Abstract
Age is known to induce remodeling and stiffening of large-conduit arteries; however, little is known of the effects of age on remodeling and mechanical properties of coronary resistance arteries. We employed a rat model of aging to investigate whether 1) age increases wall thickness and stiffness of coronary resistance arteries, and 2) exercise training reverses putative age-induced increases in wall thickness and stiffness of coronary resistance arteries. Young (4 mo) and old (21 mo) Fischer 344 rats remained sedentary or underwent 10 wk of treadmill exercise training. Coronary resistance arteries were isolated for determination of wall-to-lumen ratio, effective elastic modulus, and active and passive responses to changes in intraluminal pressure. Elastin and collagen content of the vascular wall were assessed histologically. Wall-to-lumen ratio increased with age, but this increase was reversed by exercise training. In contrast, age reduced stiffness, and exercise training increased stiffness in coronary resistance arteries from old rats. Myogenic responsiveness was reduced with age and restored by exercise training. Collagen-to-elastin ratio (C/E) of the wall did not change with age and was reduced with exercise training in arteries from old rats. Thus age induces hypertrophic remodeling of the vessel wall and reduces the stiffness and myogenic function of coronary resistance arteries. Exercise training reduces wall-to-lumen ratio, increases wall stiffness, and restores myogenic function in aged coronary resistance arteries. The restorative effect of exercise training on myogenic function of coronary resistance arteries may be due to both changes in vascular smooth muscle phenotype and expression of extracellular matrix proteins.
Keywords: Verhoeff, van Geison, nanoindentation, elastic modulus, hypertrophy
heart failure's strong association with old age (9, 28, 54) presents itself as the foremost cause of hospitalizations for patients over 65 yr of age (15), a population (13%) that will double in the next 30 yr (3, 26). Heart failure, a clinical diagnosis that is multifactorial in etiology, but nonetheless broadly characterized as being systolic or diastolic in nature, is often accompanied by an increase in left ventricular stiffness (1, 2, 7, 12, 27, 30, 46, 60, 61) and large-artery stiffening and correlates with the presence of ischemic disease (1, 8, 32). Structural remodeling of resistance arteries is an important determinant of microvascular dysfunction in both age- and diabetes-related heart failure (21, 40). Given the significance of coronary resistance arteries (50–150 μm) in distribution of oxygen and nutrient delivery to the heart (>50% of coronary vascular resistance is present in vessels of this size vs. 7% of coronary vascular resistance that resides in large-conduit arteries) (10, 20) and the association between ventricular and vascular stiffening, it is reasonable to conclude that coronary microvascular restructuring and dysfunction may accompany age-related heart failure. Although there are consistent reports of age-related reduction of elastic properties and medial degeneration in large-conduit arteries, e.g., aorta and carotid arteries (35, 43), age-induced alterations of the structure of resistance arteries do not follow the same pattern (43, 44), and little is known of the effects of age on remodeling of coronary resistance arteries. Similar to findings in age-induced vascular stiffening, individuals with diabetes mellitus demonstrate an increase in the stiffness in large-conduit arteries (1); however, Katz and colleagues (31) have recently shown that the stiffness of coronary resistance arterioles (<150 μm) declines with type 2 diabetes mellitus. Thus it cannot be assumed that the stiffness of coronary resistance arteries will increase with age, as does the stiffness of larger conduit arteries.
Exercise training has been shown to reduce stiffening of peripheral conduit arteries with age (52); however, the effects of exercise training on coronary arteriolar structure and stiffness have not been investigated. Similarly, although our laboratory has previously reported that age impairs both contractile and vasodilatory function of coronary resistance arteries (28, 37, 38), the interactive effects of age and exercise training on remodeling of coronary resistance arteries and related vasomotor function remain relatively unexplored. Therefore, the purpose of this study was to determine 1) whether aging increases the stiffness and impairs myogenic function of coronary resistance arteries, and 2) whether exercise training initiated at an advanced age reverses age-related changes in the stiffness and myogenic function of coronary resistance arteries.
MATERIALS AND METHODS
All procedures performed in this study were approved by the University of Florida Laboratory Animal Care and Use Committee. All methods employed complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (updated, 2011).
Animals.
Young (4 mo old) and old (21 mo old) male Fischer 344 rats were acquired from the National Institute on Aging. The strain was chosen because cardiovascular function decreases with age without the development of hypertension (33). The animals were housed in a temperature-controlled room (23 ± 2°C) and kept on a 12:12-h light-dark cycle with water and rat chow provided ad libitum.
Endurance exercise training.
Young and old rats were randomly assigned to a sedentary control group (YMS and OMS for young and old male sedentary, respectively) and exercise-trained group (YME and OME). Exercise-trained rats underwent exercise habituation, during which each rat walked on a motor-driven treadmill at 15 m/min (0° incline) and 5 min/day for 3 days. Posthabituation, the incline was raised to 15° for the duration of the training period, while the 15 m/min speed was maintained. In the first 5 wk of training, the time of exercise was increased by 10 min/wk until 60-min duration was reached by week 6. The exercise-trained rats continued to exercise 5 days/wk for 60 min/day for the remainder of the 10- to 12-wk training period (5). Vascular responses were determined no less than 48 h after the last exercise bout in exercise-trained rats. To determine the efficacy of the training protocol, skeletal muscle (soleus and gastrocnemius) was stored at −80°C for determination of citrate synthase activity, a measure of muscle oxidative capacity.
Microvessel preparation.
