Abstract
Cardiovascular mortality increases in cold weather in older adults, and physical activity may impart even greater cardiovascular risk than cold exposure alone. Human aging is associated with exaggerated pressor responses to whole body cooling; however, the sympathetic response to cold stress alone and in combination with isometric exercise is unknown. We hypothesized that cold stress would 1) increase muscle sympathetic nerve activity (MSNA) and 2) augment the MSNA response to isometric handgrip in older adults. Whole body cooling (water-perfused suit) was conducted in 11 young (23 ± 1 yr) and 12 healthy older adults (60 ± 2 yr). Blood pressure (BP; Finometer) and MSNA (microneurography) were measured throughout cooling and during isometric handgrip at 30% maximal voluntary contraction performed at a mean skin temperature (Tsk) of 34 and 30.5°C. MSNA was greater in older adults at Tsk = 34.0°C and throughout cooling (P < 0.05). MSNA increased during cooling in older, but not young, adults (young: Δ0 ± 1 vs. older: Δ8 ± 1 bursts/min; P < 0.05). The cooling-induced increase in BP was greater in older adults (P < 0.05). During handgrip, the increases in MSNA and BP were not different between conditions in either young (Δ14 ± 2 Tsk 34°C vs. Δ12 ± 3 Tsk 30.5°C bursts/min; Δ20 ± 3 Tsk 34°C vs. Δ19 ± 3 Tsk 30.5°C mmHg; both P > 0.05) or older adults (Δ12 ± 1 Tsk 34°C vs. Δ8 ± 1 Tsk 30.5°C bursts/min; Δ18 ± 3 Tsk 34°C vs. Δ17 ± 2 Tsk 30.5°C mmHg; both P > 0.05). In summary, MSNA increased during cold stress in older, but not young, adults. Furthermore, concomitant cold stress did not alter the sympathetic responses to isometric exercise in either age group, suggesting preserved sympathetic responsiveness during exercise in the cold in healthy aging.
Keywords: blood pressure, aging, handgrip, whole body cooling
aging is a primary risk factor for cardiovascular disease, the leading cause of death in the United States (18). Furthermore, cardiovascular-related morbidity and mortality increase at low ambient temperatures, especially in older adults (5, 38, 44, 50, 63). The acute systemic physiological responses to cold exposure include peripheral and visceral vasoconstriction, elevated plasma norepinephrine, and increased blood pressure (BP) (9, 52, 62). Therefore, alterations in the acute responses to cold may contribute to the clinical relation between cold temperatures and increased cardiovascular-related mortality in primary aging. Perturbations that further challenge systemic physiological function during cold exposure, such as static or dynamic exercise, may result in increased sympathetic activation and excessive increases in BP, imparting even greater risk for an acute cardiovascular event in older adults. As such, understanding age-related changes in the acute sympathetic and pressor responses to cold stress is clinically relevant.
Acute responses to moderate cold exposure are altered in older adults (2, 25, 28). Reflex cutaneous vasoconstriction is markedly attenuated in healthy older adults, likely mediated by impairments in both end-organ responsiveness (30, 51, 55) and the thermoregulatory control of skin sympathetic nerve activity (19). In young adults, whole body cold stress elicits a pronounced systemic pressor response (3, 4, 9, 23, 62), and this pressor response to mild cold stress (i.e., with no reduction in core body temperature) is exaggerated in aged humans (23, 60). The age-related augmentation in the pressor response to acute cold stress is associated with increased central arterial stiffness (23) and increased left ventricular preload (60). In addition, sympathetic nervous system activation likely contributes to the cold-induced increase in BP. Cold stress increases plasma norepinephrine (9, 15), yet findings from studies employing direct recordings of muscle sympathetic nerve activity (MSNA) during cold exposure are equivocal in young adults, with reports of increased MSNA during exposure to low ambient temperatures (12) or no change in MSNA during skin surface cooling via a water-perfused suit (3). However, no studies have examined the potential for alterations in the integrated MSNA response to whole body cold stress to contribute to aberrant BP regulation during cold exposure in healthy aging.
Importantly, epidemiological studies suggest that the increased incidence of cardiovascular events in the cold may be attributed, at least in part, to the increased cardiac demands that occur during physical activity in the cold (16). This effect may be mediated via impairments in the sympathetic control of the cardiovascular system and BP regulation during combined cold stress and isometric exercise. At thermoneutral temperatures (∼20–22°C), the increases in MSNA during isometric handgrip (HG) in healthy older adults have been shown to be either similar to (21, 40, 49) or less than (26, 33, 48) the increases observed in young adults. Studies of both young and older subjects exercising in cold environments are surprisingly limited. In young adults, the increases in BP and heart rate during isometric HG were not different in warm (25°C) and cold (10°C) environmental conditions (32). However, this study (32) was not designed to examine the mechanistic role of the sympathetic nervous system in mediating these responses nor to our knowledge have any studies examined the MSNA and BP responses to combined cold stress and exercise in healthy older adults.
Given this background, the purpose of the present study was to directly examine MSNA during whole body cold stress in healthy older men and women (aged 55–75 yr). We tested the hypothesis that cold stress would elicit greater increases in MSNA in older compared with young adults, contributing to an exaggerated pressor response during cooling. A secondary purpose of this investigation was to examine the sympathetic and pressor responses to isometric exercise in older adults at thermoneutrality and during cold stress. Because the cardiovascular demands of combined physical activity and cold stress are much greater than those imposed during exercise performed at neutral temperatures, we further hypothesized that cold stress would augment both the MSNA and BP responses to static HG in older, but not young, adults.
METHODS
Subjects.
All experimental procedures and protocols were approved by The Pennsylvania State University Institutional Review Board. Verbal and written consent were obtained voluntarily from all subjects before participation. The study conformed to the standards outlined in the Declaration of Helsinki. Eleven young (23 ± 1 yr) and 12 older (60 ± 2 yr) adults participated in the study. Subjects were screened for neurological, cardiovascular, and dermatological diseases and underwent a complete medical screening including a resting 12-lead electrocardiogram, physical examination, and 12-h fasting blood chemistry (Quest Diagnostics, Pittsburgh, PA). All subjects were normotensive (resting systolic BP <120 mmHg and diastolic BP <80 mmHg), nondiabetic, normally active, and not taking over-the-counter or prescription medications or supplements with primary or secondary cardiovascular effects (e.g., statins, antihypertensives, anticoagulants, antidepressants, etc). Subjects were nonobese (body mass index <30 kg/m2) and did not use tobacco products. Young women were tested during the early follicular phase of their menstrual cycle or during the placebo phase if using oral contraceptives. Women taking hormone replacement therapy or who had recently taken hormone replacement therapy were excluded. All subjects were familiarized with the equipment and experimental protocol before the testing visit.
Experimental measurements.
