Abstract
Temperature can modify neural and behavioral responses to taste stimuli that elicit “sweetness,” a perception linked to intake of calorie-laden foods. However, the role of temperature in the neural representation of sweet taste is poorly understood. Here we made electrophysiological recordings from gustatory neurons in the medulla of inbred mice to study how adjustments in taste solution temperature to cool (18°C), ambient (22°C), and warm (30°C and 37°C) values changed the magnitude and latency of gustatory activity to sucrose (0, 0.05, 0.1, 0.17, 0.31, and 0.56 M). Analysis of 22 sucrose-best neurons revealed that temperature markedly influenced responses to sucrose, which, across concentrations, were largest when solutions were warmed to 30°C. However, reducing solution temperature from warm to ambient to cool progressively steepened the slope of the sucrose concentration-response function computed across cells (P < 0.05), indicating that mean activity to sucrose increased more rapidly with concentration steps under cooling than with warming. Thus the slope of the sucrose concentration-response function shows an inverse relation with temperature. Temperature also influenced latency to the first spike of the sucrose response. Across neurons, latencies were shorter when sucrose solutions were warmed and longer, by hundreds of milliseconds, when solutions were cooled (P < 0.05), indicating that temperature is also a temporal parameter of sucrose activity. Our findings reveal that temperature systematically modifies the timing of gustatory activity to sucrose in the mammalian brain and how this activity changes with concentration. Results further highlight how oral somatosensory cues function as physiological modulators of gustatory processing.
Keywords: taste, sucrose, temperature, latency, coding
the study of neural coding involves, in part, relating features of sensory stimuli to neural activity. For taste, this pursuit has largely focused on two stimulus features: the perceptual quality of the taste chemical and its concentration while dissolved in solution. Taste quality is a descriptive characteristic of taste stimuli transmitted, in part, through substitution (cf. Stevens 1961) of responses by different neurons. For instance, exchanging a taste stimulus of one quality (e.g., “sweet”) for another (e.g., “salty”) can cause a substitutive change in the neurons that respond maximally to the stimulus, leading to an associated change in the evoked response pattern. Concentration, on the other hand, is a physical property of taste stimuli that guides the magnitude of gustatory responses.
Temperature is an additional physical property of taste stimuli that can modulate gustatory processing. Temperature strongly influences neural and behavioral responses to sweet stimuli. Gustatory activity to sucrose in peripheral nerves carrying sensory input from the tongue to the brain is greater when taste solutions are warmed than when they are cooled (Breza et al. 2006; Lu et al. 2012; Talavera et al. 2005; Yamashita and Sato 1965). This effect arises, in part, from the actions of temperature on the transient receptor potential (TRP) ion channel TRPM5, a heat-activated cation channel that plays a key role in the molecular receptor transduction cascade for sweet stimuli (Perez et al. 2002; Talavera et al. 2005, 2007; Zhang et al. 2003). In humans, the detection and perceived intensity of sweet substances can vary with change in solution, and also tongue, temperature (Bartoshuk et al. 1982; Calvino 1986; Green and Frankmann 1987, 1988; Green and Nachtigal 2012; McBurney et al. 1973), indicating that temperature importantly guides the generation of sweet taste perceptions.
Studies on the influence of temperature on gustatory responses have largely focused on peripheral and psychophysical effects, leaving only a paucity of data on the influence of temperature on neural representations of sweet stimuli in the brain. A recent electrophysiology study on gustatory neurons in the mouse nucleus of the solitary tract (NTS) showed that warming taste solutions increased responses to 0.1 M sucrose in a supralinear manner and that cooling taste solutions largely inhibited sucrose activity (Wilson and Lemon 2013). These results suggested that temperature modified the gain of the neural response to sucrose. However, a broad focus of this work on multiple taste qualities afforded only limited exploration of this hypothesis.
The goal of the present study was to quantitatively define how stimulus temperature operates on the neural representation of sucrose taste in the brain. We recorded electrophysiological responses from sucrose-best NTS neurons in inbred mice during oral delivery of six concentrations of sucrose tested at four temperatures. Concentrations included perithreshold to strong intensities, and temperatures ranged from cool to physiological warm. Analysis of neural data focused on the effect of temperature on the slope of the sucrose concentration-response function, which indexes rate of growth in response with concentration, and on latency to first spike to sucrose. The latency of a gustatory response could conceivably influence the evolution of taste perceptions during the time course of flavor (Green and Frankmann 1988) and contribute information about gustatory stimuli (Breza et al. 2010; Graham et al. 2014; Hallock and Di Lorenzo 2006). Results showed that change in temperature induced systematic change in both the slope and latency parameters of neural responses to the sucrose concentration series. Our discussion centers on how temperature operates as a parameter of the magnitude and timing of gustatory activity to sucrose. Moreover, the present data further build on the postulate that oral somatosensory cues importantly guide and modulate the operation of neurobiological substrates for taste (e.g., Green 2002; Green and Nachtigal 2012; Wilson and Lemon 2013).
MATERIALS AND METHODS
Mouse Line and Preparation
Twenty-five adult mice {8 females, mean weight = 24.8 ± 2.1 g [standard deviation (SD)]; 17 males, mean weight = 28.3 ± 1.9 g (SD)} from the C57BL/6J (B6) inbred strain (The Jackson Laboratory, Bar Harbor, ME) were used. B6 mice were selected as this line harbors the “taster” allele of the genetic locus Sac, the saccharin preference locus, and, accordingly, shows robust sensitivity to and preference for stimuli that taste “sweet” to humans (Fuller 1974; Lush 1989). Sac is associated with the T1r3 taste receptor protein involved with sweet taste (Bachmanov et al. 2001; Kitagawa et al. 2001; Max et al. 2001; Montmayeur et al. 2001; Sainz et al. 2001). Mice were housed in a vivarium that maintained a 12:12-h light-dark cycle and an air temperature of ∼23°C. All mice were naive to experimentation. Room temperature water and standard rodent chow were available in the colony ad libitum.
All procedures were performed on mice under anesthesia in accordance with National Institutes of Health guidelines and protocols reviewed and approved by the Saint Louis University Institutional Animal Care and Use Committee. Mice were acutely anesthetized with a mixture of ketamine (100 mg/kg ip) and xylazine (10 mg/kg ip) to facilitate insertion of a tracheostomy tube. Atropine (0.024 mg/kg ip) was administered to reduce bronchial secretions. Each mouse was positioned in a nontraumatic head holder, and a custom “nose cone” for gas anesthesia was placed over the open end of the tracheostomy tube, which was angled away from the mouth. The nose cone was intentionally designed not to seal onto the tracheostomy tube but to allow for continuous weak-pressure delivery and vacuum removal of anesthetic gas to and from the air space surrounding the distal opening of this tube. Mice then freely respired this gas through their tracheostomy tube, without the aid of a mechanical ventilator. Anesthesia was maintained throughout recording sessions with 1–1.5% isoflurane in oxygen, where a high-precision vaporizer (Dräger Medical, Lübeck, Germany) regulated isoflurane concentration. Other surgical and electrophysiological recording procedures from mouse NTS were as described previously (Lemon and Margolskee 2009; Wilson et al. 2012).
Stimuli
The presentation of taste solutions was carried out with a custom apparatus, as described previously (Lemon and Margolskee 2009; Wilson and Lemon 2013). Briefly, stimuli were loaded one at a time into a funnel and tubing system suspended above the preparation and drawn into the mouse oral cavity by gravity. A three-way, computer-controlled fluid valve precisely regulated the timing of taste stimulus flow and switching between taste and rinse solutions during a trial, as described below. This valve was positioned such that a small plastic elbow (∼1.5-mm inner diameter) extended the output passage of the valve into the mouse oral cavity. A small, sealable hole was punctured in the ventral end of the elbow for insertion of a fine, fast-response thermocouple probe, which continuously monitored the temperature (to 0.1°C accuracy, sampled at 1 kHz) of solution flow at the moment of oral delivery. This system facilitated broad-field application of taste and rinse solutions to multiple oral epithelia, including rostral, caudal, and lateral regions of the tongue, and also the palate.
Taste solutions included 0.3 M d-fructose (monosaccharide), 0.03 M NaCl (sodium salt), 0.003 M citric acid (organic acid), 0.01 M quinine·HCl (alkaloid; bitter), and six concentrations of sucrose (disaccharide), including 0 (purified water), 0.05, 0.1, 0.17, 0.31, and 0.56 M. Sucrose concentrations were approximate quarter-log steps selected to cover a dynamic behavioral response range in mice, from perithreshold (0.05 M) to strongly appetitive (0.31, 0.56 M; see Treesukosol and Spector 2012). Concentrations of other stimuli followed from our previous neural recording studies in mice. All taste chemicals (Sigma, St. Louis, MO) were high purity and dissolved in purified water. Taste solutions were stored in airtight glass bottles; dark glass was used for quinine, a light-sensitive compound.
