Abstract
The regulatory role of adenosine monophosphate-activated protein kinase (AMPK)-α2 on sarcoplasmic reticulum calcium-ATPase (SERCA) 1a and SERCA2a in different skeletal muscle fiber types has yet to be elucidated. Sedentary (Sed) or exercise-trained (Ex) wild-type (WT) and AMPKα2-kinase dead (KD) transgenic mice, which overexpress a mutated and inactivated AMPKα2 subunit, were utilized to characterize how genotype or exercise training influenced the regulation of SERCA isoforms in gastrocnemius. As expected, both Sed and Ex KD mice had >40% lower AMPK phosphorylation and 30% lower SERCA1a protein than WT mice (P < 0.05). In contrast, SERCA2a protein was not different among KD and WT mice. Exercise increased SERCA1a and SERCA2a protein content among WT and KD mice, compared with their Sed counterparts. Maximal SERCA activity was lower in KD mice, compared with WT. Total phospholamban protein was higher in KD mice than in WT and lower in Ex compared with Sed mice. Exercise training increased phospholamban Ser16 phosphorylation in WT mice. Laser capture microdissection and quantitative PCR indicated that SERCA1a mRNA expression among type I fibers was not altered by genotype or exercise, but SERCA2a mRNA was increased 30-fold in WT+Ex, compared with WT+Sed. In contrast, the exercise-stimulated increase for SERCA2a mRNA was blunted in KD mice. Exercise upregulated SERCA1a and SERCA2a mRNA among type II fibers, but was not altered by genotype. Collectively, these data suggest that exercise differentially influences SERCA isoform expression in type I and type II fibers. Additionally, AMPKα2 influences the regulation of SERCA2a mRNA in type I skeletal muscle fibers following exercise training.
Keywords: SERCA, AMPK, exercise training, fiber type, myosin heavy chain, MHC
skeletal muscle consists of different fiber types, each with functional characteristics. In mice, skeletal muscle is classified as being composed of either type I or type II muscle fibers, with type II fibers further subdivided into type IIa, IIx/d, IIb fibers, or intermediate (e.g., IIx/db or IIax/d) fibers (43). The gastrocnemius (gastroc) consists of 54% type IIb fibers, 19% type IIdb, 12% type IIad, 6% type IIa fibers, 6% type I fibers, and 2% type IId fibers in the C57BL/6J mouse strain (2). This nomenclature is based upon the myosin heavy-chain (MHC) isoform type and are somewhat descriptive of the contractile properties of each fiber, where type I fibers generally possess slow-twitch contractile parameters, and type II MHC fibers are considered to possess fast-twitch contractile characteristics. Although this contractile phenotype is directly influenced by MHC isoform type, the functional characteristics of muscle fibers are also regulated by other proteins. For example, tissue-specific differences in sarcoplasmic reticulum calcium-ATPase (SERCA) isoform composition exist (32, 54). Specifically, cardiac and slow-twitch skeletal (type I) muscle predominantly express the SERCA2a isoform, whereas fast-twitch (type II) muscle predominantly express SERCA1a. Moreover, differences with respect to the expression of endogenous SERCA modulator proteins, such as phospholamban (PLN) and sarcolipin, exist between the different fiber types (32, 54). Finally, type I and type IIa fibers generally have the highest mitochondrial content (10, 17) and resistance to fatigue, whereas type IIx fibers have moderate oxidative and glycolytic capacity, and type IIb fibers have a high glycolytic capacity (36, 43).
Cellular energy demand increases by ∼100-fold when transitioning from rest to exercise (41), with ∼40–50% of this increase in energy utilization being accounted for by SERCA proteins during muscle contraction (49). Repetitive contractile activity stimulates skeletal muscle to adapt in a variety of ways. For example, 10 wk of endurance exercise training (i.e., 5 times/wk; 60 min/session at ∼60% peak maximum speed) reduces the expression of SERCA1a protein content by ∼14%, but does not alter SERCA2a protein content in human skeletal muscle (13). Exercise training also increases SERCA2a and SERCA1a protein content (4, 9) and mRNA (25) in mouse skeletal muscle. In contrast, Vanderburg and Clarke (55) demonstrated that hindlimb immobilization increased SERCA1 mRNA expression and reduced SERCA2a mRNA expression in slow-twitch muscle fibers, whereas hindlimb immobilization induces a different response among fast-twitch muscle fibers, in which SERCA1 mRNA levels remained unchanged, but SERCA2a mRNA expression increased. It is not yet known why SERCA isoforms are differentially regulated in type I or type II fibers. In fact, a major limitation within the existing literature is the failure to identify the underlying mechanisms by which exercise training influences SERCA isoform expression in skeletal muscle.
Adenosine monophosphate (AMP)-activated protein kinase (AMPK) acts as an energy sensor in skeletal muscle (45) and is activated by changes in AMP during exercise or other metabolic challenges (29). Structurally, AMPK is a heterotrimeric complex, consisting of α-, β-, and γ-subunits (6, 15, 52), where the α-subunit serves as the catalytic portion of the enzyme (15, 56, 59). Two isoforms, namely AMPKα1 and AMPKα2, are coexpressed in muscle, but the AMPKα2 isoform is expressed to a greater extent (50). Studies that have used a muscle-specific AMPKα2 kinase dead transgenic mice (AMPKα2KD) or whole body AMPKα2 knockout mice (AMPKα2KO) have reported that the AMPKα2 subunit plays a critical role in regulating a variety of metabolic proteins. For example, AMPKα2 influences the contraction-stimulated activation of AMPK (21), glucose uptake (27, 34), mitochondrial enzymes (34), and FAT/CD36 translocation (20). Rockl et al. (39) have also reported that 6 wk of voluntary wheel running induces a fiber type IIb to IIa/x transition in tricep muscles, but that this fiber transition was attenuated among AMPKα2KD mice. Moreover, Lee-Young et al. (26) have demonstrated that, in human skeletal muscle, an acute bout of exercise activates AMPK in a fiber type-specific manner, where AMPKαThr172 phosphorylation was increased by ∼40% among type I and IIa fibers and by ∼60% among IIx fibers. Thus exercise differentially activates AMPK in a fiber type-specific manner, which may stimulate fiber-specific adaptations for downstream targets of AMPK.
The role of AMPK in the regulation of SERCA1a and SERCA2a expression in different skeletal muscle fiber types remains poorly understood. SERCA proteins are responsible for 40–50% energy utilization during muscle contraction (49), so it is conceivable that the regulation of SERCA isoform expression may be sensitive to the energy status of the muscle fiber. Therefore, the present study was designed to determine whether AMPKα2 regulates SERCA1a and SERCA2a expression and function in skeletal muscle from sedentary or exercise-trained C57BL/6 wild-type and AMPKα2KD transgenic mice. Recent advancements in laser capture microdissection (LCM) technology have made it possible to dissect areas of high type I or II fiber type composition from muscle cross sections (58). Therefore, we determined whether type I or type II fiber areas are differentially adapting to exercise training.
MATERIALS AND METHODS
Animals.
Animals were treated in accordance with the guidelines of the Canadian Council on Animal Care (5) and with the approval of the University of Manitoba Animal Protocol Management and Review Committee. Dr. Morris Birnbaum (University of Pennsylvania) kindly provided us with muscle-specific AMPKα2KD transgenic mice to establish a breeding colony. All mice were genotyped using tail-snip samples and PCR, using forward primer (5′-CGAGGTCGACGGTATCGATAAGCTTGATATC-3′) and reverse primer (5′-GAAGGAACCCGTTTGGAGGACTGGAGGCGAGG-3′). We made the decision to utilize this animal model because the transgenic manipulation knocks down all detectable AMPKα2 activity and significantly reduces endogenous AMPKα1 activity (35, 51). KD mice (n = 16) and their wild-type (WT; n = 16, 8 wk old) littermates were fed standard rodent chow (Test Diet product 5TJS; 12% kcal from fat, 72% kcal from carbohydrates, and 16% kcal from protein) ad libitum for a period of 5 mo.
Exercise training and testing.
Voluntary wheel running was utilized as the exercise training model because it is an effective tool for modifying AMPK activity in vivo (28, 53). To utilize this approach, one-half of the WT (n = 8) and KD mice (n = 8) were housed in standard animal cages for the duration of the project and were considered to be sedentary (Sed; i.e., nonexercise trained). The remaining animals were housed in cages with voluntary running wheels for the duration of the 5-mo protocol and were considered to be the exercise-trained (Ex) group.
