Abstract
Percutaneous electrical vestibular stimulation evokes reflexive responses in appendicular muscles that are suppressed during tasks in which the muscles are not contributing to balance control. In neck muscles, which stabilize the head on the torso and in space, it is unclear whether similar postural task dependence shapes vestibular reflexes. We investigated whether vestibulocollic reflexes are modulated during tasks in which vestibular information is not directly relevant to maintaining the head balanced on the torso. We hypothesized that vestibulocollic reflexes would be 1) evoked when neck muscles are not involved in balancing the head on the torso and 2) invariant across synergistic neck muscle contraction tasks. Muscle activity was recorded bilaterally in sternocleidomastoid and splenius capitis muscles during head-free and head-fixed conditions while subjects were exposed to stochastic electrical vestibular stimulation (±5 mA, 0–75 Hz). Significant vestibular reflex responses (P < 0.05) were observed during head-free and head-fixed trials. Response magnitude and timing were similar between head-free and head-fixed trials for sternocleidomastoid, but splenius capitis magnitudes decreased with the head fixed by ∼25% (P < 0.05). Nevertheless, this indicates that vestibulocollic responses are evoked independent of the requirement to maintain postural control of the head on the torso. Response magnitude and timing were similar across focal muscle contractions (i.e., axial rotation/flexion/extension) provided the muscle was active. In contrast, when subjects cocontracted neck muscles, vestibular-evoked responses decreased in sternocleidomastoid by ∼30–45% (P < 0.05) compared with focal muscle contractions but remained unchanged in splenius capitis. These results indicate robust vestibulocollic reflex coupling, which we suggest functions through its closed-loop influence on head posture to ensure cervical spine stabilization.
Keywords: neck postural control, reflex modulation, muscle synergy, neck muscle cocontraction, isometric contraction
whether we are engaged in a task of balance, gaze stabilization, or locomotion, vestibular information is crucial to human movement control. The vestibular contribution to movement can be probed with electrical vestibular stimulation (Fitzpatrick and Day 2004), a technique that modulates the firing rates of vestibular afferents (Goldberg et al. 1984; Kim and Curthoys 2004). Electrical vestibular stimulation elicits ocular, head, and whole body movements through vestibular reflexes projecting to motoneurons controlling the eye, axial, and appendicular musculature (Ali et al. 2003; Aw et al. 2006; Britton et al. 1993; Fitzpatrick et al. 1996; Forbes et al. 2013; Lund and Broberg 1983; Nashner and Wolfson 1974; Watson et al. 1998; Watson and Colebatch 1998). In lower limb muscles, vestibular reflexes are dependent on the postural task and contribute to balance only when vestibular information is relevant to postural control (Fitzpatrick et al. 1994). For example, vestibular reflexes are absent when upright subjects are supported by a rigid beam and balancing a mechanical-body-equivalent inverted pendulum using only their ankles (Fitzpatrick et al. 1994). In contrast to lower limb muscles, vestibuloocular reflexes (VORs) are not affected by the postural relevance of vestibular information. Stereotypical VOR responses are elicited by electrical stimuli when the head is restrained in both supine (Aw et al. 2006) and seated (MacDougall et al. 2003; Watson et al. 1998) postures. Because the VOR stabilizes the visual image on the retina in both balancing and nonbalancing conditions, a posture-independent VOR makes sense. Reflexive eye movements are even elicited in complete darkness, where the VOR is no longer functionally relevant (MacDougall et al. 2003; Watson et al. 1998). Thus, in humans, the postural task dependence of vestibular reflexes is not a universal feature for all muscles throughout the body. This raises the question of whether postural task dependence is present for the vestibulocollic reflexes (VCRs).
Neck muscles, depending on the task at hand, can be considered as part of the postural or gaze control system. Neck muscles maintain the upright orientation of the head over the torso. Therefore, VCRs may be present only when vestibular information is relevant for the postural control of the head on the torso. Neck muscles also function synergistically with extraocular muscles during large gaze shifts involving eye and head movements (see Guitton 1992 and Guitton et al. 2003 for reviews). Therefore, neck muscles exhibit short reflex latencies to vestibular stimuli, similar to those observed in the extraocular musculature [VCR: 8–10 ms (Rosengren et al. 2010; Watson and Colebatch 1998); VOR: 5–10 ms (see Cullen and Roy 2004 for review)]. If the neck muscles have a functional role closer to extraocular muscles than to postural muscles, VCR responses independent of the postural task may be expected. Partial support for this proposition can be found in the absence of VCR modulation by vision, external support, stance width (Welgampola and Colebatch 2001), and posture (Watson and Colebatch 1998). However, this evidence against postural task dependence in neck muscles is less compelling than the evidence for postural task dependence in appendicular muscles; subjects in each of these neck muscle studies held their head in an upright or elevated position that ensured vestibular information was still relevant to the postural control of the head on the torso. Here we are interested in evaluating sustained postural control tasks over a period of tens of seconds rather than active eye-head gaze shifts that can occur in under 200 ms, where in the latter task dependence (i.e., active vs. passive movements) is known to affect the VOR (see Cullen and Roy 2004 for review) and hypothesized to influence the VCR (Ezure and Sasaki 1978; Goldberg and Cullen 2011; Roy and Cullen 2001, 2004). Thus our goal was to investigate the postural task dependence of the VCR during conditions in which vestibular information was both relevant and not relevant to stabilizing the head on the torso. We hypothesized that similar to extraocular muscles, electrically evoked VCR responses would be present when the head and torso are restrained, i.e., when the neck muscles are not involved in balancing the head on the torso. To assess this hypothesis, we provide a nonpostural task comparable to that used to study responses in lower limb muscles, where vestibular reflexes are suppressed when subjects contract leg muscles while seated (Britton et al. 1993; Fitzpatrick et al. 1994).
If, as we hypothesize, the VCR remains intact despite head restraint, we then questioned whether the electrically evoked VCR is dependent on the descending motor command or simply dependent upon the neck muscle being active. Unlike lower limb muscles, neck muscles are not fixed in their agonist-antagonist relationships. For instance, bilateral neck muscles [e.g., sternocleidomastoid (SCM)] that are agonists during flexion are antagonists during axial rotation. To achieve this flexibility, vestibulospinal and reticulospinal neurons innervate multiple neck muscles to generate functional muscle synergies (Sugiuchi et al. 2004). This flexible control of the neck musculature does not lend itself well to fixed reciprocal inhibitory influences on the VCR. Therefore, we hypothesized that electrically evoked VCR responses would be present when a neck muscle is active but would not be modulated with the concurrent activation of other neck muscles (agonists-antagonists). To assess this hypothesis, we compared reflex responses in neck muscles while they were active with different combinations of agonist and antagonist neck muscles (e.g., flexion, extension, and axial rotation), as well as cocontraction of all neck muscles.
METHODS
Subjects.
Eight healthy subjects [7 men, 1 woman; age 24–48 yr, mass 76 ± 12 kg, height 1.76 ± 0.10 m (mean ± SD)] with no self-reported history of neurological disorders or injuries participated in these experiments. The experimental protocol was explained prior to the experiment, and all subjects gave written informed consent. The experiment conformed to the Declaration of Helsinki and was approved by the University of British Columbia's Clinical Research Ethics Board.
Vestibular stimuli.
Vestibular stimulation was applied to the subjects with carbon rubber electrodes (∼9 cm2) in a binaural bipolar arrangement. The electrodes were coated with Spectra 360 electrode gel (Parker Laboratories, Fairfield, NJ) and secured over the subject's mastoid processes with an elastic headband. The stimulus was delivered as an analog signal via a data acquisition board (PXI-6289, National Instruments, Austin, TX) to an isolated constant-current stimulator (STMISOL, Biopac, Goleta, CA). All subjects were exposed to the same realization of the stimulus: a 60-s filtered white noise stochastic vestibular stimulation (SVS) having a bandwidth of 0–75 Hz and a root-mean-square (RMS) current of 1.41 mA (amplitude peak ±5 mA) (Forbes et al. 2013). The signal was generated off-line with MATLAB (MathWorks, Natick, MA).
Instrumentation.