Bottles of taste solutions were placed into circulated refrigerated and warming water baths to regulate their temperature. A separate bath and group of stimulus bottles was used for each temperature condition. Solutions of one concentration of sucrose tested at multiple temperatures were generally drawn from one stock to ensure that concentration was invariant across thermal conditions. Sucrose solutions were cooled and warmed to four target temperatures: 18°C (cool), 22°C (ambient), 30°C (warm), and 37°C (warm; physiological). Temperature values were selected to provide nonnoxious cooling and warming (cf. Wilson and Lemon 2013). The actual mean temperatures measured within condition, as sampled during the last 3 s of stimulus delivery across all sucrose trials, were (±SD; in °C/ms) 17.8 ± 1.3, 21.8 ± 1.2, 30.1 ± 1.4, and 37.1 ± 1.2. Temperature was expressed in degrees Celsius per millisecond to index the stability of the actual stimulus temperature relative to the target and facilitate use of change in the thermal signal for detection of stimulus onset time, as described below. Other references to stimulus temperature in this article refer to the target values above. Fructose, NaCl, citric acid, and quinine were used only for neuronal classification and were tested at 22°C.
Oral delivery of a rinse of purified water at 22°C preceded and followed presentation of each taste stimulus and also continued between trials during data collection. This rinse aimed to maintain a stable oral adapting temperature and to adapt mechanosensory activity associated with taste stimulation. A stainless steel, brazed plate, fluid-to-fluid heat exchanger (AIC, model LA14-10X) controlled the temperature of the rinse solution, which flowed through the exchanger under light pressure. A closed-loop water supply set to 22°C fed the temperature regulation circuit of the heat exchanger. The actual mean temperature of the adapting rinse at the oral cavity, as acquired during the last 3 s of the prestimulus period across all sucrose trials, was 21.8 ± 1.2°C/ms (SD).
Data Acquisition
Electrophysiological activity was recorded with a high-impedance (2–5 MΩ) tungsten microelectrode. The NTS was located in mice as described previously (Lemon and Margolskee 2009; Wilson et al. 2012; Wilson and Lemon 2013). When searching for single units, the electrode was lowered into the nucleus and neural activity to oral delivery of 0.5 M sucrose was monitored. The use of an appetitive concentration of sucrose as the primary search stimulus was intended to purposefully target units with high sensitivity to sucrose, cells at the focus of the present work. Nonetheless, data were recorded from isolated taste-sensitive neurons, with the sucrose orientation of cells identified post hoc, as below. Unit activity in mouse NTS was digitized at 25 kHz (1401 interface and Spike2 software, CED, Cambridge, UK) and spikes from single units identified on the basis of waveform consistency (see Figs. 1 and 4). The thermocouple circuit that monitored the temperature of the rinse and stimulus flow was also linked to the data acquisition system. Thus temperature data were recorded simultaneously alongside neural activity.
Fig. 1.
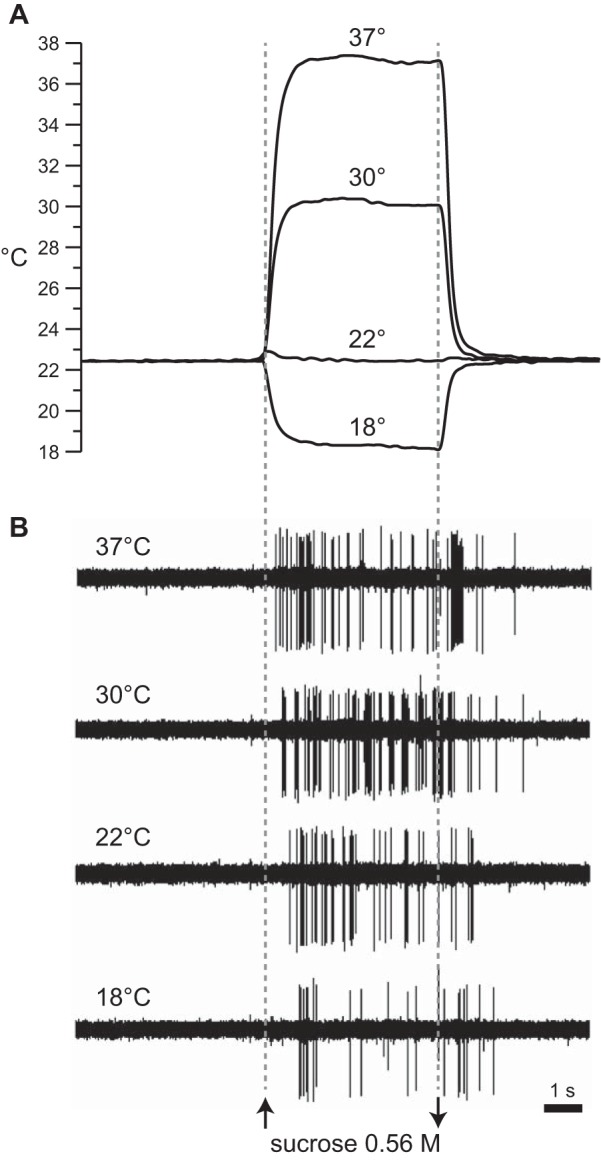
Example recordings from 1 S-type neuron. A: family of traces depicting real-time measurement of rinse and stimulus temperature, at the moment of oral delivery, on 4 separate trials where a fixed concentration of sucrose (0.56 M) was tested at 18°C, 22°C, 30°C, and 37°C. B: electrophysiological sweeps depicting trains of action potentials recorded in synchrony with temperature during each trial in A. Sweeps are aligned by time of stimulus onset (see materials and methods). Upward and downward arrows indicate stimulus onset and offset, respectively.
Fig. 4.
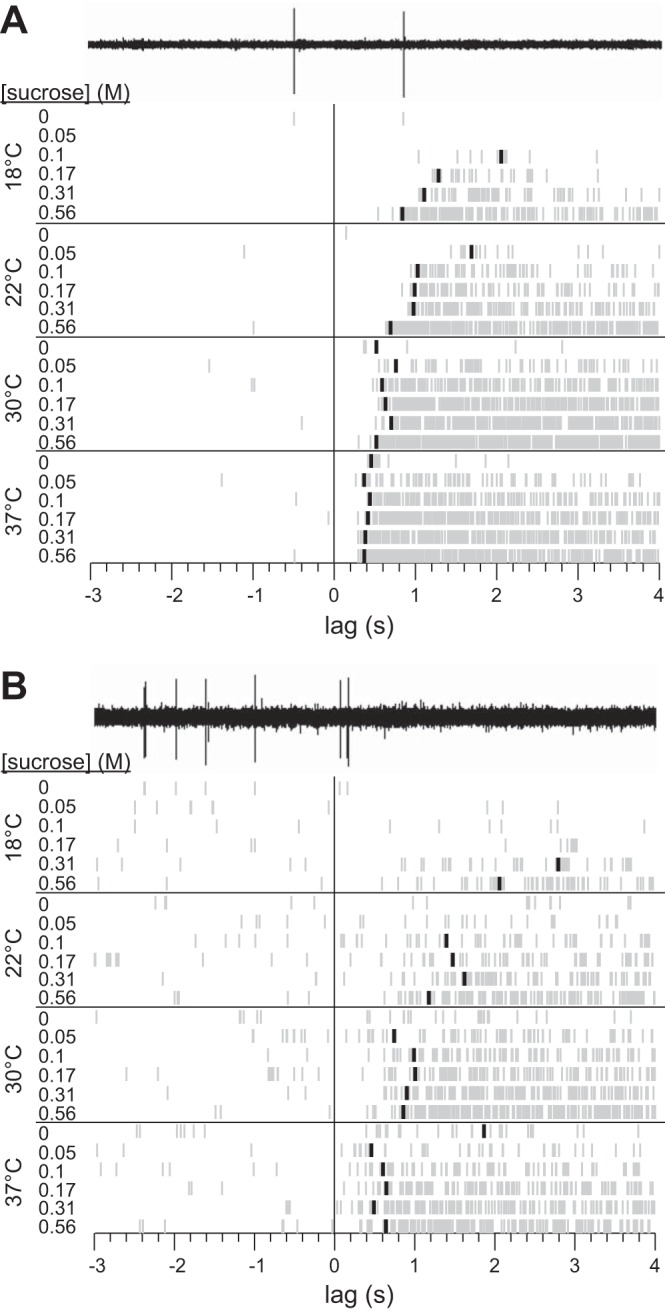
Rastergrams for 2 separately recorded S-type neurons (A and B) showing detection of latency to first spike across 24 unique temperature-concentration combination trials for sucrose. The electrophysiological sweep recorded for 0 M sucrose at 18°C is shown for each cell to demonstrate conversion of neurophysiological data to raster spikes. A blackened raster spike on a trial represents the time during stimulus delivery when the firing rate of the neuron became unusually high compared with the average prestimulus firing rate of the cell (see materials and methods). The absence of a blackened spike indicates that no significant elevation in firing was detected for that trial.
Each isolated neuron was first tested with 22°C solutions of fructose, NaCl, citric acid, and quinine, in random order. Next, neurons were tested with each of the six concentrations of sucrose adjusted to 18°C, 22°C, 30°C, and 37°C. To randomize presentation order, the four temperatures used for one sucrose concentration were grouped into a single block, and the sequence of the six concentration blocks was randomized for each cell. The ordering of temperature trials within each block was also randomized.