A graded treadmill exercise test was performed to determine whether wheel running induced training adaptations (e.g., increased maximal running speed) in the Ex mice, as previously described by Hoydal et al. (18). For baseline testing, mice were placed on a Columbus Instruments Exer-3/6 Treadmill, and the initial speed was set to 7 m/min. The speed was then increased by 1.6 m/min until fatigue. For follow-up testing, the initial treadmill speed was set to 60% of their previous maximum speed for 2 min and then increased to 70, 80, and 95% of their previous maximum speed for 2-min intervals. From this point, the treadmill speed was increased by 0.8 m/min until the animal was fatigued. Graded exercise tests were conducted monthly (i.e., baseline, 1, 2, 3, 4, and 5 mo); however, only the 5-mo data have been reported.
Tissue isolation.
After the completion of the 5-mo protocol, all animals were transferred to standard cages 2 h before being anesthetized by an intraperitoneal injection of ketamine-xylazine (150:100 mg/kg). Gastroc muscle from one leg was surgically removed. One-half of the tissue was immediately frozen in liquid N2, and the remaining tissue was homogenized for 60 s by hand using a glass mortar and pestle (Kimble Chase Glassware; 885451–0021, 885452–0021) on ice and diluted 1:10 (wt/vol) in ice-cold buffer (pH 7.5) containing 250 mmol/l sucrose, 5 mmol/l HEPES, 0.2 mmol/l phenylmethylsulfonyl fluoride, and 0.2% sodium azide (NaN3). The gastroc from the second leg was isolated and placed in a cryomold, covered with Tissue-Tek OCT compound (no. 14–373-65; Fisher Scientific) and frozen in melting isopentane on liquid nitrogen. Following removal of tissue, animals were euthanized. Tissue and tissue homogenates were stored at −80°C until future analysis. Before analysis, crude homogenates were centrifuged at low speed, after which total protein content was quantified in triplicate for each sample using the bovine serum albumin protein assay.
Western blotting.
Crude muscle homogenates were diluted with loading buffer, and 20 μg of total protein were loaded into each well. Samples were then resolved using 7.5–15% SDS-polyacrylamide gels (depending on protein size), followed by semidry transfer onto polyvinylidene difluoride membranes (Bio-Rad Laboratories). Membranes were blocked with either 5% milk or 5% bovine serum albumin in Tris-buffered saline Tween 20 buffer and then incubated in the presence of anti-SERCA1a (no. 4219, Cell Signaling Technology), anti-SERCA2a (no. 4388, Cell Signaling Technology), anti-AMPKα (no. 2532, Cell Signaling Technology), anti-phosphorylated-AMPKαThr172 (p-AMPK αThr172; no. 4188, Cell Signaling Technology), anti-PLN (No. sc-21923, Santa Cruz Biotechnology), anti-phosphorylated-PLNThr17 (p-PLNThr17; no. sc-17024-R, Santa Cruz Biotechnology), anti-phosphorylated-PLNSer16 (p-PLNSer16; no. sc-12963, Santa Cruz Biotechnology), anti-phosphorylated acetyl-CoA carboxylase (ACC) (no. 3661; Cell Signaling Technology), and anti-cytochrome-c oxidase (COX IV; no. 4844, Cell Signaling Technology) primary antibodies. All primary antibody dilutions were 1:1,000. After washing three times, membranes were incubated with secondary antibodies conjugated to horseradish peroxidase specific to the species required for each primary antibody, as indicated on the material data sheets provided by the antibody suppliers. Blots were then visualized using ECL reagent (no. 32106, Thermo Scientific, or no. RPN2232, Amersham) and characterized with the Fluor-S-Max MultiImager (Bio-Rad Laboratories). Anti-β-tubulin antibodies (no. 2128, Cell Signaling Technology) were used as gel loading controls. For each antibody, the linearity of progressive increase in protein content was established before experiments were conducted (data not shown). Relative protein levels were determined by scanning densitometry using Quantity One version 4.6.9 (Bio-Rad Laboratories), and values were expressed as a percentage (%) of standard. Values were normalized to control samples and expressed as percentage of control. All samples were analyzed in duplicate and on different gels.
Ca2+-dependent SERCA activity.
Measurement of Ca2+-dependent SERCA activity was made using crude muscle homogenates, as previously described by Simonides and van Hardeveld (46), as modified by Duhamel et al. (8) for use on a plate reader (SPECTRAmax; Molecular Devices). Cyclopiazonic acid (40 μM), which is a specific inhibitor of SERCA, was including in one reaction for each sample. SERCA-dependent ATPase activity was then calculated based on the difference between the ATP hydrolysis rate stimulated by Ca2+ in the absence and presence of cyclopiazonic acid (44). Three SERCA kinetic properties have been assessed, namely, Vmax (maximal SERCA activity), Hill coefficient, which is defined as the relationship between SERCA activity and cytosolic free Ca2+ for 10–90% of Vmax, and calcium concentration producing half-maximal activation (Ca50), which is defined as the cytosolic free Ca2+ required to activate the enzyme to 50% Vmax.
Immunohistochemistry.
Gastroc samples mounted in OCT were sectioned into 12-μm-thick cross sections at −20°C in the transverse plane using a Cryostat machine (Leica Microsystems). After sectioning, slides were fixed in 4% paraformaldehyde for 20 min, followed by three washes for 15 min in 0.1 M phosphate-buffered saline (PBS; 0.1 M phosphate buffer with 0.9% NaCl). Slides were then incubated in 0.1 M PBS and 10% normal donkey serum for 1 h at room temperature (RT), followed by an overnight incubation at RT in mouse anti-MHC-1 antibody (no. M-8421; Sigma; 1:10,000) diluted in 0.1 M PBS and 1% normal donkey serum. After three washes for 10 min in 0.1 M PBS, slides were incubated in donkey anti-mouse antibody (no. 715–065-150; Jackson Laboratories; 1:500) diluted in 0.1 M PBS and 1% normal donkey serum for 1 h at RT. Slides were washed another three times for 10 min in 0.1 M PBS, and then the tissues were treated with Vectastain ABC (no. PK-4000; Vector Laboratories) for 1 h at RT. After two additional washes for 10 min in 0.1 M PBS and one wash for 10 min in 0.05 M Tris·HCl (pH 8.0) were performed, tissues were incubated twice for 10 min each in ImmPACT DAB peroxidase substrate (Vector Laboratories). Finally, slides were washed three times for 10 min in 0.05 M Tris·HCl (pH 8.0), dehydrated through a series of increasing alcohol solutions, and then mounted. Type I muscle fibers were stained dark brown, distinguishing them from type II muscle fibers which stained a light color (Fig. 1A).
Fig. 1.