Animals were anesthetized through isoflurane inhalation (3%/O2 balance) and euthanized via excision of the heart, followed by rapid placement of the heart into 4°C physiological saline buffer solution (PSS). Resistance arteries (<150 μm) branching from the left anterior descending artery were dissected using a stereomicroscope (Olympus SZX12). In vitro experimentation was conducted on the artery to determine myogenic vasoconstrictor responses, passive pressure-diameter characteristics, and gross vascular structure. Second-order distal septal resistance arteries (<180 μm) were used to determine vascular tissue mechanics via nanoindentation methodology (53).
In vitro experimentation.
Resistance arteries were transferred to a Lucite chamber containing PSS, cannulated at each end with glass micropipettes, and secured via 11-0 ophthalmic suture (Alcon Laboratories, Fort Worth, TX). The isolated vessel chamber was transferred to the stage of an inverted microscope (Olympus IX71) interfaced in series with a video camera (Panasonic BG310), a horizontal video caliper (307A, Colorado Video, Boulder, CO), a data-acquisition system (Powerlab, AD Instruments, Colorado Springs, CO), and a video monitor (Panasonic WV-BM1410). Intraluminal pressure was set at 60 cmH2O (44 mmHg) (11), established by the heights of two independent PSS-filled reservoirs connected to the glass micropipettes. The distance between the cannulating micropipettes was adjusted so that the vessel axial length was straight but not stretched. Resistance arteries with leaks were discarded. Intraluminal diameters were continuously measured via video microscopic techniques (4, 59).
Before evaluating myogenic vasoconstriction, resistance arteries without leaks were denuded of endothelium by passing 10 ml of air through the vessel lumen (42, 58). Arteries then were equilibrated at 37°C and allowed to develop spontaneous tone. Complete lack of vasodilation to 3 × 10−5 M ACh, indicated by no change or a decrease in diameter, confirmed removal of the endothelium. To evaluate active myogenic responsiveness, intraluminal pressure was lowered to 0 cmH2O for 5 min and then increased from 0 to 140 cmH2O in increments of 10 cmH2O every 5 min by raising both PSS-fluid reservoirs simultaneously, so that all pressure changes occurred in the absence of intraluminal flow. Arteries were then incubated for 40 min in Ca2+-free PSS at an intraluminal pressure of 60 cmH2O. A passive pressure-diameter relation was then determined using identical procedures to those used for the active myogenic responsiveness, described above. A bolus dose of sodium nitroprusside (SNP; 10−4 M) was added to ensure complete vascular smooth muscle relaxation after generating the passive pressure-diameter relation (17, 42). Maximal intraluminal diameter was determined 5 min after adding SNP.
To determine the ratio of medial wall thickness to maximal intraluminal diameter, arteries without leaks were pressurized to 60 cmH2O at 37°C for 60 min in Ca2+-free PSS with 10−4 M SNP, which was replaced every 15 min to ensure complete relaxation. Maximal intraluminal diameter and medial wall thickness were measured after 60 min of incubation. Medial wall thickness was taken as the mean of three separate wall thickness measurements from positions randomly selected along the microvessel length. To measure medial wall thickness, the video caliper used to measure intraluminal diameter was positioned on the far right or far left side of the vessel. The focal plane was adjusted through the medial wall, and the smallest distance between the inner and outer medial surface was taken to be medial wall thickness.
Vascular tissue mechanics.
Nanoindentation of all distal septal resistance arteries occurred within 1 h of vessel dissection. Following dissection, the distal septal resistance arteries were opened longitudinally using microscissors and placed onto the functionalized surface of a microscope slide (Fisherbrand Superfrost Plus), creating an “en face” endothelial preparation. The microscope slides were chosen since they promote adhesion by providing enough intermolecular forces to minimize sample movement during probe loading (53). To ensure proper adhesion of the vessel to the microscope slide, a modification to a previously established protocol used to adhere hydrogels to glass slides (57) allows for the deposition of a thin layer of gluteraldehyde. To do so, the microscope slides were first etched with a diamond tip scriber, such that identification of the treated area can be established postprotocol. To wash off particulates and ensure a clean surface, slides were washed with 100% EtOH and milipore H2O and placed in a 65°C oven until dry. Subsequently, 0.1 M NaOH was applied to sufficiently cover the region identified by the etched perimeter. The slides were then placed in the 65°C oven until dry (∼30 min). Then 97% 3-aminopropyltrimethoxy silane (Sigma-Aldrich 440140) was pipetted onto the surface, completely covering the established region for 5 min. Slides were then rinsed and submerged in distilled water for 15 min. Postsubmerging, slides were rinsed again and placed in the 65°C oven to dry (5–10 min). Glutaraldehyde 0.5% (vol/vol) in phosphate-buffered saline (PBS; Cellagro 46-013-CM) was pipetted onto the slides for 30 min, followed by rinse/submerging for 10 min, and rinse/dry in oven for 5–10 min. Preparation of the slide preceded vessel dissection so that the excised vessel could immediately be placed on the slide. Approximately 40 μl of PBS were pipetted onto the surface of microscope slide, ensuring sample hydration during nanoindentation. To determine vessel location via the in situ optical microscope in the nanoindenter, a paper grid with black concentric circles and a center cross hair was attached using cyanoacrylate (superglue) to the bottom of the glass slide (53).
Nanoindentation.
Nanoindentation was performed via a TriboIndenter (Hysitron, Minneapolis, MN), as previously described (53), using a nominal 100-μm-radius diamond cono-spherical fluid cell tip (Hysitron, AA03171104), as its radius acts to minimize stress concentrations for soft samples (∼kPa range). This enables measurement within the linear stress-stain limit (elastic region) of the material (19).