Each data acquisition trial lasted 15 s. The 22°C adapting water rinse was presented during the first 5 s of a trial. Flow then immediately switched to stimulus delivery for 5 s. Flow retuned to 22°C water after the taste presentation epoch. The intertrial interval was ∼2–3 min. Mice did not ingest solutions, which fell into a drain positioned beneath the mandible.
Data analysis proceeded in two phases. Phase 1 assessed how stimulus temperature interacted with concentration to influence neuronal response magnitude to sucrose. Phase 2 assessed how stimulus temperature influenced the latency and timing of neural responses to intensity-varied solutions of sucrose. The types of data and analytic approach were unique to each phase, as follows.
Phase 1: analyses of response magnitude.
Analyses in phase 1 were performed on the magnitude of neural activity to taste solutions. For each trial, the number of action potentials that arose during the 5-s stimulus presentation period was defined as response magnitude. A “long” 5-s response window was used to capture general effects. The prestimulus firing rate for each trial was calculated as the average number of spikes that emerged in 1 s during the 5-s period that preceded taste stimulus presentation. Although indexed, prestimulus activity was not subtracted from spike counts collected during taste presentation to avoid negative responses in regression analysis of log-transformed data, as below. Nonetheless, inspection of average trends in activity to sucrose both corrected and uncorrected for prestimulus firing revealed similar effects of temperature between these conditions.
Hierarchical cluster analysis was used to sort neurons into groups with unique response profiles across stimuli and to identify neurons most responsive to sucrose. Cluster analysis was performed on a matrix of pairwise correlation distances among neurons computed from their responses to 22°C solutions of fructose, NaCl, citric acid, quinine, and all six concentrations of sucrose. Group average amalgamation was used. Scree plots determined groupings in the cluster solution. Because cluster analysis was used only to detect neurons with strong sucrose sensitivity, the clustering solution (e.g., dendrogram) was not a main finding and thus is not shown here.
Double-log regression was applied to sucrose concentration-response data at each temperature, operating on the logarithm (base 10) of response magnitude and the logarithm of concentration, to study how temperature influenced the rate by which activity to sucrose grew with concentration. The slope of the regression line fitted to sucrose responses in doubly logarithmic coordinates estimated the percent change in the response expected when concentration increased by 1% (cf. Wooldridge 2009), assuming all other variables were constant. For this analysis unit responses to sucrose were transformed as log(resp + 1), where resp is the response magnitude, and adding 1 to resp allowed the logarithm of magnitude to be taken when resp = 0. A t-test evaluated the deviation of slopes from 0 or 1.
The 95% confidence interval of the mean (95% CI) was computed for responses to sucrose and regression slopes measured under different stimulus conditions. If the 95% CIs of two estimates did not overlap at all, they were interpreted as different at the α = 0.05 level (cf. Whitlock and Schluter 2009). Where reported, 95% CIs are described as [lower bound, upper bound].
Phase 2: analyses of timing of neural activity to sucrose.
Analyses in phase 2 focused on the influence of solution temperature on the latency to the first spike of the sucrose response. To measure this effect, we estimated on each trial the time of sucrose entry into the mouth, defined as stimulus onset, and the time after onset when a statistically significant increase in neuronal discharge to sucrose occurred, defined as latency.
Time of stimulus onset during sucrose trials.
The continuous monitoring of solution temperature at the moment of oral delivery afforded the use of change in temperature from baseline as a marker of stimulus onset during presentations of cooled and warmed sucrose. For these trials, the mean temperature (in°C/ms) of the adapting prestimulus rinse, T̄adapt, was calculated, and the solution temperature sampled during each consecutive millisecond of the taste stimulation period was compared to this value with ΔT = 0.5°C as a threshold for temperature change. For trials that tested cooled (18°C) sucrose solutions, stimulus onset was defined as the time (1-ms resolution) during taste stimulation when oral solution temperature fell below T̄adapt − 0.5°C (cf. Fig. 1A). For trials that tested warmed (30°C and 37°C) solutions, onset was defined as the time at which stimulus temperature exceeded T̄adapt + 0.5°C. In contrast to cooling and warming trials, sucrose solutions tested at 22°C were isothermal with the adapting baseline temperature. Thus stimulus onset for each particular concentration of sucrose at 22°C was estimated independently for each neuron by averaging the onset times for that concentration measured at 18°C and 30°C.
Analysis of stimulus onset times revealed that solution delivery rate varied nominally with cooling and warming of taste solutions. The mean stimulus onset time for all trials tested at 18°C [5.45 ± 0.02 s (SD); min = 5.42; max = 5.47] was slightly delayed (Bonferroni-adjusted pairwise comparisons, P < 0.001; repeated-measures ANOVA, F2,42 = 519.7, P < 0.001), by ∼100 ms, relative to the mean onset times for trials at 30°C [5.35 ± 0.02 s (SD); min = 5.32; max = 5.37] and 37°C [5.34 ± 0.01 s (SD); min = 5.32; max = 5.37]. With these data and the known flow rate of the stimulus delivery system at room temperature (∼1.4 ml/s), flow rates during cooling and warming trials were estimated at 1.33 ml/s (velocity = 72.75 cm/s) and 1.47 ml/s (80.75 cm/s), respectively, with an approximate difference of 150 μl/s (8 cm/s).
The subtle variance in stimulus delivery rates across cool and warm temperature conditions likely produced only negligible, if any, effect on measurement of latency to neuronal activation. For instance, time to stimulus onset also subtly varied within each temperature-concentration condition for sucrose (average SD = 30 ms), albeit, within each condition, there was no significant correlation (P > 0.06; mean r = −0.13) between stimulus onset time and latency to neural response. Moreover, changes in temperature were found here to induce shifts in neuronal response latency to sucrose on the order of many hundreds of milliseconds (see Figs. 1, 4, and 5A). Such shifts in time were substantially larger than the observed variation in stimulus onset.
Fig. 5.
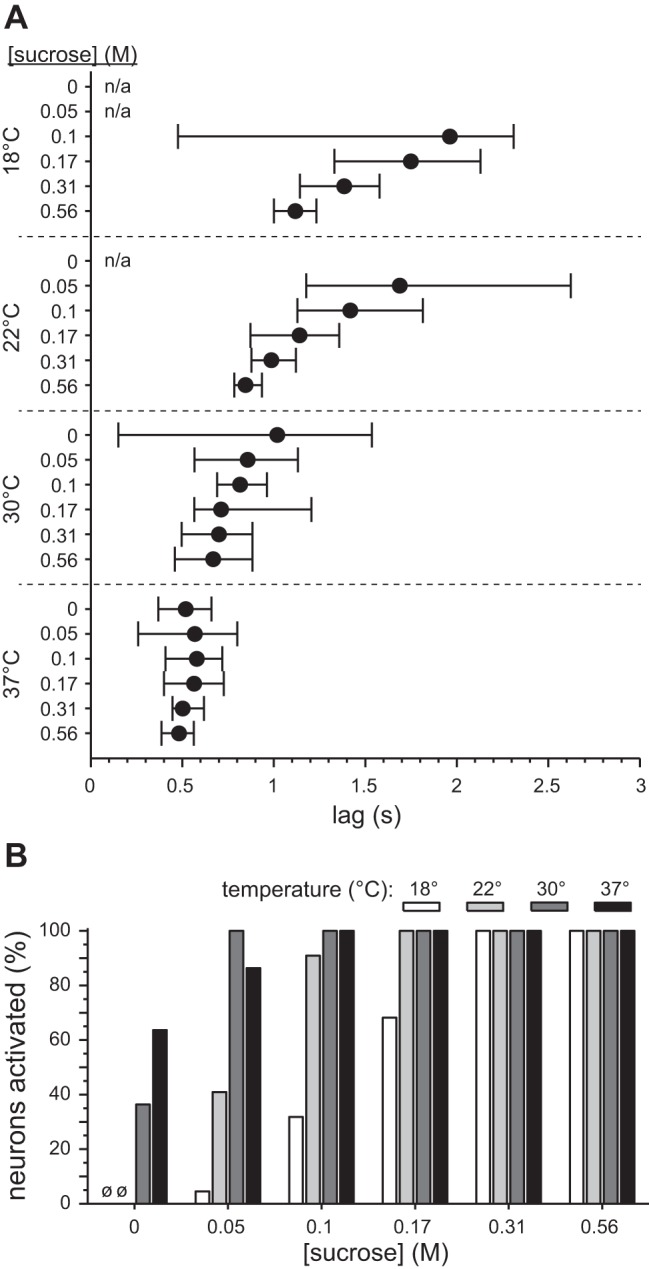
Effect of temperature on latency to first spike to sucrose across 22 S-type neurons. A: median latency (filled circles) and 95% confidence limits for the median (whiskers) for each of the 24 sucrose temperature-concentration conditions. Confidence limits were approximated with a bootstrap resampling procedure (see materials and methods). n/a, Interval not applicable: no or too few cells activated. B: % of neurons within each temperature-concentration condition for sucrose where latency to first spike could be detected (i.e., neurons that showed significant activation to sucrose). ø, No cells activated (0 M sucrose at 18°C and 22°C).