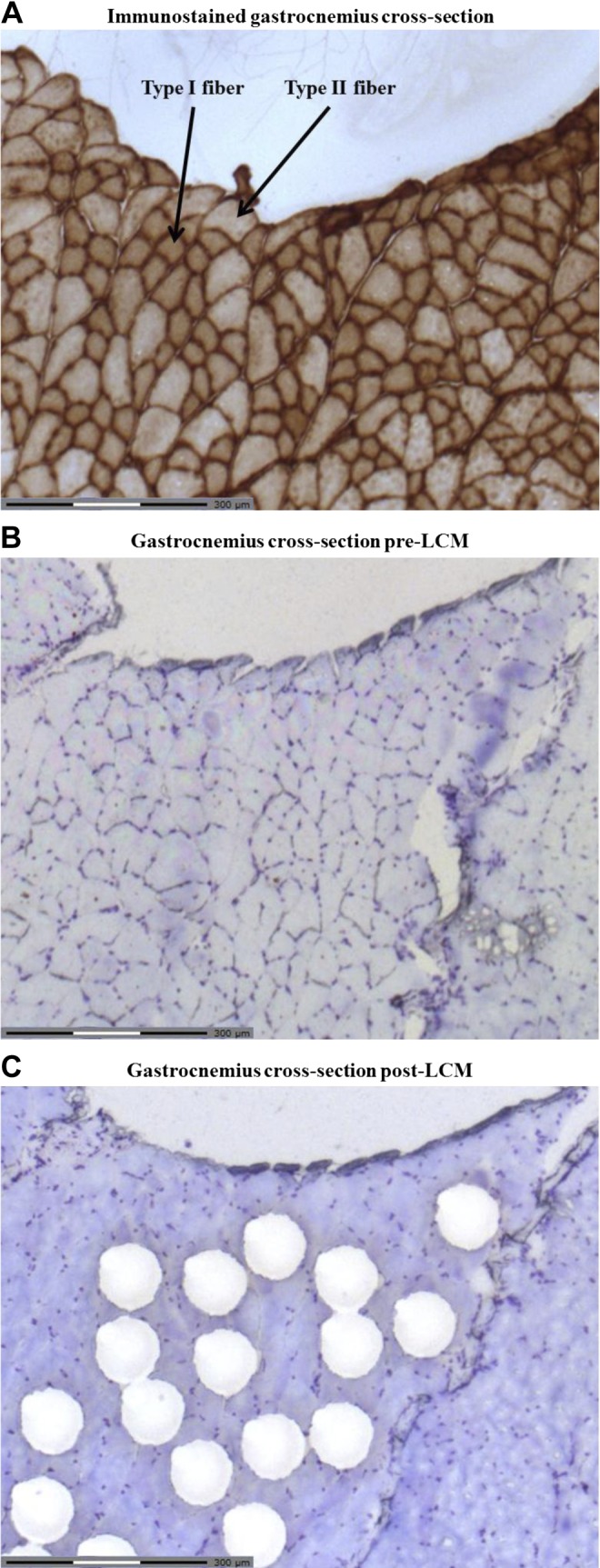
Representative gastrocnemius cross section pre- and post-laser capture microdissection (LCM). A: immunohistochemical staining of myosin heavy chain (MHC)-1β was completed on an example slice of tissue. Type I muscle fibers are stained dark, whereas type II muscle fibers are stained a lighter shade, as indicated by representative arrows. B: subsequent slices of tissue were then stained with cresyl violet stain to highlight cross-section structure. C: areas of ∼7,500 μm2 of muscle tissue, which corresponded to an area of high percentage type I muscle fibers, as identified by the immunohistochemical staining, were then dissected from each tissue slice (LCM-typeI). The same procedure was carried out for an area of high percentage type II muscle fibers (LCM-typeII).
LCM.
A Zeiss PALM MicroBeam LCM system (Carl Zeiss Canada) was utilized, as previously described by Woodrow et al. (60), with some modification. Immunohistochemical staining of MHC-1β was completed on one slice of tissue (Fig. 1A). Subsequent slices of tissue were then stained with cresyl violet stain to highlight cross-section structure (Fig. 1B). Areas of ∼7,500 μm2 of muscle tissue, which corresponded to an area of high percentage type I muscle fibers, as identified by the immunohistochemical staining, were then dissected from each tissue slice (Fig. 1C). Following LCM, 20 μl of the lysis buffer RLT (Qiagen) containing β-mercaptoethanol was added to the dissected tissue and incubated for 30 min. The sample was subsequently centrifuged at 10,000 rpm and stored at −80°C. For simplicity, we will refer to this LCM-enriched type I fiber population as “LCM-typeI.” The same procedure was carried out for an area of high percentage type II muscle fibers (LCM-typeII). Following the LCM of enriched type I and type II muscle fiber areas from a single slice, the whole tissue from the next slice in the series was dissected (LCM-whole). Enrichment of type I muscle fibers was 97–99% higher in LCM-typeI compared with LCM-typeII samples, indicating that the LCM technique was effective in selecting areas with high or low expression of type I muscle fibers. Our decision to examine LCM-typeI and LCM-typeII samples from the gastroc was made because it enables direct comparison of adaptations in muscle fibers that are located in close proximity to each other under similar recruitment patterns, albeit where motor units are activated based on the size principle during contractile activity (12, 16). Other researchers have examined fiber type-specific adaptations to exercise in mice by comparing fibers isolated from the soleus (i.e., fiber composition of 22% type I; 78% type IIa) to extensor digitorum longus (fiber composition of 12% type I; 28% type IIa, and 60% type IIx/b) (23). We decided not to use this alternate approach in our study, because the recruitment patterns for the soleus and extensor digitorum longus differ significantly during treadmill running (19, 40, 48).
RNA isolation from LCM samples.
RNA was isolated from LCM samples using the RNeasy Micro Kit (Qiagen), as per the manufacturer's protocol, with the following modifications. Microdissected samples (LCM-typeI and LCM-typeII fibers) were pooled, and volume was adjusted with buffer RLT to 300 μl. Carrier RNA (20 ng) was added to each sample, followed by 293 μl RNase free H2O and 7 μl proteinase k. The samples were incubated at 55°C for 10 min, after which 300 μl of ethanol were added to the solution. RNA from LCM-whole gastroc samples was isolated in a similar manner, except carrier RNA was not added. Following RNA isolation, RNA concentration, protein contamination, and RNA integrity of all samples were assessed using the 2100 Bioanalyzer (2100 Bioanalyzer RNA Pico 6000 kit and 2100 Expert Software; Agilent Tehnologies).
Reverse transcription and quantitative-polymerase chain reaction of LCM samples.
Total RNA (8.1 ng) was reversed transcribed using the SuperScript VILO cDNA Synthesis Kit (Invitrogen), and the resulting cDNA was preamplified (14 cycles) using the TaqMan PreAmp Master Mix Kit (Applied Biosystems) in a Master Cycler Gradient thermal cycler (Eppendorf). The preamplified cDNA was amplified by quantitative PCR using the following TaqMan probes: MHCβ, Mm01319006_g1; PGC-1α, Mm01208835_m1; SERCA1, Mm01275320_m1; SERCA2, Mm01201431_m1; RyR1, Mm01175211_m1; β-tubulin, Mm00726185_s1. Each reaction contained 1 μl of TaqMan Gene Expression Assay, 4 μl of nuclease-free water, 10 μl of iTaq Universal Probes Supermix (Bio-Rad Laboratories), and 5 μl of preamplified cDNA. Following an initial 1-min incubation at 95°C, thermal cycling was performed using 40 cycles of 95°C for 15 s and 60°C for 30 s. Changes in target mRNA expression were normalized to β-tubulin using the ΔΔCt method (30), whereby ΔCt of β-tubulin was subtracted from ΔCt of each sample gene.
Statistical analyses.
Data are presented as means ± SE. A two-way ANOVA (2 between-group comparisons) was utilized to detect differences between experimental groups. Where significant differences were found, Newman-Keuls post hoc procedures were used to compare specific means. Significance was accepted at P < 0.05.
RESULTS
Animal characteristics.
WT mice with access to the voluntary running wheels ran an average of 4 ± 1 km/day, whereas KD mice ran an average of 5 ± 1 km/day. Table 1 summarizes the general characteristics of the experimental animals. No differences in body weight, heart weight, or heart-to-body mass ratio were observed between experimental groups. As expected, maximum speeds achieved during the graded exercise treadmill test were 23% faster among WT mice compared with KD mice (P < 0.05; main effect). Likewise, maximum running speeds were 44% faster in Ex mice compared with Sed mice (P < 0.01; main effect).
Table 1.
General characteristics of the WT and KD mice
| WT+Sed | KD+Sed | WT+Ex | KD+Ex | |
|---|---|---|---|---|
| Body mass, g | 31 ± 2 | 31 ± 2 | 34 ± 3 | 31 ± 2 |
| Heart mass, mg | 88 ± 3 | 88 ± 6 | 98 ± 5 | 94 ± 6 |
| Heart mass-to- body mass ratio, mg/g | 2.9 ± 0.1 | 2.8 ± 0.1 | 3.0 ± 0.2 | 3.0 ± 0.1 |
| Grade exercise test maximum speed, m/min | 18 ± 1 | 14 ± 1 † | 25 ± 2 ‡ | 21 ± 2 †‡ |
Values are means ± SE; n = 8 mice/group. WT+Sed, wild-type mice housed in standard cages (sedentary condition); KD+Sed, adenosine monophosphate-activated protein kinase (AMPK) α2 kinase dead mice housed in standard cages (sedentary condition); WT+Ex, wild-type mice housed in cages with voluntary exercise wheels (exercised condition); KD+Ex, AMPKα2 kinase dead mice housed in cages with voluntary exercise wheels (exercised condition).
Main effect of genotype, where WT > KD (P < 0.05).
Main effect of exercise was observed, where Sed < Ex (P < 0.01).
AMPKα phosphorylation.