Before the experimental session, subjects abstained from eating for 4 h, caffeinated and alcoholic beverages for 12 h, and strenuous physical activity for 24 h. The protocol was performed in a thermoneutral laboratory (22°C).
Whole body mean skin temperature (Tsk) was controlled using a water-perfused suit that covered the entire body except for the head, hands, feet, and lower left leg. Copper-constantan thermocouples were affixed to the surface of the skin at six sites (calf, thigh, abdomen, chest, shoulder, and back), and an unweighted average of these sites was used for continuous measurement of mean Tsk. To obtain an index of cutaneous blood flow, cutaneous red blood cell flux was continuously measured directly on the dorsum of the foot with a laser-Doppler flowmetry probe placed in a local heating unit (Moor-Lab, Temperature Monitor, SHO2; Moor Instruments, Devon, UK). Cutaneous vascular conductance (CVC) was calculated as red blood cell flux divided by mean arterial pressure (MAP). Local Tsk around the laser-Doppler probe was clamped at 33°C throughout baseline and whole body cooling to ensure that changes in cutaneous blood flow were systemic reflex in origin.
Arterial BP was obtained on a beat-to-beat basis using photoplethysmography (Finapres; Finapres Medical Systems, Amsterdam, The Netherlands) from the middle finger of the left hand held at heart level. Automated brachial artery BPs (Cardiocap; GE Healthcare) were measured every 3 min and used to verify absolute finger BP measurements. Heart rate was measured via a single-lead electrocardiogram (Cardiocap; GE Healthcare). Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position over the abdomen (Pneumotrace; UFI, Morro Bay, CA) to ensure that subjects did not inadvertently perform Valsalva maneuvers during the protocol.
Multiunit postganglionic MSNA was recorded using standard microneurographic techniques, as previously described (20, 21, 56). Briefly, a unipolar tungsten microelectrode was inserted percutaneously into muscle fascicles of the peroneal nerve near the fibular head of the left leg. Neural signals were amplified, filtered (bandwidth; 700–2,000 Hz), rectified, and integrated (time constant 0.1 s) to obtain mean voltage neurograms (Nerve Traffic Analyzer; University of Iowa Bioengineering, Iowa City, IA). MSNA recordings were identified by the presence of spontaneously occurring busts with characteristic pulse synchronicity, responsiveness to an end-expiratory breath hold, and lack of response to arousal or skin stimulation. MSNA burst frequency at rest has been demonstrated to be equivalent between contralateral limbs (59) as well as between the arm and leg (47).
Experimental protocol.
All subjects were tested in the supine position. The maximal voluntary contraction (MVC) of the right hand was tested by having subjects squeeze a commercially-available device (ADInstruments, Bella Vista, NSW, Australia) at maximal effort three to five times; the highest value was used as MVC. The MVC was used to calculate the relative work rate of 30% for the experimental protocol. After subject instrumentation and obtainment of a suitable nerve recording, baseline data were collected for 10 min. Throughout baseline, mean Tsk was held at thermoneutral by perfusing ∼32°C water through the suit. Each subject then performed static HG at 30% MVC for 2 min. During HG, ratings of perceived exertion were obtained using the standard 6–20 Borg scale (1). Subjects were provided with visual feedback of force production during exercise. All subjects were able to complete the full 2 min of HG. Force production was recorded to compare the exercise stimulus between groups and conditions. After the HG trial, subjects rested quietly for 15–20 min until MSNA and BP returned to resting baseline values. Thereafter, cool water was perfused through the suit to gradually lower mean Tsk from 34 to 30.5°C (∼30 min). This target mean Tsk is above the threshold for shivering for most adults (6), and shivering was not observed in either subject group in the present study. Because of the brevity of the skin surface cooling protocol, core temperature does not change significantly (25, 55). Mean Tsk was then clamped at 30.5°C, and a second bout of static HG was performed, as described above. During the HG trials, acceptable MSNA recordings were obtained in eight young adults and eight older adults.
Data analysis.
All data were recorded at 1,000 Hz (Powerlab and LabChart; ADInstruments). The MSNA signal was calibrated by assigning the voltage of the three largest bursts during baseline the value of 100 arbitrary units (AU), and all other bursts within a trial were normalized with respect to this value. MSNA was analyzed using a custom-designed LabVIEW program (13, 14), which generated synchronized beat-by-beat data of all recorded variables gated by the R wave of the electrocardiogram. Sympathetic activity was quantified using standard measures, including burst frequency (bursts/min), burst incidence (bursts/100 heartbeats), total MSNA (AU/beat), and total activity (AU/min). However, caution must be used when interpreting differences in indexes of burst area between subject groups. The absolute area of a burst is critically dependent on the location of the microelectrode in relation to the nerve fibers that are being recorded, and because this cannot be determined, direct comparisons of total MSNA and total activity between groups are not typically performed. Multiple indexes of MSNA are presented in an effort to be complete; however, our main study conclusions are based on sympathetic burst frequency, which is reproducible over time in the same subject and comparable between groups (29). Sympathetic and cardiovascular variables were calculated as mean values over an initial 5-min thermoneutral baseline and at each 0.5°C decrease in mean Tsk during whole body cooling (∼1–2 min). For each HG trial, data were averaged during an additional 2 min of baseline before the onset of exercise and during the last minute of static HG.
Arterial baroreflex control of MSNA was evaluated during the initial thermoneutral baseline and also at mean Tsk 30.5°C by analyzing the relation between spontaneously occurring variations in diastolic BP and MSNA, as previously described in detail (21, 22, 42, 53), which allows for the quantification of sympathetic baroreflex sensitivity around the operating point of the arterial baroreflex. This technique is highly correlated with sympathetic baroreflex sensitivity derived using the more invasive modified Oxford approach (i.e., bolus intravenous administration of sodium nitroprusside and phenylephrine) (22). Briefly, the diastolic BP for each cardiac cycle within each analysis region of interest was grouped into 2-mmHg pressure bins. Total MSNA was determined for each pressure bin by calculating the total area of all MSNA bursts, relative to the number of cardiac cycles; burst incidence for each pressure bin was calculated by determining the percentage of heartbeats that were associated with a sympathetic burst. The slope of the relation between MSNA variables and diastolic BP was identified using weighted linear regression analysis. To account for the number of cardiac cycles within each pressure bin, all data were weighted to remove bias due to bins containing fewer cardiac cycles (21, 42). Pressure bins containing zero values for MSNA were included in the linear regression analysis to maintain consistency between conditions and groups. A minimal r value of 0.5 was used as criterion for accepting slopes, which is consistent with previous literature using this analysis technique (21, 42, 43). One young subject and two older subjects did not fit this criterion and were excluded from the analysis. The mean r value of the accepted slopes for the initial baseline was −0.80 ± 0.02 (range, 0.514–0.991) and for mean Tsk 30.5°C was −0.77 ± 0.02 (range, 0.509–0.968).