Intramuscular electromyography (EMG) was recorded bilaterally in the SCM and splenius capitis (SPL) with indwelling electrodes. Pairs of 0.05-mm wires (Stablohm 800A, California Fine Wire, Grover Beach, CA) were inserted under ultrasound guidance (Blouin et al. 2007) (Micromaxx, Sonosite, Bothell, WA). One of the two wires of each electrode had 2–3 mm of exposed wire to allow the recording of multiunit EMG potentials. Each wire was placed near the center of its muscle's horizontal cross section. In the SCM muscles, the wire always remained superficial to the readily identifiable cleidomastoid subvolume (Kamibayashi and Richmond 1998). All EMG signals were amplified (×200; NeuroLog, Digitimer, Welwyn Garden City, UK) and band-pass filtered (0.05–1,000 Hz) before digitization. Isometric neck forces and moments were measured with a six-axis load cell (JR3 E-Series, JR3, Woodland, CA). EMG, forces, moments, and vestibular stimuli data were digitized and recorded at 2,000 Hz via a digital acquisition board (PXI-4495, National Instruments) using a custom LabVIEW software program (National Instruments).
Protocol.
Subjects were seated with their eyes closed throughout all experiments and performed neck muscle contraction tasks in a head-free or -fixed (i.e., isometric) condition. Subjects had their torso firmly strapped to a rigid vertical seatback for all trials, ensuring that stabilization of the head was always maintained relative to a stationary torso. During head-fixed conditions subjects had their head firmly clamped to the overhead load cell via a helmet (Pro-tec, Vans, Cypress, CA). The experiment was divided into six blocks: two blocks to examine the effects of the postural control task and four blocks to examine the effects of descending motor command (see Fig. 1). Each block comprised four 60-s trials, where the first two trials were without stimulation and the next two trials were with SVS stimulation. The presentation order of trials with and without stimulation was not randomized because the trials without stimulation were required to define the target force or moment for the stimulation trials (see below).
Fig. 1.
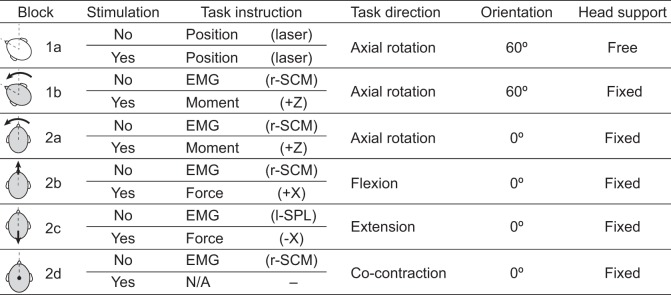
Experimental test matrix summarizing task instruction and direction and head orientation and support. Head-free and head-fixed conditions are represented as open and shaded heads, respectively. Accompanying arrows indicate the direction of load applied by subjects in fixed conditions. For task instruction, information provided in parentheses indicates the source of the defined target. For EMG tasks, muscle activity was matched to the head-free position task (block 1a). r-SCM, right sternocleidomastoid; l-SPL, left splenius capitis.
Subjects first performed a head-free task (block 1a in Fig. 1), in which they were instructed to rotate their head 60° to the left to activate the right (r-)SCM and left (l-)SPL muscles and extend their head and neck to orient the Reid's plane 18° up from horizontal. This head position maximizes the perception of head roll during electrical vestibular stimulation (Day and Fitzpatrick 2005; Fitzpatrick and Day 2004). Head orientation was maintained throughout the trials with a headgear-mounted laser and verbal feedback from the experimenter. The head-free condition without stimulation was used to define the target muscle activity for all five head-fixed conditions. Target muscle activity was calculated as the RMS of the 50-Hz high-pass filtered (2nd-order Butterworth) EMG signals for each muscle separately over each trial and then averaged across the two nonstimulation trials.
To examine the effect of the postural control task on the VCR, subjects then performed a second block of trials (block 1b in Fig. 1) with the head fixed at the same orientation (60° left axial rotation, Reid's plane at 18° extension). Subjects were asked to generate a yaw moment to the left and were given verbal instructions throughout the trial to increase or decrease the moment to ensure that r-SCM muscle activity remained equivalent to the target muscle activity derived from the head-free yaw task. During trials without stimulation, the r-SCM EMG was processed in real time by first high-pass filtering (50 Hz), then calculating the RMS (20 ms window), and then low-pass filtering (1 Hz) the RMS EMG for presentation to the experimenter. During the equivalent condition with electrical vestibular stimulation, online EMG could not be used because of stimulus-induced artifact. Therefore, the mean yaw moment obtained from the two preceding trials without stimulation was used to calculate a target moment—defined as the mean moment across both trials—for the stimulation trials. During both trial types (with and without stimulation), horizontal forces were monitored and subjects were given verbal instructions throughout the experiments to maintain these close to zero.
To examine whether the VCR is similar across variations in the descending motor command, subjects performed four additional blocks: three blocks of isometric trials (blocks 2a–2c in Fig. 1) in which they contracted their neck muscles to generate a yaw moment, a flexion moment, and an extension moment (measured as anterior and posterior forces at the load cell location) and a fourth block (block 2d in Fig. 1) used for comparison in which subjects were instructed to cocontract all neck muscles while maintaining zero net forces and moments. The three arrangements of isometric contraction (yaw, flexion, and extension) were chosen to determine whether the agonist-antagonist relationship of two contracting muscles influenced the vestibular reflex. The fourth cocontraction trial was chosen to examine whether muscle activity without a focal muscle contraction was sufficient to evoke the reflex response. In all trials within these four blocks, the head was fixed, facing forward, and oriented with Reid's plane at 18°. These four blocks were performed in a random order. During isometric muscle contractions (i.e., yaw, flexion, or extension; blocks 2a–2c) without stimulation, subjects applied forces/moments that generated the same RMS muscle activity observed during the head-free task (block 1a). Equivalent activity in the r-SCM muscle was used for the yaw and flexion tasks, whereas equivalent activity in the l-SPL muscle was used for the extension task. During the subsequent stimulation trials, subjects generated forces/moments equivalent to those of the preceding trials without stimulation. For blocks 2a–2c, subjects were instructed to minimize forces/moments along the other axes. During the cocontraction task (block 2d), subjects were instructed to contract all neck muscles while simultaneously generating r-SCM muscle activity equivalent to that of the head-free task and maintaining zero forces and moments. During the cocontraction task with stimulation, subjects were instructed to replicate to the best of their ability the contraction levels of the preceding trials without stimulation.
Signal and statistical analyses.
EMG data were first high-pass filtered with a phaseless 8th-order Butterworth digital filter (−3 dB at 100 Hz) to remove the stimulation artifact (Forbes et al. 2013). Repeated trials within each subject were concatenated to create 120-s data records and analyzed to examine the responses on a subject-by-subject basis. Data from all subjects were then concatenated to create a single pooled data set for each condition (960 s). Prior to the pooled concatenation, the filtered EMG of each trial was normalized by its vector norm to ensure equal contributions from all subjects to the pooled response. Both the individual and pooled EMG data were full-wave rectified.
To confirm that there was no difference in the level of muscle activity with and without stimulation and across all tasks, we evaluated the RMS of the filtered EMG using repeated-measures ANOVAs for the r-SCM and l-SPL separately (2 stimulation conditions × 5 contraction tasks). For the r-SCM the five contraction tasks included all conditions except extension, and for the l-SPL the five contraction tasks included all conditions except flexion. An additional paired t-test was performed to confirm that similar muscle activity was achieved in bilateral muscle pairs during symmetrical contraction tasks (i.e., SCMs during flexion and SPLs during extension). To confirm that subjects generated neck muscle cocontraction during the cocontraction trials with stimulation, we calculated a cocontraction index (Forbes et al. 2011) to estimate the level of activity of measured muscles during trials with the head oriented forward (blocks 2a–2d). The four filtered EMG signals during stimulation trials were first normalized to the separate RMS obtained from the flexion trials without stimulation for SCM muscles and the extension trials without stimulation for SPL muscles. Flexion and extension conditions were used because they theoretically involve equivalent muscle activity of bilateral muscle pairs. The cocontraction index was then calculated as the mean of the normalized RMSs of the four muscles (Forbes et al. 2011), where a value of 1 indicates effective neck muscle cocontraction and a value of 0 indicates inactivity of all muscles. We expected that the cocontraction index would be close to 0.5 during isometric muscle contractions (i.e., yaw, flexion, and extension) and close to 1 during muscle cocontraction. Differences between isometric muscle contractions and cocontraction trials were assessed with three paired t-tests.