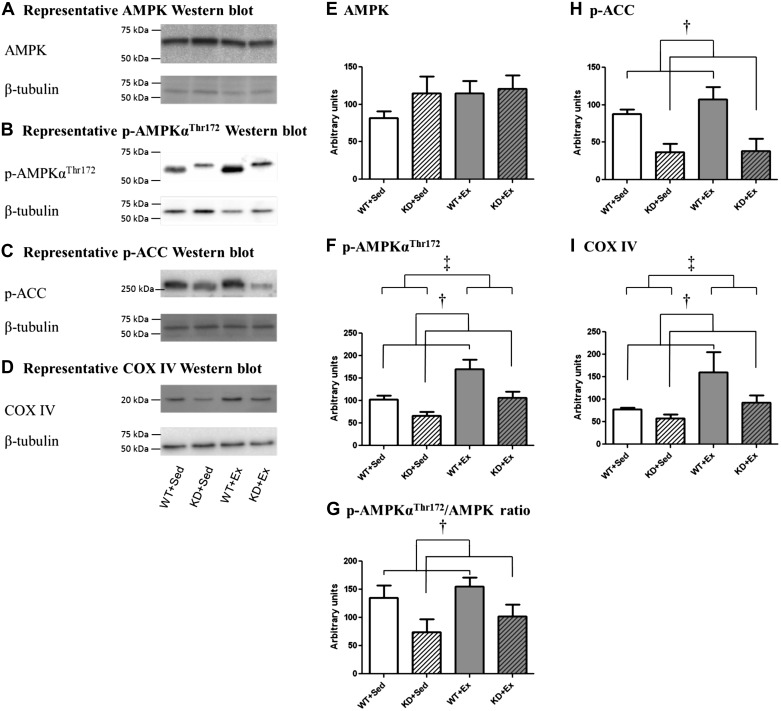
To verify that AMPK activation was modified in the transgenic animal model as well as by exercise training, we assessed the relative levels of total AMPK and phosphorylated AMPKαThr172 (Fig. 2). We also assessed the relative levels of phosphorylated ACC (p-ACC), which is a downstream target activated by AMPK, and COX IV, which is a marker of mitochondrial content. Although total AMPK content in gastroc muscle was not statistically different between WT and KD mice or modified by exercise training, a main effect of genotype was observed for p-AMPKαThr172, p-AMPKαThr172-to-AMPK ratio, p-ACC, and COX IV, where WT > KD (P < 0.05). Likewise, there was a main effect of exercise observed for p-AMPKαThr172 and COX IV, where Sed < Ex (P < 0.05). In contrast, exercise training did not alter p-ACC in either WT or KD mice.
Fig. 2.
Representative Western blots and graphs depicting left gastrocnemius protein expression of adenosine monophosphate-activated protein kinase (AMPK), phosphorylated (p)-AMPKαThr172, p-AMPKαThr172-to-AMPK ratio, p-acetyl-CoA carboxylase (ACC), and cytochrome-c oxidase (COX IV) from sedentary (Sed) and exercise-trained (Ex) animals. Graphs indicated the means ± SE (n = 5 mice/group). WT+Sed, wild-type mice, sedentary group; KD+Sed, AMPKα2 kinase dead mice, sedentary group; WT+Ex, wild-type mice, exercise-trained group; KD+Ex, AMPKα2 kinase dead mice, exercise-trained group. A: AMPK. B: p-AMPKαThr172. C: p-ACC. D: COX IV. E: AMPK. F: p-AMPKThr172. G: p-AMPKThr172-to-AMPK ratio. H: p-ACC. I: COX IV. †Main effect of genotype was observed for p-AMPKThr172, p-AMPKThr172-to-AMPK ratio, p-ACC, and COX IV, where WT > KD (P < 0.05). ‡Main effect of exercise was observed for p-AMPKThr172 and COX IV, where Sed < Ex (P < 0.05).
Exercise training enhanced SERCA1a and SERCA2a protein content in gastroc muscle.
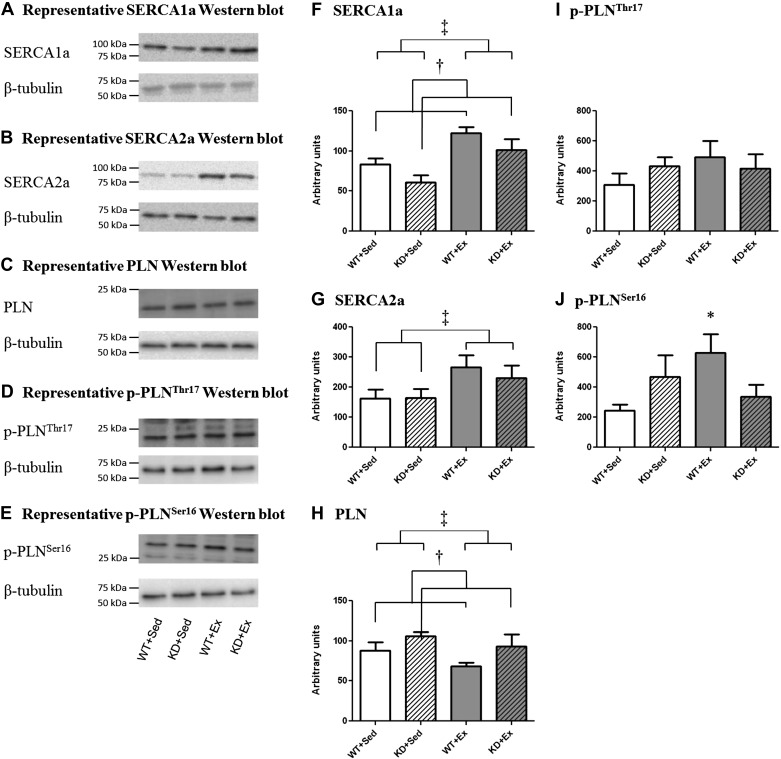
Western blotting was used to characterize relative changes in SERCA1a and SERCA2a protein content in gastroc muscle samples isolated from Sed and Ex WT and KD mice (Fig. 3). Notably, SERCA1a protein content was ∼30% lower (P < 0.05; main effect) among KD mice, compared with their WT littermates. A main effect of exercise training was also observed, where SERCA1a protein content was ∼40% higher (P < 0.05) among Ex mice, compared with their Sed littermates. In contrast, SERCA2a protein content was not different among KD mice, compared with WT mice. However, exercise training did increase SERCA2a protein by ∼25% among Ex mice, compared with their Sed littermates (P < 0.05; main effect).
Fig. 3.
Representative Western blots and graphs depicting left gastrocnemius protein expression of sarcoplasmic reticulum calcium-ATPase (SERCA) 2a, phospholamban (PLN), p-PLNThr17, and p-PLNSer16 from Sed and Ex animals. Graphs indicated the means ± SE (n = 5 mice/group). A: SERCA1a. B: SERCA2a. C: PLN. D: p-PLNThr17. E: p-PLNSer16. F: SERCA1a. G: SERCA2a. H: PLN. I: p-PLNThr17. J: p-PLNSer16. †Main effect of genotype was observed for SERCA1a, where WT > KD (P < 0.05) and for PLN, where WT < KD (P < 0.05). ‡Main effect of exercise was observed for SERCA1a and SERCA2a, where Sed < Ex (P < 0.05) and for PLN, where Sed > Ex (P < 0.05). *Different from Sed of same genotype (P < 0.05).
Changes in PLN protein content were characterized as PLN is expressed in type I muscle fibers isolated from mice (47). PLN is not expressed in type II fibers isolated from mice (47). Total PLN protein content was ∼20% higher (P < 0.05; main effect) among gastroc muscle isolated from KD mice, compared with their WT littermates. A main effect of exercise training was also observed, where total PLN protein content was ∼20% lower (P < 0.05) among Ex mice, compared with their Sed littermates. Likewise, changes in PLN phosphorylation were examined because this protein inhibits SERCA activity when in its unphosphorylated form (3). No differences between any groups were detected for p-PLNThr17 phosphorylation levels. In contrast, p-PLNSer16 phosphorylation increased by ∼158% in Ex WT mice compared with their Sed counterparts (P < 0.05). However, exercise training did not alter p-PLNSer16 phosphorylation in KD mice.
