Abstract
Although xenon is classically taught to be a “perfusion-limited” gas, 129Xe in its hyperpolarized (HP) form, when detected by magnetic resonance (MR), can probe diffusion limitation. Inhaled HP 129Xe diffuses across the pulmonary blood-gas barrier, and, depending on its tissue environment, shifts its resonant frequency relative to the gas-phase reference (0 ppm) by 198 ppm in tissue/plasma barrier and 217 ppm in red blood cells (RBCs). In this work, we hypothesized that in patients with idiopathic pulmonary fibrosis (IPF), the ratio of 129Xe spectroscopic signal in the RBCs vs. barrier would diminish as diffusion-limitation delayed replenishment of 129Xe magnetization in RBCs. To test this hypothesis, 129Xe spectra were acquired in 6 IPF subjects as well as 11 healthy volunteers to establish a normal range. The RBC:barrier ratio was 0.55 ± 0.13 in healthy volunteers but was 3.3-fold lower in IPF subjects (0.16 ± 0.03, P = 0.0002). This was caused by a 52% reduction in the RBC signal (P = 0.02) and a 58% increase in the barrier signal (P = 0.01). Furthermore, the RBC:barrier ratio strongly correlated with lung diffusing capacity for carbon monoxide (DLCO) (r = 0.89, P < 0.0001). It exhibited a moderate interscan variability (8.25%), and in healthy volunteers it decreased with greater lung inflation (r = −0.78, P = 0.005). This spectroscopic technique provides a noninvasive, global probe of diffusion limitation and gas-transfer impairment and forms the basis for developing 3D MR imaging of gas exchange.
Keywords: hyperpolarized 129Xe, gas-transfer spectroscopy, idiopathic pulmonary fibrosis, diffusion limitation
the introduction of hyperpolarized (HP) 129Xe has provided a sustainable replacement for the dwindling 3He supply to enable noninvasive imaging of pulmonary structure and function.1 Much like 3He magnetic resonance imaging (MRI), 129Xe MRI can be used for breath-hold high-resolution 3D imaging of pulmonary ventilation (30), and imaging of microstructural changes using diffusion-weighted methods (13). However, perhaps the most powerful and intriguing properties of 129Xe are its solubility in pulmonary blood and tissues combined with associated changes in its signal frequency. When 129Xe is inhaled, the bulk of the gas remains in the airspaces where it exhibits its primary, gas-phase resonance of f0 = 17.66 MHz at 1.5 T. However, a portion of the gas also quickly diffuses into and saturates the alveolar septa where it exhibits two additional distinct resonances. 129Xe dissolved in interstitial tissues and plasma increases its frequency by 3.48 kHz (at 1.5 T) compared with gaseous 129Xe remaining in the airspaces. This shifted signal, referred to as the barrier resonance, is also commonly described in field-independent terms as being shifted by 198 ppm relative to the gas-phase. When 129Xe reaches the red blood cells (RBCs), it transiently [20 ms (2)] binds with hemoglobin, distorting the xenon electron cloud, and further deshielding the 129Xe nucleus from the applied magnetic field (4). This further shifts the 129Xe frequency to 3.83 kHz (217 ppm) above the gas phase and enables 129Xe in RBCs to be uniquely identified. This 129Xe transfer pathway to the RBCs is identical to that followed by oxygen, making 129Xe a sensitive probe of not just ventilation, but also diffusive gas-transfer.
Imaging 129Xe in this “dissolved-phase” is challenging because its maximum signal is only ∼2% of that remaining in the alveolar spaces. However, because these compartments are in dynamic exchange, the continuous replenishment of dissolved-phase magnetization by 129Xe atoms diffusing in from the airspaces makes it possible to use nearly all of the inhaled magnetization to generate 3D images of dissolved 129Xe in a single breath-hold (5, 19). Initial human imaging of the dissolved-phase 129Xe distribution demonstrated the feasibility of this technique and confirmed that the gas-transfer distribution varied with subject posture (14). However, this work was limited in that both the barrier and RBC resonances were imaged together as a single entity. This makes it difficult to untangle the competing effects of regional changes in perfusion, interstitial thickness, and alveolar-capillary surface area available for gas exchange. Hence, harnessing the properties of 129Xe to more fully evaluate regional gas exchange requires the ability to detect 129Xe transfer to the RBCs separately from transfer to the barrier.
When 129Xe signal in the barrier and RBCs is detected by magnetic resonance, the radiofrequency (RF) pulses applied to excite the 129Xe magnetization also consume it. It is replenished when 129Xe atoms and their associated magnetization diffuse back into these compartments from the airspaces. Because those atoms must first diffuse through the barrier before reaching the RBCs, we hypothesized that the ratio of 129Xe signal in RBCs vs. barrier would be reduced in patients with interstitial lung disease and could detect their diffusion impairment. To test this hypothesis we sought to employ simple 129Xe gas-transfer spectroscopy (without spatial localization) to evaluate gas-transfer impairment averaged over the entire lung. Given the relative simplicity of implementing and executing 129Xe transfer spectroscopy, and the small volumes of gas required, this measurement was easily added to the calibration sequence of the broader 129Xe MRI protocol.
129Xe spectra were collected in patients with idiopathic pulmonary fibrosis (IPF), a particularly devastating form of interstitial lung disease, with an estimated incidence of 10.7/100,000 people (23). IPF is characterized by decreased fibroblast death, which results in excessive collagen deposition that thickens the interstitial barrier tissue in the lungs, causing diffusion limitation (26). IPF has a poor prognosis, with a mean survival of ∼3 years from diagnosis (24). This is largely attributable to a lack of viable therapeutic options that can reverse or halt disease progression (23). However, numerous therapies are now under investigation for the treatment of IPF (16, 25), and these clinical trials are in need of sensitive biomarkers to monitor their potential benefits and efficacy. Given the continuing need to develop noninvasive biomarkers of gas-transfer impairment, and that this patient population has known diffusion limitation (1), this group is well suited for initial evaluation of 129Xe gas-transfer spectroscopy.
The goal of this proof-of-concept study was to establish a preliminary range of normal values for the RBC:barrier signal ratio in healthy subjects, understand the short- and intermediate-term reproducibility, and establish the degree to which 129Xe gas-transfer spectroscopy correlates with conventional metrics of gas exchange such as lung diffusion capacity for carbon monoxide (DLCO). Moreover, we sought to test the hypothesis that 129Xe gas-transfer spectroscopy can detect impaired gas-transfer in patients with IPF compared with healthy volunteers.
METHODS
Subject Recruitment
The study was approved by the Duke Institutional Review Board, and written informed consent was obtained from all subjects prior to recruitment to the protocol. 129Xe spectra were acquired in 6 subjects with IPF (mean age = 66.8 ± 12.3 yr, all men) and 11 healthy volunteers (mean age = 41.3 ± 17.8 yr, 10 men, 1 woman). One hour prior to MR studies, all subjects underwent baseline pulmonary function testing and body plethysmography to obtain functional residual capacity (FRC) and total lung capacity (TLC) measurements by methods previously described (14), and DLCO by the single-breath method (7), to characterize lung function and lung diffusing capacity of the subject cohorts and to aid in interpreting the 129Xe transfer spectra.
