Abstract
Sleep is involved in memory consolidation. Current theories propose that sleep-dependent memory consolidation requires active communication between the hippocampus and neocortex. Indeed, it is known that neuronal activities in the hippocampus and various neocortical areas are correlated during slow-wave sleep. However, transitioning from wakefulness to slow-wave sleep is a gradual process. How the hippocampal-cortical correlation is established during the wakefulness-sleep transition is unknown. By examining local field potentials and multiunit activities in the rat hippocampus and visual cortex, we show that the wakefulness-sleep transition is characterized by sharp-wave ripple events in the hippocampus and high-voltage spike-wave events in the cortex, both of which are accompanied by highly synchronized multiunit activities in the corresponding area. Hippocampal ripple events occur earlier than the cortical high-voltage spike-wave events, and hippocampal ripple incidence is attenuated by the onset of cortical high-voltage spike waves. This attenuation leads to a temporary weak correlation in the hippocampal-cortical multiunit activities, which eventually evolves to a strong correlation as the brain enters slow-wave sleep. The results suggest that the hippocampal-cortical correlation is established through a concerted, two-step state change that first synchronizes the neuronal firing within each brain area and then couples the synchronized activities between the two regions.
Keywords: high-voltage spike waves, ripples, sleep, hippocampus, memory consolidation
a large body of literature supports the view that sleep plays an important role in memory consolidation [see Diekelmann and Born (2010) and Stickgold (2005) for reviews]. The neural mechanism underlying this role is not well-understood. A leading hypothesis in modern memory theories is that neuronal activities representing specific memory traces are repetitively reactivated in the hippocampus and neocortex during sleep (Buzsáki 1998; McClelland et al. 1995; Teyler and Rudy 2007; Wilson and McNaughton 1994). This repetitive reactivation is proposed to induce the strengthening of cortical memory traces and, as a result, gives rise to the formation of stable cortical memory representations that can be retrieved independently of the hippocampus (Aton et al. 2009; Frankland and Bontempi 2005; Goshen et al. 2011; Rosanova and Ulrich 2005). There are several lines of evidence that support this hypothesis. First, human dreams during sleep and at sleep onset are highly related to past or recent experiences (Nielsen and Stenstrom 2005; Stickgold et al. 2000), indicating memory reactivation during those periods. Second, the presence of sensory cues during sleep appear to promote memory reactivation and enhance human memory performance after the sleep period (Rasch et al. 2007; Rudoy et al. 2009). Third, neuronal firing patterns encoding specific experiences in rats, such as running through a particular trajectory of a maze, are replayed during slow-wave sleep (SWS) in the hippocampus (Bendor and Wilson 2012; Lee and Wilson 2002; Nádasdy et al. 1999; Skaggs and McNaughton 1996; Wilson and McNaughton 1994) and in a number of neocortical areas (Euston et al. 2007; Hoffman and McNaughton 2002; Ji and Wilson 2007; Peyrache et al. 2009; Qin et al. 1997; Ribeiro et al. 2004). Importantly, the replays in the hippocampus and in the visual cortex are coordinated, suggesting an active interaction during sleep across the two brain areas (Ji and Wilson 2007). Finally, disrupting sleep activity patterns related to memory reactivation impairs later memory performance in rats (Ego-Stengel and Wilson 2010; Girardeau et al. 2009).
A key component in the sleep-dependent memory consolidation hypothesis is that the neuronal activities between the hippocampus and cortex are coordinated during sleep. Indeed, there is strong evidence that this is the case, at least, during SWS. First, local-field potential (LFP) events between the hippocampus and various cortical areas are correlated during SWS. Hippocampal sharp-wave ripples (“ripples”), 100- to 250-Hz oscillations in the LFP at the CA1 pyramidal layer of the hippocampus (Buzsáki et al. 1992; Csicsvari et al. 2000), are correlated with cortical spindles, delta waves, and slow oscillations in the prefrontal (Mölle et al. 2006; Siapas and Wilson 1998), somatosensory (Sirota et al. 2003), and visual cortices (Ji and Wilson 2007). There is also evidence that hippocampal and cortical slow waves are correlated during SWS (Wolansky et al. 2006). Second, activity patterns of neuronal populations across the cortex and hippocampus are coordinated. At a gross level, hippocampal and cortical neuronal firing activities are highly correlated (Ji and Wilson 2007; Siapas and Wilson 1998; Sirota et al. 2003). A more elaborate picture emerges when their firing activities are examined in detail. A well-established phenomenon is that cortical neurons alternate between UP and DOWN states (Sanchez-Vives and McCormick 2000; Steriade et al. 1993, 2001). Almost all cortical neurons, at least within the local population, are synchronized in transitioning between these UP and DOWN states (Amzica and Steriade 1995a,b; Petersen et al. 2003; Steriade and Amzica 1998; Volgushev et al. 2006). As such, the cortical population displays an alternation between active and inactive periods (Battaglia et al. 2004; Ji and Wilson 2007). Although the majority of hippocampal neurons do not display clear UP and DOWN states, their activities are still synchronized during periods of strong corticohippocampal interaction in SWS (Hahn et al. 2006, 2007; Isomura et al. 2006), and the hippocampal population also alternates between active and inactive periods at this time (Ji and Wilson 2007). Interestingly, the hippocampus and visual cortex tend to switch between active and inactive periods together (Ji and Wilson 2007). This coordination provides strong evidence that hippocampal and cortical firing activities are correlated at a fine time scale and that this interregional correlation is achieved through coordination between highly synchronized neuronal activities within each of these brain regions.
