshi A, Willey JC, Malhotra D, Xie Z, Shapiro JI. Effect of chronic renal failure on cardiac contractile function, calcium cycling, and gene expression of proteins important for calcium homeostasis in the rat. J Am Soc Nephrol 14: 90–97, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Knap B, Buturovic-Ponikvar J, Ponikvar R, Bren AF. Regular exercise as a part of treatment for patients with end-stage renal disease. Ther Apher Dial 9: 211–213, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kuczmarski JM, Martens CR, Lennon-Edwards SL, Edwards DG. Cardiac function and tolerance to ischemia-reperfusion injury in chronic kidney disease. Nephrol Dial Transplant 29: 1514–1524, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 107: 15565–15570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libonati JR. Cardiac effects of exercise training in hypertension. ISRN Hypertension 2013: 9, 2013 [Google Scholar]
- 30.Loupal G, Url A, Skalicky M, Viidik A. Physical exercise retards the development of chronic nephropathy in the ageing rat as efficiently as food restriction does. Gerontology 51: 83–93, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Martens CR, Kuczmarski JM, Lennon-Edwards S, Edwards DG. Impaired L-arginine uptake but not arginase contributes to endothelial dysfunction in rats with chronic kidney disease. J Cardiovasc Pharmacol 63: 40–48, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 93: 388–398, 2003 [DOI] [PubMed] [Google Scholar]
- 33.McMahon AC, Naqvi RU, Hurst MJ, Raine AE, MacLeod KT. Diastolic dysfunction and abnormality of the Na+/Ca2+ exchanger in single uremic cardiac myocytes. Kidney Int 69: 846–851, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Michea L, Villagran A, Urzua A, Kuntsmann S, Venegas P, Carrasco L, Gonzalez M, Marusic ET. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and prevents oxidative stress in uremic rats. Hypertension 52: 295–300, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Moinuddin I, Leehey DJ. A comparison of aerobic exercise and resistance training in patients with and without chronic kidney disease. Adv Chronic Kidney Dis 15: 83–96, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Moningka NC, Sindler AL, Muller-Delp JM, Baylis C. Twelve weeks of treadmill exercise does not alter age-dependent chronic kidney disease in the Fisher 344 male rat. J Physiol 589: 6129–6138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, Schulz R, Butany J, Stewart DJ, Husain M. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest 109: 735–743, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naito K, Anzai T, Yoshikawa T, Anzai A, Kaneko H, Kohno T, Takahashi T, Kawamura A, Ogawa S. Impact of chronic kidney disease on postinfarction inflammation, oxidative stress, and left ventricular remodeling. J Card Fail 14: 831–838, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Nardi E, Cottone S, Mule G, Palermo A, Cusimano P, Cerasola G. Influence of chronic renal insufficiency on left ventricular diastolic function in hypertensives without left ventricular hypertrophy. J Nephrol 20: 320–328, 2007 [PubMed] [Google Scholar]
- 40.Parfrey PS, Foley RN. Ischemic heart disease in chronic uremia. Blood Purif 14: 321–326, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11: 1277–1285, 1996 [PubMed] [Google Scholar]
- 42.Pehrsson SK, Jonasson R, Lins LE. Cardiac-performance in various stages of renal-failure. Br Heart J 52: 667–673, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powers SK, Quindry JC, Kavazis AN. Exercise-induced cardioprotection against myocardial ischemia-reperfusion injury. Free Radic Biol Med 44: 193–201, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Reddy V, Bhandari S, Seymour AM. Myocardial function, energy provision, and carnitine deficiency in experimental uremia. J Am Soc Nephrol 18: 84–92, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Sasser JM, Moningka NC, Tsarova T, Baylis C. Nebivolol does not protect against 5/6 ablation/infarction induced chronic kidney disease in rats - comparison with angiotensin II receptor blockade. Life Sci 91: 54–63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Seddon M, Shah AM, Casadei B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc Res 75: 315–326, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Stamler JS, Loh E, Roddy MA, Currie KE, Creager MA. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation 89: 2035–2040, 1994 [DOI] [PubMed] [Google Scholar]
- 48a.US Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease, and End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 49.Wu IW, Hung MJ, Chen YC, Hsu HJ, Cherng WJ, Chang CJ, Wu MS. Ventricular function and all-cause mortality in chronic kidney disease patients with angiographic coronary artery disease. J Nephrol 23: 181–188, 2010 [PubMed] [Google Scholar]