The time of stimulus onset on each trial was set as the zero point along the timescale of the neuronal spiking response. All spike times on a trial were expressed relative to this zero point, as was the latency to the first spike of the sucrose response.
Latency to neural response to sucrose.
Latency to first spike during sucrose presentation was defined as the time of the first significant elevation in firing rate from the prestimulus rate. Only significant increases in responding were assessed, as some cells had low or zero prestimulus activity, precluding detection of inhibition. A binless algorithm (cf. Bair and Koch 1996; Chase and Young 2007; Schumacher et al. 2011) quantified latency on individual sucrose trials. For description, let us assume that s represents a counter for spikes that arose in series following stimulus onset. For each of these spikes considered in sequence, a Poisson-based statistical model estimated the probability that a firing rate equal to or greater than s was due to lingering prestimulus activity at time ts, which was the time lag (precision = 0.1 ms) from stimulus onset to spike s. This probability was given by
where λ is the mean prestimulus firing rate (spikes/s) for the neuron under consideration, as averaged across all 24 temperature-concentration combination trials for sucrose. When iteratively computing Pr[≥s], λ was multiplied by the period, in seconds, of the current spike count window, ts. This scaled λ according to the size of the potential response window (cf. Chase and Young 2007). If Pr[≥s] became <10−6, a firing rate of s spikes at time ts was unusually high relative to prestimulus firing (Bair and Koch 1996; Chase and Young 2007; Schumacher et al. 2011) and the time of spike s (i.e., ts) was taken as latency to first spike. If this criterion was not met for spikes falling within 4 s of stimulus onset, response latency was left undefined. This statistical criterion for latency worked reasonably well for NTS gustatory units, as based on visual inspection of all latencies plotted against spike rastergrams (e.g., Fig. 4).
In part because of imbalance in the number of latencies obtained between certain temperature-concentration conditions, a bootstrap-t resampling procedure (cf. Wilcox 2003) was used to approximate the sampling distribution of median response latency for each condition, and to estimate the confidence interval for the median. To do this, n replicates of the actual n latencies measured for one thermo-concentration condition were randomly resampled, with replacement, 1,000 times. After each resample, a studentized bootstrap-t (T*) was computed as given by T* = (m* − m)/s*, where m* is the median of the current bootstrap resample, m is the median of the actual latency data, and s* is the standard deviation of the medians of 100 nested bootstrapped resamples of the current bootstrap resample. The resulting distribution of T* values was used to calculate a 95% bootstrapped confidence interval (95% CI*) for median latency to response. The 95% CI* for sample median m was given by × SM* + m, × SM* + m], where and are the respective 2.5th and 97.5th percentile T* values and SM* is the standard deviation of the distribution of medians for the 1,000 bootstrap-generated resamples of latency. Confidence intervals were compared between different temperature-concentration conditions to assess trends in the effect of temperature on latency to first spike to sucrose.
ANOVAs were performed with SPSS (version 20, IBM, Somers, NY). All other mathematical, statistical, and plotting procedures were carried out with custom code and proprietary functions in MATLAB (version 8.1, The MathWorks, Natick, MA). Regression and bootstrap resampling procedures were performed with methods included with the MATLAB statistics toolbox (version 8.2).
RESULTS
Trains of action potentials were recorded from 35 NTS neurons in B6 mice. Cluster analysis of responses to all stimuli tested at 22°C revealed that 22 of these cells responded “best” to 0.56 M sucrose; these cells were called S-type neurons. Other identified neuron types included cells that responded best to quinine (n = 3), those that responded best to NaCl (n = 9), and one acid-best unit. When collapsed across all concentrations excluding 0 M, sucrose tested at 22°C induced significantly larger responses (nonoverlapping 95% CIs) in S-type neurons (mean response magnitude = 66.5 spikes, 95% CI [56.4, 76.5]) compared with Na+-best (mean response magnitude = 16.4 spikes, 95% CI [12.7, 20.2]) or quinine-best (mean response magnitude = 32.6 spikes, 95% CI [12.5, 52.7]) cells. Because this study focused on the neural signaling of sucrose taste, all analyses reported here involved response data from only S-type units, which composed 63% of recorded cells. In NTS, S-type cells arise at a reduced frequency in mouse lines with relatively lower behavioral sensitivity to sucrose (McCaughey 2007) and do not emerge in mice genetically deficient for the T1r3 taste receptor protein (Lemon and Margolskee 2009), which is a component of the neural circuit that guides normal behavioral preference for sucrose (Damak et al. 2003; Zhao et al. 2003). Thus S-type neurons likely play a key role in the central processing of sucrose taste in mice. Digital oscilloscope sweeps depicting responses by one S-type neuron to 0.56 M sucrose tested at cool, ambient, and warm temperatures are shown in Fig. 1. Each of the 22 S-type neurons was tested with all six concentrations of sucrose at all four temperatures, which made 528 trials for sucrose available for analysis. S-type cells displayed low activity during the prestimulus period of all sucrose trials [mean spiking rate = 0.62 ± 1 Hz (SD)].
Temperature Influences the Slope of the Concentration-Response Function to Sucrose in S-Type Neurons
We first studied how variation in stimulus temperature would impact the magnitude of the neuronal response to sucrose. Figure 2A depicts the mean concentration-response functions for sucrose, in linear coordinates, measured across temperature conditions. Visual inspection of this plot revealed that at each temperature, activity to sucrose generally, and expectedly, increased monotonically with increasing concentration steps. However, the magnitude of the sucrose concentration-response function varied markedly across temperatures. This observation was statistically explored through a two-way repeated-measures ANOVA on responses to 0.1, 0.17, 0.31, and 0.56 M sucrose tested at 18°C, 22°C, 30°C, and 37°C; responses to 0 and 0.05 M sucrose violated assumptions of ANOVA and were not included in this analysis. Following a significant concentration × temperature interaction (F9,189 = 7, P < 0.001), tests of simple effects of temperature revealed that neuronal responses to sucrose, at all concentrations considered, systematically increased with warming from 18°C to 22°C to 30°C, then decreased with further warming to 37°C (Bonferroni-adjusted pairwise comparisons, P < 0.04). Thus under the present conditions augmentation of neural activity to sucrose by warming must asymptote near a temperature of 30°C, as further increases in temperature began to reduce responses (cf. Wilson and Lemon 2013). In fact, the response to 0.56 M sucrose, albeit increased by warming from 22°C to 30°C, did not differ between 22°C and 37°C (Bonferroni-adjusted pairwise comparison, P = 1).
Fig. 2.
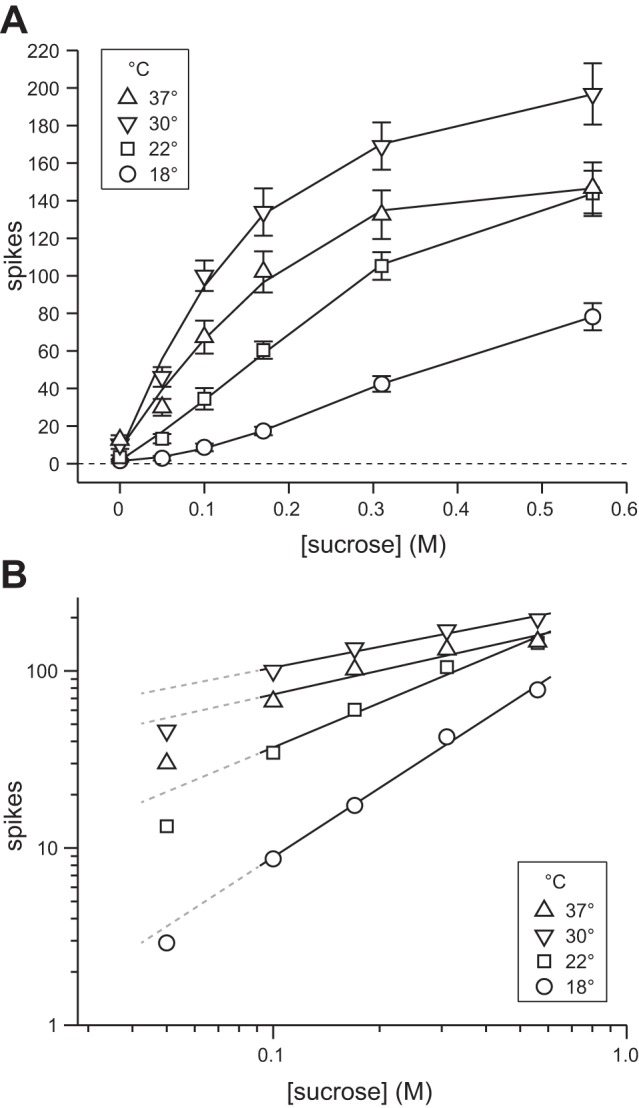
Effect of temperature on averaged concentration-response functions to sucrose. A: mean (±SE) responses across 22 S-type neurons to 0, 0.05, 0.1, 0.17, 0.31, and 0.56 M sucrose tested at 18°C, 22°C, 30°C, and 37°C. Data are plotted in linear coordinates. Solid lines represent quadratic fit of mean responses at each temperature. B: mean response values in A, except for 0 M, plotted in doubly logarithmic (base 10) coordinates. Solid lines represent least-squares fits applied to mean response values for 0.1, 0.17, 0.31, and 0.56 M sucrose at each temperature, operating on the logarithm of the mean response and the logarithm of concentration. Dashed lines extend fits for each temperature to allow visual comparison against activity to 0.05 M sucrose.