Coherence and cumulant density estimates were calculated with the individual and pooled data to evaluate the correlation between the input SVS and output EMG (Dakin et al. 2007, 2010, 2011). Coherence is used as a measure of the linear relationship between the two signals at the frequencies considered (Pintelon and Schoukens 2001) and is significant when exceeding the 95% confidence limit as derived from the number of disjoint segments (Halliday et al. 1995). Coherence was estimated with concatenated data for each trial condition within each participant as well as concatenated pooled data for each trial across all subjects. Individual-subject coherence data were assessed to ensure that responses were exceeding significance and consistent with the pooled data. Cumulant density estimates provide a time-domain representation of the input-output relationship and are calculated by taking the inverse Fourier transform of the cross-spectrum (Halliday et al. 1995). In the lower limb, cumulant density estimates are equivalent to vestibulomuscular reflex responses evoked by square-wave stimuli (Dakin et al. 2007). The cumulant density estimates were normalized by the product of the vector norms of the input and output to provide coefficients of correlation (r) ranging between −1 and +1 (Dakin et al. 2010), thus facilitating comparison between subjects. A normalized 95% confidence interval was calculated for individual-subject cumulant density estimates to indicate where responses were significant (Halliday et al. 1995). By convention, anode right/cathode left currents represent a positive vestibular signal. Thus a positive cumulant density indicates that an anode right/cathode left current induced an excitation of the muscle activity and an anode left/cathode right current induced an inhibition. For statistical analysis of the cumulant density estimates, dependent variables were extracted from the responses with concatenated data from each subject, whereas cumulant density functions estimated with concatenated data across all subjects were used for illustrative purposes only.
Coherence and cumulant density were analyzed with a resolution of 4 Hz (0.25 s/segment). This resolution was chosen because we were interested in evaluating differences in coherence between conditions across the entire bandwidth (0–75 Hz): at 4-Hz resolution the number of comparisons is reduced and identifies significant differences spanning broad frequency bandwidths rather than at individual frequencies (see below). Furthermore, coherence and cumulant density estimates improve with the number of segments used (individual subjects: 480 segments; pooled: 3,840 segments).
To evaluate the postural task dependence of the VCR, responses during the head-fixed trials were examined to determine whether the coherence and cumulant density estimates exceeded the 95% confidence interval for the r-SCM and l-SPL muscles. Significant differences between head-fixed and head-free trials for the pooled coherence were determined with difference of coherence (DoC) tests (Amjad et al. 1997) and for cumulant density responses with paired t-tests. These latter two tests were performed to determine whether the responses (coherence and cumulant density) we predicted to occur during head-fixed trials were different from head-free trials. The DoC test was applied on the Fisher transform (tanh−1) of the coherency (square root of the coherence) values and compared to a χ2-distribution with k − 1 degrees of freedom (k is the number of conditions included in the comparison; k = 2). Cumulant density waveform shapes were muscle dependent, being triphasic for the SCM and biphasic for the SPL. Because the difference between the first two peaks (i.e., peak-to-peak amplitude) is commonly used to evaluate vestibular evoked myogenic potentials in SCM muscles with square-wave stimuli (Rosengren et al. 2010; Watson and Colebatch 1998), we limited our analysis to the peak-to-peak amplitude of the first two peaks for both the r-SCM and l-SPL muscles. To evaluate the effect of the different focal descending motor commands on the VCR, DoC tests of the pooled coherence and paired t-tests of the cumulant density peak-to-peak amplitude were also performed for the r-SCM (yaw vs. flexion) and l-SPL (yaw vs. extension) muscle responses. We also assessed whether simple activation of the muscle was sufficient to evoke a reflex response. Here we expected to see no reflex response in conditions where a muscle was not contributing to the contraction task (extension task for r-SCM and flexion task for l-SPL) and reflex responses in all muscles during the cocontraction task. A significance level of 0.05 was used for all analyses.
RESULTS
Patterns of neck muscle activity.
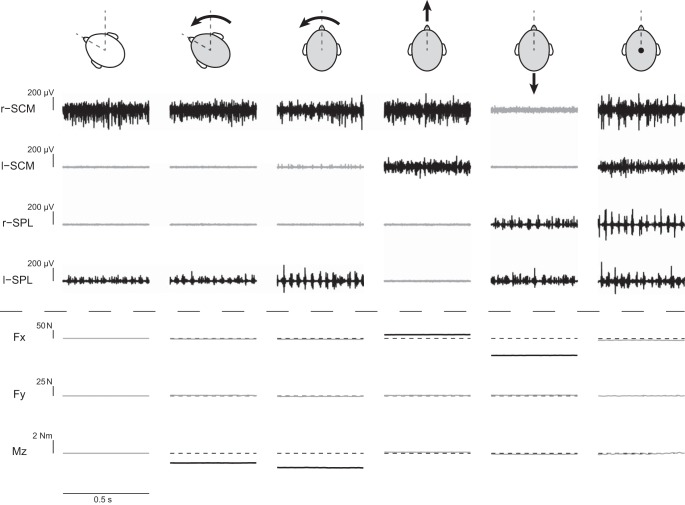
All subjects exhibited muscle activation patterns (Fig. 2) that depended upon the direction of applied force. Left yaw moments—both head free and fixed—were generated by contralateral r-SCM and l-SPL activation, whereas horizontal flexion and extension forces were generated by bilateral SCM and SPL activation, respectively, and cocontraction was achieved by activating both SCM and SPL muscles bilaterally (Fig. 2). Although we were unable to directly control for muscle contraction levels during stimulation trials, muscle activity (i.e., RMS of filtered EMG) did not vary between electrical stimulation conditions or between contraction tasks for r-SCM (stimulation: F1,7 = 0.0, P = 0.908; contraction task: F4,28 = 0.8, P = 0.418) and l-SPL (stimulation: F1,7 = 0.7, P = 0.434; contraction task: F4,28 = 2.5, P = 0.128) muscles. Thus electrical activity was similar during focal isometric tasks and the cocontraction task for both the r-SCM and l-SPL muscles. In addition, muscle activity in bilateral muscles was not significantly different in the SCM during flexion (t7 = 1.1, P = 0.295) and the SPL during extension (t7 = 1.6, P = 0.159).
Fig. 2.
Recorded data from a single subject: 0.5 s of filtered EMG and force collected during the different tasks with stimulation. Darker lines represent the muscles and loads (force and moments) related to the task performed. Fx and Fy are horizontal forces applied in the x (+ve forward)- and y (+ve rightward)-directions, respectively. Mz is the moment generated about the z-axis (+ve down) during left yaw axial rotation tasks in fixed conditions. Head-free and -fixed conditions are represented as open and shaded heads, respectively. Accompanying arrows indicate the direction of load applied by the subject in fixed conditions.
The cocontraction index was higher for the cocontraction trials than for any of the isometric contraction trials [0.89 ± 0.34 (cocontraction) vs. 0.60 ± 0.16 (yaw), 0.59 ± 0.13 (flexion) and 0.54 ± 0.11 (extension); multiple t7 > 2.6, P < 0.05]. In addition, subjects generated near-zero mean forces and moments during cocontraction trials {−0.2 ± 6.0 N [force in x-direction (Fx)], 1.3 ± 3.8 N [force in y-direction (Fy)], and −0.14 ± 0.10 Nm [moment generated about z-axis (Mz)]}, which were one to two orders of magnitude below the mean forces and moments generated during the flexion, extension, and yaw trials [20.4 ± 6.9 N (Fx-flexion), −62.8 ± 22.1 N (Fx-extension), and −1.42 ± 0.51 Nm (Mz-yaw)]. Although on average subjects did not perfectly cocontract all muscles to achieve a cocontraction index of 1, the increased cocontraction index compared with isometric muscle contractions in one direction, as well as the minimization of applied forces and moments, indicates that subjects effectively cocontracted their neck muscles.
Frequency response of the VCR.