Statistical analysis.
Subject characteristics were compared using unpaired t-tests (SPSS 19.0). Comparisons of neural cardiovascular variables during whole body cooling and the gain of arterial baroreflex control of MSNA between groups were conducted using two-way mixed model repeated-measures ANOVA. A three-way mixed model repeated-measures ANOVA was conducted to detect temperature and condition (i.e., HG) differences in neural cardiovascular variables between groups. When appropriate, Bonferroni post hoc comparisons were performed and corrected for multiple comparisons. Results are reported as means ± SE, and the alpha level was set at P < 0.05.
RESULTS
Subject characteristics.
Subject characteristics are presented in Table 1. The groups were well-matched for anthropometric characteristics, resting BP, and blood biochemistry. Body mass index (BMI) was greater in older adults. Fasting total cholesterol and low-density lipoproteins, as well as HbA1c, were elevated in older compared with young adults; however, these values were within the normal recommended ranges for all older subjects. As expected, MSNA at rest was significantly elevated in older adults (P < 0.05).
Table 1.
Subject characteristics
| Baseline Characteristic | Young | Older |
|---|---|---|
| n (M/F) | 11 (6/5) | 12 (7/5) |
| Age, yr | 23 ± 1 | 60 ± 2 |
| Height, cm | 175 ± 4 | 174 ± 3 |
| Mass, kg, | 71 ± 3 | 81 ± 4 |
| BMI, kg/m2 | 23.0 ± 0.8 | 26.2 ± 0.7* |
| Systolic BP, mmHg | 112 ± 2 | 117 ± 2 |
| Diastolic BP, mmHg | 71 ± 2 | 70 ± 2 |
| Heart rate, beats/min | 60 ± 2 | 62 ± 2 |
| MSNA burst frequency, bursts/min | 12 ± 2 | 30 ± 2* |
| MSNA burst incidence, bursts/100 heartbeats | 22 ± 3 | 51 ± 4* |
| Blood biochemistry | ||
| Hemoglobin, g/dl | 14.3 ± 0.4 | 14.2 ± 0.2 |
| Hematocrit, % | 41.9 ± 1.3 | 41.8 ± 0.8 |
| Serum sodium, mmol/l | 139.0 ± 0.5 | 139.5 ± 0.6 |
| Serum potassium, mmol/l | 4.2 ± 0.1 | 4.5 ± 0.1 |
| Serum chloride, mmol/l | 105.5 ± 0.7 | 105.2 ± 0.5 |
| Serum creatinine, mg/dl | 1.0 ± 0.1 | 0.9 ± 0.04 |
| HbA1c, % | 5.1 ± 0.1 | 5.5 ± 0.1* |
| Fasting total cholesterol, mg/dl | 151.5 ± 8.3 | 193.3 ± 8.6* |
| Fasting HDL, mg/dl | 60.5 ± 3.7 | 63.5 ± 6.7 |
| Fasting LDL, mg/dl | 79.1 ± 9.5 | 107.5 ± 8.0* |
| Fasting triglycerides, mg/dl | 92.4 ± 12.9 | 114.8 ± 9.4 |
Values are means ± SE.
M/F, male/female; BMI, body mass index; BP, blood pressure; MSNA, muscle sympathetic nerve activity; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < 0.05 vs. young.
Sympathetic and cardiovascular responses to whole body cooling.
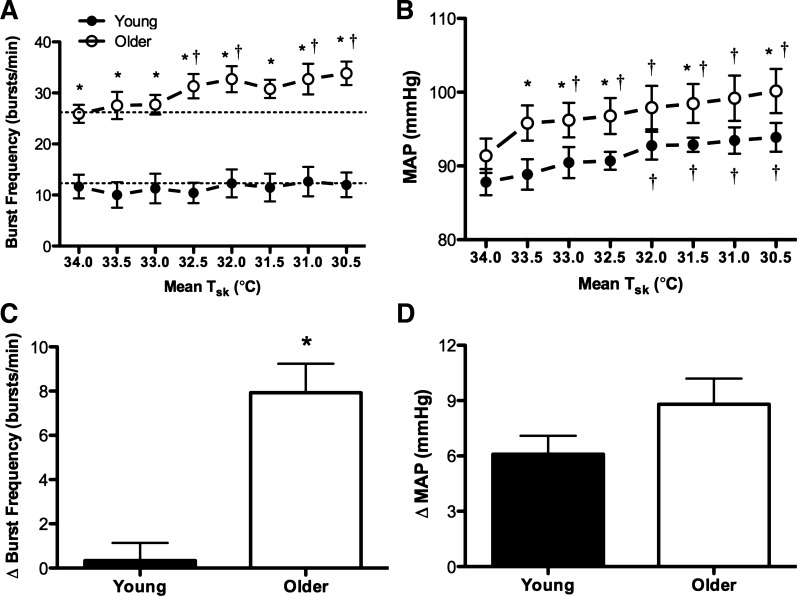
Baseline mean Tsk was not different between groups (young: 34.5 ± 0.2 vs. older: 34.2 ± 0.1°C; P > 0.05). By study design, whole body cooling decreased mean Tsk to 30.5°C in all subjects. Reflex cutaneous vasoconstriction during whole body cooling was blunted in older adults (young: ΔCVC −0.046 ± 0.01 vs. older: ΔCVC −0.013 ± 0.004 flux·mmHg−1; young: %ΔCVCbase −36.7 ± 5 vs. older: %ΔCVCbase −12.1 ± 3.8 flux·mmHg−1; P < 0.05 for both). Original recordings of heart rate, BP, and MSNA at mean Tsk 34.0°C and Tsk 30.5°C in one young and one older adult are presented in Figure 1. Group summary data for absolute MSNA are presented in both Fig. 2 and Table 2. MSNA burst frequency was greater in older adults at Tsk 34.0°C and at each 0.5°C decrease in mean Tsk throughout the protocol (Fig. 2A). Additionally, MSNA increased during cold stress in older but not young adults (Fig. 2, A and C; Table 2). MAP increased during cooling in both groups (Fig. 2B). By study design, BP was not different between the groups at baseline, but absolute MAP was greater in older adults throughout cooling (Fig. 2). The increases from baseline in both MAP (young: Δ6 ± 1 vs. older: Δ9 ± 1 mmHg; P = 0.14; Fig. 2D) and systolic BP (young: Δ5 ± 2 vs. older: Δ14 ± 2 mmHg; Table 2) were exaggerated in older adults. Interestingly, the threshold at which MAP began to increase relative to baseline occurred at a higher mean Tsk (33.0°C) in older adults (Fig. 2B). Similar findings were noted for systolic BP (Table 2). The heart rate response to whole body cooling was not different between groups (Table 2).