In the present study, we asked how the hippocampal-neocortical coordination during SWS is established. In cats (Steriade et al. 2001) and rodents (Wierzynski et al. 2009), a typical, complete wakefulness-sleep cycle consists of a wakeful state followed by a period of SWS, a subsequent brief period of rapid-eye-movement (REM) sleep, and finally a return to the wakeful state. However, the act of falling asleep, the transition from wakefulness to SWS, is not an instantaneous event but more of a gradual process lasting from seconds to minutes. In the rat cortex, a prominent phenomenon at the wakefulness-sleep transition are high-voltage spike waves (HVS), a high-amplitude, 7- to 12-Hz LFP event typically lasting for many seconds (Jandó et al. 1995). When HVS occur, cortical neurons are extremely synchronized (Kandel and Buzsáki 1997; Sakata et al. 2005). HVS are considered a rodent model of absence seizure (McCormick and Contreras 2001; Noebels and Sidman 1979; Panayiotopoulos 2008; Shaw 2004; Vergnes et al. 1990). However, given that HVS occur naturally in many strains of rats, it has been proposed that HVS may serve normal, nonpathophysiological functions (Nicolelis and Fanselow 2002). In the hippocampus, ripple events coincide with synchronous firing of CA1 neurons during both SWS and wakeful states (Buzsáki et al. 1992; Csicsvari et al. 2000). Given the power of hippocampal ripples and cortical HVS in synchronizing hippocampal and cortical neurons, respectively, we investigated the emergence and progression of hippocampal ripples and cortical HVS events and how they interact to coordinate neuronal firing in these two areas during the transitional period from wakefulness to sleep.
We recorded LFPs and multiunit activities (MUAs) from the CA1 region of the hippocampus and from various layers of the primary visual cortex as freely behaving rats transitioned from wakefulness to SWS. Electromyography (EMG) was also recorded from the neck muscle to clarify further the behavioral states. We found that hippocampal ripples occur earlier than cortical HVS events. However, hippocampal ripple incidence and MUA are both reduced at the onset of HVS events. Within HVS events, hippocampal and cortical MUA exhibit only a relatively weak interregional correlation, which, over time, evolves into a stronger correlation during SWS. These results suggest that, as rats transition from wakefulness to sleep, the hippocampus and, subsequently, the cortex enter a synchronized state independently of one another and only later become correlated with each other in SWS. Furthermore, these findings also suggest that, during the wakefulness-sleep transitional period, cortical HVS may play a role in building up the cortical-hippocampal correlation in SWS, potentially for the purpose of memory consolidation.
MATERIALS AND METHODS
Animals and behavioral tasks.
Six male, 3- to 6-mo-old, Long-Evans rats were used in the electrophysiological recording experiments. The data analyzed in this study were acquired while the animals were placed on an elevated plate in an enclosed cage for 2–3 h (a “sleep session”). The animals were given no tasks and naturally fell asleep on their own. The sleep sessions took place either before or after the rat had performed tasks related to a separate study. The behavioral procedure and the electrophysiological recordings were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine and followed the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health.
Surgery.