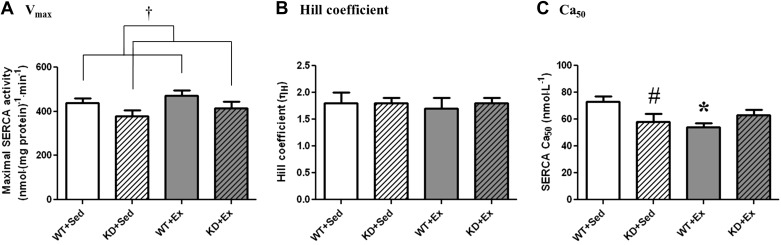
Maximal SERCA activity was lower among KD mice.
Maximal Ca2+-stimulated SERCA activity (Fig. 4) was ∼14% lower (P < 0.05; main effect) among KD mice, compared with their WT littermates. However, exercise training did not alter Vmax in either WT or KD mice. The Hill coefficient was not different between any of the experimental groups. In contrast, an interaction effect (P < 0.05) was observed for Ca50, where KD+Sed mice had ∼21% lower Ca50 values, compared with WT+Sed mice. Among WT mice, Ca50 decreased in exercise vs. Sed mice (73 ± 4 nmol/l in WT-Sed vs. 54 ± 3 nmol/l in WT-Ex), indicating an exercise-induced enhancement of SERCA Ca2+ sensitivity. However, exercise training did not alter Ca50 among KD mice. Finally, Ca50 was not different when comparisons were made between WT+Ex and KD+Ex mice.
Fig. 4.
Calcium-dependent SERCA activity from left gastrocnemius samples isolated from Sed and Ex animals. Graphs indicate the means ± SE (n = 8 mice/group). A: maximal SERCA activity (Vmax). B: Hill coefficient (ηH). C: calcium concentration producing half-maximal activation (Ca50). †Main effect of genotype for Vmax, where WT > KD (P < 0.05). *Different from Sed of same genotype (P < 0.05). #Different from WT from same training condition (P < 0.05).
mRNA expression from laser captured samples.
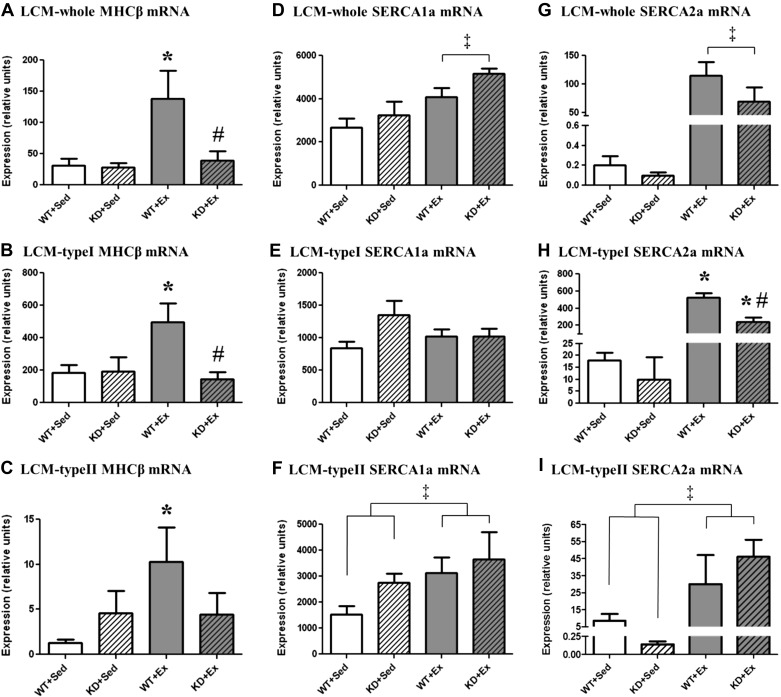
To determine whether type I or type II muscle fibers were differentially influenced by genotype or exercise training, the mRNA levels of MHC-1β, SERCA1a, and SERCA2a were examined (Fig. 5). The relative expression level of MHC-1β was 40- to 200-fold higher in LCM-typeI tissue, compared with LCM-typeII tissue. Exercise-training increased MHC-1β mRNA by 345% in LCM-whole tissue samples isolated from WT mice, compared with WT Sed mice (P < 0.05). However, exercise training did not alter the expression of MHC-1β in LCM-whole tissue samples isolated from KD mice. A similar response was observed for MHC-1β among both LCM-typeI and LCM-typeII samples isolated from WT mice, where exercise training increased MHC-1β mRNA by ∼1.7- and 10-fold, compared with WT Sed mice (P < 0.05). However, exercise training was without effect in either tissue isolated from KD mice.
Fig. 5.
Quantitative PCR data characterizing mRNA expression of laser capture microdissected gastrocnemius tissue isolated from Sed and Ex WT and KD mice. Graphs indicated the means ± SE (n = 5 mice/group). A: LCM-whole MHC-1β mRNA. B: LCM-typeI MHC-1β mRNA. C: LCM-typeII MHC-1β mRNA. D: LCM-whole SERCA1a mRNA. E: LCM-typeI SERCA1a mRNA. F: LCM-typeII SERCA1a mRNA. G: LCM-whole SERCA2a mRNA. H: LCM-typeI SERCA2a mRNA. I: LCM-typeII SERCA2a mRNA. ‡A main effect of exercise (P < 0.05) was observed for SERCA1a among LCM-whole and LCM-typeII, where Sed < Ex, and for SERCA2a among LCM-whole and LCM-typeII, where Sed < Ex. *Different from Sed of same genotype (P < 0.05). #Different from WT from same training condition (P < 0.05).
The relative expression level of SERCA1a mRNA was two- to fourfold lower in LCM-typeI tissue, compared with LCM-typeII tissue. For SERCA1a mRNA, there was no effect of genotype in LCM-whole tissue samples (Fig. 5). However, a main effect of exercise training was observed for SERCA1a mRNA expression in LCM-whole tissue, where Sed < Ex (P < 0.05). In contrast, SERCA1a mRNA expression was not altered by genotype or exercise training in LCM-typeI tissue. Although SERCA1a mRNA was not altered by genotype, a main effect of exercise was observed, where Sed < Ex (P < 0.05) in LCM-typeII tissue.
The relative expression level of SERCA2a mRNA was 20- to 600-fold higher in LCM-typeI tissue, compared with LCM-typeII tissue. For SERCA2a mRNA, there was no effect of genotype in LCM-whole tissue samples. However, a main effect of exercise training was observed for SERCA2a mRNA expression, where Sed < Ex (P < 0.05) in LCM-whole tissue samples. An interaction effect was observed for SERCA2a mRNA in LCM-typeI tissue. Specifically, SERCA2a mRNA expression was not different when comparisons were made between WT+Sed and KD+Sed mice. However, SERCA2a mRNA increased by 30-fold in Ex WT mice, compared with their Sed counterparts (P < 0.01). In contrast, the exercise-stimulated response was blunted in KD mice, where exercise training stimulated a ninefold increase for SERCA2a mRNA in LCM-typeI tissue (P < 0.01). In fact, SERCA2a mRNA expression in LCM-typeI tissue was ∼54% lower among KD+Ex mice, compared with WT+Ex mice (P < 0.01). Finally, the pattern of change for SERCA2a mRNA in LCM-typeII tissue was similar to that observed for LCM-whole tissue, where genotype had no effect, but a main effect of exercise increased SERCA2a mRNA by more than fourfold (P < 0.05).
DISCUSSION
Our data indicates that KD mice have lower AMPK phosphorylation, lower SERCA1a protein, higher levels of PLN protein, and lower maximal SERCA activity, compared with their WT littermates. Moreover, exercise training enhanced AMPK phosphorylation and upregulated SERCA1a, SERCA2a, and PLN protein content among both WT and KD mice, compared with their Sed counterparts. We are the first to report that exercise training induced a 30-fold increase for SERCA2a mRNA expression among type I fibers isolated from WT mice, but that this exercise-stimulated effect was blunted among KD mice. There was a parallel exercise-stimulated increase in MHC-1β expression among type I fibers isolated from WT mice and a blunting of this exercise-stimulated response among type I fibers isolated from KD mice, which is consistent with the fiber type transition and a change in SERCA2a transcriptional regulation. In contrast, SERCA1a mRNA expression was not altered by genotype or exercise among type I fibers. Exercise training also upregulated SERCA1a and SERCA2a mRNA among type II fibers, but genotype did not modify this effect. Collectively, these data suggest that exercise training differentially influences SERCA isoform expression in type I and type II fibers.