The treated microscope slide with sample was attached to an iron block (∼1 × 2 × 3 cm) via cyanoacrylate and loaded into the nanoindentation system. The sample was located using an in situ optical microscope, calibrated to allow indentation at identified position (6). Indents were made in the displacement-control mode of the instrument with a maximum displacement of 4,000 nm. A trapezoidal displacement cycle was used with a loading rate of 400 nm/s to maximum displacement, followed by a 5-s hold, followed by an unloading rate of 400 nm/s. All indents were performed at 25°C with the sample submerged in PBS. Surface detection was determined at 2 μN following calibration in the fluid reservoir to account for the forces the fluid exerted onto the tip. A minimum of five indents were done on the vessel within 1 h of excision. Indents spanned the length of the sample (spacing ≥ 60 μm). A total of 33 samples were tested (OME, n = 9; OMS, n = 8; YME, n = 9; YMS, n = 7). Methanol-soaked (99.8%) cotton swabs were used to clean the tip. Cleaning of the tip and the addition of PBS to the sample was done ad libitum.
Immunohistochemical analysis of elastin and collagen content.
Arteries were cannulated, pressurized, and fixed in Bouin's solution. Fixed arteries were placed in optimal cutting temperature compound and stored at −80°C. Five-micrometer-thick cryosections were cut for analysis of collagen and elastin. Sections were stained with Verhoeff-van Gieson (Scytek Laboratories, ETS-1) for elastin (Verhoeff) and collagen (van Gieson) (25). Collagen and elastin measurements were determined via a color threshold in MATLAB (Mathworks, Natick, MA). A total pixel measurement for each vessel was done via Image J using a set threshold for all images. To accommodate for cross staining of both elastin and vessel nuclei by the Verhoeff stain, an analysis of nuclei per cross-sectional area was done via 4,6-diamidino-2-phenylindole and green fluorescent protein using an overlap identification with color threshold in MATLAB. The analysis concluded no significant difference among groups; as such, the data presented herein also includes the nuclei cross-sectional area.
Solutions.
PSS contained (in mM) 145 NaCl, 4.7 KCl, 1.2 NaH2PO4, 1.17 MgSO4, 2.0 CaCl2, 5.0 glucose, 2.0 pyruvate, 0.025 EDTA, and 3.0 MOPS with a pH of 7.4. PSS was supplemented with bovine serum albumin (1 g/100 ml; USB, Cleveland, OH) and passed through a 0.22-μm cellulose acetate filter (430015, Corning). Ca2+-free PSS buffer preparation was identical except for the addition of 2 mM EDTA, the replacement of CaCl2 with 2.0 mM NaCl, and the exclusion of bovine serum albumin (53).
Statistical and data analysis.
The development of spontaneous tone was expressed as the percent constriction relative to maximal diameter, and was calculated as:
where IDmax is the maximal inner diameter recorded in Ca2+-free PSS at a pressure of 60 cmH2O, and IDB is the starting baseline diameter.
Active and passive responses to pressure changes were normalized to the maximal diameter according to the formula:
where IDS is the steady-state inner diameter recorded after each pressure change.
The contact stiffness (S) and effective elastic modulus (Eeff) of each indent was measured by the method of Oliver and Pharr (48), as previously described (53). In accordance with this method (48), S is determined from the derivative of the unloading portion of the load-displacement curve. In our experiments, the S was calculated from the slope of the loading portion of the curve, an adaptation suitable due to the absence of inelasticity in the samples and preferred to minimize errors encountered upon unloading due to sample-tip adhesion. Analysis of the indents was done via IGOR Pro (WaveMetrics, Portland, OR) and MATLAB by using a power-law fit to the loading curve, the derivative of which determined S. Eeff was determined by:
where Ac is the contact area at maximum force.
Active myogenic and passive pressure-response curves were evaluated by three-way (age, exercise training, and intraluminal pressure) ANOVA with two comparisons between factors (age and exercise training) and one within factor with repeated measurements (intraluminal pressure). Pairwise comparisons were conducted at each intraluminal pressure to determine whether differences existed between groups. Two-way ANOVA was used to detect group differences in developed spontaneous tone, maximal diameter, medial wall thickness, wall-to-lumen ratio, citrate synthase activity, heart-to-body weight ratio, left ventricle-to-body weight ratio, Eff, stiffness, %collagen, %elastin, C/E, and body mass. For all analyses conducted, a value of P < 0.05 was used for significance, and all data are presented as means ± SE.
RESULTS
Animals.
Body mass increased with age (Table 1). Exercise training reduced body mass in old rats. A significant difference in body mass was not observed for young rats with exercise training (Table 1). Heart-to-body weight ratio increased with exercise training in young and old rats. No difference in the left ventricle-to-heart weight ratio was determined between groups (Table 1). Exercise training increased citrate synthase activity by 46.5% in the young rats, and 82.2% in the old rats (Table 1), confirming the efficacy of the exercise training regimen, as previously demonstrated (51).
Table 1.