A microelectrode array (tetrode drive) with 2 cannulae, each containing 12 independently adjustable tetrodes, was surgically implanted to the rat's skull. The tetrode drive was custom-designed and manufactured by three-dimensional (3-D) printing (American Precision Prototyping). Tetrodes were made by twisting 4 fine Nichrome wires (diameter 13 μm; Sandvik Palm Coast, Palm Coast, FL). The animal was anesthetized with 0.5–3% of the inhalation anesthetic isoflurane (the concentration usually started high, 2–3%, and then was reduced to ∼1.2% to maintain stable heartbeat and breathing rates). Two exposures, one at the coordinates anteroposterior (AP) −3.8 mm, mediolateral (ML) 2.5 mm from bregma and the other at AP −6.8 mm and ML 4.5 mm, were made to target the brain areas CA1 and primary visual cortex, respectively. The tetrode drive was lowered so that its two cannulae fitted into the exposures. A bipolar electrode, made by twisting two stainless steel wires (0.11-mm diameter; A-M Systems), was inserted to the neck muscle to record EMG. The drive was then anchored to the skull using stainless steel screws and dental cement. The analgesic ketoprofen (≥5 mg/kg) was administrated by subcutaneous injection before the animal was allowed to recover from the surgery.
Tetrode recording.
Electrophysiological recordings, using a Digital Lynx system (Neuralynx), were performed as described previously (Ji and Wilson 2007, 2008). Briefly, during the 2–4 wk postsurgery, tetrodes were slowly moved to the CA1 and primary visual cortex. Once stable units were obtained, electrophysiological recordings were carried out as animals slept on an elevated plate. For the recording of LFPs, tetrode signals were sampled at 2 kHz with a broad-band filter (0.1 Hz to 9 kHz). For the recording of MUA activity, tetrode signals were filtered with a band pass of 600 Hz to 9 kHz. Spikes (action potentials) were identified by a preset threshold of 50–70 μV and recorded at a sampling rate of 32 kHz. All of the spikes crossing the threshold from a tetrode were included in the MUA of that tetrode. Individual spikes were not sorted in this study.
Histology.
After the tetrode recording, the animal was killed using pentobarbital overdose (≥120 mg/kg). An electrical lesion was created at the tip of each tetrode by passing a current of 30 μA for ∼15 s. The brain was dissected and fixed in 10% formalin for at least 24 h. The fixed brain was then sectioned at 50- or 100-μm thickness and stained with 0.2% cresyl violet. The recording sites were verified from the lesion marks in the stained sections (data not shown). Only the data recorded by tetrodes that were located in primary visual cortex (layers 2–6) and the pyramidal layer of CA1, according to the standard rat brain atlas (Paxinos and Watson 2007), were included in the analysis.
Behavioral state classification.
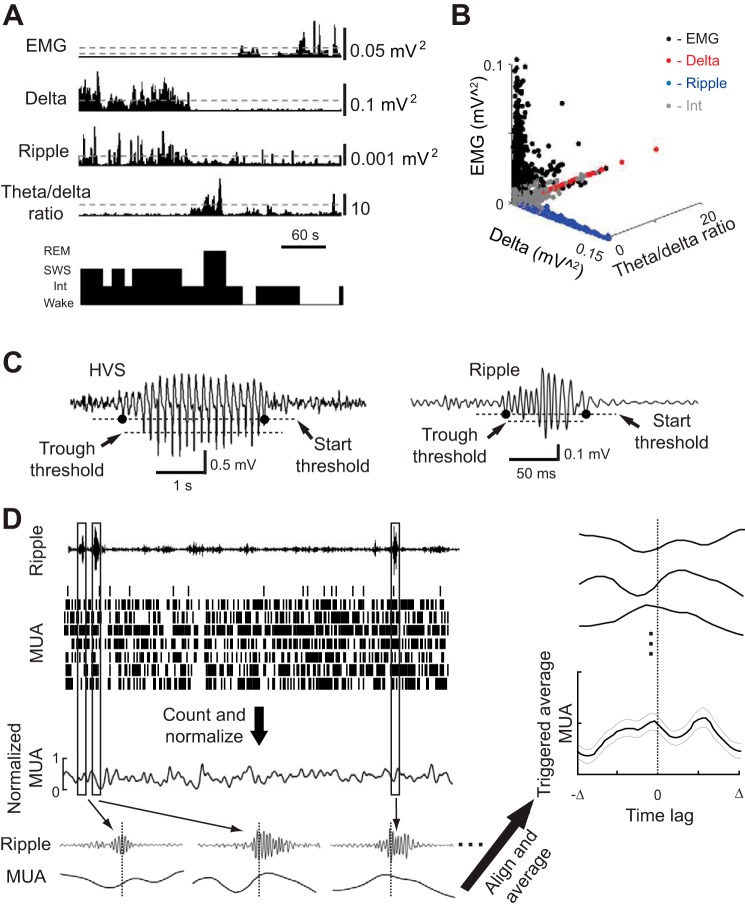
The animal's behavior at each second was classified to one of the four states of vigilance: wakefulness, SWS, REM, and a nonspecific intermediate state. The classification was based on the cortical and hippocampal LFPs and the EMG (Fig. 1, A and B) as previously described (Ji and Wilson 2007). Briefly, we computed the EMG power, the hippocampal LFP power in the ripple band (100–250 Hz) and the power ratio between theta band (5–10 Hz) and delta band (1–4 Hz), and the cortical LFP power in the delta band for each second of a sleep session. Thresholds were defined on these power (ratio) traces (Fig. 1A). The wakeful state was defined by high EMG power (larger than a threshold, same below), low hippocampal ripple power and low cortical delta power. SWS was defined by high hippocampal ripple power, high cortical delta power, and low hippocampal theta-to-delta ratio. REM was defined by low EMG power (lower than a 2nd EMG threshold; Fig. 1A), low hippocampal ripple power, low cortical delta power, and high hippocampal theta-to-delta