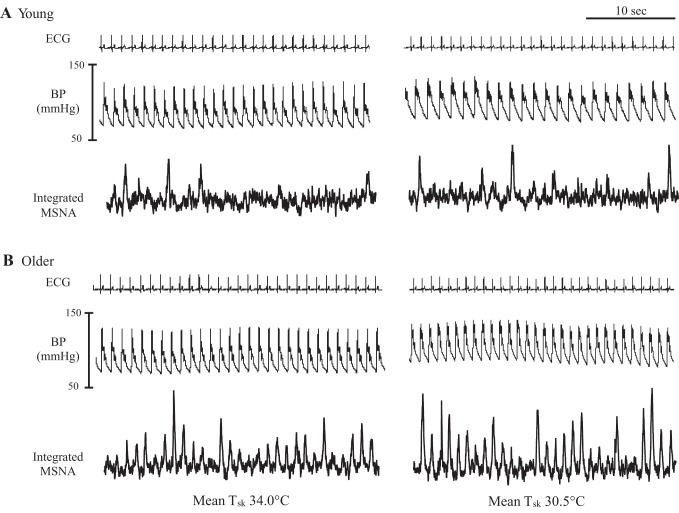
Fig. 1.
Segments of original records showing heart rate, blood pressure (BP), and muscle sympathetic nerve activity (MSNA) at mean skin temperature (Tsk) 34°C and Tsk 30.5°C in a young (A) and older subject (B). Older adults exhibited a robust increase in MSNA during whole body cold stress.
Fig. 2.
Group summary data for absolute muscle sympathetic nerve activity (MSNA; burst frequency; ANOVA: condition P < 0.01, group P < 0.01, and interaction P < 0.01) and mean arterial pressure (MAP; ANOVA: condition P < 0.01, group P < 0.05, and interaction P = 0.835) at each 0.5°C decrease in mean Tsk are shown in A and B. The increases (delta value) in MSNA and MAP from baseline (i.e., mean Tsk 34°C) to mean Tsk 30.5°C are shown in C and D. ●, Young adults; ○, older adults. *P < 0.05 vs. young; †P < 0.05 vs. mean Tsk 34°C.
Table 2.
Cardiovascular and sympathetic responses to whole body cooling
| Mean Tsk 34.0°C | Mean Tsk 30.5°C | Δ | |
|---|---|---|---|
| Systolic BP, mmHg | |||
| Young | 120 ± 3 | 125 ± 3† | 5 ± 2 |
| Older | 122 ± 3 | 136 ± 4*† | 14 ± 2* |
| Diastolic BP, mmHg | |||
| Young | 72 ± 2 | 79 ± 2† | 7 ± 1 |
| Older | 76 ± 2 | 81 ± 3† | 6 ± 1 |
| Heart rate, beats/min | |||
| Young | 59 ± 2 | 59 ± 3 | 0 ± 2 |
| Older | 59 ± 2 | 59 ± 2 | 0 ± 1 |
| Burst incidence, bursts/100 beats | |||
| Young | 21 ± 5 | 22 ± 5 | 1 ± 2 |
| Older | 46 ± 4* | 58 ± 4*† | 12 ± 2* |
| Total MSNA, AU/beat | |||
| Young | 15.1 ± 2.5 | 15.0 ± 2.4 | −0.1 ± 0.8 |
| Older | 28.6 ± 2.3* | 39.9 ± 3.3*† | 11.4 ± 2.7* |
| Total activity, AU/min | |||
| Young | 840 ± 124 | 828 ± 118 | −12 ± 51 |
| Older | 1,623 ± 115* | 2,459 ± 186*† | 836 ± 161* |
Values are means ± SE.
Tsk, skin temperature; AU, arbitrary units.
P < 0.05 vs. young;
P < 0.05 vs. mean Tsk 34.0°C.
The slope of the relation between MSNA burst incidence and diastolic BP did not change during cold stress in either subject group (young: −3.2 ± 0.5 Tsk 34.0°C vs. −4.3 ± 0.8 Tsk 30.5°C bursts·100 heartbeats−1·mmHg−1; older: −5.0 ± 0.8 Tsk 34.0°C vs. −5.0 ± 0.5 Tsk 30.5°C bursts·100 heartbeats−1·mmHg−1; P > 0.05 for all comparisons). Similarly, the slope of the relation between total MSNA and diastolic BP was not different during whole body cooling in either young or older adults (young: −2.5 ± 0.4 Tsk 34.0°C vs. −3.7 ± 0.7 Tsk 30.5°C AU·beat−1·mmHg−1; older: −3.7 ± 0.6 Tsk 34.0°C vs. −4.1 ± 0.3 Tsk 30.5°C AU·beat−1·mmHg−1; P > 0.05 for all comparisons). There were no group differences in sympathetic baroreflex sensitivity for either condition (P > 0.05 for both). From mean Tsk 34.0°C to Tsk 30.5°C, the linear relation between MSNA and diastolic BP was shifted rightwards in young subjects, indicating a resetting of arterial baroreflex control of MSNA to the higher BP elicited by whole body cold stress; in older subjects, this relation shifted rightwards and upwards to account for the cold-induced increases in both MSNA and BP.
Sympathetic and cardiovascular responses to combined cold stress and isometric exercise.
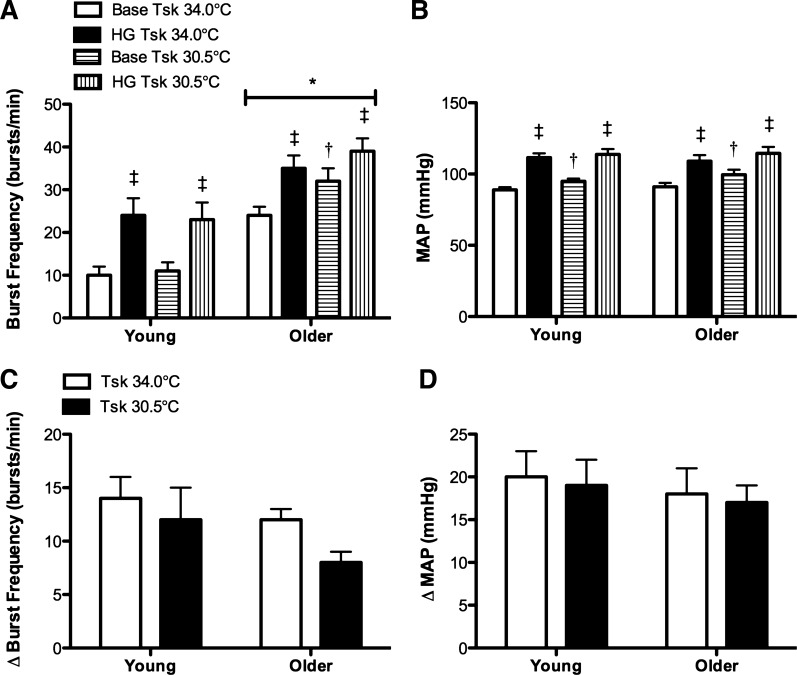
MSNA was greater in older adults at baseline and during HG at both Tsk 34.0 and 30.5°C (Fig. 3A). There was no age-related difference in the magnitude of the increase in MSNA during static HG at either mean Tsk 34.0°C or Tsk 30.5°C (Fig. 3, A and C; Table 3). Furthermore, contrary to our original hypothesis, whole body cooling did not affect the MSNA response to isometric HG in either group (Fig. 3, A and C; Table 3).