Single-subject and pooled coherence estimates between SVS and EMG indicated significant reflex responses for all muscles in conditions where muscle activity was present (see Fig. 3A and Fig. 4A). The r-SCM exhibited significant coherence across the entire 0–75 Hz bandwidth, with distinct peaks between 4 and 12 Hz and 48 and 56 Hz and a dip around 20 Hz. The l-SPL exhibited significant coherence primarily at low frequencies (4–20 Hz), although sporadic coherence was detected at high frequencies in the pooled data. This latter observation was found to originate from some of the subjects (n = 3) where coherence exceeded significance at high frequencies (see insets in Figs. 3A and 4A). These results are presented in more detail together with cumulant density responses in Polarity and latency of the VCR.
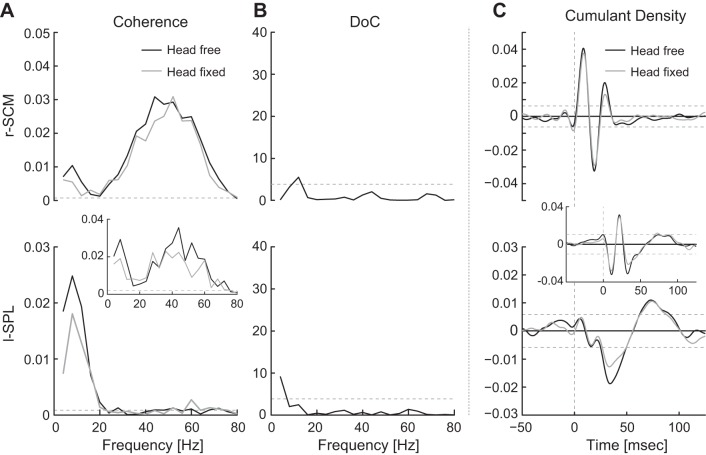
Fig. 3.
Comparison of head-free and head-fixed conditions (blocks 1a and 1b). A: pooled (n = 8, r-SCM; n = 7, l-SPL) coherence plots. Pooled coherences (n = 3) for l-SPL using data from 3 subjects are included as an inset to detail high-frequency coherence for these subjects. Horizontal dashed lines indicate 95% confidence limits. B: difference of coherence (DoC) test comparing free and fixed conditions. Horizontal dashed lines represent significance level for the χ2-distribution (P = 0.05). C: pooled (n = 8, r-SCM; n = 7, l-SPL) cumulant density estimate showing a triphasic r-SCM and a biphasic l-SPL response. Pooled cumulant density estimates (n = 3) for l-SPL using data from 3 subjects are included as an inset to detail the additional short-latency biphasic waveform for these subjects. Horizontal dashed lines represent 95% confidence interval.
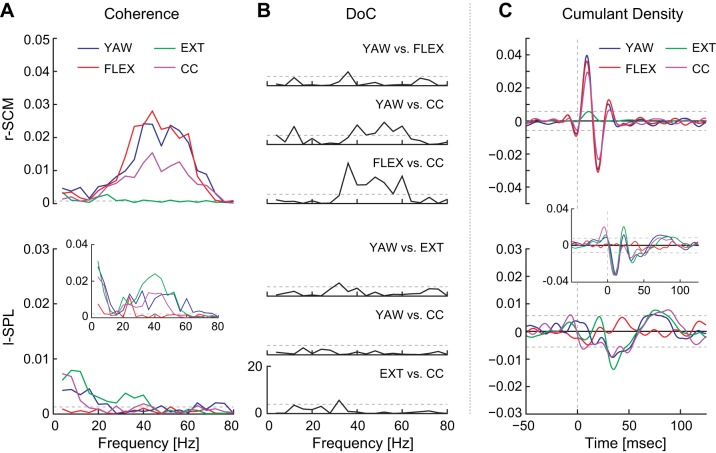
Fig. 4.
Comparison of descending motor command for r-SCM (top) and l-SPL (bottom) during yaw, flexion, extension, and cocontraction tasks with the head forward (blocks 2a–2d). A: pooled (n = 8, r-SCM; n = 7, l-SPL) coherence plots. Pooled coherences (n = 3) for l-SPL using data from 3 subjects are included as an inset to detail high-frequency coherence for these subjects. Horizontal dashed lines indicate 95% confidence limits. B: DoC test comparing key combinations of descending motor command. Horizontal dashed lines represent significance level for the χ2-distribution (P = 0.05). C: pooled (n = 8, r-SCM; n = 7, l-SPL) cumulant density estimate showing a triphasic r-SCM and a biphasic l-SPL response. Pooled cumulant density estimates (n = 3) for l-SPL using data from 3 subjects are included as an insets to detail the additional short-latency biphasic waveform for these subjects. Horizontal dashed lines represent 95% confidence interval. YAW, yaw moment; FLEX, flexion force; EXT, extension force; CC, cocontraction.
Polarity and latency of the VCR.
The cumulant density profiles were similarly muscle dependent (see Figs. 3C and 4C). The r-SCM exhibited a triphasic response (positive-negative-positive) with peaks exceeding the confidence interval for all subjects and occurring at ∼9.2 ± 1.3, 20.1 ± 2.1, and 29.0 ± 2.6 ms when averaged across subjects (n = 8) and conditions (n = 5). The l-SPL muscle exhibited a longer-latency biphasic response with peaks at 34.7 ± 4.4 and 75.8 ± 8.2 ms when averaged across subjects (n = 7; for 1 subject, the l-SPL waveform remained below the confidence interval for all conditions) and conditions (n = 5). However, for some subjects (n = 3), an additional short-latency biphasic waveform appeared in the l-SPL (see insets in Figs. 3C and 4C) with similar timing but opposing polarity to that of the first two peaks in the pooled r-SCM response [8.6 ± 2.9 and 21.3 ± 3.0 ms (l-SPL) vs. 9.2 ± 1.3 and 20.1 ± 2.1 ms (r-SCM)]. In each subject presenting this response, coherence was above significance in the high-frequency region (see insets in Figs. 3A and 4A). This high-frequency correlation was not as prominent in the pooled responses.
For both the SCM and SPL muscles, the polarity of the cumulant density response was unaffected by the contraction task being performed. Bilateral comparison of cumulant density responses, on the other hand, revealed inverted responses; right-sided muscles were initially facilitated and then inhibited, whereas left-sided muscles were initially inhibited and then facilitated. In conditions where bilateral pairs were active (i.e., flexion and extension), muscles demonstrated similar peak-to-peak amplitudes [flexion: 0.069 ± 0.028 (r-SCM) vs. 0.068 ± 0.020 (l-SCM); extension: 0.025 ± 0.006 (r-SPL) vs. 0.027 ± 0.008 (l-SPL)] and peak latencies [flexion (1st, 2nd, 3rd): 9.4 ± 1.2, 20.5 ± 2.1, and 31.1 ± 2.1 ms (r-SCM) vs. 9.5 ± 1.3, 20.8 ± 2.1, and 32.8 ± 4.9 ms (l-SCM); extension (1st, 2nd): 35.8 ± 4.3 and 64.7 ± 6.3 ms (r-SPL) vs. 35.1 ± 7.6 and 76 ± 4.7 ms (l-SPL)]. These results indicate a mirrored bilateral response to the input stimulus.
Effect of postural task on the VCR.
We compared the effects of restraining the head at an orientation of 60° to determine whether neck muscles need to be engaged in controlling the upright posture of the head on top of the torso to evoke VCRs. A clear response to the input stimulus was evoked when the head was fixed, as shown by individual subjects (SCM n = 8, SPL n = 7) and pooled coherence and cumulant density responses exceeding the confidence intervals for both muscles (Fig. 3). Coherence was nearly equivalent across the head-free and -fixed conditions (Fig. 3A), and the DoC test revealed significant effects of head restraint at only one frequency each in the r-SCM and l-SPL (Fig. 3B). This was associated with no significant change in the cumulant density peak-to-peak amplitudes of SCM and a 25 ± 19% decrease of l-SPL when the head was fixed (r-SCM: t7 = 1.7, P = 0.14; l-SPL: t6 = 3.5, P = 0.014; see Fig. 5). For the responses from the three subjects demonstrating a short-latency l-SPL cumulant density waveform, only the longer-latency biphasic waveform appeared to be affected by head fixation (see Fig. 3, A and C, insets). Overall, these results indicate that vestibular reflexes remain present when the necessity to maintain the upright orientation of the head is eliminated (i.e., head fixed).