Xenon Polarization and Delivery
Isotopically enriched 129Xe (85%, Linde Gases, Stewartsville, NJ) was polarized using Rb-vapor spin-exchange optical pumping in a commercially available polarizer (model 9800, Polarean, Durham, NC). 129Xe transfer spectra were acquired using a 200-ml dose of 129Xe, which was cryogenically accumulated and subsequently dispensed into a Tedlar bag (Jensen Inert Products, Coral Springs, FL), typically polarized between 10 and 14%. This dose was then mixed with 800 ml of ultra-high-purity nitrogen and administered to the subject. Prior to inhalation, subjects were coached to inhale to TLC and exhale to FRC twice. Following this, the subjects inhaled the contents of the bag through a 6-mm-ID Tygon tube, and held their breath for 4–8 s, during which time 129Xe spectra were acquired (see below). The subject's heart rate and oxygen saturation were monitored using an MR-compatible monitoring system (GE Healthcare, Helsinki, Finland).
Dissolved-Phase Spectroscopy
Acquisition.
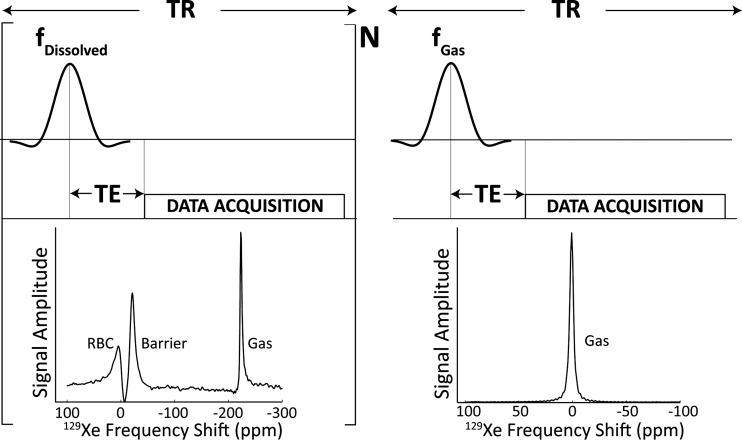
Studies were conducted on a 1.5-T MRI scanner (GE Healthcare, 15M4 EXCITE). Subjects were fitted with a quadrature 129Xe vest coil tuned to the gas-phase xenon Larmor frequency of 17.660445 MHz (Clinical MR Solutions, Brookfield, WI). Spectra were acquired in a single breath-hold with the following parameters: dissolved-phase 129Xe was selectively excited by applying a 3-lobe sinc pulse of 1,200-μs duration to the 129Xe-RBC resonance, 3,832 Hz above the gas phase. Spectra were collected with echo time (TE) = 0.932 ms, repetition time (TR) = 20/40 ms, receiver bandwidth = 8.06/15.63 kHz, flip-angle = 15–20°, and number of free induction decays (FIDs) = 200. Subsequently, in the same breath-hold, the transmit/receive frequency was switched to the gas-phase resonance, and with otherwise identical pulse parameters, a single gas-phase reference spectrum was acquired. The acquisition is graphically illustrated in Fig. 1. For a subset of healthy subjects (n = 7), spectra were acquired again using the same protocol ∼30 min later to study the short-term reproducibility. To analyze the interscan variability over a longer time period (t = 2.2 ± 1.8 mo), 9 healthy volunteers participated in a follow-up scan, and 129Xe transfer spectra were again acquired as described above.
Fig. 1.
Acquisition scheme for the dissolved and gas-phase 129Xe spectra. First, 200 (N) dissolved-phase 129Xe spectra were acquired by pulsing and acquiring on the red blood cell (RBC) resonance, +3,832 Hz above the gas-phase resonance. Then, with identical acquisition parameters, transmit and receive frequencies were lowered to match the gas-phase 129Xe resonance, and a single reference spectrum was acquired. TR, repetition time; TE, echo time.
Processing and analysis.
129Xe spectra were processed in MATLAB (MathWorks, Natick, MA) as follows. Because the first 129Xe spectra in the series occurred an arbitrary time after starting inhalation, they were expected to contain 129Xe signal that had accumulated “downstream” of the pulmonary capillary beds in the larger vasculature. Therefore, the first 100 FIDs of the series were discarded. However, their associated 100 RF pulses ensured that any 129Xe signal from the larger vasculature should be depleted to ∼1% of its starting value. Thus, signal in the remaining spectra originated entirely from 129Xe within the capillary beds and represented a diffusive steady state. The remaining 100 FIDs were averaged together and processed as follows. The averaged FID underwent 50-Hz line broadening using an exponential apodizing function. Such techniques are commonly used in NMR spectroscopy to improve the signal-to-noise ratio (SNR) of spectra (15). The FID was then Fourier transformed to yield a 129Xe spectrum consisting primarily of dissolved phase 129Xe signal, with only a small amount of gas-phase 129Xe signal to provide a reference frequency. As recently shown by Chang et al. (3), the complex spectrum was curve fit using a nonlinear least-squares optimization algorithm to a sum of complex frequency-dependent Lorentzian functions for the three resonances (RBC, barrier, and gas), allowing each to have its own amplitude, central frequency, line width, and phase, according to
| (1A) |
where each complex Lorentzian is given by
| (1B) |
and f is frequency, Aj is the amplitude of a given resonance, fj its resonant frequency, Δfj its full width at half-maximum (FWHM), and ϕi its phase.
Typical fits of the real and imaginary channels of a 129Xe transfer spectrum are shown in Fig. 2, top and middle, while Fig. 2, bottom, shows the three fitted resonances with phase differences removed. The same fitting procedure was applied to the single gas-phase reference spectrum. The resulting fit parameters were used to compute the area under the curve (AUC) of each resonance, which for Lorentzian functions is simply the product of peak amplitude and FWHM. From these AUCs the ratio of the RBC to barrier (RBC:barrier) was calculated and used as the primary metric of gas-transfer. To further investigate whether changes in RBC:barrier ratio were caused by changing RBC or barrier signals, the ratios of each resonance to the dedicated gas-phase reference spectrum, RBC:gas and barrier:gas, were also computed.
Fig. 2.
Curve fitting the dissolved-phase 129Xe spectra. The real and imaginary spectra were fit separately. The imaginary fit was rephased by 90° and averaged with the real spectrum to generate the final fit parameters, including amplitude, frequency, and spectral width for each peak.
A Wilcoxon Rank-sum test was used to test whether the various ratios, frequencies, and peak widths were significantly different between the two groups. Reproducibility was assessed using Bland-Altman plots, and a pairwise, two-tailed t-test was used to check for significant differences between the scans. Potential associations between the subject's RBC:barrier ratio and lung volume, age, and DLCO were also evaluated. To examine the impact of the fixed dose volume on our measurements, a metric to estimate lung inflation during 129Xe signal acquisition was defined as (FRC + 1 liter)/TLC, and correlated against the spectroscopic ratios for the healthy volunteers. The significance of the correlations was examined using a two tailed t-test.