In addition to its influence on magnitude, temperature appeared to markedly influence the shape of the sucrose concentration-response function (Fig. 2A), which reflects the degree of change in spike discharge induced by stepping concentration. This observation was quantitatively explored through log transformation of the data. Plotting neural activity to sucrose against concentration in doubly logarithmic coordinates, using the logarithm of the response and the logarithm of concentration, yielded points for averaged activity to 0.1, 0.17, 0.31, and 0.56 M sucrose that tightly adhered to a least-squares line (0.91 < r2 < 1) with a slope that appeared to differ between cool, ambient, and warm temperatures (Fig. 2B). As an aside, the response to 0.05 M sucrose at each temperature clearly fell below each line (Fig. 2B). This suggests that the relationship between response growth, sucrose concentration, and temperature may markedly shift when transitioning from perithreshold concentrations to intermediate or greater intensities (cf. Calvino 1986).
It is noteworthy that the slope of a least-squares line computed in doubly logarithmic coordinates estimates the percent growth in the dependent variable expected while the predictor is increased by 1%. Following this, we performed least-squares regression on individual responses to 0.1, 0.17, 0.31, and 0.56 M sucrose at each temperature, operating on the logarithms of firing rate and concentration (Fig. 3), to determine how temperature influenced the rate of growth of the sucrose concentration-response function. Regression slopes were significantly greater than 0 at each temperature (1-sample t-tests, t20 > 5.5, P < 0.001) and significantly steepened with reductions in sucrose temperature from warm (30°C or 37°C) to ambient (22°C) to cool (18°C) values (nonoverlapping 95% CIs between conditions; Table 1). At 18°C, the slope of the sucrose concentration-response function was significantly greater than 1 (1-sample t-test, t20 = 3.5, P = 0.002), predicting that under cooling a 1% increase in concentration would cause, on average, a >1% increase in neuronal firing. On the other hand, the slope fell to significantly less than 1 at 30°C (1-sample t-test, t20 = −8.5, P < 0.001) and 37°C (1-sample t-test, t20 = −5.6, P < 0.001), predicting that under warming a 1% increase in sucrose concentration would increase neuronal firing by <1%, on average. The slope of the regression line fit to responses to sucrose at ambient temperature did not differ from 1 (1-sample t-test, t20 = −0.6, P = 0.6), reflecting relatively intermediate growth in activity with concentration.
Fig. 3.
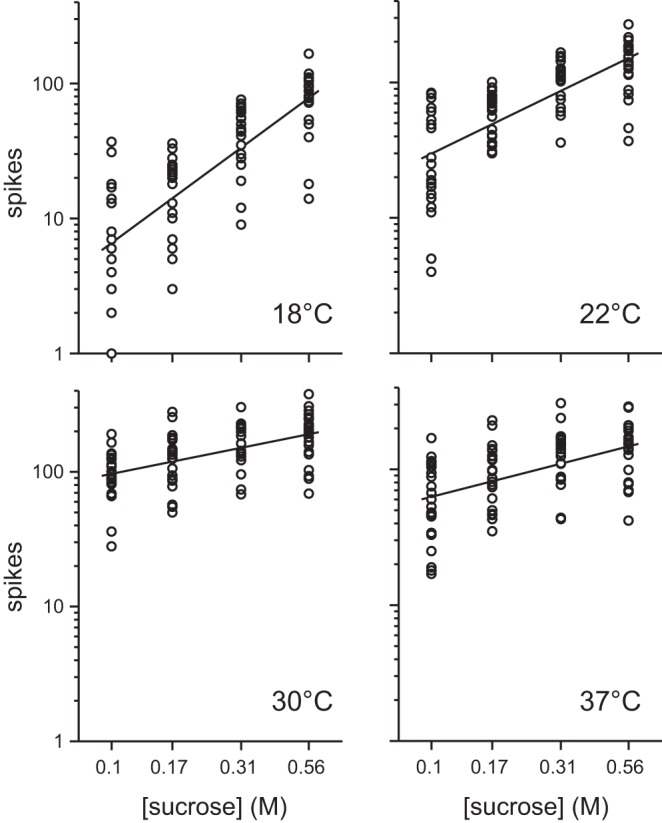
Responses by each of 22 S-type neurons to 0.1, 0.17, 0.31, and 0.56 M sucrose tested at 18°C, 22°C, 30°C, and 37°C. Data are plotted in doubly logarithmic (base 10) coordinates. Solid lines represent least-squares fits applied to all individual responses to these concentrations at each temperature, operating on the logarithm of neural activity and the logarithm of concentration. See materials and methods for details and Table 1 for regression results.
Table 1.
Results of least-squares regression applied to responses by 22 S-type neurons to 0.1, 0.17, 0.31, and 0.56 M sucrose at each temperature
| °C | Slope [95% CI lower, 95% CI upper] | Intercept | r2 | Group |
|---|---|---|---|---|
| 18 | 1.44 [1.18, 1.70] | 2.25 | 0.61 | a |
| 22 | 0.94 [0.74, 1.15] | 2.42 | 0.52 | b |
| 30 | 0.39 [0.25, 0.54] | 2.38 | 0.26 | c |
| 37 | 0.50 [0.31, 0.68] | 2.29 | 0.26 | c |
Values are results of least-squares regression applied to responses by 22 S-type neurons to 0.1, 0.17, 0.31, and 0.56 M sucrose at each temperature (Fig. 3). Calculations operated on the logarithm of the spiking response and the logarithm of concentration. All regression slopes were significantly greater than 0 (P < 0.001). The Group column indicates whether there was separation, denoted by different characters, between the 95% confidence intervals (95% CIs) for 2 slopes.
The results above show that, for 0.1 M and higher intensities, temperature systematically modified the slope of the concentration-response function to sucrose observed across S-type neurons. This slope was flattest with warming and steepest with cooling, and holding sucrose at ambient temperature produced a relatively intermediate slope. Thus steps in concentration induced the largest relative change in mean activity to sucrose under cooling.
Temperature Influences Latency to Respond to Sucrose in S-Type Neurons
We were able to observe during recordings a strong influence of temperature on latency to first spike to sucrose. This effect was readily seen in S-type neurons with zero prestimulus activity, as illustrated in Fig. 1. For this cell, increasing the temperature of the sucrose solution, while holding concentration constant, systematically decreased the latency to first spike. Latencies across the four temperatures were 18°C, 917 ms; 22°C, 658 ms; 30°C, 451 ms; and 37°C, 278 ms. The shortest and longest lags across these trials differed by 639 ms, indicating that temperature could markedly impact the time at which this cell generated its response to sucrose.
Unlike the example unit in Fig. 1, most neurons produced some spikes during the prestimulation period of sucrose trials, presumably reflecting, in part, “spontaneous” activity commonly reported in taste-sensitive units. The presence of prestimulus activity during a trial raises the question of whether a spike observed just after the onset of stimulus presentation reflects lingering spontaneous drive or the beginning of a taste response. Given this uncertainty, latency to response on each sucrose trial was detected with a statistical algorithm (materials and methods). Figure 4 demonstrates application of this algorithm to estimate, for two neurons, latency to first spike to temperature- and also concentration-varied solutions of sucrose. As shown in Fig. 4, sucrose temperature strongly influenced latency to first spike, which showed a general trend across sucrose concentrations to decrease with warming. Increasing sucrose concentration could also reduce latency in these cells, particularly for the unit in Fig. 4A, albeit this effect was obvious only at cool (18°C) and ambient (22°C) temperatures. For both neurons, warming solutions to 30°C and also 37°C markedly reduced the dependence of latency on concentration. Warming also yielded the shortest latencies measured across all thermo-concentration conditions. Multiple concentrations of sucrose at 30°C and 37°C induced latencies in these units that were visibly shorter than lags measured to the highest concentration of sucrose tested at ambient temperature (Fig. 4), the standard stimulus temperature used in many taste neurophysiology studies.
Statistical assessment of latency data from all S-type neurons recapitulated the effects observed in the example units above. Across units, temperature ubiquitously modified the dependence of latency on sucrose concentration. For instance, at 18°C latencies for 0.56 M [median latency (m) = 1.12 s] were quicker than latencies measured for 0.17 M (m = 1.75 s), as evidenced by nonoverlapping 95% CI*s between concentrations (Fig. 5A). At 22°C, latencies for 0.31 M (m = 0.99 s) and 0.56 M (m = 0.85 s) were shorter than latencies for 0.1 M (m = 1.42 s) or 0.05 M (m = 1.69 s), as suggested by lack of overlap between 95% CI*s (Fig. 5A). On the other hand, warming solutions to 30°C or 37°C reduced all latencies such that median latency for each concentration generally overlapped with the 95% CI* of the median for every other concentration (Fig. 5A). Thus concentration can modulate first spike latency to sucrose at cool and ambient temperatures but has little to no influence on latency when sucrose is warmed.