Fig. 5.
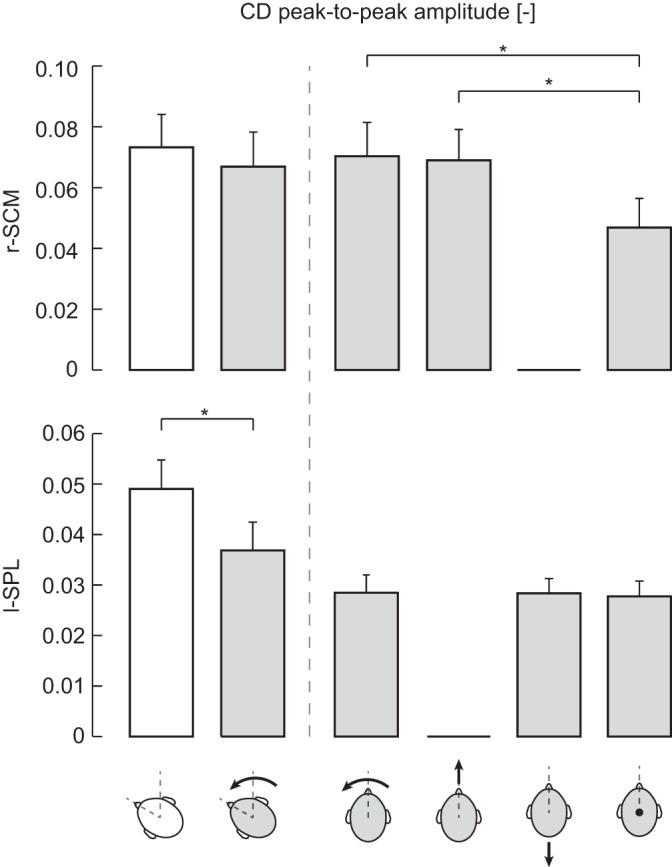
Mean cumulant density (CD) peak-to-peak amplitudes for the r-SCM (top) and l-SPL (bottom) muscles for all conditions. Error bars represent SE (r-SCM: n = 8; l-SPL: n = 7). Extension for r-SCM and flexion for l-SPL are not plotted because no significant cumulant density responses were detected. Head-free and -fixed conditions are represented as open and shaded heads, respectively. Accompanying arrows indicate the direction of load applied by subjects in fixed conditions.
Effect of descending motor command on the VCR.
As mentioned above, significant reflex responses were only detected when a muscle was contributing to the isometric force/moment production task. Therefore, both coherence and cumulant density responses were below significance thresholds in all single-subject and pooled estimates when the muscle was not active; reflex responses were absent in SCM muscles during extension and SPL muscles during flexion (Fig. 4, A and C). In contrast, during the cocontraction task, where all muscles were active, reflex responses were observed in all muscles (Fig. 4, A and C).
Having shown that vestibular input was not suppressed during head fixation, we assessed the effects of descending motor commands by comparing responses across direction-specific (yaw, flexion, and extension) and cocontraction tasks with the head facing forward. Comparing the direction-specific tasks, no significant difference between the two muscle-specific tasks was found for either the r-SCM (yaw vs. flexion) or the l-SPL (yaw vs. extension) in the DoC (Fig. 4, A and B) and cumulant density peak-to-peak amplitudes [r-SCM: 0.070 ± 0.031 (yaw) vs. 0.069 ± 0.029 (flexion); l-SPL: 0.028 ± 0.009 (yaw) vs. 0.028 ± 0.008 (extension)] (Figs. 4C and 5).
An unexpected outcome of the cocontraction task was the decrease in coherence relative to the focal isometric tasks (see Fig. 4A). To examine this further, we performed additional DoC tests on the coherence and t-tests on the cumulant density to determine whether responses obtained during cocontraction in each muscle were significantly different from their respective focal direction tasks. In the pooled responses, the DoC test revealed that this was significant only in the r-SCM and not the l-SPL. Coherence decreased during cocontraction between 36 and 60 Hz (Fig. 4B), with an average decrease of 49 ± 12% and 45 ± 6% relative to flexion and yaw, respectively. The decrease in pooled coherence during cocontraction was further confirmed in individual responses from seven of eight subjects. Comparisons of the cocontraction cumulant density peak-to-peak amplitude against direction-specific tasks were also significant, but once again only for the r-SCM. During the cocontraction task, the amplitude decreased by 31 ± 25% relative to yaw (t7 = 3.1, P = 0.018) and 30 ± 24% relative to flexion (t7 = 2.8, P = 0.028; see Fig. 5).
For the responses from the three subjects demonstrating a short-latency l-SPL cumulant density waveform, similar decreases in high-frequency coherence and short-latency peak-to-peak magnitude were observed during cocontraction (see Fig. 4, A and C, insets). Because of the small number of subjects, this was not confirmed with a statistical test. Overall, these results indicate that activation of the muscle, regardless of contraction task, can evoke a reflex response, albeit the response was decreased in the r-SCM muscle during neck muscle cocontraction.
DISCUSSION
The first aim of this study was to determine whether VCRs are present only when vestibular information is relevant for the postural control of the head on the torso. Our results demonstrate that restraining the head, thereby eliminating the need to maintain upright postural control of the head on the torso, does not suppress vestibular evoked responses in the SCM and SPL muscles. Each muscle exhibited coupling with the input stimulus: the SCM responses were composed primarily of high frequencies, while the SPL responses were composed primarily of low frequencies. The second aim of this study was to determine whether neck muscles needed to be active to exhibit a vestibular reflex and whether descending neck muscle contraction commands modify the magnitude of VCRs. We observed VCRs only in active muscles, and these reflexes were unaffected by the contraction task except during cocontraction, in which VCRs were suppressed but not extinguished relative to isometric focal contractions. Suppression occurred primarily at high frequencies (>30 Hz) and affected mainly the SCM. Overall, these findings indicate robust vestibular reflexes in the human neck muscles.
Head immobilization does not suppress vestibulo-motor pathways in neck muscles.
In the present study, vestibular reflexes were not suppressed in neck muscles (i.e., VCR) when the head was restrained. This contrasts with findings in the appendicular muscles, where evoking electrically induced vestibular reflexes depends on the presence of two factors: 1) that vestibular information is relevant to the postural control task (Britton et al. 1993; Fitzpatrick et al. 1994) and 2) that the motor behavior contributes to the control of posture (Luu et al. 2012). Despite neither factor being present in our head-fixed condition, the VCR was present in the neck muscles we examined provided the muscle was active. Our results thus parallel the observations of Welgampola and Colebatch (2001), who found that VCR responses are unaffected by additional sensory feedback (vision and touch) or variations in stance width, and expand these prior findings by showing that they hold even when the head is restrained and the neck muscles are not required to maintain the upright postural control of the head.
Our results lead to a question of why this postural task dependence does not occur in neck muscles. One possible explanation is that the central nervous system (CNS) is unable to parse for neck muscles the two factors (i.e., irrelevance of both vestibular information and motor behavior on postural control) that seem to permit vestibular suppression in lower limb muscles. This explanation, however, seems unlikely given that vestibular information undergoes complex and selective processing in the vestibular nuclei to dissociate self-generated from passively applied head motion (McCrea et al. 1999; Roy and Cullen 2002, 2004) prior to transmission to neck motoneurons. The results of the present study therefore imply that unsuppressed vestibular input to neck muscles contributes to their control, regardless of the necessity to control the posture of the head on the torso. From an engineering perspective, a preserved vestibular input to neck muscles seems logical given the VCR's closed-loop nature, in which its output, i.e., neck muscle-driven head motion, directly affects the subsequent vestibular input. This tight coupling ensures a highly effective response in the event that stabilization of the head-neck is compromised and a sudden compensatory response is necessary. Furthermore, the reversal in cumulant density polarity across bilateral muscles indicates a neck system response that is coordinated to counter the perceived roll motion induced by the electrical stimulus, whereby muscles on the right are initially facilitated and then inhibited whereas muscles on the left are initially inhibited and then facilitated.