RESULTS
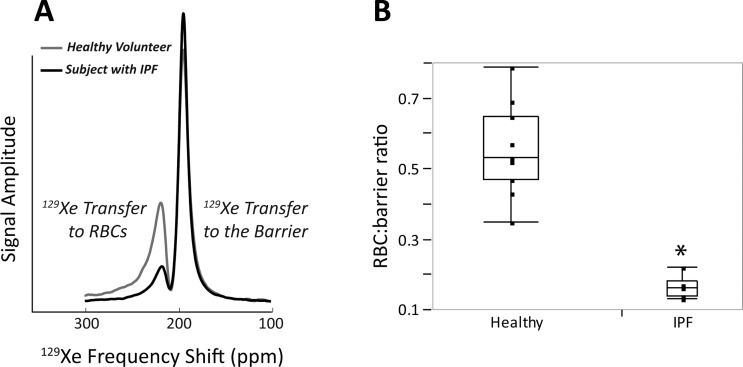
129Xe gas-transfer spectra were successfully acquired in all subjects, whose demographics and pulmonary function test data are summarized in Table 1. Figure 3A shows a 129Xe transfer spectrum from a typical healthy volunteer compared with that of an age-matched subject with IPF. For this healthy volunteer, the RBC:barrier ratio was 0.49, whereas for the IPF subject, it was 0.17, nearly threefold lower.
Table 1.
Subject demographics and spectroscopic ratios
| Ratiosb |
|||||||
|---|---|---|---|---|---|---|---|
| Subjecta | Age, yr | RBC:barrier | TLCc, liters | DLCOc, ml·min−1·mmHg−1 | RBC:gas | Barrier:gas | Lung Inflation, % |
| Healthy | |||||||
| 1 | 59 | 0.47 | 0.002 | 0.005 | 7.6 | 23.3 | 59.1 |
| 2 | 65 | 0.47 | 0.003 | 0.006 | 7.2 | 28.1 | 62.6 |
| 3 | 51 | 0.35 | 0.002 | 0.007 | 5.7 | 21.5 | 74.9 |
| 4 | 24 | 0.79 | 0.003 | 0.004 | 6.7 | 26.6 | 66.3 |
| 5 | 33 | 0.57 | 0.003 | 0.005 | 8.0 | 31.8 | 68.6 |
| 6 | 29 | 0.43 | 0.003 | 0.007 | 6.3 | 27.8 | 61.7 |
| 7 | 27 | 0.65 | 0.005 | 0.008 | 7.2 | 42.7 | 54.6 |
| 8 | 23 | 0.53 | 0.007 | 0.014 | 7.3 | 31.9 | 46.7 |
| 9 | 22 | 0.69 | 0.006 | 0.009 | 9.2 | 29.8 | 54.9 |
| 10 | 56 | 0.52 | 0.004 | 0.007 | 6.6 | 25.7 | 70.3 |
| 11 | 65 | 0.53 | 0.004 | 0.008 | 7.0 | 30.1 | 59.7 |
| IPF | |||||||
| 1 | 74 | 0.13 | 0.002 | 0.012 | 4.2 | 10.8 | |
| 2 | 83 | 0.22 | 0.003 | 0.013 | 4.7 | 12.6 | |
| 3 | 54 | 0.17 | 0.003 | 0.016 | 3.8 | 11.2 | |
| 4 | 51 | 0.14 | 0.002 | 0.011 | 3.3 | 7.2 | |
| 5 | 72 | 0.16 | 0.001 | 0.008 | 4.1 | 9.4 | |
| 6 | 67 | 0.16 | 0.001 | 0.009 | 3.8 | 9.4 | |
All subjects were male, except subject 3. bRatios were measured in the supine position. cPulmonary function data were measured in the upright position. IPF, idiopathic pulmonary fibrosis; RBC, red blood cell; TLC, total lung capacity; DLCO, lung diffusing capacity for carbon monoxide.
Fig. 3.
A: a truncated dissolved-phase spectrum from a representative healthy volunteer and a subject with idiopathic pulmonary fibrosis (IPF). Compared with the healthy volunteer, this IPF subject exhibits a 2.9-fold reduction in the RBC:barrier ratio. B: for all subjects the RBC:barrier ratio was 0.55 ± 0.13 in the healthy volunteers but was 3.3-fold lower in the IPF patients (0.16 ± 0.03; *P = 0.0002).
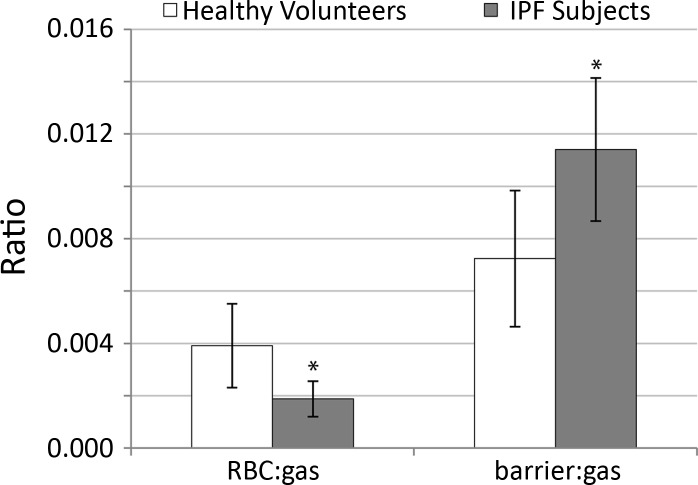
The RBC:barrier ratio was consistently diminished in the entire IPF group relative to the healthy volunteers. As shown in Fig. 3B, the mean RBC:barrier ratio for all healthy volunteers was 0.55 ± 0.13 compared with a more than 3-fold smaller value of 0.16 ± 0.03 for IPF subjects (P = 0.0002). Further investigation revealed that the reduced RBC:barrier ratio in IPF subjects was attributable to a 2.1-fold reduction in the RBC:gas (P = 0.02) and a 1.6-fold increase in barrier:gas (P = 0.01) (Fig. 4). These ratios are summarized in Table 1.
Fig. 4.
Comparison of the RBC:gas ratio and the barrier:gas ratio in healthy volunteers and IPF subjects. The RBC:gas ratio (left) was reduced ∼2-fold in IPF vs. healthy subjects (*P = 0.02), and the barrier:gas ratio (right) was increased ∼1.6-fold in IPF subjects (*P = 0.01). These two effects together reduced the overall RBC:barrier ratio in the IPF subjects.
In addition to their significantly lower RBC:barrier ratios, the IPF subjects also exhibited differences in their RBC resonance frequencies and widths. As shown in Table 2, the RBC resonance frequency was 342.2 ± 15.5 Hz above the barrier resonance in healthy volunteers, but the RBC frequency was 43.3 Hz lower in patients with IPF (298.9 ± 21.7 Hz, P = 0.003). By contrast, the frequencies of the barrier and gas-phase resonances did not differ significantly between the groups. However, the spectral widths of all three resonances were significantly different between the two groups. The mean width of the RBC resonance was 166.5 ± 16.5 Hz (with line broadening subtracted) in the healthy volunteers, but significantly narrower in the IPF subjects (125.8 ± 16.8 Hz, P = 0.0002). This was true for the barrier resonance as well, with the healthy volunteers exhibiting a width of 144.4 ± 10.4 Hz, and the IPF subjects exhibiting a barrier width of only 124.7 ± 6.0 Hz (P = 0.001). The width of the gas-phase resonance in IPF subjects was ∼62% of that in the healthy volunteers (18.6 ± 6.0 vs. 30.2 ± 9.6 Hz, P = 0.01).