The reduced latencies with warming were the shortest observed among all thermo-concentration conditions. For example, latencies to 0.31 M (m = 0.50 s), 0.17 M (m = 0.56 s), and 0.1 M (m = 0.58 s) sucrose warmed to 37°C were shorter than latencies for even the highest concentration of sucrose, 0.56 M, tested at ambient temperature (m = 0.85 s), as suggested by nonoverlapping 95% CI*s (Fig. 5A). What is more, latencies for particular concentrations of sucrose systematically decreased with warming. For instance, latencies for 0.56 M sucrose progressively fell as temperature was raised from 18°C (m = 1.12 s) to 22°C (m = 0.85 s) to 37°C (m = 0.48 s), as revealed by nonoverlapping 95% CI*s between temperatures (Fig. 5A). Latencies for 0.56 M at 30°C (m = 0.67 s) and 37°C were also significantly reduced relative to 18°C (Fig. 5A). These data indicate that the “speed” of neural activation to sucrose is importantly controlled by stimulus temperature. Warming systematically speeds up neural activation to sucrose, and cooling slows it down.
Finally, warming significantly increased the percentage of units activated (i.e., units with defined latencies) by perithreshold (0.05 M; cf. Treesukosol and Spector 2012) and low (0.1 M) concentrations of sucrose (χ2 > 8, df = 3, P < 0.04; Fig. 5B). It is noteworthy that only one S-type neuron responded to 0.05 M sucrose at 18°C, albeit all S-class cells responded to this concentration tested at 30°C. Thus the ability of S-type neurons to register the presence of weakly sweet solutions of sucrose is augmented by warmth.
Our previous work indicated that many NTS units can respond to warmed water applied to oral epithelia, in the absence of taste input (Wilson and Lemon 2013). Thus we explored whether the early phase of reduced-latency firing to warmed sucrose represented a bona fide chemosensory response or the appearance of an initial “warming” response induced solely by temperature. Using data from S-type neurons, spike trains for all concentrations of sucrose tested at 30°C and 37°C were divided into 50-ms bins, where each bin held a spike count. Median spike counts for synchronized bins were compared, within temperature, between trials for warmed water (0 M sucrose) and each sucrose concentration to address whether firing to warmed sucrose surpassed that to warmth alone in early response windows.
Analysis of binned data revealed that firing to sucrose became greater than activity to warmed water by 600–800 ms after stimulus onset. Specifically, at 30°C binned activity to 0.1, 0.17, 0.31, and 0.56 M sucrose significantly exceeded, under a conservative α, activity to water at 800, 700, 750, and 750 ms, respectively, poststimulus (Bonferroni-adjusted sign tests, P < 0.003; Fig. 6A). At 37°C, firing to 0.1, 0.17, 0.31, and 0.56 M sucrose became significantly greater than activity to water at 700, 600, 650, and 600 ms, respectively, poststimulus (Bonferroni-adjusted sign tests, P < 0.003; Fig. 6B). For each temperature-concentration condition noted, the time bin where activity to sucrose rose above activity to warmed water was within or immediately followed the 95% CI* for latency to first spike to sucrose (Fig. 5A). Thus reductions in latency with warming appeared to result from a reduction in lag to a chemosensory response to sucrose rather than the emergence of an early, purely thermal signal.
Fig. 6.
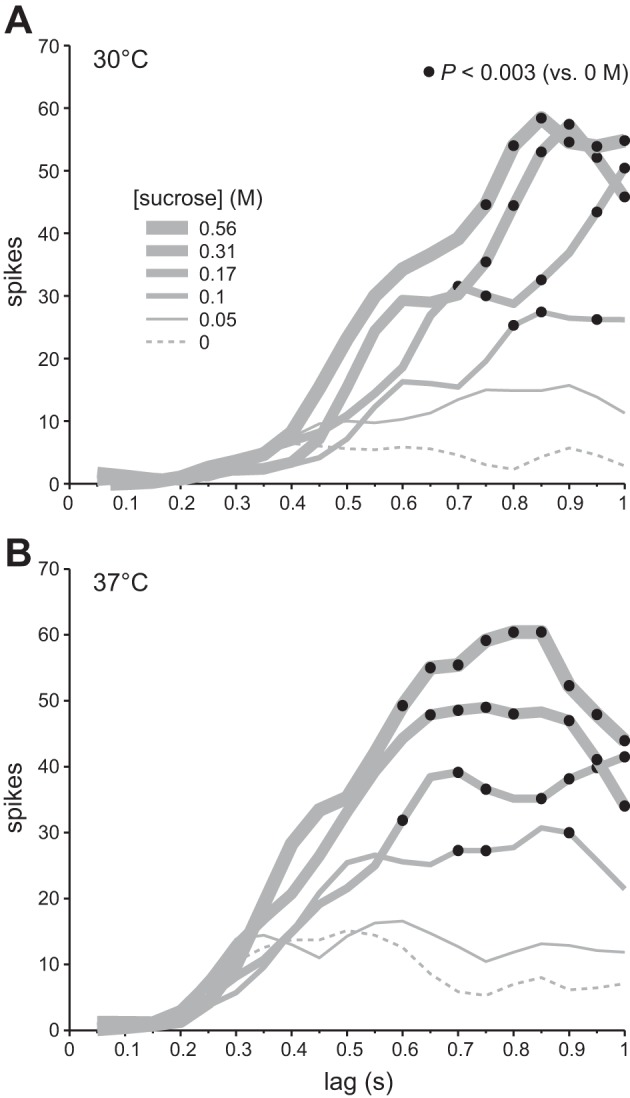
Time course of neuronal responses to 0, 0.05, 0.1, 0.17, 0.31, and 0.56 M sucrose tested at 30°C (A) and 37°C (B). In each panel, a family of traces (gray lines) depicts time-evolved activity by 22 S-type neurons to multiple sucrose concentrations, where traces for different concentrations are denoted by line thickness. To construct each plot, individual spike trains were binned (50 ms) and spike counts in time-aligned bins were summed across cells. Each trace connects points for sequential response bins for 1 concentration. Bins are referenced along the x-axis by their time of closure (e.g., data for the 0–0.05 s bin are plotted at 0.05 s on x-axis). Filled circles denote bins where median activity to sucrose was significantly greater than median activity to isothermal water (i.e., 0 M sucrose). Response traces represent smoothed data (lowess method), although statistical comparisons were made with raw spike counts.
DISCUSSION
Here we report two novel effects of temperature on neural responses to sucrose. Recordings from S-type neurons in mouse NTS revealed that cooling and warming solutions of sucrose induced marked change in 1) the slope of the concentration-response function to this stimulus and 2) the time of onset of neural activation. Temperature systematically modified these parameters of sucrose activity, with both slope and latency showing an inverse relation with temperature. These results reveal that temperature is an operational parameter of the central code for sucrose taste, yet few studies have considered temperature in the analysis of central responses to sweet, or other, taste stimuli (cf. Wilson and Lemon 2013).
Cooling and Concentration Effects
Change in solution temperature changed the slope of the response function for 0.1–0.56 M sucrose, as assessed by log-log regression. Decreasing temperature from warm (30°C or 37°C) to ambient (22°C) to cool (18°C) systematically steepened the regression slope (Figs. 2 and 3). Because this slope is proportional to the percent change in response expected, on average, for a given concentration step, neural responses to sucrose increased more rapidly with concentration under cooling compared with warming. This effect associates with mathematical trends reported in human psychophysical studies on the temperature dependence of sucrose taste perception. For instance, the slope of the psychometric function for sucrose is steeper when the tongue is cooled to 20°C than when held at 36°C (Green and Frankmann 1987), and this slope progressively steepens when solution temperature is stepped from 44°C down to 12°C (Bartoshuk et al. 1982). Thus the perceived sweetness of sucrose also appears to increase more rapidly with concentration when solutions are cooled than when they are warmed (see also Calvino 1986). This trend and the present data converge on the postulate that rate of growth in the brain's taste response to increasing concentrations of sucrose is augmented by cooling.