Coronary vessel and animal characteristics
| Characteristic | YMS | OMS | YME | OME |
|---|---|---|---|---|
| Body weight, g | 368 ± 5 (43) | 460 ± 7* (28) | 367 ± 7 (29) | 416 ± 5*† (30) |
| Maximal diameter, μm | 157.7 ± 5.8 (39) | 154.6 ± 5.4 (36) | 151.5 ± 8.6 (23) | 168.8 ± 6.7 (39) |
| Wall thickness, μm | 11.8 ± 0.6 (23) | 14.6 ± 0.9* (19) | 10.0 ± 0.6 (11) | 12.8 ± 0.8* (21) |
| Spontaneous tone, % | 25.70 ± 1.46 (10) | 22.65 ± 2.70 (10) | 35.47 ± 4.46† (6) | 26.40 ± 1.54* (11) |
| Citrate synthase activity, μmol·min−1·g wet wt−1 | 16.03 ± 0.73 (25) | 12.45 ± 0.40* (21) | 23.49 ± 1.64† (20) | 22.69 ± 0.71† (25) |
| HW/BW, mg/g | 2.43 ± 0.05 (42) | 2.51 ± 0.05 (30) | 2.65 ± 0.06† (29) | 2.82 ± 0.06*† (28) |
| LV/HW, mg/g | 0.72 ± 0.02 (36) | 0.72 ± 0.03 (24) | 0.70 ± 0.03 (29) | 0.76 ± 0.02 (28) |
Values are means ± SE; nos. in parentheses, no. of rats. YMS, young male sedentary; OMS, old male sedentary; YME, young male exercise trained; OME, old male exercise trained; HW/BW, heart weight-to-body weight ratio; LV/HW, left ventricle weight-to-heart weight ratio.
P < 0.05 vs. corresponding young group. †P < 0.05 vs. age-matched sedentary group.
Characteristics of isolated vessels.
Maximal diameter of resistance arteries was not altered by age or exercise training status (Table 1). Initial development of spontaneous tone during equilibration at a pressure of 60 cmH2O was greater in arteries from YME rats compared with all other groups (Table 1). Medial wall thickness was greater in arteries from old rats, regardless of training status (Table 1). Aging increased the wall-to-lumen ratio; exercise training reduced wall-to-lumen ratio in arteries from old rats to a level comparable to that of arteries from YMS rats (Fig. 1). Exercise training also decreased wall-to-lumen ratio in arteries from young rats.
Fig. 1.
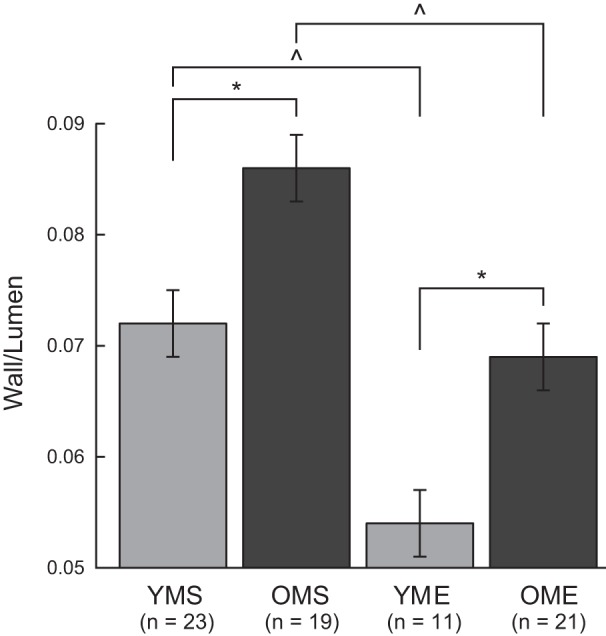
Wall-to-lumen ratio (W/L) of resistance arteries from young and old male sedentary (YMS and OMS, respectively) and exercise-trained (YME and OME, respectively) rats. Exercise training decreased W/L. Aging increases W/L; exercise training ameliorates effects of aging to ratio to a level comparable to YMS. Values are means ± SE; n, no. of rats. *P < 0.05 vs. corresponding old group. ^P < 0.05 vs. age-matched sedentary group.
Myogenic responses.
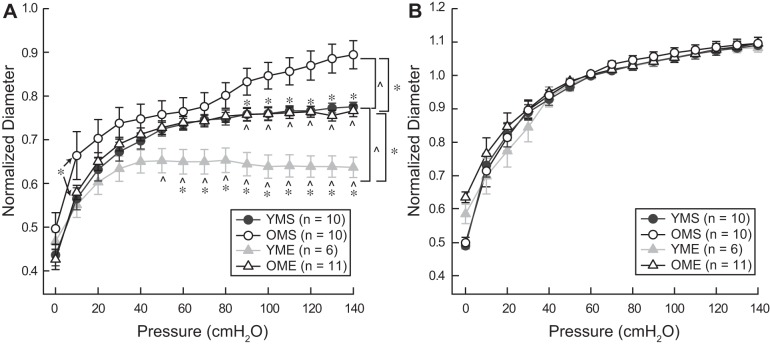
Myogenic constriction to increasing intraluminal pressure was reduced in coronary resistance arteries from old rats (Fig. 2A). Age did not alter passive responses to increasing pressure (Fig. 2B). Exercise training increased active myogenic responsiveness in coronary resistance arteries from both young and old rats (Fig. 2A). Following exercise training, myogenic responses in coronary resistance arteries from old rats no longer differed from the responses of arteries from YMS rats. Passive pressure-diameter responses were not altered by exercise training (Fig. 2B).
Fig. 2.
Active (A) and passive (B) pressure-diameter responses in resistance arteries from YMS, OMS, YME, and OME rats. Active responses were recorded in the presence of 2 mM extracellular Ca2+. Passive responses were recorded in the absence of extracellular Ca2+. Active responses demonstrated significant differences for corresponding old groups and age-matched sedentary groups. No differences in the passive responses were detected between groups. Values are means ± SE; n, no. of rats. *P < 0.05 vs. corresponding old group. ^P < 0.05 vs. age-matched sedentary group.
Mechanical properties.
Eeff and S declined with age in arteries from sedentary rats. In arteries from old rats, Eeff and S increased with exercise training. Load-displacement curves demonstrate a linear loading segment, indicative of an elastic response (Fig. 3C). A lower slope indicates a lower stiffness. A higher curvature of the upper 20% of the unloading segment is often observed and is indicative of sample-tip adhesion, commonly observed in the nanoindentation of soft biological tissue (39). This tip-sample adhesion is also the cause of the loading curve to return to a negative force rather than 0. Exercise training reduced the Eeff (Fig. 3A) and S (Fig. 3B) in the young rats to a level comparable to OMS, whereas an increase in Eeff and S was observed with exercise training in arteries from old rats.