Table 2.
Frequencies and widths of resonances in healthy volunteers and subjects with IPF
| Frequency, Hza |
Width, Hz |
|||||
|---|---|---|---|---|---|---|
| RBC (ppm) | Barrier (ppm) | Gas | RBC | Barrier | Gas | |
| Healthy | 342.2 ± 15.5 (217.4) | 0.0 ± 11.0 (198.0) | −3497.0 ± 6.5 | 166.5 ± 16.5 | 144.4 ± 10.4 | 30.2 ± 9.6 |
| IPF | 298.9 ± 21.7 (215.1) | −5.0 ± 9.7 (198.2) | −3500.2 ± 13.8 | 125.8 ± 16.8 | 124.7 ± 6.0 | 18.6 ± 6.0 |
| P value | 0.003 | 0.18 | 0.18 | 0.0002 | 0.0009 | 0.01 |
Values are means ± SD.
Frequencies are referenced to the mean barrier resonance frequency in the healthy volunteers. Chemical shift (ppm) referenced to the gas phase frequency of 17.66 MHz.
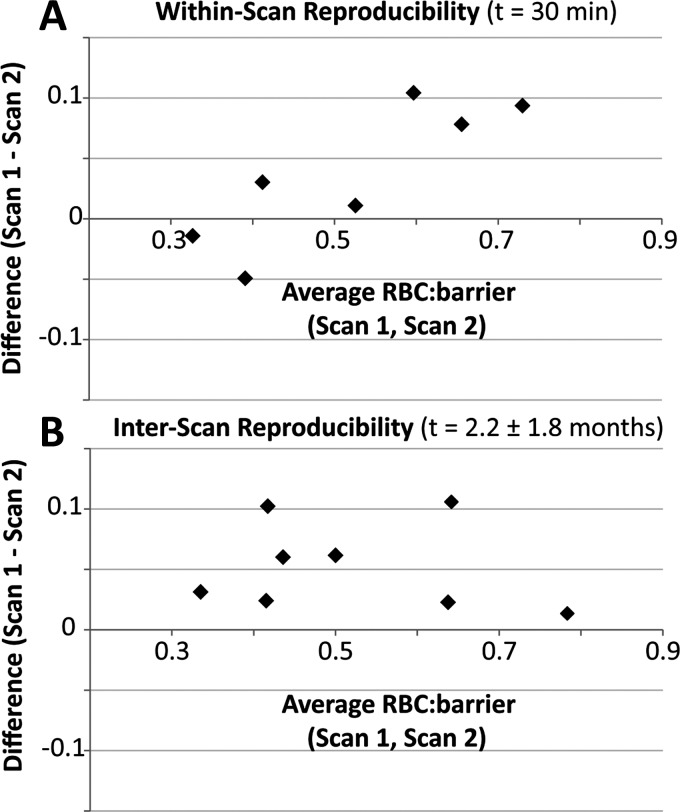
As shown in Fig. 5A, the RBC:barrier ratio obtained in healthy volunteers during a given MRI session did not change significantly. The mean difference between scans 1 and 2 was 0.036 ± 0.06 or 6.6% (P = 0.15) as seen in Table 3. While RBC:barrier was highly reproducible within a given imaging session, it did change significantly over scans conducted on different days. On average, the RBC:barrier ratio acquired on the second day of scanning was 8.25% lower than on day one, and this was significant (P = 0.01). As shown in Table 4, the bulk of this variability was contributed by two subjects whose RBC:barrier ratios were reduced by 24.5% and 16.5% (subjects 2 and 9) between scan and rescan. The RBC:gas and barrier:gas metrics, however, showed no significant short-term or interscan change (P > 0.1). The same was true for the frequencies and widths of all three resonances, all of which were highly reproducible (P > 0.1).
Fig. 5.
Bland-Altman plots showing the reproducibility of the RBC:barrier ratio. A: within a given session, RBC:barrier had a variability of 6.6%, which was not significant. B: over different scanning sessions, RBC:barrier was significantly reduced in the follow-up session (P = 0.01), with a mean variability of 8.25%.
Table 3.
Within-scan variability of the spectroscopic ratios
| Ratios |
||||||
|---|---|---|---|---|---|---|
| RBC:barrier |
RBC:gas |
Barrier:gas |
||||
| Subject | Scan 1 | Scan 2 | Scan 1 | Scan 2 | Scan 1 | Scan 2 |
| 2 | 0.37 | 0.42 | 0.003 | 0.004 | 0.006 | 0.010 |
| 3 | 0.32 | 0.33 | 0.003 | 0.002 | 0.007 | 0.007 |
| 4 | 0.78 | 0.68 | 0.003 | 0.003 | 0.004 | 0.005 |
| 6 | 0.43 | 0.40 | 0.003 | 0.003 | 0.007 | 0.007 |
| 7 | 0.65 | 0.55 | 0.005 | 0.004 | 0.008 | 0.007 |
| 8 | 0.53 | 0.52 | 0.007 | 0.008 | 0.014 | 0.014 |
| 9 | 0.70 | 0.62 | 0.006 | 0.005 | 0.009 | 0.008 |
Table 4.
Interscan variability of the spectroscopic ratios
| Ratios |
||||||
|---|---|---|---|---|---|---|
| RBC:barrier |
RBC:gas |
Barrier:gas |
||||
| Subject | Scan 1 | Scan 2 | Scan 1 | Scan 2 | Scan 1 | Scan 2 |
| 1 | 0.47 | 0.41 | 0.0024 | 0.0026 | 0.0050 | 0.0065 |
| 2 | 0.47 | 0.37 | 0.0030 | 0.0026 | 0.0064 | 0.0070 |
| 3 | 0.36 | 0.32 | 0.0025 | 0.0020 | 0.0070 | 0.0063 |
| 4 | 0.80 | 0.78 | 0.0029 | 0.0032 | 0.0036 | 0.0041 |
| 6 | 0.43 | 0.40 | 0.0030 | 0.0034 | 0.0069 | 0.0085 |
| 7 | 0.65 | 0.63 | 0.0050 | 0.0059 | 0.0077 | 0.0095 |
| 8 | 0.53 | 0.47 | 0.0072 | 0.0091 | 0.0136 | 0.0194 |
| 9 | 0.70 | 0.59 | 0.0063 | 0.0035 | 0.0091 | 0.0060 |
| 11 | 0.53 | 0.54 | 0.0042 | 0.0041 | 0.0080 | 0.0078 |
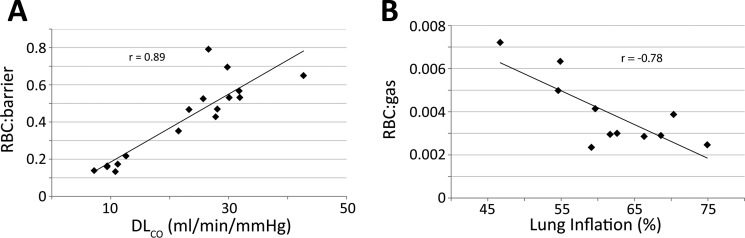
RBC:barrier across all subjects studied, correlated to a high degree with DLCO (r = 0.89, P < 0.0001, Fig. 6A). There was also evidence that for healthy subjects, total RBC signal depended on lung inflation level during acquisition (Table 1). Since the subjects received an identical fixed volume of gas, those with larger TLC were scanned at a lower level of lung expansion. As shown in Fig. 6B, we found a robust association between the RBC:gas ratio and the lung inflation parameter (r = −0.78, P = 0.005). The barrier:gas ratio also correlated with lung inflation (r = −0.67, P = 0.025); however, the RBC:barrier ratio did not (r = −0.28, P = 0.40).