Fig. 3.
Group summary data for absolute MSNA (burst frequency; ANOVA: condition P < 0.01, group P < 0.01, and interaction P = 0.194) and MAP (ANOVA: condition P < 0.01, group P = 0.552, and interaction P = 0.679) during baseline (base) and during the last minute of moderate intensity static handgrip (HG) at mean Tsk 34°C and Tsk 30.5°C in young and older adults are shown in A and B. The increases (delta value) in MSNA (ANOVA: condition P = 0.10, group P = 0.179, and interaction P = 0.178) and MAP (ANOVA: condition P = 0.470, group P = 0.705, and interaction P = 0.329) from baseline to the last minute of static HG are presented in C and D. *P < 0.05 vs. young; †P < 0.05 vs. mean Tsk 34°C; ‡P < 0.05 vs. base.
Table 3.
Cardiovascular and sympathetic responses to static handgrip
|
P Values |
|||||
|---|---|---|---|---|---|
| Mean Tsk 34.0°C | Mean Tsk 30.5°C | Group | Condition | Interaction | |
| ΔSystolic BP, mmHg | |||||
| Young | 29 ± 4 | 22 ± 4 | 0.599 | 0.249 | 0.436 |
| Older | 24 ± 5 | 23 ± 3 | |||
| ΔDiastolic BP, mmHg | |||||
| Young | 17 ± 2 | 17 ± 3 | 0.187 | 0.835 | 0.328 |
| Older | 15 ± 3 | 13 ± 2 | |||
| ΔHeart rate, beats/min | |||||
| Young | 11 ± 3 | 11 ± 2 | 0.143 | 0.882 | 0.995 |
| Older | 7 ± 1 | 7 ± 2 | |||
| ΔBurst incidence, bursts/100 beats | |||||
| Young | 16 ± 2 | 13 ± 3 | 0.077 | 0.158 | 0.293 |
| Older | 11 ± 2 | 7 ± 3 | |||
| ΔTotal MSNA, AU/beat | |||||
| Young | 12.7 ± 2.8 | 18.1 ± 4.8 | 0.328 | 0.618 | 0.040 |
| Older | 14.2 ± 3.9 | 10.4 ± 1.9* | |||
| ΔTotal activity, AU/min | |||||
| Young | 1,136 ± 252 | 1,458 ± 457 | 0.426 | 0.843 | 0.067 |
| Older | 1,216 ± 273 | 904 ± 121 | |||
Values are means ± SE.
P < 0.05 vs. young.
The BP and heart rate responses to exercise are presented in Fig. 3 and Table 3. There were no group differences in absolute MAP at baseline or during HG at Tsk 34.0°C and Tsk 30.5°C (Fig. 3B). Similar to the MSNA responses to exercise, there were no group differences in the increase in BP during isometric HG during either thermoneutral or cold stress conditions (Fig. 3, B and D; Table 3). In addition, there were no temperature-mediated differences in the pressor response to exercise in either group (Fig. 3, B and D; Table 3). There were no group differences in the heart rate responses to cold stress or HG (Table 3). MVC was not different between groups (young: 432 ± 53 vs. older: 350 ± 43 Newtons; P > 0.05). Reported rates of perceived exertion during static HG were not different between groups or conditions (young: 16 ± 1 Tsk 34.0 vs. 17 ± 1 Tsk 30.5 Borg units; older: 15 ± 1 Tsk 34.0 vs. 16 ± 1 Tsk 30.5 Borg units; P > 0.05 for all comparisons).
DISCUSSION
The principle novel finding of the current study was that the MSNA response to acute whole body cold stress is augmented in healthy older adults. However, contrary to our initial hypothesis, neither the MSNA nor the BP responses to static HG during cold stress were exaggerated in older adults, suggesting that sympathetic responsiveness during isometric exercise is preserved in healthy aging. Collectively, these data indicate that marked increases in sympathetic outflow likely contribute to the greater increases in arterial BP during cold exposure in the older population. Alterations in the acute sympathetic and pressor responses to cold may contribute to the clinical relation between cold temperatures and increased cardiovascular mortality in primary aging.
Limited reports in young adults have demonstrated either no change (3) or increases (12) in MSNA during cold exposure elicited via a whole body water-perfused suit and facial cooling during exposure to low ambient temperatures, respectively. Although the reason for these disparate findings remains unclear, differences may be related to varied methodology of the cooling perturbation, because the intensity and populations of thermal and/or pain receptors stimulated by the cooling paradigm are known to influence sympathetic outflow. To this end, the present investigation examined sympathetic nervous system activation in healthy young and older adults using a standardized whole body skin surface cooling protocol via water-perfused suit (3, 30, 51, 55). Consistent with a wide body of literature examining MSNA at rest in older adults at thermoneutrality (27, 39, 53), older adults in the present study exhibited increases in basal muscle sympathetic outflow. Furthermore, whole body cold stress elicited pronounced increases in MSNA burst frequency and strength in older, but not young, adults. One previous study examined cold-induced increases in plasma norepinephrine in young and older adults, reporting blunted increases in plasma norepinephrine in older adults during reductions in core body temperature elicited via cold intravenous saline infusion (15). Importantly, the current study employed a direct measure of central sympathetic outflow (MSNA), whereas plasma norepinephrine measures are indirect indicators of sympathetic activity and depend on the rate of removal of norepinephrine in addition to sympathetic outflow and norepinephrine release. Moreover, because we were interested in the precise effect of primary aging on the neurocirculatory responses to cold exposure, we used a milder cold stress (i.e., no change in core temperature and designed not to induce shivering) to isolate a vasoconstrictor response. Exposure to this magnitude of cooling is likely more indicative of the typical cold stress experienced by older adults (23) and may therefore have greater clinical relevance.