Fig. 3.
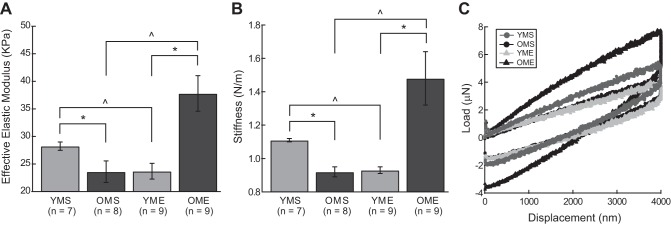
Effective elastic modulus (Eeff; KPa; A), calculated stiffness (S; N/m; B), and representative load-displacement curves for second degree septal arteries (<180 μm; C). Exercise training reduced the Eeff and S in the young group to a level comparable to OMS, whereas an increase in Eeff and S was observed with exercise training in the old group. Values are means ± SE; n, no. of rats. *P < 0.05 vs. corresponding old group. ^P < 0.05 vs. age-matched sedentary group.
Elastin and collagen content.
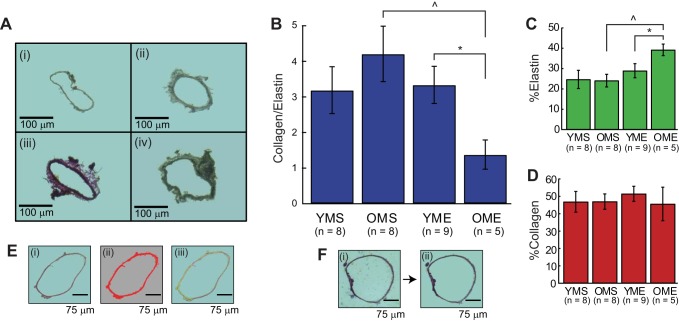
Representative cross sections demonstrating collagen and elastin staining in 1) YMS, 2) OMS, 3) YME, and 4) OME are shown in Fig. 4A. The C/E was highest in arteries from OMS rats; however, there was not a significant increase above the C/E in arteries from YMS rats (Fig. 4B). Exercise training reduced the C/E in arteries from old, but not young, rats. The percent elastin, as a function of cross-sectional area, was higher in OME compared with all other groups (Fig. 4C). No significant difference in percent collagen as a function of cross-sectional area was detected among groups (Fig. 4D). Figure 4E, ii and iii, shows a representative figure for cross-sectional area, as done via Image J, and elastin and collagen in a given cross section as done via a color threshold in MATLAB, respectively. Figure 4F is a representative figure showing the background subtraction process.
Fig. 4.
A: representative 5-μm-thick cross sections for YMS (i), OMS (ii), YME (iii), and OME (iv) rats. B: collagen-to-elastin ratio (C/E) for YMS, OMS, YME, and OME rats, measured in pixels/total pixels. The C/E is most uniform (approaches 1) for OME, which is significantly different from its age-matched sedentary group (OMS) and corresponding young group (YME). C: percent elastin (%E) as a function of cross-sectional area, measured in pixels/total pixels. %E demonstrates the highest concentration of elastin in the OME group, significantly different from OMS and YME. D: percent collagen (%C) as a function of cross-sectional area, measured in pixels/pixels. No differences in %C were detected between groups. E: representative cross section (i) demonstrating which pixels were counted for cross-sectional area (ii), and which pixels were counted for collagen and elastin (iii). With respect to iii, red pixels indicate collagen, green pixels elastin, and the yellow pixels are the regions where the two overlap. F: representative cross section demonstrating background subtraction: i → ii. Values are means ± SE; n, no. of rats. *P < 0.05 vs. corresponding old group. ^P < 0.05 vs. age-matched sedentary group.
DISCUSSION
In large peripheral conduit arteries, aging induces intimal-medial thickening and an increase in stiffness (34, 45). Arterial stiffness depends on intrinsic stress-strain relationships that are determined by structural properties of the blood vessel wall and by smooth muscle tone. In resistance arteries, structural changes and alterations in vascular smooth muscle function could lead to changes in both stiffness and intrinsic pressure-induced tone development, i.e., myogenic responsiveness. We tested the hypothesis that age would increase the stiffness of coronary resistance arteries, contributing to greater myogenic constriction in response to transmural pressure. In contrast to our hypothesis, the results demonstrate that age induced hypertrophic remodeling of the medial wall, but reduced the stiffness of coronary resistance arteries. Myogenic responses to increasing transmural pressure were impaired in the less stiff coronary resistance arteries from old rats. Ten weeks of treadmill exercise training induced hypotrophic remodeling of the medial wall in coronary resistance arteries from both young and old rats; this remodeling was accompanied by an increase in stiffness of coronary resistance arteries from old rats, and a decrease in stiffness in arteries from young rats. Exercise training restored myogenic responsiveness in coronary resistance arteries of old rats to a level not different than that of YMS rats, and exercise training also enhanced myogenic responsiveness of coronary resistance arteries from young rats. C/E did not change in coronary resistance arteries with age, but decreased in aged arteries with exercise training. These data suggest that age-induced hypertrophic remodeling of coronary resistance arteries may result from smooth muscle hypertrophy, with concomitant loss of smooth muscle contractile function. Exercise training appears to restore vascular smooth muscle contractile function and increase elastin content in aged coronary resistance arteries; however, the increase in elastin content does not prevent stiffening of the aged vascular wall with exercise training.