Fig. 6.
Correlations of gas-transfer metrics. A: RBC:barrier was strongly correlated with lung diffusing capacity of carbon monoxide (DLCO) (r = 0.89, P < 0.001). B: in healthy subjects, the RBC:gas metric was significantly reduced by greater lung inflation (r = −0.78, P = 0.005).
DISCUSSION
Measuring Diffusion Limitation with a Perfusion-Limited Gas
The ability to use 129Xe to measure diffusion limitation may appear counterintuitive given that xenon, like other inert gases, is traditionally considered to be “perfusion-limited.” This apparent paradox was recently explained by Cleveland et al. (6), who pointed out the fundamental differences between MR-based detection of hyperpolarized 129Xe magnetization and traditional diffusing capacity measurements. Standard physiological measurements of gas transfer exploit the physical consumption of a tracer (V) in accordance with Fick's law, V = [Ad(PA − PC)t]/x (Ref. 8), where A is the surface area, d is the diffusion constant, PA is the alveolar partial pressure of the tracer, PC is the capillary partial pressure of the tracer, t is the measurement time, and x is the thickness of the membrane. This consumption is driven by the alveolar-capillary partial pressure gradient (PA − PC). Since inert gases like xenon quickly saturate the alveolar septum and cause this partial pressure gradient to vanish, the only means to remove more xenon from the alveolar spaces during a breath hold is to increase perfusion. This is why xenon is considered to be “perfusion-limited.”
To measure diffusion limitation by traditional means, carbon monoxide (CO) is typically used because it binds strongly to hemoglobin such that the pulmonary blood acts as an “infinite sink” and the alveolar-capillary partial pressure gradient is maintained (32). In this way, the bulk consumption of CO during a breath-hold is primarily limited by its rate of diffusion across the blood-gas barrier. If the blood-gas barrier thickness increases, the flux of CO across it is reduced and less CO is taken up by the capillary blood before it exits the gas-exchange region.
However, when HP 129Xe is combined with MR-based detection and destruction of magnetization, it can detect diffusion limitation. It should be noted that while the net flux of xenon atoms across the barrier is zero, the net flux of 129Xe magnetization into these compartments is nonzero. This is because the RF pulses used to detect dissolved 129Xe magnetization also consume it. This depletion can only be replenished by new magnetization from the xenon diffusing in from the alveolar compartment (20). Because the barrier is adjacent to the alveoli, magnetization within this compartment is replenished first. By contrast, the RBC compartment cannot be replenished until 129Xe magnetization has traversed the barrier. Thus 129Xe magnetization in the barrier and RBCs is “pinned” to a depleted, nonequilibrium value by continuous RF irradiation. With a thickened barrier, the magnetization flux to the RBCs will be particularly diminished, as the time it takes for the magnetization to diffuse across a barrier (τ) scales as the square of its thickness (Δx) as τ ∼ (Δx)2/2D, where D is the diffusion coefficient (Ref. 10). Such thickening only impacts the replenishment of the RBC resonance, since only this compartment is separated from the airspaces by the barrier tissue. By contrast, the barrier signal is not diminished by thickening, and depending on its chemical composition, this signal can even be increased by inflammation or fibrosis. Hence, with the proper choice of acquisition parameters, interrogating HP 129Xe signal in the RBCs relative to the 129Xe signal in the barrier can detect diffusion impairment.
In this small subject population, the global RBC:barrier ratio exhibited a considerable dynamic range. For example, one IPF subject exhibited an RBC:barrier more than 5-fold lower than the largest ratio seen in a healthy volunteer (0.79). This supports our hypothesis that 129Xe transfer spectroscopy can serve as a marker of diffusion impairment. This is further bolstered by a strong correlation between RBC:barrier and the well-established measure of pulmonary gas exchange, DLCO. Although these two techniques differ substantially in their implementation, they are able to measure similar effects.
Gas Transfer in Healthy Volunteers
The mean RBC:barrier ratio in healthy volunteers was 0.55 ± 0.13, but exhibited a relatively broad range (0.35–0.79), even in this relatively small subject population. The lowest ratio observed was 0.35 or 64% of the population mean, whereas the largest value of 0.79 was 144% of the population mean. In healthy volunteers, we assume that the blood-gas barrier is of normal thickness, and there exists no diffusive limitation to 129Xe reaching the RBCs. Thus the remaining source of variability appears to be contributed by individual differences in the resting capillary blood volume. If a greater fraction of the capillary bed is recruited and perfused at rest, this provides a larger pool of RBCs for 129Xe to enter, and the RBC:barrier ratio will increase accordingly. Additionally, as noticed by Qing et al. (22), our healthy volunteers with the largest lung capacity also exhibited the greatest RBC:barrier ratio. This is likely because for these subjects, inhaling 1 liter of gas from FRC left them at the lower lung inflation during data acquisition, and this resulted in a larger capillary blood volume during MR acquisition (18).
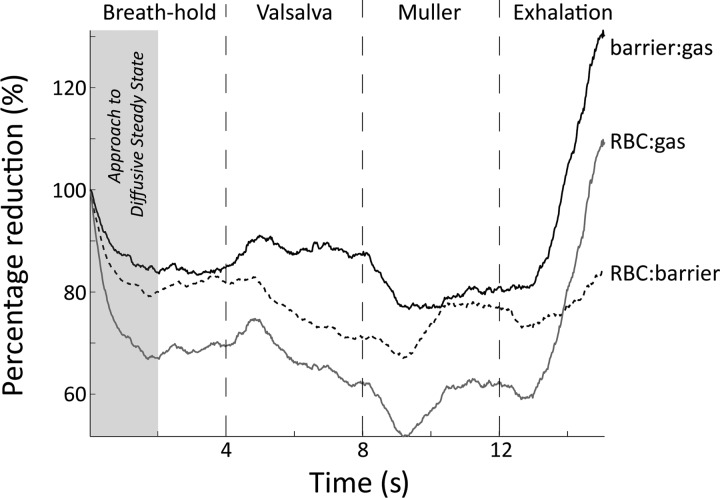
The potential sensitivity of RBC:barrier to lung inflation was illustrated in one healthy volunteer who conducted a series of breathing maneuvers during continuous acquisition of 129Xe spectra (Fig. 7). With the same acquisition parameters described in methods, 803 spectra were acquired over a 16-s time interval. During this time, the subject performed a 4-s breath hold, followed directly by a 4-s Valsalva maneuver (positive pulmonary pressure caused by forced exhalation against a closed airway), a 4-s Müller maneuver (negative pulmonary pressure caused by forced inhalation against a closed airway), and finally an exhale to FRC during the remaining 4 s. During the initial 2 s of the breath-hold, 129Xe magnetization reaches a diffusive steady state as 129Xe magnetization in the larger vasculature (downstream signal) is depleted by RF pulsing. Then the Valsalva maneuver increases alveolar pressure, which decreased capillary blood volume, estimated to be 17.2% over 5 s by Smith and Rankin (27). This corresponds reasonably well to our observed 11% reduction in RBC:barrier over 4 s during the Valsalva maneuver. In contrast, during the Müller maneuver Smith and Rankin (27) demonstrated that capillary blood volume increases by 6.1% over 5 s. This again is consistent with the 5.4% increase in RBC:barrier that we observed. Last, during exhalation, as the alveolar pressure is greatly reduced, the transfer of 129Xe to both the barrier and the RBCs increased dramatically. Although this is an anecdotal study, it illustrates that the lung inflation and alveolar pressure during the breath-hold can affect these gas-transfer metrics.