KD mice are characterized by reduced AMPK signaling, but are still able to adapt to exercise training.
Our data support previous reports indicating that KD mice are characterized by ∼46% lower p-AMPKαThr172/AMPK (14, 20, 34), ∼60% lower p-ACC (14), and reduced exercise capacity compared with WT mice (11). Even so, KD mice were able to improve their maximal running speed following 5 mo of exercise training. Exercise training also upregulated COX IV protein levels by ∼60% in skeletal muscle isolated from KD mice, albeit at a lower level than that observed among WT mice. These data support a previous publication that reported that impaired AMPKα2 activity partially blunts the contraction-stimulated activation of AMPK (21) and mitochondrial enzymes (34). Thus it appears that exercise training activates multiple pathways to influence energy homeostasis. Alternatively, it is possible that the residual phosphorylation of AMPK observed among KD mice in the present study is sufficient to stimulate adaptations to exercise training.
Regulation of SERCA1a and SERCA2a in skeletal muscle.
We are the first to report that Sed KD mice are characterized by a ∼27% lower SERCA1a protein content in gastroc, compared with Sed WT mice. In contrast, an effect of genotype was not observed for SERCA2a in gastroc. Even so, maximal SERCA activity (Vmax) was reduced by ∼12–14% in Sed and Ex KD mice, compared with their WT counterparts. This pattern of change is similar to that observed for SERCA1a protein content. Based on the fact that SERCA1a has a much higher ATP hydrolysis rate than does SERCA2a (31), it is possible that the lower expression of SERCA1a may explain the observed pattern of change for Vmax in KD mice, compared with WT mice. It is important to indicate that the Ca2+-dependent SERCA activity assay employed in this study cannot be used to assess the activity of specific SERCA protein isoforms. Thus we cannot determine whether changes in SERCA1a or SERCA2a protein content contributed to the observed changes in Vmax.
It has previously been demonstrated that exercise training enhances AMPK activity (37, 38) and increases SERCA1a and SERCA2a isoform content and function in skeletal muscle (4, 8, 9, 13, 25). In the present study, exercise training enhanced SERCA1a protein content in the gastroc isolated from both WT (∼47% increase) and KD (∼66% increase) mice, compared with their Sed counterparts. Exercise training also enhanced SERCA2a protein content in skeletal muscle. It is possible that exercise training enhanced SERCA1a and SERCA2a protein content by lowering the posttranslational rate of SERCA2a protein turnover. In fact, Kho et al. (22) reported that the colocalization of small ubiquitin-related modifier (SUMO1) to SERCA2a can inhibit SERCA2a protein degradation in a model of heart failure. It is not yet known if SUMO1 binds SERCA1a in skeletal muscle, or if exercise training may enhance the colocalization of SUMO1 with SERCA1a or SERCA2a in skeletal muscle as a strategy to reduce protein turnover, which we speculate might occur. The observed changes in SERCA1a and SERCA2a protein content stimulated by exercise training were not accompanied by similar changes in Vmax. This discrepancy is difficult to explain. It is possible that the number of functional SERCA proteins was modified by exercise. For example, Schertzer et al. (42) has previously reported that an acute bout of treadmill running followed by 45 min of passive recovery enhances the number of functional SERCA proteins in sarcoplasmic reticulum vesicles isolated from rat skeletal muscle. Tissue limitations prevented us from performing a similar assessment using mouse skeletal muscle in the present study. The discrepancy between the observed changes in SERCA1a and SERCA2a protein content and Vmax cannot be explained by the observed changes in PLN protein content, as PLN reduces the specific activity of SERCA2a at submaximal concentrations of Ca2+ (33). Even so, exercise training enhanced p-PLNSer16 phosphorylation by ∼158% and Ca50 by ∼26% in Ex WT mice, compared with WT+Sed mice. This outcome would be expected to increase SERCA activity at submaximal calcium concentrations. In contrast, exercise did not influence Ca50 among KD mice. However, this response may be explained by the fact that Ca50 was already ∼21% lower among KD+Sed mice, compared with WT+Sed mice.
Fiber type-specific regulation of SERCA isoform mRNA expression.
A previous study by Lee-Young et al. (26) reported that exercise training up regulates AMPKαThr172 phosphorylation in a fiber type-specific manner. Thus we decided to utilize the LCM technique to enrich areas of high and low type I fiber content based on the expression pattern of MHC-1β. This laser capture method has been employed in previous studies to examine mitochondrial adaptations in different muscle fibers (7), electron transport system abnormalities (57), and changes in fiber type distribution in obese and diabetic animals (1). In the present study, we utilized immunohistochemistry and nonnormalized MHC-1β mRNA values to confirm the selection of enriched slow-twitch and fast-twitch muscle fibers from the different LCM tissue types (i.e., whole, type I, and type II). It was notable that exercise training stimulated a threefold increase in MHC-1β mRNA expression in LCM-whole tissue isolated from WT mice compared with Sed WT mice. Similar changes for MHC-1β mRNA levels were observed in LCM-typeI as well as LCM-typeII tissue samples. These data support previous observations made by Klitgaard et al. (24), where they reported a greater increase of MHC-1β protein levels in vastus lateralis muscle in subjects who regularly exercised (i.e., walking, cycling, and jogging). Our novel data also indicate that exercise training did not alter MHC-1β mRNA expression in the LCM-whole or enriched LCM-typeI or LCM-typeII tissue isolated from KD mice. We interpret these observations as an indication that the AMPKα2 subunit has a role for regulating exercise-induced changes of MHC-1β mRNA in skeletal muscle. This observation supports the previous work reported by Rockl et al. (39), indicating that AMPKα2 influences the regulation of MHC isoforms.
Vanderburg and Clarke (55) have utilized LCM to determine that hindlimb immobilization differentially regulates SERCA1a and SERCA2a mRNA expression among type I and type II fibers isolated from gastroc muscle. Even so, to our knowledge, the present study is the first to utilize the LCM technique to examine changes in mRNA expression of various proteins following exercise training. A novel observation generated using the LCM technique was the identification of a main effect of exercise training for both SERCA1a and SERCA2a mRNA expression in LCM-whole and LCM-typeII tissue. In contrast, exercise training did not influence the expression of SERCA1a mRNA among LCM-typeI tissue. Notably, the KD genotype did not influence the exercise-stimulated response for SERCA1a in any of the LCM tissue samples. SERCA2a mRNA increased 30-fold in WT+Ex, compared with WT+Sed, but this exercise-stimulated effect was blunted among KD mice. Based on these observations, it appears that AMPKα2 influences the regulation of SERCA2a mRNA in type I muscle fibers, but does not directly influence mRNA expression of SERCA1a in type I muscle fibers or either isoform in type II muscle fibers. It is unclear to us why this fiber type-specific difference was observed; however, it may have been influenced by the observed upregulation of MHC-1β among both LCM-typeI and typeII fiber samples induced by exercise training. The up regulation of SERCA2a mRNA would be consistent with an exercise-induced transition from fast- to slow-twitch fiber types. The observed exercise-stimulated fiber type-specific difference in SERCA isoform expression may also be explained, at least theoretically, based on the size principle, which indicates that type I fibers are recruited initially on contraction to remain active until they become fatigued.
Limitations.
A limitation of this work exists within the use of LCM. This method was somewhat limited in its ability to select slow- and fast-twitch muscle fibers. We used stained slices to identify areas with a high concentration of either type I or type II muscle fibers. Unfortunately, this approach does not allow the specific selection of single type I or type II fibers alone. As a result, we were forced to isolate an enriched area of mostly type I or mostly type II fibers within each LCM sample. Our results would be more specific if we would have used immunohistochemistry to identify type IIa, type IIx, and type IIb muscle fibers rather than simply using the approach employed to stain type I fibers based on MHC-1β isoform content. Even with this limitation, our LCM mRNA expression data indicate that the approach we used to isolate MHC-1β expression was 200-fold lower in LCM-typeII tissue, compared with LCM-typeI tissue samples. Vanderburg and Clarke (55) previously utilized a similar LCM approach and pooled samples of a particular type together to demonstrate that hindlimb immobilization enhanced SERCA1 mRNA expression and reduced SERCA2a mRNA expression in type I fibers, but increased SERCA2a mRNA expression in type II fibers.