Our laboratory (50) has previously reported an impairment of contractile responses in intact coronary resistance arteries of aged Fischer 344 rats and found evidence that endothelial mechanisms, potentially both endothelium-dependent constrictors and/or dilators, contributed to the reduction of myogenic tone development. In the present work, we evaluated myogenic responsiveness in the absence of the endothelium; consistent with our previous work, we found myogenic responses to be impaired in denuded coronary resistance arteries from old rats, indicating that age-induced changes in the vascular smooth muscle also contribute to the blunting of myogenic responses. Indeed, we found that this impairment of myogenic responsiveness manifested as a loss of myogenic gain over the range of pressures that constitute the autoregulatory range (70–140 cmH2O).
Our application of nanoindentation technology indicates that the mechanical properties of the arteriolar wall change with age and exercise training, independent of geometric remodeling. It is important to note that our purpose was to compare the stiffness of the arteriolar wall between young and old groups, and between age-matched sedentary and exercise-trained groups. Our approach, previously used to document reduced stiffness in cerebral vessels of space-flown mice (53), was uniformly applied to vessel segments adhered to glass slides in an en face presentation to avoid substrate variability; however, our results do not allow us to determine whether these material changes are paralleled by changes in arteriolar compliance at physiological blood pressure levels. The reported differences in stiffness could be due to changes in the endothelium, smooth muscle, or extracellular matrix components, and further study will be required to determine how specific changes in the material components of the arteriolar wall contribute to alterations in myogenic behavior.
Surprisingly, wall stiffness was reduced in the resistance arteries from old rats, a finding that is directionally opposite from reports in large-conduit arteries (34, 45). With advancing age, vascular smooth muscle has been reported to transform to a more secretory phenotype in large arteries, contributing to changes in the extracellular matrix and a proinflammatory environment in the vascular wall (13, 58). In coronary resistance arteries, switching of the phenotype of vascular smooth muscle could contribute to the change in C/E observed in the present study. In addition, a transition away from a contractile smooth muscle phenotype could contribute to the loss of myogenic contractile function in these resistance arteries (Fig. 2A).
The composition of the extracellular matrix is regulated by the vascular smooth muscle and fibroblasts, and, in turn, the function of both the endothelium and the vascular smooth muscle are regulated by interactions with extracellular matrix proteins. In coronary resistance arteries from old rats, wall-to-lumen ratio and the C/E increased; however, the stiffness of resistance arteries from OMS rats decreased, consistent with the idea that this reduction was related to decreased stiffness of the vascular smooth muscle, as opposed to changes in extracellular matrix components. Our previous data (36, 50) and the current assessment of myogenic responsiveness in aged coronary resistance arteries indicate a loss of contractile function in the smooth muscle, suggesting that aging may promote a transition from a contractile vascular smooth muscle to a less stiff smooth muscle. In contrast, the stiffness of aortic smooth muscle cells from monkeys increased with age, as assessed by atomic force microscopy of isolated cells (49); however, it should also be considered that reports of enhanced, unchanged, and diminished contractile function have been reported in the aged aorta (16, 23, 41, 47).
Exercise training reduced the C/E in arteries from aged rats, primarily due to an increase in the elastin content of the vessel wall. Interestingly, this increase in elastin was accompanied by increased wall stiffness, possibly due to stiffer vascular smooth muscle cells. Although total collagen content did not change with exercise training, collagen cross-linking may have altered the mechanical properties of the vascular wall in a manner that could not be reversed by the increase in elastin content that occurred with exercise training. Collagen cross-linking has been reported to be a major determinant of increasing ventricular stiffness and a key contributor to age-related diastolic dysfunction (3, 4, 9). The possibility of an exercise training-induced transition to a contractile (and possibly stiffer) smooth muscle phenotype is supported by the increase in myogenic responsiveness that we found in aged coronary resistance arteries post-exercise training. Further study will need to be performed to evaluate the effects of age and exercise training on vascular smooth muscle phenotype and collagen cross-linking in coronary resistance arteries.
Exercise training induced a reduction of wall-to-lumen ratio in coronary resistance arteries from both young and old rats. This finding is consistent with reports of exercise training-induced outward remodeling in the aorta and in coronary and skeletal muscle resistance arteries (22, 55). This remodeling effect, which may be mediated through long-term exercise-induced increases in flow (21), appears to be preserved with age. These results further suggest that the hypertrophic remodeling of the medial wall that occurred in arteries from OMS rats may be related to decreases in physical activity (4) and a subsequent reduction in the shear stimulus on the vascular wall.
The physiological mechanisms that underlie age-induced adaptations of the vascular wall were not investigated in this study; however, our results demonstrate some similarity with models of obesity and diabetes, suggesting potential common mechanisms. Coronary arterioles from type 2 diabetic mice undergo hypertrophic remodeling and demonstrate decreased stiffness (31). Similarly, hypertrophic remodeling is accompanied by reduced stiffness in coronary microvessels from pigs with metabolic syndrome (56). Microvascular remodeling in these models and in our model of aging may be driven by increased blood pressure, oxidant stress, or alterations in neurohumoral or inflammatory factors. Elevation of blood pressure often occurs in both type 2 diabetes and the metabolic syndrome; however, blood pressure does not increase with age in Fischer 344 rats (14, 18), suggesting that age-induced hypertrophic remodeling of coronary arterioles is blood pressure independent. In contrast, age-related endothelial dysfunction is accompanied by reduction of antioxidant protein levels in coronary arterioles (28), and endothelial dysfunction may exacerbate remodeling related to vascular smooth muscle hypertrophy. In the aged heart (24), as in the hearts of pigs with metabolic syndrome (56), inadequate coronary flow reserve may lead to ischemic conditions and increase the expression or cross-linking of collagen in coronary microvessels as well as in the myocardium. The physiological contributors to age-induced coronary microvascular remodeling remain to be identified. Similarly, the ability of exercise training to mitigate the effects of these signals will require further study.