Fig. 7.
Changes in the RBC:gas, RBC:barrier, and barrier:gas ratios observed during a breath-hold. Spectra were processed using a “sliding-window” processing technique to provide a pseudotemporal depiction. During the breath-hold, RBC:gas and RBC:barrier ratio are initially reduced by depletion of 129Xe magnetization in the larger vasculature (downstream signal). During the Valsalva maneuver RBC:gas diminishes while barrier:gas remains stable, resulting in diminishing RBC:barrier ratio. This is attributable to a reduction in capillary blood volume during the maneuver. Conversely, the Müller maneuver increases capillary blood volume, and hence the associated ratios. Exhalation increases overall gas transfer, but also maximally increases capillary blood volume, which is reflected in an increasing RBC:barrier.
The reproducibility of the RBC:barrier ratio during repeat testing provides additional insights into this measurement. While intrascan repeat testing showed RBC:barrier to remain stable, this ratio decreased significantly over different days (t = 2.2 ± 1.8 mo, 8.3%, P = 0.01). A large portion of this variability was contributed by subjects 2 and 9, whose RBC:barrier ratios were reduced by 24.5% and 16.5% on follow-up (Table 3). The variation in subject 9 may have been caused in part by a higher heart rate during the first session relative to the second (74 to 55 beats/min). This higher heart rate, and presumed greater cardiac output, may increase capillary recruitment and thereby present a larger capillary blood volume into which 129Xe can diffuse. However, heart rate cannot explain the change in RBC:barrier seen in subject 2 (52 to 49 beats/min). It may be the case that this subject had a lower resting capillary blood volume at scan 2. This could be attributable to differences in lung inflation, as previously discussed. As we did not control for subject exercise prior to the MRI study, the variability in the RBC:barrier may also be caused by differences in the subject's activity profile prior to scans.
Given these observations in healthy subjects, it appears that controlling lung inflation will be important to maximize reproducibility of measurements. However, the optimal means to do so within a 129Xe MRI study is still somewhat unclear. One possibility is to inhale 129Xe and continue on to TLC as is done for DLCO. However, at such inflations it appears from our data that 129Xe gas-transfer and capillary blood volume are minimized and SNR is reduced. Alternatively, Diaz et al. (9) demonstrated excellent reproducibility of 3He apparent diffusion coefficient measurements, by tailoring inhaled volumes to 15% of subject TLC. However, this requires a priori knowledge of lung volumes from plethysmography and requires subjects reliably starting their inhalation from FRC. An alternative approach may be to make measurements at end-expiration as is common in contrast-enhanced perfusion MRI (11). Although this involves exhaling 15–20% of the 129Xe magnetization, it may be the most reliable means to minimize intersubject variability of gas-transfer measurements.
Gas-Transfer in Subjects with IPF
129Xe gas-transfer to RBCs was dramatically reduced in the IPF subject group. In aggregate, these patients exhibited an RBC:barrier ratio that was only 29% of the mean value seen in healthy volunteers. In fact, the lowest ratio observed in IPF was only 24% of the mean in healthy volunteers and represented just 17% of the highest ratio observed in a healthy subject. Although RBC:barrier can vary significantly in healthy subjects, and this variability is likely attributable to differences in resting perfused capillary blood volume, this does not seem a plausible explanation for the low gas-transfer in IPF. In healthy subjects, RBC:barrier exhibited a mean variability of 8.3%, with individual excursions of 20% being somewhat rare. Thus it seems that the more than 3-fold reduction in RBC:barrier observed in IPF subjects cannot simply be attributed to a 3-fold reduction in perfused capillary blood volume. Instead, the reduced gas-transfer to RBCs must be caused, at least in part, by thickening of the blood-gas barrier. Such thickening delays the diffusive replenishment of 129Xe magnetization to the RBCs. Moreover, depending on 129Xe solubility within the thickened barrier, the barrier signal can actually be increased. Both of these effects work together to reduce the RBC:barrier ratio. In fact, to significantly impede the transfer of 129Xe magnetization requires only 10 μm of interstitial thickening (10). Thus we suggest that significant areas of the IPF lung remain perfused even in the presence of a thickened blood-gas barrier. These areas would contribute significantly to diffusion limitation, which we observe both with 129Xe spectroscopy and conventional DLCO. This notion is further supported by pulmonary ventilation/perfusion imaging using high-resolution CT showing that, even in injured or inflamed lung, perfusion can be maintained to continue resolving the insult, despite the deleterious effect on O2 saturation (12).
Changes in the Frequency and Width of 129Xe Resonances
While the amplitudes of the 129Xe RBC and barrier resonances are of primary interest, their frequency and spectral width convey additional insights regarding capillary blood oxygenation and hemoglobin levels (33, 34). A somewhat surprising finding was that the frequency of the RBC resonance was ∼43 Hz (2.4 ppm) lower in patients with IPF than in healthy volunteers (Table 2). This may be understood by considering the work of Wolber et al. (33), who reported in vitro studies showing that the frequency of the RBC resonance increases with oxygen saturation. The RBC frequency increases by 5 ppm (88 Hz at 1.5 T) over an oxygen saturation range of 30–100%. (33). Interestingly, our observed RBC frequency of 217.4 ppm in healthy subjects lies below the 221 ppm in vitro value reported by Wolber et al. for fully oxygenated blood and suggests that average SpO2 in our healthy population is only ∼0.95. The 2.4 ppm lower RBC frequency in our IPF patients suggests an average SpO2 of only 0.60. However, pulse oximetry in all our subjects revealed that healthy subjects had an SpO2 above 95%, and even IPF patients had SpO2 above 90%. This apparent discrepancy can be reconciled by recognizing that SpO2 is measured in the distal vasculature, whereas 129Xe spectroscopy reports from within the alveolar capillary bed. Hence, even in healthy subjects, a fraction of the 129Xe-RBC resonance arises from cells that are early in their transit through the capillary bed and are not yet fully oxygenated. However, in healthy subjects, such oxygenation is largely complete within 1/3 of the blood transit time, and thus 129Xe spectra are derived from roughly 2/3 fully oxygenated blood and 1/3 partially oxygenated blood. By contrast, the diffusion limitation in IPF means that maximal oxygenation is only achieved towards the very end of alveolar-capillary transit. Hence, 129Xe spectra in IPF are more heavily weighted toward deoxygenated RBCs. Thus the reduced 129Xe-RBC frequency in IPF appears to be a second indicator of diffusion limitation. Interestingly, as interstitial thickness will vary across the lung, along with their attendant oxygen saturation times, it may become possible to crudely image such regional oxygenation, by employing chemical shift imaging and measuring RBC frequencies locally (28). By contrast, the frequency of the barrier resonance did not differ significantly between IPF and healthy subjects, consistent with literature reports that it is independent of blood oxygenation (33).