A robust vestibular input to neck muscles would further provide advantages for complex motor behaviors such as rapid gaze shifts, which are generated via a coordinated activation of neck and extraocular muscles (Guitton 1992). For example, when humans transition to a state in which vestibular information becomes relevant to the postural control task, the CNS takes ∼900 ms to reassociate the balancing task with postural activity and generate lower-limb muscle contributions in response to the ongoing vestibular perturbations (Luu et al. 2012). Certainly, if vestibular control of neck muscles depended upon the postural task, then such large delays to reestablish vestibular control would impair rapid head-eye gaze shifts during head perturbations. Our results parallel the response of VORs to electrical vestibular stimulation, where reflexes are generated regardless of the postural control requirement or functional state of the head and body (Aw et al. 2006). Considering that neck and oculomotor systems receive similar descending input from the superior colliculus (Corneil et al. 2002; Freedman et al. 1996) and the frontal eye fields (Elsley et al. 2007; Knight and Fuchs 2007; Monteon et al. 2010; Tu and Keating 2000), it seems plausible that these two systems have evolved together to generate vestibulomuscular responses that do not depend on their functional state.
Our results showed a significant decrease in peak-to-peak amplitude of l-SPL but not r-SCM, with head support (see Fig. 5). This effect was attributed to a decrease in low-frequency coherence that was observed in both r-SCM and l-SPL muscles. It was also confirmed in a subset of three subjects who demonstrated an additional short-latency l-SPL waveform, where only the longer-latency, and presumably low-frequency, peak-to-peak amplitude appeared to be affected by head fixation. The presence of this effect in the l-SPL only is therefore likely due to the dominance of low frequencies in generating the later muscle response (Dakin et al. 2011) and not necessarily a difference in the vestibular control between these two muscles. Regardless of how the effect is observed in each neck muscle, vestibular control of the neck muscles was present (although showing minimal inhibition for the later response) when the head was restrained.
Descending motor commands can modulate vestibulocollic reflexes.
In both the SCM and SPL muscles, the magnitude of the VCR did not change across focal neck muscle contraction tasks used to generate forces/moments in different directions, although the responses were absent in muscles not contributing to the focal task. Together with the observed VCR in all muscles during the cocontraction tasks, these results indicate that an electrically evoked VCR is observed only in the presence of neck muscle activity and is not influenced by the descending motor command or synergies generating muscle activity during focal tasks. With the exception of cocontraction, the VCR remained intact regardless of the combination of neck muscles contributing the output forces and moments. This further highlights the flexibility in neck muscle control where activation of a group of muscles does not have strong reciprocal connection to other muscles. The observation that the VCR response remained below significance in quiescent muscles could arguably be a result of recurrent inhibition from antagonist muscles. Although we did not measure VCR during a purely relaxed state, and therefore cannot directly eliminate this possibility, our results are similar to observations by Watson and Colebatch (1998), who reported a linear relationship between reflex magnitude and background muscle activity with a zero intercept. In their “relaxed” conditions, subjects were lying semireclined with their head supported.
The scaling of neck vestibular responses, which is also observed in lower limb muscles (Fitzpatrick et al. 1994), is argued to be similar to the scaling of muscle stretch reflexes (Colebatch and Rothwell 2004). Muscles undergo “automatic gain compensation” to generate a muscle activation appropriate reflex response (Marsden et al. 1976; Matthews 1986). In contrast, neck muscles responses are measured during whole body and head-only transient perturbations in which neck muscles are relaxed prior to the onset of the perturbation (Aoki et al. 2001; Ito et al. 1995). The need for background neck muscle activity to elicit a reflex to an electrical vestibular input is therefore most likely related to the weak electrical stimulus used in our study (±5 mA over a 75-Hz bandwidth). The head motion equivalent of a 1-mA constant current is ∼1–6°/s with an acceleration of ∼0.33–3°/s2 (Day and Cole 2002; Fitzpatrick et al. 2002; Schneider et al. 2002). These motion equivalents are well below peak head rotational velocities and accelerations recorded during dynamic equilibrium tasks (>25°/s and >400°/s2; Pozzo et al. 1995), locomotor tasks (>30°/s; Grossman et al. 1988; Hirasaki et al. 1999), voluntary head oscillations (>500°/s2; Siegmund et al. 2007), and ballistic head movements (>320°/s and 4,500°/s2; Siegmund et al. 2001).
When subjects cocontracted their neck muscles, however, both VCR measures (coherence and cumulant density) decreased in the r-SCM neck muscle compared with direction-specific contractions. These variations were not attributed to changes in the magnitude of neck muscle contractions, since muscle activity was confirmed to be similar across all conditions. While the inhibition of VCRs during neck muscle cocontraction was unexpected, it may function to facilitate head-on-torso stabilization. Muscle cocontraction contributes to spinal stabilization by increasing stiffness (Gardner-Morse and Stokes 1998; Granata and Orishimo 2001; Lee et al. 2006) and is used during unpredictable whole body perturbations (Danna-Dos-Santos et al. 2007). However, because vestibular reflexes respond in a direction opposing the global head movement (Baker et al. 1985; Ezure et al. 1978; Goldberg and Peterson 1986; Wilson et al. 1990), a large response originating from vestibular reflexes during neck muscle cocontraction while perturbing the torso would be detrimental to head-on-torso stabilization. This behavior is similar to the decrease in H reflexes during the cocontraction of muscles acting on limb joints, which is facilitated through increased presynaptic and disynaptic reciprocal Ia inhibition (Nielsen and Kagamihara 1992, 1993). Such a mechanism may also function to ensure neck spinal stabilization during conditions requiring neck muscle cocontraction, and may be particularly relevant in light of our first observation that VCR is present even when vestibular information is irrelevant and there is no longer a necessity to maintain the upright orientation of the head.
The two primary outcomes of our study are seemingly at odds with each other: a retained VCR regardless of the postural requirement of the task indicates a lack of central control, while a reduced VCR during cocontraction indicates at least partial influence of central mechanisms. Welgampola and Colebatch (2001) argued that postural task irrelevance in neck muscles may be due to the short-latency three-neuron reflex arcs mediating the VCR (Shinoda et al. 2006; Wilson and Maeda 1974; Wilson and Yoshida 1969b), whereas limb vestibulomuscular responses are mediated by polysynaptic pathways having significant central conduction delays (Ali et al. 2003; Britton et al. 1993). However, the more complicated polysynaptic circuitry contributing to the VCR response (Ezure et al. 1978; Ezure and Sasaki 1978; Peterson et al. 1980; Thomson et al. 1995) may be subject to descending and central control (Wilson and Yoshida 1969a), and could therefore be responsible for the modulation of vestibular reflexes observed during muscle cocontraction. Considering that interneurons and commissural neurons in midcervical segments receive monosynaptic input from vestibulospinal neurons (Alstermark et al. 1987; Rose et al. 1992; Schor et al. 1986; Sugiuchi et al. 1995), it is plausible that presynaptic mechanisms shape the VCR responses based on the ongoing motor command (Bussieres and Dubuc 1992; Pflieger and Dubuc 2004). The motor commands generating neck muscle synergies are thought to be controlled by equivalent innervation of vestibulospinal and reticulospinal neurons on functional sets of neck muscles (Kakei et al. 1994; Sugiuchi et al. 2004). These characteristics therefore argue for a specific cocontraction motor command, mediated by reticulospinal input that may function to inhibit descending vestibular signals. This is supported by the interaction between these two pathways: vestibular stimulation elicits excitatory and inhibitory responses in reticulospinal neurons (Peterson and Felpel 1971), and reticulospinal signals converge on spinal interneurons and commissural interneurons that mediate vestibular signals (Alstermark et al. 1987; Krutki et al. 2003). The absence of variation between the isometric focal contraction trials (yaw vs. flexion vs. extension) indicates a cocontraction-specific inhibition mechanism.
Study limitations.