The observed increase in slope while cooling, with similar intercept across temperatures (Table 1), suggests that raising the concentration of sucrose progressively weakened the effect of temperature on neural activity (cf. Green and Frankmann 1988). Indeed, temperature induced larger effects on responses to low concentrations of sucrose. For instance, increasing temperature from 18°C to 30°C caused a 1,049% increase in the mean response to 0.1 M sucrose but only a 152% increase in mean activity to 0.56 M sucrose (Fig. 2A). What is more, systematic increases in slope due to cooling predict that responses to sucrose should show independence from temperature at high stimulus concentrations (cf. Green and Frankmann 1988). Here this was observed, in part, for neuronal concentration-response functions to sucrose at 23°C and 37°C, where responses at these temperatures increased in similarity as concentration was raised and became indistinguishable between 0.31 and 0.56 M (Fig. 2). An analogous effect was reported in human psychophysical studies, where the perception of sucrose intensity begins to operate independently of temperature at concentrations greater than ∼0.4 M (Bartoshuk et al. 1982; Calvino 1986; Green and Frankmann 1987). Although comparison of data across species and levels of analysis can be performed only with caution, similarities between prior psychometric data and the present neurometric data suggest that the ability of temperature to modulate responses to sucrose in the mammalian brain depends on concentration, and that greater modulation arises at low to weak than high concentrations. Moreover, the similar quantitative effects of temperature noted in humans and mice suggest that neurophysiological mechanisms for sucrose taste in the mouse partly model these mechanisms in humans.
Only a few peripheral electrophysiological studies have measured responses to sucrose across several concentrations and temperatures. These studies have focused on the chorda tympani (CT) nerve, which innervates taste receptors on only the anterior tongue. Neurons in rostral regions of the NTS can receive convergent input from afferents innervating gustatory epithelia located on different regions of the tongue and mouth (Corson and Erisir 2013; Grabauskas and Bradley 1996; Travers et al. 1986; Travers and Norgren 1995), which were broadly bathed with solutions by our taste stimulation method (Wilson et al. 2012). Along this line, there exist similarities and differences between prior peripheral data and the present central data on thermo-concentration effects on gustatory activity to sucrose. For instance, recordings from the CT nerve in B6 mice showed that responses to 0.1 M sucrose were elevated with warming, as opposed to cooling, and appeared similar when solutions were tested at warm temperatures ranging from 29°C to 38°C (Lu et al. 2012). As found here, warming also augmented responses to 0.1 M sucrose by central S-type neurons in B6 mice, albeit responses to this concentration, and others, were significantly larger when sucrose solutions were warmed to 30°C compared to 37°C (Fig. 2A). The range of warm temperatures that maximizes activity to sucrose may be narrower in the CNS (cf. Wilson and Lemon 2013). What is more, the present finding that the influence of temperature on gustatory responses to sucrose weakens with increasing concentration (Fig. 2) was not uniformly found across studies of CT nerve activity to temperature- and concentration-varied solutions of sucrose (Lu et al. 2012; Talavera et al. 2005). Although methodological discrepancies between studies may account for these differences, it is certainly possible that input from multiple nerves and central processing may contribute to the effects of temperature on gustatory activity to sucrose in the NTS.
Our understanding of how temperature influences orosensory behavioral responses to sucrose in rodents is nascent. Torregrossa et al. (2012) described complex effects of temperature on sucrose intake in rats, with dependence on experimental procedure. Inspection of their data (Fig. 4D, p. 288) suggests that water-replete rats performing in brief-access intake tests can, under certain conditions, show greater licking responses to warmed (e.g., 30°C) than cooled (e.g., 10°C) sucrose, yet cooling appears to cause greater percent increase in the rate of licking when sucrose concentration is raised. In addition to its effects on sucrose-guided behavior, temperature can serve as salient cue in oral sensory learning (Smith et al. 2010) and also regulate preference toward water (Gold et al. 1973; Kapatos and Gold 1972) in rats. As with humans, temperature appears to importantly guide oral sensation in rodents, albeit more work is needed in this area.
Temperature and Timing
Prior studies on peripheral neurons by Marowitz and Halpern (1977) and Breza et al. (2010) showed that latency to response to sodium salts inversely followed concentration. The present analysis revealed an inverse relationship between concentration and response latency to sucrose in central neurons, and showed that this relationship was temperature dependent. Change in sucrose concentration could modulate latency for solutions held at cool and ambient temperatures but largely had no effect on latency when solutions were warmed (Fig. 5A). Moreover, increasing solution temperature from cool or ambient to warm, while holding concentration constant, generally decreased lag to response to sucrose (Figs. 1, 4, and 5A). The longest latencies (e.g., >1,200 ms) were found with cooling, and the shortest (e.g., <500 ms) were obtained under warming. The shortened latencies for warmed sucrose were unprecedented by latencies measured for sucrose at ambient temperature, the common stimulus temperature used in neurophysiological taste studies.
The ability of temperature to modify latency has implications for gustatory coding. For one, latency was proposed to convey neural information used to represent taste stimuli (Breza et al. 2010; Hallock and Di Lorenzo 2006). However, the present data introduce complications for this hypothesis for sucrose. Median latencies for select concentrations of sucrose varied by hundreds of milliseconds with cooling and warming (e.g., for 0.1 M, median lag was 1,418 ms at 22°C but 580 ms at 37°; Fig. 5A), indicating that latency was a poor marker of concentration. Although latencies generally decreased with warming, lags for low and high concentrations of sucrose were sometimes indifferent at different temperatures (e.g., 0.56 M at 22°C and 0.05 M at 30°C showed overlapping medians/95% CI*s; Fig. 5A), suggesting that latency does not encode temperature. Although latency is evidenced in other systems to signal features of sensory stimuli (e.g., Chase and Young 2007; Gollisch and Meister 2008), it is unclear whether latency could serve as a marker for information about sucrose. Differences in neuronal response latency were reported for stimuli of different gustatory qualia (Breza et al. 2010; Pritchard and Scott 1982; Yamamoto et al. 1984), albeit this awaits evaluation with temperature-varied stimuli.
Alternatively, the present data suggest that temperature will impact the timing and evolution of responses to sucrose across central neurons, and may also influence the synchrony of sucrose activity with that for other qualia bound with sucrose in a mixture. Temperature-imbued change in the speed of the signal for sucrose relative to input about another tastant, delivered alongside, could change which message reaches the central network first. Differences in the timing and order of delivery of sucrose and other taste stimuli can modulate firing to sucrose in NTS units (Di Lorenzo et al. 2003; Di Lorenzo and Lemon 2000), and this modulation may partly originate through a central mechanism (Lemon and Di Lorenzo 2002). Although the influence of temperature on responses to taste mixtures that include sucrose awaits investigation, temperature does uniquely modify unit responses in NTS to sucrose compared with sodium salt, acidic, and bitter tastants (Wilson and Lemon 2013). Moreover, taste receptors for sucrose (Talavera et al. 2005) and other stimuli, such as sodium salts (Askwith et al. 2001), can show inverse sensitivity to a thermal gradient. This predicts that temperature change, in one direction, would simultaneously enhance and attenuate features of mixture responses to sucrose and select stimuli.
Considerations and Conclusions
There are several conditions to the present data to consider for interpretation. For one, we used a statistical threshold to define extracellular spiking latency to sucrose for single units, which may only approximate the time lag to an actual physiological change in the recorded neurons. Considering the delayed latencies noted under cool and ambient temperatures, it is possible that there exist earlier stimulus-induced perturbations in, for example, the membrane potential of the recorded cells that precede significant spiking activity but are undetected by our recording or statistical methods. The present definition of latency might reflect, in part, the influence of oral temperature, and stimulus concentration, on the efficiency or speed by which taste transduction processes can convert the chemical energy of sucrose to a set of electrical impulses, rather than a time point of absolute change in physiology. However, although our neuronal data likely do incorporate the upstream influence of temperature on taste receptors for sucrose (e.g., Lu et al. 2012; Talavera et al. 2005, 2007), the exact neurobiological mechanism contributing to the present effects and the potential contribution of central processing remain unknown. It is noteworthy that long latencies to respond to sucrose under cool and ambient temperatures, as shown in the present study (Figs. 1, 4, and 5), are not atypical for medullary neurons. Other studies of NTS units in mice and rats have shown latencies to response, and to maximal activation, to room temperature sucrose of near 1 s from stimulus onset (Chen and Di Lorenzo 2008; McCaughey 2007). Considering the present effects of cooling on latency, these lags would be expected to further increase with reduction in solution temperature.
It is also important to consider that our measurements of response latency were made relative to when stimuli entered the mouth space and are an overestimate of latency relative to time of receptor activation. Moreover, latencies, and spiking responses, were “static” in that our measurements could not account for any dynamic modulations in activity imposed by tongue and mouth movements in awake animals. On the other hand, anesthesia promoted recording of sensory responses to sucrose across multiple thermo-concentration conditions in the absence of nonspecific influences, such as behavioral differences across animals (Chapuis and Wilson 2011). Finally, the rinse temperature (22°C, ambient) used, albeit common in taste neurophysiology studies, is probably lower than the normal oral temperature of an awake mouse (cf. Wilson and Lemon 2013), and other adapting temperatures need to be tested in future studies.
Caveats notwithstanding, the present results agree with prior data suggesting that the taste of sucrose is strongly sensitive to temperature and delineate how temperature operates as a systematic parameter of the gustatory neural representation of sucrose. Our results are part of a growing body of data that argue that temperature is equally important to stimulus concentration and quality as a parameter of gustatory coding, and should be included as a standard factor in neurophysiological studies on sweet and other tastes (Breza et al. 2006; Lu et al. 2012; Lundy and Contreras 1999; Talavera et al. 2005; Wilson and Lemon 2013; Yamashita and Sato 1965). Temperature is an omnipresent property of taste stimuli that can modify taste experience. Thus, elucidating thermodynamic principles of gustatory processing may greatly facilitate our understanding of neural substrates for taste (Talavera et al. 2007).