Another limitation is that KD mice do not have a complete inactivation of AMPK. In our study, AMPK phosphorylation was reduced by ∼46% in skeletal muscle. Thus the residual p-AMPK activity would be expected to stimulate some AMPK-dependent signaling pathways. For example, we provide data indicating that p-ACC, which is a downstream target of p-AMPK, was not completely inhibited. We did consider using muscle-specific AMPKα1 and AMPKα2 KO mice; however, they were not commercially available. Whole body AMPKα1 and AMPKα2 KO animals would complicate our experimental design, because the physiological functions of a variety of tissues other than skeletal muscle are altered.
Summary.
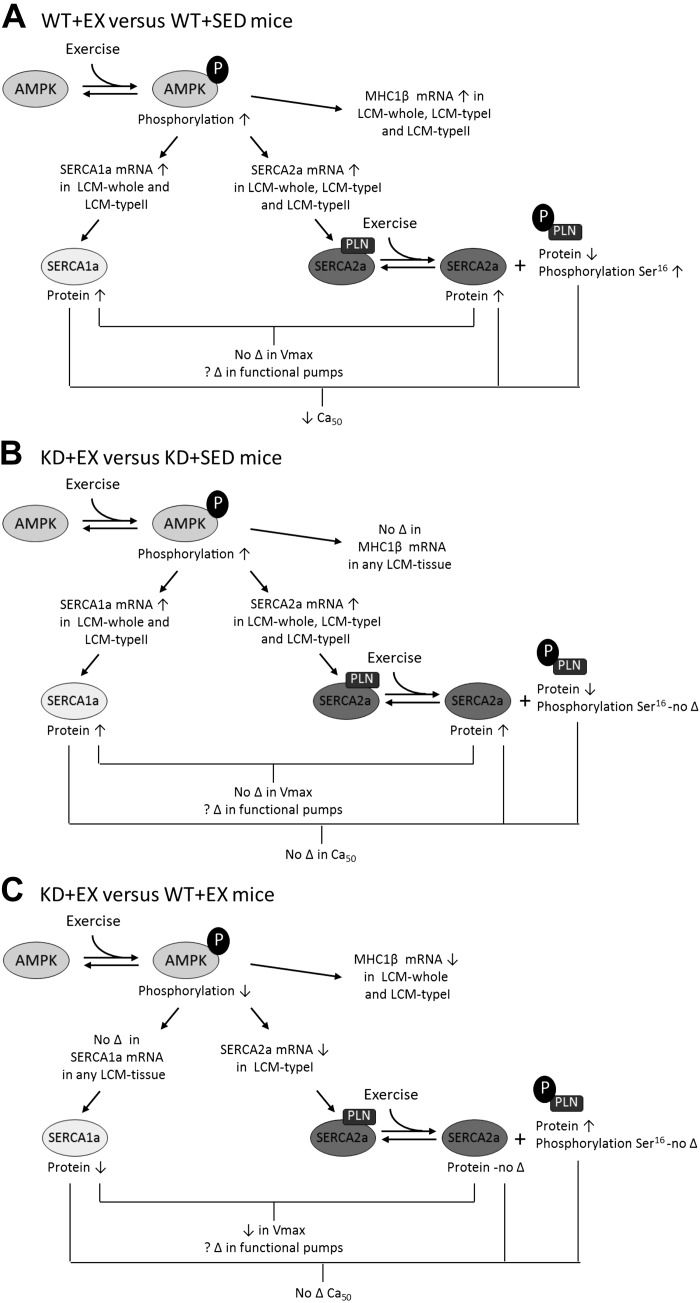
The present study examined whether AMPKα2 regulates SERCA1a and SERCA2a expression and function in skeletal muscle isolated from Sed or Ex WT or KD mice. Our findings, as summarized in Fig. 6, indicate that SERCA1a protein content and SERCA Vmax is downregulated in whole gastroc muscle isolated from KD mice, compared with WT mice. Moreover, our data indicate that SERCA1a and SERCA2a mRNA expression are differentially regulated in response to exercise training based on the specific fiber type assessed. Finally, the exercise-stimulated upregulation of SERCA2a mRNA among type I fibers is blunted in muscle isolated from KD mice, compared with WT mice. Based on these data, we suggest that AMPKα2 has a role for regulating SERCA isoform expression in skeletal muscle.
Fig. 6.
A schematic representation summarizing the effects of exercise training and reduced AMPKα2 activity on SERCA expression and function. A: under exercise conditions, WT mice show increased phosphorylation of AMPK. In LCM-whole and LCM-typeII fibers, SERCA1a mRNA increased, while mRNA levels of SERCA2a increased in LCM-whole, LCM-typeI, and LCM-typeII fibers. Exercise elevated the mRNA level of MHC-1β in LCM-whole tissue and LCM-typeI and LCM-typeII fibers. Protein levels of both SERCA1a and SERCA2a also increased as a result of exercise. The Ca2+ sensitivity of SERCA decreased as a result of exercise, but the Vmax does not. Exercise caused increased phosphorylation of PLN at Ser16 but decreased protein levels of PLN. B: under exercise conditions, KD mice show increased phosphorylation of AMPK. In LCM-whole tissue and LCM-typeII fibers, SERCA1a mRNA increased, while mRNA levels of SERCA2a increased in LCM-whole tissue and LCM-typeI and LCM-typeII fibers. MHC-1β mRNA levels did not change as a result of exercise in KD mice. Protein levels of both SERCA1a and SERCA2a also increased as a result of exercise. In KD mice, exercise did not cause any changes in the Ca2+ sensitivity or Vmax of SERCA. Exercise caused a reduction of protein levels of PLN, but had no effect on its phosphorylation. C: under exercise conditions, differences between WT and KD mice exist. KD mice show decreased levels of p-AMPK following exercise compared with WT mice. Although there was no difference in the effect of exercise on mRNA levels of SERCA1a, KD mice exhibited lower levels of SERCA2a in LCM-typeI fibers compared with WT mice. Exercise reduced the mRNA level of MHC-1β in LCM-whole tissue and LCM-typeI fibers in KD mice compared with WT mice. Protein levels of SERCA1a were reduced, whereas there was no change in SERCA2a protein levels. In KD mice, exercise did not change the Ca2+ sensitivity compared with WT mice, but Vmax of SERCA was reduced. Exercise increased the protein levels of PLN in KD mice compared with WT, but had no effect on its phosphorylation. ↓, Decrease; ↑, increase; ?, undetermined; Δ, change.
GRANTS
This project was supported by an operating grant from the Canadian Institutes of Health Research held by T. A. Duhamel. M. P. Morissette was supported by an National Sciences and Engineering Research Council Studentship. S. E Susser was supported by a St. Boniface Hospital Institute of Cardiovascular Sciences Studentship. A. N. Stammers was supported by a University of Manitoba Undergraduate Research Award. T. A. Duhamel was supported by a Manitoba Health Research Council Establishment Grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.P.M., S.E.S., P.F.G., P.S., and T.A.D. conception and design of research; M.P.M., S.E.S., A.N.S., K.A.O., P.S., and T.L.M. performed experiments; M.P.M., S.E.S., A.N.S., K.A.O., T.L.M., and T.A.D. analyzed data; M.P.M., S.E.S., P.F.G., and T.A.D. interpreted results of experiments; M.P.M., S.E.S., K.A.O., and T.A.D. prepared figures; M.P.M. and T.A.D. drafted manuscript; M.P.M., S.E.S., A.N.S., K.A.O., P.F.G., P.S., T.L.M., and T.A.D. edited and revised manuscript; M.P.M., S.E.S., A.N.S., K.A.O., P.F.G., P.S., T.L.M., and T.A.D. approved final version of manuscript.