A limitation regarding the interpretation of this study was the variability of responses across subjects in the SPL muscle. Within one group of subjects (n = 4), the effect of cocontraction was limited to the SCM muscle (extending across a wide range of frequencies) while the SPL demonstrated no high-frequency coupling regardless of contraction condition. In the remaining subjects (n = 3), however, high-frequency coupling between SVS and SPL muscle activity, similar to the SCM, was observed along with evidence of cocontraction modulation in the SPL pooled response. These between-subject variations in the VCR frequency response of the SPL may be partly due to neural control mechanisms that vary between subjects for that muscle (Blouin et al. 2007; Keshner et al. 1989). Although the latter observations may further indicate that cocontraction affects primarily the high frequencies of the vestibular reflex in neck muscles, this could not be confirmed statistically on account of the low number of subjects presenting the high-frequency SPL responses.
Conclusions.
The present study has shown that vestibular reflexes in neck muscles are retained during conditions in which there is no longer a requirement to balance the head and vestibular information is no longer directly relevant to neck muscle postural control. In addition, VCR magnitudes decrease, but are not completely suppressed, during neck muscle cocontraction relative to isometric neck muscle contractions generating a specific moment. However, VCRs evoked by electrical vestibular stimulation are only found in active muscles. Together, these findings indicate a robust vestibular influence over active neck muscles with cocontraction task-dependent variations that may function to ensure neck spinal stabilization.
GRANTS
This research was supported by the Dutch Technology Foundation STW, which is part of the Netherlands Organization for Scientific Research (NWO) and partly funded by the Ministry of Economic Affairs, Agriculture and Innovation (P. A. Forbes, R. Happee, and A. C. Schouten) and the Natural Sciences and Engineering Research Council of Canada Discovery (J.-S. Blouin and G. P. Siegmund) and Research Tools and Instruments (J.-S. Blouin, M. G. Carpenter, and J. T. Inglis) programs, the Michael Smith Foundation for Health Research, the Canadian Chiropractic Research Foundation, and the Canadian Institutes of Health Research (J.-S. Blouin and G. P. Siegmund).
DISCLOSURES
G. P. Siegmund owns shares in a consulting company, and both he and the company may derive benefit from being associated with this work.
AUTHOR CONTRIBUTIONS
Author contributions: P.A.F., G.P.S., A.C.S., and J.-S.B. conception and design of research; P.A.F., G.P.S., and J.-S.B. performed experiments; P.A.F. analyzed data; P.A.F., G.P.S., R.H., A.C.S., and J.-S.B. interpreted results of experiments; P.A.F. prepared figures; P.A.F. drafted manuscript; P.A.F., G.P.S., R.H., A.C.S., and J.-S.B. edited and revised manuscript; P.A.F., G.P.S., R.H., A.C.S., and J.-S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Barrie Forbes and Susan Forbes for assistance with data collection.
REFERENCES
- Ali AS, Rowen KA, Iles JF. Vestibular actions on back and lower limb muscles during postural tasks in man. J Physiol 546: 615–624, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A, Pinter M, Sasaki S. Vestibular effects in long C3-C5 propriospinal neurons. Brain Res 404: 389–394, 1987 [DOI] [PubMed] [Google Scholar]
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods 73: 69–79, 1997 [DOI] [PubMed] [Google Scholar]
- Aoki M, Matsunami K, Han XY, Yamada H, Muto T, Ito Y. Neck muscle responses to abrupt vertical acceleration in the seated human. Exp Brain Res 140: 20–24, 2001 [DOI] [PubMed] [Google Scholar]
- Aw ST, Todd MJ, Halmagyi GM. Latency and initiation of the human vestibuloocular reflex to pulsed galvanic stimulation. J Neurophysiol 96: 925–930, 2006 [DOI] [PubMed] [Google Scholar]
- Baker J, Goldberg J, Peterson B. Spatial and temporal response properties of the vestibulocollic reflex in decerebrate cats. J Neurophysiol 54: 735–756, 1985 [DOI] [PubMed] [Google Scholar]
- Blouin JS, Siegmund GP, Carpenter MG, Inglis JT. Neural control of superficial and deep neck muscles in humans. J Neurophysiol 98: 920–928, 2007 [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res 94: 143–151, 1993 [DOI] [PubMed] [Google Scholar]
- Bussieres N, Dubuc R. Phasic modulation of transmission from vestibular inputs to reticulospinal neurons during fictive locomotion in lampreys. Brain Res 582: 147–153, 1992 [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Rothwell JC. Motor unit excitability changes mediating vestibulocollic reflexes in the sternocleidomastoid muscle. Clin Neurophysiol 115: 2567–2573, 2004 [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. II. Gaze shift initiation and volitional head movements. J Neurophysiol 88: 2000–2018, 2002 [DOI] [PubMed] [Google Scholar]
- Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol 91: 1919–1933, 2004 [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Inglis JT, Blouin JS. Short and medium latency muscle responses evoked by electrical vestibular stimulation are a composite of all stimulus frequencies. Exp Brain Res 209: 345–354, 2011 [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Luu BL, van den Doel K, Inglis JT, Blouin JS. Frequency-specific modulation of vestibular-evoked sway responses in humans. J Neurophysiol 103: 1048–1056, 2010 [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Son GM, Inglis JT, Blouin JS. Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol 583: 1117–1127, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna-Dos-Santos A, Degani AM, Latash ML. Anticipatory control of head posture. Clin Neurophysiol 118: 1802–1814, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Cole J. Vestibular-evoked postural responses in the absence of somatosensory information. Brain 125: 2081–2088, 2002 [DOI] [PubMed] [Google Scholar]
- Day BL, Fitzpatrick RC. Virtual head rotation reveals a process of route reconstruction from human vestibular signals. J Physiol 567: 591–597, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsley JK, Nagy B, Cushing SL, Corneil BD. Widespread presaccadic recruitment of neck muscles by stimulation of the primate frontal eye fields. J Neurophysiol 98: 1333–1354, 2007 [DOI] [PubMed] [Google Scholar]
- Ezure K, Sasaki S. Frequency-response analysis of vestibular-induced neck reflex in cat. I. Characteristics of neural transmission from horizontal semicircular canal to neck motoneurons. J Neurophysiol 41: 445–458, 1978 [DOI] [PubMed] [Google Scholar]
- Ezure K, Sasaki S, Uchino Y, Wilson VJ. Frequency-response analysis of vestibular-induced neck reflex in cat. II. Functional significance of cervical afferents and polysynaptic descending pathways. J Neurophysiol 41: 459–471, 1978 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol 478: 363–372, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol 76: 3994–4008, 1996 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol 96: 2301–2316, 2004 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Marsden J, Lord SR, Day BL. Galvanic vestibular stimulation evokes sensations of body rotation. Neuroreport 13: 2379–2383, 2002 [DOI] [PubMed] [Google Scholar]
- Forbes PA, Dakin CJ, Vardy AN, Happee R, Siegmund GP, Schouten AC, Blouin JS. Frequency response of vestibular reflexes in neck, back, and lower limb muscles. J Neurophysiol 110: 1869–1881, 2013 [DOI] [PubMed] [Google Scholar]
- Forbes PA, Happee R, van der Helm FC, Schouten AC. EMG feedback tasks reduce reflexive stiffness during force and position perturbations. Exp Brain Res 213: 49–61, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol 76: 927–952, 1996 [DOI] [PubMed] [Google Scholar]
- Gardner-Morse MG, Stokes IA. The effects of abdominal muscle coactivation on lumbar spine stability. Spine 23: 86–91, 1998 [DOI] [PubMed] [Google Scholar]
- Goldberg J, Peterson BW. Reflex and mechanical contributions to head stabilization in alert cats. J Neurophysiol 56: 857–875, 1986 [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Cullen KE. Vestibular control of the head: possible functions of the vestibulocollic reflex. Exp Brain Res 210: 331–345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol 51: 1236–1256, 1984 [DOI] [PubMed] [Google Scholar]
- Granata KP, Orishimo KF. Response of trunk muscle coactivation to changes in spinal stability. J Biomech 34: 1117–1123, 2001 [DOI] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res 70: 470–476, 1988 [DOI] [PubMed] [Google Scholar]