Studies in both animal models and humans indicate an age-related impairment of norepinephrine-mediated vasoconstriction in multiple vascular beds, including muscle and cutaneous thermoregulatory circulations (8, 24, 41, 54, 58). MSNA and plasma norepinephrine concentrations are elevated with advancing age (15, 24, 27, 39, 53); therefore, impairments in norepinephrine-mediated vasoconstriction have generally been attributed to sustained agonist-mediated desensitization in α-adrenergic receptor responsiveness (24). Conversely, the age-related increase in MSNA during cooling may be a compensatory response as a result of age-associated reductions in α-adrenergic receptor responsiveness. Intriguingly, studies also suggest an age-related impairment in tonic nitric oxide “buffering” of α2-adrenergic receptor responsiveness (31) or perhaps diminished α2-adrenergic receptor responsiveness reflective of more generalized reductions in nitric oxide bioavailability with aging (8). Furthermore, while the sympathetic cotransmitters NPY and ATP contribute to reflex cutaneous vasoconstriction in young adults, these mechanisms are functionally absent in healthy aging; therefore, older adults instead rely entirely on impaired adrenergic-mediated vasoconstriction during cold stress (55). Clearly, the interaction between sympathetic outflow and adrenergic receptor function during whole body cooling in the context of healthy aging requires further investigation.
Whole body cold stress elicits a robust systemic pressor response (3, 4, 9, 23, 62) that is exaggerated in aged adults, evidenced by this and other (23, 60) studies. The augmented pressor response to skin surface cooling in older adults is likely not mediated by an increase in cardiac output, as cardiac output derived via transthoracic echocardiography remains unchanged throughout cooling in older adults (60). The results of the present study indicate that pronounced increases in MSNA during cold stress in older adults may contribute to the greater pressor response. Indeed, the exaggerated increases in sympathetic outflow noted in the present study were accompanied by greater increases in BP throughout cold exposure, presumably by increases in systemic vascular resistance. Importantly, whole body cooling elicits significant visceral (celiac, superior mesenteric, and renal vascular conductance indexes) and peripheral (brachial artery, total forearm, and CVC) vasoconstriction that contributes to sustained increases in BP throughout cooling in young adults (62). Reflex peripheral cutaneous vasoconstriction is significantly impaired in aged adults (30, 51, 55). While the visceral vasoconstrictor responses to cold stress have not been examined in older adults, it is interesting to note that the splanchnic vasoconstrictor responses to upright tilt are greater in older compared with young subjects (36). Conceivably, greater visceral vasoconstriction during cold exposure, concomitant with greater increases in sympathetic outflow, may further contribute to augmented pressor responses in older adults and warrants further investigation.
The age-related alterations in the acute responses to cold stress observed in the present study may underlie increased cardiovascular risk in older adults exposed to cold environments (5, 38, 44, 50, 63). Because isometric exercise is accompanied by robust increases in MSNA and BP (34, 37), and coupled with the noted impairments in the sympathetic and pressor responses to cooling, we reasoned that physical exertion in the cold may impart greater challenges to sympathetic control of the cardiovascular system compared with cold exposure alone. Importantly, many physical activities of daily living for healthy older adults that may occur in the cold have brief and submaximal components in the forearm musculature (e.g., gripping snow shovels, scraping ice, etc.). While these brief bouts of isometric contractions are likely occurring in the context of dynamic whole body exercise, they presumably result in transient increases in MSNA and BP, and these repeated responses may increase both short- and long-term cardiovascular risk (45). Therefore, understanding the acute physiological responses to isometric forearm muscle contractions in the cold is clinically relevant. Studies of adults exercising in cold environments are few and generally limited to those using varied methodology exploring either the control of cutaneous blood flow during exercise (46) or the performance-related effects of cold acclimation (17, 57, 61). At thermoneutral temperatures, older adults exhibit either similar (21, 40, 49) or blunted (26, 33, 48) increases in MSNA and BP during static HG compared with the increases demonstrated in young adults. Consistent with these previous studies, we report no age-related differences in the sympathetic and pressor responses to static HG at baseline (i.e., mean Tsk 34.0°C).
To our knowledge, only one previous study has examined cardiovascular function during combined isometric exercise and whole body cold exposure in young adults (32). These authors reported that the increases in BP and heart rate during isometric HG were not different in warm (25°C) and cold (10°C) ambient environmental conditions (32). The results of the present investigation are similar, as the increases in BP during HG were not different during combined cold stress in young adults. Furthermore, we extend the findings of this previous report by demonstrating that increases in MSNA during isometric HG in the cold are not different from the responses at thermoneutral temperatures in young healthy adults. Moreover, our novel findings also demonstrate that there are no temperate-related differences in the sympathetic and pressor responses to isometric exercise in healthy older adults. Interestingly, there also were no age-related differences in the pressor or sympathetic burst frequency responses to HG in the cold. Although the increase in indexes of MSNA burst area during HG in the cold condition were less in the older adults, perhaps suggesting blunted sympathetic activation during combined isometric exercise and cold stress, when considered collectively with burst frequency, the sympathetic responses are clearly not augmented in older adults. Nevertheless, our finding of no difference in the sympathetic and pressor responses to HG between temperature conditions in the older subjects, which was contrary to our initial hypothesis, instead suggests preserved sympathetic responsiveness during exercise in the cold in healthy aging. These results were somewhat surprising given the clear increases in sympathetic outflow during cooling. However, it is plausible that higher absolute MSNA may impose a ceiling effect for further increasing MSNA during exercise, especially as some older adults approached maximal burst frequency during exercise in the cold. Previous studies (7) of normotensive older adults with basal MSNA similar to that reported in the older adults during cooling in this study (i.e., ∼31 bursts/min) have demonstrated increases in MSNA during sympathoexcitatory maneuvers above those which were noted during combined exercise and cold stress in this investigation. In addition to the aforementioned ceiling effect, similar sympathetic and pressor responses to HG in the cold in older adults may also reflect preserved buffering capacity of the arterial baroreflex during exercise. However, it is important to note that this study was not designed to investigate specific reflex mechanisms governing BP regulation during exercise (e.g., exercise pressor reflex, baroreflex, etc.). Future studies more directly assessing the contribution of alterations in these individual reflex mechanisms are necessary.