GRANTS
Supported by National Institutes of Health (NIH) Grant DC-011579 to C. H. Lemon. D. M. Wilson was supported by NIH Training Grant GM-008306.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.M.W. and C.H.L. conception and design of research; D.M.W. performed experiments; D.M.W. and C.H.L. analyzed data; D.M.W. and C.H.L. interpreted results of experiments; D.M.W. and C.H.L. edited and revised manuscript; D.M.W. and C.H.L. approved final version of manuscript; C.H.L. prepared figures; C.H.L. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Barry Green for valuable comments and suggestions on this manuscript.
REFERENCES
- Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci USA 98: 6459–6463, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 26: 925–933, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair W, Koch C. Temporal precision of spike trains in extrastriate cortex of the behaving macaque monkey. Neural Comput 8: 1185–1202, 1996 [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Rennert K, Rodin J, Stevens JC. Effects of temperature on the perceived sweetness of sucrose. Physiol Behav 28: 905–910, 1982 [DOI] [PubMed] [Google Scholar]
- Breza JM, Curtis KS, Contreras RJ. Temperature modulates taste responsiveness and stimulates gustatory neurons in the rat geniculate ganglion. J Neurophysiol 95: 674–685, 2006 [DOI] [PubMed] [Google Scholar]
- Breza JM, Nikonov AA, Contreras RJ. Response latency to lingual taste stimulation distinguishes neuron types within the geniculate ganglion. J Neurophysiol 103: 1771–1784, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvino AM. Perception of sweetness: the effects of concentration and temperature. Physiol Behav 36: 1021–1028, 1986 [DOI] [PubMed] [Google Scholar]
- Chapuis J, Wilson DA. Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nat Neurosci 15: 155–161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase SM, Young ED. First-spike latency information in single neurons increases when referenced to population onset. Proc Natl Acad Sci USA 104: 5175–5180, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JY, Di Lorenzo PM. Responses to binary taste mixtures in the nucleus of the solitary tract: neural coding with firing rate. J Neurophysiol 99: 2144–2157, 2008 [DOI] [PubMed] [Google Scholar]
- Corson JA, Erisir A. Monosynaptic convergence of chorda tympani and glossopharyngeal afferents onto ascending relay neurons in the nucleus of the solitary tract: a high-resolution confocal and correlative electron microscopy approach. J Comp Neurol 521: 2907–2926, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Lemon CH. The neural code for taste in the nucleus of the solitary tract of the rat: effects of adaptation. Brain Res 852: 383–397, 2000 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Lemon CH, Reich CG. Dynamic coding of taste stimuli in the brainstem: effects of brief pulses of taste stimuli on subsequent taste responses. J Neurosci 23: 8893–8902, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller JL. Single-locus control of saccharin preference in mice. J Hered 65: 33–36, 1974 [DOI] [PubMed] [Google Scholar]
- Gold RM, Kapatos G, Prowse J, Quackenbush PM, Oxford TW. Role of water temperature in the regulation of water intake. J Comp Physiol Psychol 85: 52–63, 1973 [DOI] [PubMed] [Google Scholar]
- Gollisch T, Meister M. Rapid neural coding in the retina with relative spike latencies. Science 319: 1108–1111, 2008 [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Bradley RM. Synaptic interactions due to convergent input from gustatory afferent fibers in the rostral nucleus of the solitary tract. J Neurophysiol 76: 2919–2927, 1996 [DOI] [PubMed] [Google Scholar]
- Graham DM, Sun C, Hill DL. Temporal signatures of taste quality driven by active sensing. J Neurosci 34: 7398–7411, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG. Studying taste as a cutaneous sense. Food Qual Prefer 14: 99–109, 2002 [Google Scholar]
- Green BG, Frankmann SP. The effect of cooling the tongue on the perceived intensity of taste. Chem Senses 12: 609–619, 1987 [Google Scholar]
- Green BG, Frankmann SP. The effect of cooling on the perception of carbohydrate and intensive sweeteners. Physiol Behav 43: 515–519, 1988 [DOI] [PubMed] [Google Scholar]
- Green BG, Nachtigal D. Somatosensory factors in taste perception: effects of active tasting and solution temperature. Physiol Behav 107: 488–495, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock RM, Di Lorenzo PM. Temporal coding in the gustatory system. Neurosci Biobehav Rev 30: 1145–1160, 2006 [DOI] [PubMed] [Google Scholar]
- Kapatos G, Gold RM. Tongue cooling during drinking: a regulator of water intake in rats. Science 176: 685–686, 1972 [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun 283: 236–242, 2001 [DOI] [PubMed] [Google Scholar]
- Lemon CH, Di Lorenzo PM. Effects of electrical stimulation of the chorda tympani nerve on taste responses in the nucleus of the solitary tract. J Neurophysiol 88: 2477–2489, 2002 [DOI] [PubMed] [Google Scholar]
- Lemon CH, Margolskee RF. Contribution of the T1r3 taste receptor to the response properties of central gustatory neurons. J Neurophysiol 101: 2459–2471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Breza JM, Nikonov AA, Paedae AB, Contreras RJ. Leptin increases temperature-dependent chorda tympani nerve responses to sucrose in mice. Physiol Behav 107: 533–539, 2012 [DOI] [PubMed] [Google Scholar]
- Lundy RF, Jr, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J Neurophysiol 82: 2970–2988, 1999 [DOI] [PubMed] [Google Scholar]
- Lush IE. The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genet Res 53: 95–99, 1989 [DOI] [PubMed] [Google Scholar]
- Marowitz L, Halpern B. Gustatory neural response of the chorda tympani to lick-duration stimuli. Chem Sens Flav 2: 457–485, 1977 [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28: 58–63, 2001 [DOI] [PubMed] [Google Scholar]
- McBurney DH, Collings VB, Glanz LM. Temperature dependence of human taste responses. Physiol Behav 11: 89–94, 1973 [DOI] [PubMed] [Google Scholar]
- McCaughey SA. Taste-evoked responses to sweeteners in the nucleus of the solitary tract differ between C57BL/6ByJ and 129P3/J mice. J Neurosci 27: 35–45, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4: 492–498, 2001 [DOI] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5: 1169–1176, 2002 [DOI] [PubMed] [Google Scholar]
- Pritchard T, Scott T. Amino acids as taste stimuli. II. Quality coding. Brain Res 253: 93–104, 1982 [DOI] [PubMed] [Google Scholar]
- Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem 77: 896–903, 2001 [DOI] [PubMed] [Google Scholar]
- Schumacher JW, Schneider DM, Woolley SM. Anesthetic state modulates excitability but not spectral tuning or neural discrimination in single auditory midbrain neurons. J Neurophysiol 106: 500–514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Smith JC, Houpt TA. Interactions of temperature and taste in conditioned aversions. Physiol Behav 99: 324–333, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SS. The psychophysics of sensory function. In: Sensory Communication, edited by Rosenblith WA. New York: Wiley, 1961, p. 1–33 [Google Scholar]
- Talavera K, Ninomiya Y, Winkel C, Voets T, Nilius B. Influence of temperature on taste perception. Cell Mol Life Sci 64: 377–381, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- Torregrossa AM, Bales MB, Breza JM, Houpt TA, Smith JC, Contreras RJ. Water restriction and fluid temperature alter preference for water and sucrose solutions. Chem Senses 37: 279–292, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of the rat. J Neurophysiol 73: 2144–2162, 1995 [DOI] [PubMed] [Google Scholar]
- Travers SP, Pfaffmann C, Norgren R. Convergence of lingual and palatal gustatory neural activity in the nucleus of the solitary tract. Brain Res 365: 305–320, 1986 [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Spector AC. Orosensory detection of sucrose, maltose, and glucose is severely impaired in mice lacking T1R2 or T1R3, but Polycose sensitivity remains relatively normal. Am J Physiol Regul Integr Comp Physiol 303: R218–R235, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock M, Schluter D. The Analysis of Biological Data. Greenwood Village, CO: Roberts, 2009 [Google Scholar]
- Wilcox R. Applying Contemporary Statistical Techniques. San Diego, CA: Academic, 2003 [Google Scholar]
- Wilson DM, Boughter JD, Jr, Lemon CH. Bitter taste stimuli induce differential neural codes in mouse brain. PLoS One 7: e41597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, Lemon CH. Modulation of central gustatory coding by temperature. J Neurophysiol 110: 1117–1129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge JM. Introductory Econometrics: a Modern Approach. Mason, OH: South-Western Cengage Learning, 2009 [Google Scholar]
- Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. I. Response characteristics. J Neurophysiol 51: 616–635, 1984 [DOI] [PubMed] [Google Scholar]
- Yamashita S, Sato M. The effects of temperature on gustatory response of rats. J Cell Comp Physiol 66: 1–18, 1965 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003 [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003 [DOI] [PubMed] [Google Scholar]