Of further note is that the spectral widths (FWHM) of all three 129Xe resonances are significantly narrower in subjects with IPF than in healthy volunteers (Table 2). For example, the width of the barrier resonance was 144.4 ± 10.4 Hz in healthy volunteers but was reduced to 124.7 ± 6.0 Hz in the IPF subjects (P = 0.001). The width of 129Xe resonances is driven by exchange of 129Xe between the different frequency environments. For the simple case of 129Xe in whole blood, the width of the barrier (plasma) resonance is known to increase linearly with hematocrit (34) as 129Xe exchanges between the RBCs and plasma. The nominal barrier width is ∼127 Hz (T2 ≈ 2.5 ms) for whole blood with hematocrit of 38 ± 2% (34). The diminished barrier width we observed in IPF may indicate that a greater fraction of the barrier resonance is contributed by 129Xe further removed from the RBCs in the now thickened interstitium, where exchange with RBCs is absent. Like the barrier resonance, the RBC resonance was also considerably broader in the healthy volunteers (166.5 ± 16.5 Hz) than in IPF patients (125.8 ± 16.8 Hz, P = 0.0002). The RBC resonance in healthy volunteers was only slightly broader than reported in pure RBCs in vitro, which have a width of ∼152 Hz [T2 ≈ 2.1 ms (34)]. Thus, while the 129Xe-RBC resonance frequency is affected by oxygenation, its narrower width in IPF may indicate that those RBCs experience fewer susceptibility gradients as fibrotic processes keep them more distal from the air-tissue interfaces. Finally, the gas-phase resonance was also broader in the healthy volunteers (30.2 ± 9.6 Hz) than in IPF subjects (18.6 ± 6.0 Hz, P = 0.01). This may indicate that some portion of the gas-phase linewidth in healthy volunteers is contributed by exchange into the dissolved-phase compartments, and this exchange may be impeded in the presence of fibrosis.
Study Limitations
There are several technical limitations that must be considered when interpreting the RBC:barrier and other ratios reported here. First, the measurement is likely to depend on the choice of RF pulse TR and flip angle. If a lower flip angle and/or longer TR were used, 129Xe magnetization in RBCs would not be depleted as much and significant RBC signal might arise from larger vasculature to increase the RBC:barrier ratio. Similarly, the opposite case of larger flip and/or shorter TR would limit RBC replenishment and reduce the RBC:barrier ratio. The flip angles used to acquire spectra in our study varied between 15.3° and 19.6° because they were taken prior to transmitter gain calibration. This variability could explain some degree of variability seen in the RBC:barrier on repeat scans. Second, the ratio calculation is predicated on equal excitation of the RBC and barrier resonances. But this can be undermined by distortion of the RF pulse frequency profile due to nonlinearity of the power amplifier. In our system we estimate this to result in application of a marginally (6%) lower flip angle on the RBC than the barrier resonance. However, the impact of such imperfection is not immediately clear as it causes less depletion of the RBC magnetization, but also less excitation, and these two effects would cancel. In any case, it should be possible to create a distortion-free selective RF frequency profile, as demonstrated by Leung et al. (17), using phase modulated composite RF pulses. However, none of the RF effects discussed above are expected to change the relative differences we observed in IPF vs. healthy volunteers; they would only impact the absolute magnitude of RBC:barrier obtained across different MRI systems. Furthermore, in this preliminary study, subject hematocrit was not measured. We speculate that the hematocrit will positively correlate with the RBC:barrier ratio. Thus, future studies are needed to consider correcting spectroscopic metrics for potential disease-induced variations in hematocrit (31), and ensure that the RBC:barrier ratio is truly indicative of diffusion limitation. Last, we note that RBC:barrier in patients with significant lung disease could be affected by heterogeneity of ventilation (29) and diffusion-perfusion ratios (21). The heterogeneity in ventilation has been shown to change DLCO measurements by ∼6% (29). Also, much like the uptake of CO is dictated by the regional diffusion-perfusion ratio (21), changes in regional perfusion will impact the uptake of 129Xe in the RBCs.
Conclusions
This study has confirmed our hypothesis that spectroscopic interrogation of dissolved-phase HP 129Xe can detect diffusion impairment in subjects with IPF. These patients exhibited an RBC:barrier that was 3.3-fold lower than for healthy volunteers, and this ratio was strongly correlated with the well-established DLCO measure of gas exchange. Given its excellent sensitivity to diffusion impairment, this metric can lend itself to longitudinal assessment of lung function and analyze response to a potential therapy. Moreover, the ability to detect variations in RBC:barrier dynamically, during breath-hold and exhalation, provides a fundamentally novel means to study gas exchange dynamics. Additionally, 129Xe transfer spectroscopy provides intriguing complementary information, via the oxygenation-dependent frequency of the RBC resonance, as well as the line widths of all three resonances. However, further studies are needed to better understand the degree to which RBC:barrier and all other parameters reported here depend on lung inflation and capillary blood volume. Methods must be developed to ensure that the ratio is measured in a way that is maximally reproducible, not only at a given imaging site, but across centers. While the present work measures gas exchange on a global basis, we anticipate this measurement will provide the fundamental basis for future work that remains to be done that focuses on three-dimensional imaging of the transfer of 129Xe exclusively to the RBC.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant R01-HL-105643; partly by Center for In Vivo Microscopy Grant NIBIB-P41-EB-015897; by Duke Clinical and Translational Science Award (CTSA) UL1-RR-024128 from the National Center for Research Resources; and by National Institutes of Health Grant AI-081672.
DISCLOSURES
B. Driehuys is the founder and Chief Technology Officer of Polarean, Inc.
AUTHOR CONTRIBUTIONS
Author contributions: S.S.K., J.E.R., W.M.F., C.R.R., H.P.M., and B.D. conception and design of research; S.S.K., M.S.F., and J.V.S. performed experiments; S.S.K., S.W.Y., M.G.L., and B.D. analyzed data; S.S.K., S.W.Y., and B.D. interpreted results of experiments; S.S.K. prepared figures; S.S.K. and B.D. drafted manuscript; S.S.K., M.S.F., S.W.Y., J.E.R., W.M.F., C.R.R., H.P.M., and B.D. edited and revised manuscript; S.S.K., M.S.F., S.W.Y., M.G.L., J.V.S., J.E.R., W.M.F., C.R.R., H.P.M., and B.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. Davies for subject monitoring, Dr. Z. I. Cleveland for helpful discussions, and S. Zimney for carefully proofreading this manuscript.