In summary, changes in both structural components and possibly in vascular smooth muscle function contribute to a reduction of arteriolar stiffness and impaired myogenic responsiveness in coronary resistance arteries with advancing age. Exercise training enhances myogenic responsiveness and mitigates the increase in wall-to-lumen ratio in aged coronary resistance arteries. The coronary resistance vasculature is subjected to constant intermittent compressive forces, and regulation of flow distribution is critical to the maintenance of diastolic flow to the endocardium in particular. The age-related shift to more compliant resistance arteries in which myogenic function is impaired could contribute to the maldistribution of coronary blood flow and an increased risk for ischemic events in the aged myocardium. Exercise training, even at an advanced age, may restore both mechanical and functional capacity of the vascular smooth muscle and the vascular wall of coronary resistance arteries, promoting improved distribution of coronary blood flow and oxygen delivery in the endocardial and epicardial microcirculation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-77224 and R01-HL-90937.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.E.H., C.R.T., B.J.B., M.D.D., and J.M.M.-D. conception and design of research; M.E.H., B.C., H.-S.L., J.J.M., C.R.K., and J.M.M.-D. performed experiments; M.E.H., C.R.T., B.C., H.-S.L., J.J.M., C.R.K., and J.M.M.-D. analyzed data; M.E.H., C.R.T., B.C., H.-S.L., J.J.M., C.R.K., B.J.B., M.D.D., and J.M.M.-D. interpreted results of experiments; M.E.H. prepared figures; M.E.H. drafted manuscript; M.E.H., B.C., H.-S.L., J.J.M., B.J.B., M.D.D., and J.M.M.-D. edited and revised manuscript; M.E.H., C.R.T., B.C., H.-S.L., J.J.M., C.R.K., B.J.B., M.D.D., and J.M.M.-D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Xueling Teng and Shige Tsuda for technical support.
REFERENCES
- 1.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21: 3–12, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Badenhorst D, Maseko M, Tsotetsi OJ, Naidoo A, Brooksbank R, Norton GR, Woodiwiss AJ. Cross-linking influences the impact of quantitative changes in myocardial collagen on cardiac stiffness and remodelling in hypertension in rats. Cardiovasc Res 57: 632–641, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr 22: 309–323, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Behnke BJ, Prisby RD, Lesniewski LA, Donato AJ, Olin HM, Delp MD. Influence of ageing and physical activity on vascular morphology in rat skeletal muscle. J Physiol 575: 617–626, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behnke BJ, Ramsey MW, Stabley JN, Dominguez JM, Davis RT, McCullough DJ, Muller-Delp JM, Delp MD. Effects of aging and exercise training on skeletal muscle blood flow and resistance artery morphology. J Appl Physiol 113: 1699–1708, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourne GR. A Methodology for Tribological Examination of Thin Films in the Nanodisplacement Regime. Gainesville, FL: University of Florida, 2006 [Google Scholar]
- 7.Brower GL, Gardner JD, Forman MF, Murray DB, Voloshenyuk T, Levick SP, Janicki JS. The relationship between myocardial extracellular matrix remodeling and ventricular function. Eur J Cardiothorac Surg 30: 604–610, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol 32: 1221–1227, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Chen MA. Heart failure with preserved ejection fraction in older adults. Am J Med 122: 713–723, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Chilian WM. Microvascular pressures and resistances in the left-ventricular subepicardium and subendocardium. Circ Res 69: 561–570, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779–H788, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Choi SY, Chang HJ, Choi SI, Il Kim K, Cho YS, Youn TJ, Chung WY, Chae IH, Choi DJ, Kim HS, Kim CH, Oh BH, Kim MH. Long-term exercise training attenuates age-related diastolic dysfunction: association of myocardial collagen cross-linking. J Korean Med Sci 24: 32–39, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci 67: 811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis RT, Stabley JN, Dominguez JM, Ramsey MW, McCullough DJ, Lesniewski LA, Delp MD, Behnke BJ. Differential effects of aging and exercise on intra-abdominal adipose arteriolar function and blood flow regulation. J Appl Physiol (1985) 114: 808–815, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13: 1–209, 2007 [PubMed] [Google Scholar]
- 16.Delp MD, Brown M, Laughlin MH, Hasser EM. Rat aortic vasoreactivity is altered by old age and hindlimb unloading. J Appl Physiol (1985) 78: 2079–2086, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Delp MD, Colleran PN, Wilkerson MK, McCurdy MR, Muller-Delp J. Structural and functional remodeling of skeletal muscle microvasculature is induced by simulated microgravity. Am J Physiol Heart Circ Physiol 278: H1866–H1873, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Delp MD, Evans MV, Duan C. Effects of aging on cardiac output, regional blood flow, and body composition in Fischer-344 rats. J Appl Physiol 85: 1813–1822, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Ebenstein DM, Kuo A, Rodrigo JJ, Reddi AH, Ries M, Pruitt L. A nanoindentation technique for functional evaluation of cartilage repair tissue. J Mater Res 19: 273–281, 2004 [Google Scholar]
- 20.Feigl EO. Coronary physiology. Physiol Rev 63: 1–205, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med 330: 1431–1438, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Gu Q, Wang B, Zhang XF, Ma YP, Liu JD, Wang XZ. Contribution of hydrogen sulfide and nitric oxide to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Mol Cell Biochem 375: 199–206, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Gurdal H, Cai G, Johnson MD. Alpha 1-adrenoceptor responsiveness in the aging aorta. Eur J Pharmacol 274: 117–123, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Hachamovitch R, Wicker P, Capasso JM, Anversa P. Alterations of coronary blood flow and reserve with aging in Fischer 344 rats. Am J Physiol Heart Circ Physiol 256: H66–H73, 1989 [DOI] [PubMed] [Google Scholar]