REFERENCES
- 1.Adachi T, Kikuchi N, Yasuda K, Anahara R, Gu N, Matsunaga T, Yamamura T, Mori C, Tsujimoto G, Tsuda K, Ishihara A. Fibre type distribution and gene expression levels of both succinate dehydrogenase and peroxisome proliferator-activated receptor-gamma coactivator-1alpha of fibres in the soleus muscle of Zucker diabetic fatty rats. Exp Physiol 92: 449–455, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Augusto V, Padovani CR, Campos GER. Skeletal muscle fiber types in C57BL6J mice. Braz J Morphol Sci 21: 89–94, 2004 [Google Scholar]
- 3.Bhupathy P, Babu GJ, Periasamy M. Sarcolipin and phospholamban as regulators of cardiac sarcoplasmic reticulum Ca2+ ATPase. J Mol Cell Cardiol 42: 903–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bueno CR, Jr, Ferreira JC, Pereira MG, Bacurau AV, Brum PC. Aerobic exercise training improves skeletal muscle function and Ca2+ handling-related protein expression in sympathetic hyperactivity-induced heart failure. J Appl Physiol 109: 702–709, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Canadian Council on Animal Care in Science. Guidelines for the Ethical Use and Care of Animals in Science. Ottawa, Ontario, Canada: Canadian Council on Animal Care in Science, 2012 [Google Scholar]
- 6.Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci 67: 3407–3423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Z, Wanagat J, McKiernan SH, Aiken JM. Mitochondrial DNA deletion mutations are concomitant with ragged red regions of individual, aged muscle fibers: analysis by laser-capture microdissection. Nucleic Acids Res 29: 4502–4508, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duhamel TA, Stewart RD, Tupling AR, Ouyang J, Green HJ. Muscle sarcoplasmic reticulum calcium regulation in humans during consecutive days of exercise and recovery. J Appl Physiol 103: 1212–1220, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Ferreira JC, Bacurau AV, Bueno CR, Jr, Cunha TC, Tanaka LY, Jardim MA, Ramires PR, Brum PC. Aerobic exercise training improves Ca2+ handling and redox status of skeletal muscle in mice. Exp Biol Med (Maywood) 235: 497–505, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Fujii N, Seifert MM, Kane EM, Peter LE, Ho RC, Winstead S, Hirshman MF, Goodyear LJ. Role of AMP-activated protein kinase in exercise capacity, whole body glucose homeostasis, and glucose transport in skeletal muscle: insight from analysis of a transgenic mouse model. Diabetes Res Clin Pract 77, Suppl 1: S92–S98, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Gordon T, Thomas CK, Munson JB, Stein RB. The resilience of the size principle in the organization of motor unit properties in normal and reinnervated adult skeletal muscles. Can J Physiol Pharmacol 82: 645–661, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Green HJ, Ballantyne CS, MacDougall JD, Tarnopolsky MA, Schertzer JD. Adaptations in human muscle sarcoplasmic reticulum to prolonged submaximal training. J Appl Physiol (1985) 94: 2034–2042, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Habets DD, Coumans WA, El HM, Zarrinpashneh E, Bertrand L, Viollet B, Kiens B, Jensen TE, Richter EA, Bonen A, Glatz JF, Luiken JJ. Crucial role for LKB1 to AMPKalpha2 axis in the regulation of CD36-mediated long-chain fatty acid uptake into cardiomyocytes. Biochim Biophys Acta 1791: 212–219, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motorneurons. J Neurophysiol 28: 560–580, 1965 [DOI] [PubMed] [Google Scholar]
- 17.Hoppeler H. Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med 7: 187–204, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Hoydal MA, Wisloff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil 14: 753–760, 2007 [DOI] [PubMed] [Google Scholar]
- 19.James RS, Altringham JD, Goldspink DF. The mechanical properties of fast and slow skeletal muscles of the mouse in relation to their locomotory function. J Exp Biol 198: 491–502, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Jeppesen J, Albers PH, Rose AJ, Birk JB, Schjerling P, Dzamko N, Steinberg GR, Kiens B. Contraction-induced skeletal muscle FAT/CD36 trafficking and FA uptake is AMPK independent. J Lipid Res 52: 699–711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of AMPKalpha2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab 292: E331–E339, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477: 601–605, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinnunen S, Manttari S. Specific effects of endurance and sprint training on protein expression of calsequestrin and SERCA in mouse skeletal muscle. J Muscle Res Cell Motil 33: 123–130, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Klitgaard H, Bergman O, Betto R, Salviati G, Schiaffino S, Clausen T, Saltin B. Co-existence of myosin heavy chain I and IIa isoforms in human skeletal muscle fibres with endurance training. Pflügers Arch 416: 470–472, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Kubo H, Libonati JR, Kendrick ZV, Paolone A, Gaughan JP, Houser SR. Differential effects of exercise training on skeletal muscle SERCA gene expression. Med Sci Sports Exerc 35: 27–31, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lee-Young RS, Canny BJ, Myers DE, McConell GK. AMPK activation is fiber type specific in human skeletal muscle: effects of exercise and short-term exercise training. J Appl Physiol 107: 283–289, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Lefort N, St-Amand E, Morasse S, Cote CH, Marette A. The alpha-subunit of AMPK is essential for submaximal contraction-mediated glucose transport in skeletal muscle in vitro. Am J Physiol Endocrinol Metab 295: E1447–E1454, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1alpha is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab 294: E463–E474, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Li C, Keaney JF., Jr AMP-activated protein kinase: a stress-responsive kinase with implications for cardiovascular disease. Curr Opin Pharmacol 10: 111–115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem 267: 14483–14489, 1992 [PubMed] [Google Scholar]
- 32.MacLennan DH, Rice WJ, Green NM. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases. J Biol Chem 272: 28815–28818, 1997 [DOI] [PubMed] [Google Scholar]
- 33.MacLennan DH, Rice WJ, Green NM. The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases. J Biol Chem 272: 28815–28818, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry 11: 2627–2633, 1972 [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen BB, Hancock CR, Winder WW. Postexercise recovery of skeletal muscle malonyl-CoA, acetyl-CoA carboxylase, and AMP-activated protein kinase. J Appl Physiol 85: 1629–1634, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen BB, Winder WW. Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. J Appl Physiol 83: 1104–1109, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Rockl KSC, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 56: 2062–2069, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol (1985) 70: 2522–2529, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Sahlin K, Tonkonogi M, Soderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand 162: 261–266, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Schertzer JD, Green HJ, Duhamel TA, Tupling AR. Mechanisms underlying increases in SR Ca2+-ATPase activity after exercise in rat skeletal muscle. Am J Physiol Endocrinol Metab 284: E597–E610, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem 264: 17816–17823, 1989 [PubMed] [Google Scholar]
- 45.Shirwany NA, Zou MH. AMPK in cardiovascular health and disease. Acta Pharmacol Sin 31: 1075–1084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonides WS, van Hardeveld C. An assay for sarcoplasmic reticulum Ca2+-ATPase activity in muscle homogenates. Anal Biochem 191: 321–331, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Slack JP, Grupp IL, Ferguson DG, Rosenthal N, Kranias EG. Ectopic expression of phospholamban in fast-twitch skeletal muscle alters sarcoplasmic reticulum Ca2+ transport and muscle relaxation. J Biol Chem 272: 18862–18868, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Sloniger MA, Cureton KJ, Prior BM, Evans EM. Lower extremity muscle activation during horizontal and uphill running. J Appl Physiol (1985) 83: 2073–2079, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Smith IC, Bombardier E, Vigna C, Tupling AR. ATP consumption by sarcoplasmic reticulum Ca2+ pumps accounts for 40–50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PLos One 8: e68924, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J Biol Chem 271: 611–614, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Steinberg GR, Watt MJ, Ernst M, Birnbaum MJ, Kemp BE, Jorgensen SB. Ciliary neurotrophic factor stimulates muscle glucose uptake by a PI3-kinase-dependent pathway that is impaired with obesity. Diabetes 58: 829–839, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem 281: 32207–32216, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Thomson DM, Porter BB, Tall JH, Kim HJ, Barrow JR, Winder WW. Skeletal muscle and heart LKB1 deficiency causes decreased voluntary running and reduced muscle mitochondrial marker enzyme expression in mice. Am J Physiol Endocrinol Metab 292: E196–E202, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Tupling AR. The sarcoplasmic reticulum in muscle fatigue and disease: role of the sarco(endo)plasmic reticulum Ca2+-ATPase. Can J Appl Physiol 29: 308–329, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Vanderburg CR, Clarke MS. Laser capture microdissection of metachromatically stained skeletal muscle allows quantification of fiber type specific gene expression. Mol Cell Biochem 375: 159–170, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol 45: 276–295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J 15: 322–332, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Wanagat JF, Cao ZF, Pathare PF, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J 15: 322–332, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Wojtaszewski JF, Birk JB, Frosig C, Holten M, Pilegaard H, Dela F. 5′AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. J Physiol 564: 563–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woodrow L, Sheppard P, Gardiner PF. Transcriptional changes in rat alpha-motoneurons resulting from increased physical activity. Neuroscience 255: 45–54, 2013 [DOI] [PubMed] [Google Scholar]