The baroreceptors operate as a negative feedback control system that responds to beat-to-beat changes in BP by reflexively adjusting autonomic outflow to modulate cardiac output and systemic vascular resistance. Therefore, the baroreflex is critically important in rapid reflex adjustments that accompany acute cardiovascular stressors (10), such as whole body cooling and isometric exercise. Interestingly, in young adults, the sensitivity of arterial baroreflex control of MSNA (i.e., the slope of the relation between MSNA and diastolic BP) assessed during pharamacological manipulations in BP was not altered during whole body cooling (3). However, the operating point of the baroreflex curve, defined as the mean MSNA and BP for both thermoneutral and cold conditions, was shifted rightward to operate around the cooling-induced increase in BP (3). Presumably, this shift allows adequate baroreflex-mediated buffering if BP was further increased during cold stress. The current study was not intended to examine age-related differences in baroreflex function, at rest or during exercise, during cold stress. However, it is plausible that potential age-related impairments in baroreflex function could result in a reduced ability to appropriately buffer increases in MSNA and BP during cooling, resulting in the exaggerated neurocirculatory responses to cold stress noted in the present study. To begin to address this possibility, we quantified sympathetic baroreflex sensitivity around the operating point of the arterial baroreflex by analyzing the relation between spontaneously occurring variations in diastolic BP and MSNA. Similar to the previous report in young adults (3), there was no change in the gain of arterial baroreflex control of MSNA during whole body cooling, for either burst incidence or total MSNA, in older adults, which is indicative of preserved sympathetic baroreflex function during cold exposure in healthy aging. However, future studies including a more robust investigation of baroreflex function are warranted, both during cold exposure alone and also in combination with exercise, to more appropriately examine potential age- or temperature-related alterations in the role of the arterial baroreflex in contributing to neurocirculatory modulation.
Limitations.
By study design, only apparently healthy older adults were included in this study, and all older subjects were normotensive, nondiabetic, and nonobese. Despite the strict study inclusion criteria, older adults still exhibited robust increases in MSNA and BP during whole body cold stress. Importantly, these exaggerated responses to cold exposure occur even in the absence of overt cardiovascular disease. In addition, no direct quantification of muscle mass was obtained in subjects participating in this study. Potential age-related differences in body composition may influence the vasoconstrictor responses to cold exposure. Importantly, the mean age of the older subjects was ∼60 yr; therefore, because sarcopenia would likely be a much larger potential confounding factor to consider in an elderly population (35), decrements in muscle mass likely minimally affected muscle vascular resistance in this group of healthy older adults. However, the acute sympathetic and pressor responses to whole body cooling remain to be examined in at-risk populations with underlying cardiovascular pathology, including the elderly.
It is important to note that no quantification of cold or pain perception was obtained in the present study. In regards to the relation between cold-induced pain and the stimulation of MSNA, there appears to be a weak, but statistically significant, correlation between ratings of perceived pain and the increase in MSNA during a 1-min cold pressor test (11). These findings may reflect specificity for reflex stimulation of MSNA within the broad range of cutaneous nociceptive fibers. We are unaware of any reports examining age-related changes in cold-sensitive afferent fibers. Nevertheless, for a given decrease in mean Tsk (i.e., 34 to 30.5°C), older adults clearly demonstrated robust increases in MSNA. We acknowledge that the observed responses may be specific to the cooling paradigm utilized and results may differ if the methodology for cooling is altered. Lastly, it is important to note that most physical activity in the cold involves dynamic whole body exercise that employs larger muscle groups (e.g., shoveling snow), and therefore, conclusions regarding exercise in the cold from the present study are limited to brief submaximal isometric forearm contractions. Potential age-related differences in the responses to dynamic exercise remain to be prospectively examined.
Perspectives and Significance
In conclusion, the primary finding of this study was that acute whole body cold stress increased MSNA in older, but not young, adults. Secondly, in both young and older adults, the sympathetic and pressor responses to combined cold stress and isometric exercise were not different from the responses to exercise during a thermoneutral condition. This finding was contrary to our initial hypothesis that cold stress would augment the responses to isometric HG in older adults and instead indicates that older adults do not exhibit exaggerated sympathetic responsiveness to cardiovascular challenges in cold environments. Taken together, these findings have significant clinical implications and provide further insight into the mechanisms underlying the association between cold temperature and increased cardiovascular mortality in healthy aging.
GRANTS
This research was supported by National Institutes of Health Grants HL-120471-01 (to J. L. Greaney), AG-007004-23 (to W. L. Kenney), and HL-093-238-04 (to L. M. Alexander).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.G., W.L.K., and L.M.A. conception and design of research; J.L.G. and A.E.S. performed experiments; J.L.G. analyzed data; J.L.G., A.E.S., W.L.K., and L.M.A. interpreted results of experiments; J.L.G. prepared figures; J.L.G. drafted manuscript; J.L.G., A.E.S., W.L.K., and L.M.A. edited and revised manuscript; J.L.G., A.E.S., W.L.K., and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The time and effort expended by all the volunteer subjects are greatly appreciated. We thank Dr. Paul J. Fadel for the use of the analysis program. We are grateful for the assistance of Jessica L. Kutz, Susan Slimak, and Jane Pierzga.
REFERENCES
- 1.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982 [PubMed] [Google Scholar]
- 2.Brody GM. Hyperthermia and hypothermia in the elderly. Clin Geriatr Med 10: 213–229, 1994 [PubMed] [Google Scholar]
- 3.Cui J, Durand S, Crandall CG. Baroreflex control of muscle sympathetic nerve activity during skin surface cooling. J Appl Physiol (1985) 103: 1284–1289, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Cui J, Durand S, Levine BD, Crandall CG. Effect of skin surface cooling on central venous pressure during orthostatic challenge. Am J Physiol Heart Circ Physiol 289: H2429–H2433, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Danet S, Richard F, Montaye M, Beauchant S, Lemaire B, Graux C, Cottel D, Marecaux N, Amouyel P. Unhealthy effects of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary deaths. A 10-year survey: the Lille-World Health Organization MONICA project (Monitoring trends and determinants in cardiovascular disease). Circulation 100: E1–7, 1999 [DOI] [PubMed] [Google Scholar]
- 6.DeGroot DW, Havenith G, Kenney WL. Responses to mild cold stress are predicted by different individual characteristics in young and older subjects. J Appl Physiol (1985) 101: 1607–1615, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Durand S, Cui J, Williams KD, Crandall CG. Skin surface cooling improves orthostatic tolerance in normothermic individuals. Am J Physiol Regul Integr Comp Physiol 286: R199–R205, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97: 39–50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagius J, Karhuvaara S, Sundlof G. The cold pressor test: effects on sympathetic nerve activity in human muscle and skin nerve fascicles. Acta Physiol Scand 137: 325–334, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Fagius J, Kay R. Low ambient temperature increases baroreflex-governed sympathetic outflow to muscle vessels in humans. Acta Physiol Scand 142: 201–209, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairfax ST, Padilla J, Vianna LC, Holwerda SH, Davis MJ, Fadel PJ. Influence of spontaneously occurring bursts of muscle sympathetic nerve activity on conduit artery diameter. Am J Physiol Heart Circ Physiol 305: H867–H874, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank SM, Raja SN, Bulcao C, Goldstein DS. Age-related thermoregulatory differences during core cooling in humans. Am J Physiol Regul Integr Comp Physiol 279: R349–R354, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Franklin BA, Hogan P, Bonzheim K, Bakalyar D, Terrien E, Gordon S, Timmis GC. Cardiac demands of heavy snow shoveling. JAMA 273: 880–882, 1995 [PubMed] [Google Scholar]