- Guitton D. Control of eye-head coordination during orienting gaze shifts. Trends Neurosci 15: 174–179, 1992 [DOI] [PubMed] [Google Scholar]
- Guitton D, Bergeron A, Choi WY, Matsuo S. On the feedback control of orienting gaze shifts made with eye and head movements. Prog Brain Res 142: 55–68, 2003 [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data: theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278, 1995 [DOI] [PubMed] [Google Scholar]
- Hirasaki E, Moore ST, Raphan T, Cohen B. Effects of walking velocity on vertical head and body movements during locomotion. Exp Brain Res 127: 117–130, 1999 [DOI] [PubMed] [Google Scholar]
- Ito Y, Corna S, von Brevern M, Bronstein A, Rothwell J, Gresty M. Neck muscle responses to abrupt free fall of the head: comparison of normal with labyrinthine-defective human subjects. J Physiol 489: 911–916, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei S, Muto N, Shinoda Y. Innervation of multiple neck motor nuclei by single reticulospinal tract axons receiving tectal input in the upper cervical spinal cord. Neurosci Lett 172: 85–88, 1994 [DOI] [PubMed] [Google Scholar]
- Kamibayashi LK, Richmond FJ. Morphometry of human neck muscles. Spine 23: 1314–1323, 1998 [DOI] [PubMed] [Google Scholar]
- Keshner EA, Campbell D, Katz RT, Peterson BW. Neck muscle activation patterns in humans during isometric head stabilization. Exp Brain Res 75: 335–344, 1989 [DOI] [PubMed] [Google Scholar]
- Kim J, Curthoys IS. Responses of primary vestibular neurons to galvanic vestibular stimulation (GVS) in the anaesthetised guinea pig. Brain Res Bull 64: 265–271, 2004 [DOI] [PubMed] [Google Scholar]
- Knight TA, Fuchs AF. Contribution of the frontal eye field to gaze shifts in the head-unrestrained monkey: effects of microstimulation. J Neurophysiol 97: 618–634, 2007 [DOI] [PubMed] [Google Scholar]
- Krutki P, Jankowska E, Edgley SA. Are crossed actions of reticulospinal and vestibulospinal neurons on feline motoneurons mediated by the same or separate commissural neurons? J Neurosci 23: 8041–8050, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PJ, Rogers EL, Granata KP. Active trunk stiffness increases with co-contraction. J Electromyogr Kinesiol 16: 51–57, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand 117: 307–309, 1983 [DOI] [PubMed] [Google Scholar]
- Luu BL, Inglis JT, Huryn TP, Van der Loos HF, Croft EA, Blouin JS. Human standing is modified by an unconscious integration of congruent sensory and motor signals. J Physiol 590: 5783–5794, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall HG, Brizuela AE, Curthoys IS. Linearity, symmetry and additivity of the human eye-movement response to maintained unilateral and bilateral surface galvanic (DC) vestibular stimulation. Exp Brain Res 148: 166–175, 2003 [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Servo action in human thumb. J Physiol 257: 1–44, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Observations on the automatic compensation of reflex gain on varying the preexisting level of motor discharge in man. J Physiol 374: 73–90, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Gdowski GT, Boyle R, Belton T. Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol 82: 416–428, 1999 [DOI] [PubMed] [Google Scholar]
- Monteon JA, Constantin AG, Wang H, Martinez-Trujillo J, Crawford JD. Electrical stimulation of the frontal eye fields in the head-free macaque evokes kinematically normal 3D gaze shifts. J Neurophysiol 104: 3462–3475, 2010 [DOI] [PubMed] [Google Scholar]
- Nashner LM, Wolfson P. Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of human labyrinth. Brain Res 67: 255–268, 1974 [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of disynaptic reciprocal Ia inhibition during cocontraction of antagonistic muscles in man. J Physiol 456: 373–391, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during cocontraction of antagonistic muscles in man. J Physiol 464: 575–593, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BW, Felpel LP. Excitation and inhibition of reticulospinal neurons by vestibular, cortical and cutaneous stimulation. Brain Res 27: 373–376, 1971 [DOI] [PubMed] [Google Scholar]
- Peterson BW, Fukushima K, Hirai N, Schor RH, Wilson VJ. Responses of vestibulospinal and reticulospinal neurons to sinusoidal vestibular stimulation. J Neurophysiol 43: 1236–1250, 1980 [DOI] [PubMed] [Google Scholar]
- Pflieger JF, Dubuc R. Vestibulo-reticular projections in adult lamprey: their role in locomotion. Neuroscience 129: 817–829, 2004 [DOI] [PubMed] [Google Scholar]
- Pintelon R, Schoukens J. System Identification: a Frequency Domain Approach. New York: IEEE Press, 2001 [Google Scholar]
- Pozzo T, Levik Y, Berthoz A. Head and trunk movements in the frontal plane during complex dynamic equilibrium tasks in humans. Exp Brain Res 106: 327–338, 1995 [DOI] [PubMed] [Google Scholar]
- Rose PK, Wainwright K, Neuberhess M. Connections from the lateral vestibular nucleus to the upper cervical spinal-cord of the cat: a study with the anterograde tracer PHA-L. J Comp Neurol 321: 312–324, 1992 [DOI] [PubMed] [Google Scholar]
- Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin Neurophysiol 121: 636–651, 2010 [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci 21: 2131–2142, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol 87: 2337–2357, 2002 [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci 24: 2102–2111, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E, Glasauer S, Dieterich M. Comparison of human ocular torsion patterns during natural and galvanic vestibular stimulation. J Neurophysiol 87: 2064–2073, 2002 [DOI] [PubMed] [Google Scholar]
- Schor RH, Suzuki I, Timerick SJ, Wilson VJ. Responses of interneurons in the cat cervical cord to vestibular tilt stimulation. J Neurophysiol 56: 1147–1156, 1986 [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sugiuchi Y, Izawa Y, Hata Y. Long descending motor tract axons and their control of neck and axial muscles. In: Neuroanatomy of the Oculomotor System, edited by Büttner-Ennever JA. Amsterdam: Elsevier, 2006, p. 527–563 [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Blouin JS, Brault JR, Hedenstierna S, Inglis JT. Electromyography of superficial and deep neck muscles during isometric, voluntary, and reflex contractions. J Biomech Eng 129: 66–77, 2007 [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Inglis JT, Sanderson DJ. Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol 535: 289–300, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiuchi Y, Izawa Y, Shinoda Y. Trisynaptic inhibition from the contralateral vertical semicircular canal nerves to neck motoneurons mediated by spinal commissural neurons. J Neurophysiol 73: 1973–1987, 1995 [DOI] [PubMed] [Google Scholar]
- Sugiuchi Y, Kakei S, Izawa Y, Shinoda Y. Functional synergies among neck muscles revealed by branching patterns of single long descending motor-tract axons. Prog Brain Res 143: 411–421, 2004 [DOI] [PubMed] [Google Scholar]
- Thomson DB, Ikegami H, Wilson VJ. Effect of MLF transection on the vertical vestibulocollic reflex in decerebrate cats. J Neurophysiol 74: 1815–1818, 1995 [DOI] [PubMed] [Google Scholar]
- Tu TA, Keating EG. Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. J Neurophysiol 84: 1103–1106, 2000 [DOI] [PubMed] [Google Scholar]
- Watson SR, Brizuela AE, Curthoys IS, Colebatch JG, MacDougall HG, Halmagyi GM. Maintained ocular torsion produced by bilateral and unilateral galvanic (DC) vestibular stimulation in humans. Exp Brain Res 122: 453–458, 1998 [DOI] [PubMed] [Google Scholar]
- Watson SR, Colebatch JG. Vestibulocollic reflexes evoked by short-duration galvanic stimulation in man. J Physiol 513: 587–597, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Vestibulospinal reflexes: quantitative effects of sensory feedback and postural task. Exp Brain Res 139: 345–353, 2001 [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Maeda M. Connections between semicircular canals and neck motoneurons in cat. J Neurophysiol 37: 346–357, 1974 [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yamagata Y, Yates BJ, Schor RH, Nonaka S. Response of vestibular neurons to head rotations in vertical planes. III. Response of vestibulocollic neurons to vestibular and neck stimulation. J Neurophysiol 64: 1695–1703, 1990 [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Comparison of effects of stimulation of Deiters' nucleus and medial longitudinal fasciculus on neck, forelimb, and hindlimb motoneurons. J Neurophysiol 32: 743–758, 1969a [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Monosynaptic inhibition of neck motoneurons by medial vestibular nucleus. Exp Brain Res 9: 365–380, 1969b [DOI] [PubMed] [Google Scholar]