- 25.Hayenga HN, Hu JJ, Meyer CA, Wilson E, Hein TW, Kuo L, Humphrey JD. Differential progressive remodeling of coronary and cerebral arteries and arterioles in an aortic coarctation model of hypertension. Front Physiol 3: 420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen LA, Kent M, Lee M, Mather M. America's aging population. Popul Bull 66: 2–16, 2011 [Google Scholar]
- 27.Jyothirmayi GN, Soni BJ, Masurekar M, Lyons M, Regan TJ. Effects of metformin on collagen glycation and diastolic dysfunction in diabetic myocardium. J Cardiovasc Pharmacol Ther 3: 319–326, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kass DA, Bronzwaer JGF, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res 94: 1533–1542, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Katz PS, Trask AJ, Souza-Smith FM, Hutchinson KR, Galantowicz ML, Lord KC, Stewart JA, Cismowski MJ, Varner KJ, Lucchesi PA. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res Cardiol 106: 1123–1134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction–Implications for systolic and diastolic reserve limitations. Circulation 107: 714–720, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Lakatta EG. Cardiovascular aging research: the next horizons. J Am Geriatr Soc 47: 613–625, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. I. Aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am 93: 583–604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leblanc AJ, Chen B, Dougherty PJ, Reyes RA, Shipley RD, Korzick DH, Muller-Delp JM. Divergent effects of aging and sex on vasoconstriction to endothelin in coronary arterioles. Microcirculation 20: 365–376, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leblanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, Muller-Delp JM. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 297: R1713–R1723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol 295: H2280–H2288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin DC, Horkay F. Nanomechanics of polymer gels and biological tissues: a critical review of analytical approaches in the Hertzian regime and beyond. Soft Matter 4: 669–682, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Liu YP, Gutterman DD. Vascular control in humans: focus on the coronary microcirculation. Basic Res Cardiol 104: 211–227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marin J, Rodrıǵuez-Martıńez A. Age-related changes in vascular responses. Exp Gerontol 34: 503–512, 1999 [DOI] [PubMed] [Google Scholar]
- 42.McCurdy MR, Colleran PN, Muller-Delp J, Delp MD. Effects of fiber composition and hindlimb unloading on the vasodilator properties of skeletal muscle arterioles. J Appl Physiol (1985) 89: 398–405, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43: 1239–1245, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Norton GR, Tsotetsi J, Trifunovic B, Hartford C, Candy GP, Woodiwiss AJ. Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation 96: 1991–1998, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Olah ME, Rahwan RG. Age-related changes in responsiveness of the rat aorta to depolarizing and receptor-mediated contractile stimuli and to calcium antagonism. Pharmacology 35: 163–173, 1987 [DOI] [PubMed] [Google Scholar]
- 48.Oliver WC, Pharr GM. Measurement of hardness and elastic modulus by instrumented indentation: advances in understanding and refinements to methodology. J Mater Res 19: 3–20, 2004 [Google Scholar]
- 49.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res 107: 615–619, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res 66: 374–383, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Taylor CR, Hanna M, Behnke BJ, Stabley JN, McCullough DJ, Davis RT, Ghosh P, Papadopoulos A, Muller-Delp JM, Delp MD. Spaceflight-induced alterations in cerebral artery vasoconstrictor, mechanical, and structural properties: implications for elevated cerebral perfusion and intracranial pressure. FASEB J 27: 2282–2292, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas S, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Heart Fail Clin 3: 381–387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trask AJ, Delbin MA, Katz PS, Zanesco A, Lucchesi PA. Differential coronary resistance microvessel remodeling between type 1 and type 2 diabetic mice: impact of exercise training. Vascul Pharmacol 57: 187–193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trask AJ, Katz PS, Kelly AP, Galantowicz ML, Cismowski MJ, West TA, Neeb ZP, Berwick ZC, Goodwill AG, Alloosh M, Tune JD, Sturek M, Lucchesi PA. Dynamic micro- and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. J Appl Physiol 113: 1128–1140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tse JR, Engler AJ. Preparation of hydrogel substrates with tunable mechanical properties. Curr Prot Cell Biol 10: 10.–16., 2010 [DOI] [PubMed] [Google Scholar]
- 58.Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens 19: 201–207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkerson MK, Lesniewski LA, Golding EM, Robert M, Bryan J, Amin A, Wilson E, Delp MD. Simulated microgravity enhances cerebral artery vasoconstriction and vascular resistance through endothelial nitric oxide mechanism. Am J Physiol Heart Circ Physiol 288: H1652–H1661, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Willemsen S, Hartog JWL, Hummel YM, van Ruijven MHI, van der Horst ICC, van Veldhuisen DJ, Voors AA. Tissue advanced glycation end products are associated with diastolic function and aerobic exercise capacity in diabetic heart failure patients. Eur J Heart Fail 13: 76–82, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Woodiwiss AJ, Tsotetsi OJ, Sprott S, Lancaster EJ, Mela T, Chung ES, Meyer TE, Norton GR. Reduction in myocardial collagen cross-linking parallels left ventricular dilatation in rat models of systolic chamber dysfunction. Circulation 103: 155–160, 2001 [DOI] [PubMed] [Google Scholar]