- 17.Galoza P, Sampaio-Jorge F, Machado M, Fonseca R, Silva PA. Resistance exercise inter-set cooling strategy: effect on performance and muscle damage. Int J Sports Physiol Perform 6: 580–584, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129: e28–e292, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassi G, Seravalle G, Turri C, Bertinieri G, Dell′Oro R, Mancia G. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation 108: 729–735, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL, Farquhar WB. Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: role of purinergic receptors. Am J Physiol Heart Circ Physiol 306: H132–H141, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Greaney JL, Schwartz CE, Edwards DG, Fadel PJ, Farquhar WB. The neural interaction between the arterial baroreflex and muscle metaboreflex is preserved in older men. Exp Physiol 98: 1422–1431, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol 298: H816–H822, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol (1985) 107: 1076–1082, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogikyan RV, Supiano MA. Arterial α-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol Endocrinol Metab 266: E717–E724, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol (1985) 109: 1538–1544, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houssiere A, Najem B, Pathak A, Xhaet O, Naeije R, Van De Borne P. Chemoreflex and metaboreflex responses to static hypoxic exercise in aging humans. Med Sci Sports Exerc 38: 305–312, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Iwase S, Mano T, Watanabe T, Saito M, Kobayashi F. Age-related changes of sympathetic outflow to muscles in humans. J Gerontol 46: M1–5, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Kenney WL, Munce TA. Invited review: aging and human temperature regulation. J Appl Physiol (1985) 95: 2598–2603, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang JA, Jennings JD, Holowatz LA, Kenney WL. Reflex vasoconstriction in aged human skin increasingly relies on Rho kinase-dependent mechanisms during whole body cooling. Am J Physiol Heart Circ Physiol 297: H1792–H1797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lembo G, Iaccarino G, Vecchione C, Barbato E, Izzo R, Fontana D, Trimarco B. Insulin modulation of an endothelial nitric oxide component present in the alpha2- and beta-adrenergic responses in human forearm. J Clin Invest 100: 2007–2014, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makinen TM, Mantysaari M, Paakkonen T, Jokelainen J, Palinkas LA, Hassi J, Leppaluoto J, Tahvanainen K, Rintamaki H. Autonomic nervous function during whole body cold exposure before and after cold acclimation. Aviat Space Environ Med 79: 875–882, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Markel TA, Daley JC, 3rd, Hogeman CS, Herr MD, Khan MH, Gray KS, Kunselman AR, Sinoway LI. Aging and the exercise pressor reflex in humans. Circulation 107: 675–678, 2003 [DOI] [PubMed] [Google Scholar]
- 34.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melton LJ, 3rd, Khosla S, Riggs BL. Epidemiology of sarcopenia. Mayo Clinic Proc 75, Suppl: S10–12; discussion S12–13, 2000 [PubMed] [Google Scholar]
- 36.Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol Regul Integr Comp Physiol 276: R203–R212, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Mitchell JH. J.B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc 22: 141–154, 1990 [PubMed] [Google Scholar]
- 38.Morabito M, Modesti PA, Cecchi L, Crisci A, Orlandini S, Maracchi G, Gensini GF. Relationships between weather and myocardial infarction: a biometeorological approach. Int J Cardiol 105: 288–293, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol Heart Circ Physiol 267: H344–H353, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Nielsen H, Hasenkam JM, Pilegaard HK, Aalkjaer C, Mortensen FV. Age-dependent changes in α-adrenoceptor-mediated contractility of isolated human resistance arteries. Am J Physiol Heart Circ Physiol 263: H1190–H1196, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H2202–H2209, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Ogoh S, Fisher JP, Young CN, Raven PB, Fadel PJ. Transfer function characteristics of the neural and peripheral arterial baroreflex arcs at rest and during postexercise muscle ischemia in humans. Am J Physiol Heart Circ Physiol 296: H1416–H1424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panagiotakos DB, Chrysohoou C, Pitsavos C, Nastos P, Anadiotis A, Tentolouris C, Stefanadis C, Toutouzas P, Paliatsos A. Climatological variations in daily hospital admissions for acute coronary syndromes. Int J Cardiol 94: 229–233, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Parati G, Ochoa JE, Bilo G. Blood pressure variability, cardiovascular risk, and risk for renal disease progression. Curr Hypertens Rep 14: 421–431, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Pergola PE, Johnson JM, Kellogg DL, Jr, Kosiba WA. Control of skin blood flow by whole body and local skin cooling in exercising humans. Am J Physiol Heart Circ Physiol 270: H208–H215, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol (1985) 66: 2778–2781, 1989 [DOI] [PubMed] [Google Scholar]
- 48.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol 528: 407–417, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seals DR, Taylor JA, Ng AV, Esler MD. Exercise and aging: autonomic control of the circulation. Med Sci Sports Exerc 26: 568–576, 1994 [PubMed] [Google Scholar]
- 50.Sheth T, Nair C, Muller J, Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol 33: 1916–1919, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin augments reflex vasoconstriction in aged human skin through noradrenergic mechanisms. J Appl Physiol (1985) 115: 1025–1031, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stocks JM, Taylor NA, Tipton MJ, Greenleaf JE. Human physiological responses to cold exposure. Aviat Space Environ Med 75: 444–457, 2004 [PubMed] [Google Scholar]
- 53.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson CS, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am J Physiol Regul Integr Comp Physiol 288: R1108–R1113, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol 558: 697–704, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 57.Vieira A, Oliveira AB, Costa JR, Herrera E, Salvini TF. Cold modalities with different thermodynamic properties have similar effects on muscular performance and activation. Int J Sports Med 34: 873–880, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Vila E, Vivas NM, Tabernero A, Giraldo J, Arribas SM. Alpha 1-adrenoceptor vasoconstriction in the tail artery during ageing. Br J Pharmacol 121: 1017–1023, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallin BG, Burke D, Gandevia S. Coupling between variations in strength and baroreflex latency of sympathetic discharges in human muscle nerves. J Physiol 474: 331–338, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson TE, Gao Z, Hess KL, Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol 298: R1627–R1633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson TE, Johnson SC, Petajan JH, Davis SL, Gappmaier E, Luetkemeier MJ, White AT. Thermal regulatory responses to submaximal cycling following lower-body cooling in humans. Eur J Appl Physiol 88: 67–75, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Wilson TE, Sauder CL, Kearney ML, Kuipers NT, Leuenberger UA, Monahan KD, Ray CA. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol (1985) 103: 1257–1262, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Wolf K, Schneider A, Breitner S, von Klot S, Meisinger C, Cyrys J, Hymer H, Wichmann HE, Peters A. Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation 120: 735–742, 2009 [DOI] [PubMed] [Google Scholar]