REFERENCES
- 1.Agustí AG, Roca J, Gea J, Wagner PD, Xaubet A, Rodriguez-Roisin R. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Am Rev Respir Dis 143: 219–225, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Bifone A, Song YQ, Seydoux R, Taylor RE, Goodson BM, Pietrass T, Budinger TF, Navon G, Pines A. NMR of laser-polarized xenon in human blood. Proc Natl Acad Sci USA 93: 12932–12936, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang YV, Quirk JD, Ruset IC, Atkinson JJ, Hersman FW, Woods JC. Quantification of human lung structure and physiology using hyperpolarized 129Xe. Magn Reson Med 71: 339–344, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Cherubini A, Bifone A. Hyperpolarised xenon in biology. Prog Nucl Magn Reson Spectrosc 42: 1–30, 2003 [Google Scholar]
- 5.Cleveland ZI, Cofer GP, Metz G, Beaver D, Nouls J, Kaushik SS, Kraft M, Wolber J, Kelly KT, McAdams HP, Driehuys B. Hyperpolarized Xe-129 MR imaging of alveolar gas uptake in humans. Plos One 5: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleveland ZI, Virgincar RS, Qi Y, Robertson SH, Degan S, Driehuys B. 3D MRI of impaired hyperpolarized 129Xe uptake in a rat model of pulmonary fibrosis. NMR Biomed 2014 May 12. 10.1002/nbm.3127 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotes JE, Chinn DJ, Miller MR. Lung Function: Physiology, Measurement and Application in Medicine. New York: Wiley, 2009 [Google Scholar]
- 8.Crank J. The Mathematics of Diffusion. New York: Oxford University Press, 1975 [Google Scholar]
- 9.Diaz S, Casselbrant I, Piitulainen E, Pettersson G, Magnusson P, Peterson B, Wollmer P, Leander P, Ekberg O, Akeson P. Hyperpolarized He-3 apparent diffusion coefficient MRI of the lung: reproducibility and volume dependency in healthy volunteers and patients with emphysema. J Magn Reson Imaging 27: 763–770, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Driehuys B, Cofer GP, Pollaro J, Mackel JB, Hedlund LW, Johnson GA. Imaging alveolar-capillary gas transfer using hyperpolarized Xe-129 MRI. Proc Natl Acad Sci USA 103: 18278–18283, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fink C, Ley S, Risse F, Eichinger M, Zaporozhan J, Buhmann R, Puderbach M, Plathow C, Kauczor HU. Effect of inspiratory and expiratory breathhold on pulmonary perfusion: assessment by pulmonary perfusion magnetic resonance imaging. Invest Radiol 40: 72–79, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Fuld MK, Halaweish AF, Haynes SE, Divekar AA, Guo J, Hoffman EA. Pulmonary perfused blood volume with dual-energy CT as surrogate for pulmonary perfusion assessed with dynamic multidetector CT. Radiology 267: 747–756, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Hsia CCW. Alveolo-capillary diffusion of hyperpolarized 129Xe as a marker of pulmonary fibrosis. J Appl Physiol. 10.1152/japplphysiol.00688.2014 [DOI] [PubMed] [Google Scholar]
- 13.Kaushik SS, Cleveland ZI, Cofer GP, Metz G, Beaver D, Nouls J, Kraft M, Auffermann W, Wolber J, McAdams HP, Driehuys B. Diffusion-weighted hyperpolarized Xe-129 MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med 65: 1155–1165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaushik SS, Freeman MS, Cleveland ZI, Davies J, Stiles J, Virgincar RS, Robertson SH, He M, Kelly KT, Foster WM, McAdams HP, Driehuys B. Probing the regional distribution of pulmonary gas exchange through single-breath gas- and dissolved-phase 129Xe MR imaging. J Appl Physiol 115: 850–860, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeler J. Understanding NMR Spectroscopy. New York: Wiley, 2013 [Google Scholar]
- 16.King TE, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Leung G, Norquay G, Schulte RF, Wild JM. Radiofrequency pulse design for the selective excitation of dissolved 129Xe. Magn Reson Med 2014. January 6. 10.1002/mrm.25089 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Miller J, Johnson R., Jr Effect of lung inflation on pulmonary diffusing capacity at rest and exercise. J Clin Invest 45: 493, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mugler JP, Altes TA, Ruset IC, Dregely IM, Mata JF, Miller GW, Ketel S, Ketel J, Hersman FW, Ruppert K. Simultaneous magnetic resonance imaging of ventilation distribution and gas uptake in the human lung using hyperpolarized xenon-129. Proc Natl Acad Sci USA 107: 21707–21712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patz S, Muradyan I, Hrovat MI, Dabaghyan M, Washko GR, Hatabu H, Butler JP. Diffusion of hyperpolarized Xe-129 in the lung: a simplified model of Xe-129 septal uptake and experimental results. New J Phys 13: 015009, 2011. ( 10.1088/1367-2630/13/1/015009) [DOI] [Google Scholar]
- 21.Piiper J, Sikand R. Determination of DCO by the single breath method in inhomogeneous lungs: theory. Respir Physiol 1: 75–87, 1966 [DOI] [PubMed] [Google Scholar]
- 22.Qing K, Ruppert K, Jiang Y, Mata JF, Miller GW, Shim YM, Wang C, Ruset IC, Hersman FW, Altes TA, Mugler JP. Regional mapping of gas uptake by blood and tissue in the human lung using hyperpolarized xenon-129 MRI. J Magn Reson Imaging 39: 346–359, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafii R, Juarez MM, Albertson TE, Chan AL. A review of current and novel therapies for idiopathic pulmonary fibrosis. J Thorac Dis 5: 48–73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ. An Official ATS/ERS/JRS/ALAT Statement. Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med 183: 788–824, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N Engl J Med 370: 2071–2082, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Selman Ms King JTE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134: 136–151, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Smith TC, Rankin J. Pulmonary diffusing capacity and the capillary bed during Valsalva and Muller maneuvers. J Appl Physiol 27: 826–833, 1969 [DOI] [PubMed] [Google Scholar]
- 28.Swanson SD, Rosen MS, Coulter KP, Welsh RC, Chupp TE. Distribution and dynamics of laser-polarized Xe-129 magnetization in vivo. Magn Reson Med 42: 1137–1145, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Thompson B, Verbanck S, Schuermans D, Van Malderen S, Vincken W. The effect of conductive ventilation heterogeneity on. J Appl Physiol 104: 1094–1100, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Virgincar RS, Cleveland ZI, Kaushik SS, Freeman MS, Nouls J, Cofer G, Martinez-Jimenez S, He M, Kraft M, Wolber J, McAdams HP, Driehuys B. Quantitative analysis Of hyperpolarized 129Xe ventilation imaging in healthy volunteers and subjects with chronic obstructive pulmonary disease. NMR Biomed 424–435, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weil JV, Jamieson G, Brown DW, Grover RF. The red cell mass-arterial oxygen relationship in normal man: application to patients with chronic obstructive airway disease. J Clin Invest 47: 1627–1639, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West JB. Diffusion. In: Respiratory Physiology—The Essentials, edited by Coryell PA. Baltimore, MD: Williams and Wilkins, 1995 [Google Scholar]
- 33.Wolber J, Cherubini A, Leach MO, Bifone A. Hyperpolarized 129Xe NMR as a probe for blood oxygenation. Magn Reson Med 43: 491–496, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Wolber J, Cherubini A, Santoro D, Payne GS, Leach MO, Bifone A. Linewidths of hyperpolarized 129Xe NMR spectra in human blood at 1.5T. In: Proc Intl Soc Magn Reson Med 8: 2000, p. 970 [Google Scholar]