ratio. Those states that could not be precisely defined as wakefulness, SWS, or REM according to the criteria above were assigned to the intermediate state. The intermediate state included both the transitional state from SWS to REM (Gervasoni et al. 2004; Gottesmann 1996; Sullivan et al. 2014) and the transitional state between Wake and SWS (Fig. 2A). Because our classification method employed the commonly used delta band in the cortical LFPs and ignored the HVS band of 7–12 Hz (Gilmour et al. 2010; Robert et al. 1999), some of the intermediate states at the transition between Wake and SWS were accompanied with high power of cortical LFP at 7–12 Hz (Fig. 2B), suggesting that these states are similar to the “whisker-twitching” behavior defined in previous studies (Gervasoni et al. 2004; Nicolelis et al. 1995). Because the power values depended on the precise recording locations (for example, the cortical layer), the thresholds were manually defined for each animal. The state classification was generated automatically from these thresholds by a custom program and later manually verified, similarly to many other classification methods (Gilmour et al. 2010; Robert et al. 1999). Following a previous study (Gervasoni et al. 2004), we visualized the final classification results of a sleep session in a 3-D space of EMG power, hippocampal theta-to-delta ratio, and cortical delta power (Fig. 1B). The clusters of Wake, SWS, and REM states were well-separated along the three axes. Replacing the cortical delta power of the 3-D plot with the hippocampal ripple power yielded similar results. All clusters, including intermediate clusters, had an isolation distance >17, demonstrating that the clusters were well-separated from the rest of the data points in a multidimensional space (Schmitzer-Torbert et al. 2005). The analysis in this study focused on the transitional period between wakefulness and the first sustained SWS episode that lasted >60 s.
Fig. 1.
Methods for behavioral state classification, high-voltage spike wave (HVS) and ripple event detection, and computing triggered multiunit activity (MUA) averages. A: an animal's behavior at every second of a 6-min time window was classified into wakefulness (Wake), rapid-eye-movement sleep (REM), slow-wave sleep (SWS), or intermediate (Int) states based on the electromyography (EMG) power, cortical delta power, hippocampal ripple power, and hippocampal theta-to-delta power ratio. Dashed lines: thresholds for state classification (see materials and methods for classification criteria). Two thresholds were defined for EMG, 1 for defining Wake state and 1 for REM. B: the animal's behavioral states during a 1-h session are displayed in a state space defined by EMG power, cortical delta power, and hippocampal theta-to-delta ratio. Each dot represents a state. Note that Wake, REM, and SWS states are well-separated along the 3 axes. C: cortical HVS and hippocampal ripple events were detected from band-pass filtered (6–12 Hz for HVS, 100–250 Hz for ripple) local-field potentials (LFPs). A detected event had at least 1 value below a “trough threshold.” The start and end times (black dots) of the event was determined by a “start threshold.” D: an example of computing the MUA average triggered by ripple trough times. MUA spikes recorded from all of the tetrodes in a brain area (each row of the MUA raster plot represents a tetrode) were counted in each time bin, smoothed, and then normalized (see materials and methods for details). For each detected ripple event i, we identified the time ti of its most negative trough. The triggered MUA with a trigger range [−Δ Δ] was a segment of the normalized MUA within the time interval [ti−Δ ti+Δ]. The triggered MUA average was obtained by stacking the MUA segments triggered by all of the ripple events centered at time 0 and computing the mean value (thick line) and standard error (thin lines) at each time lag from 0.
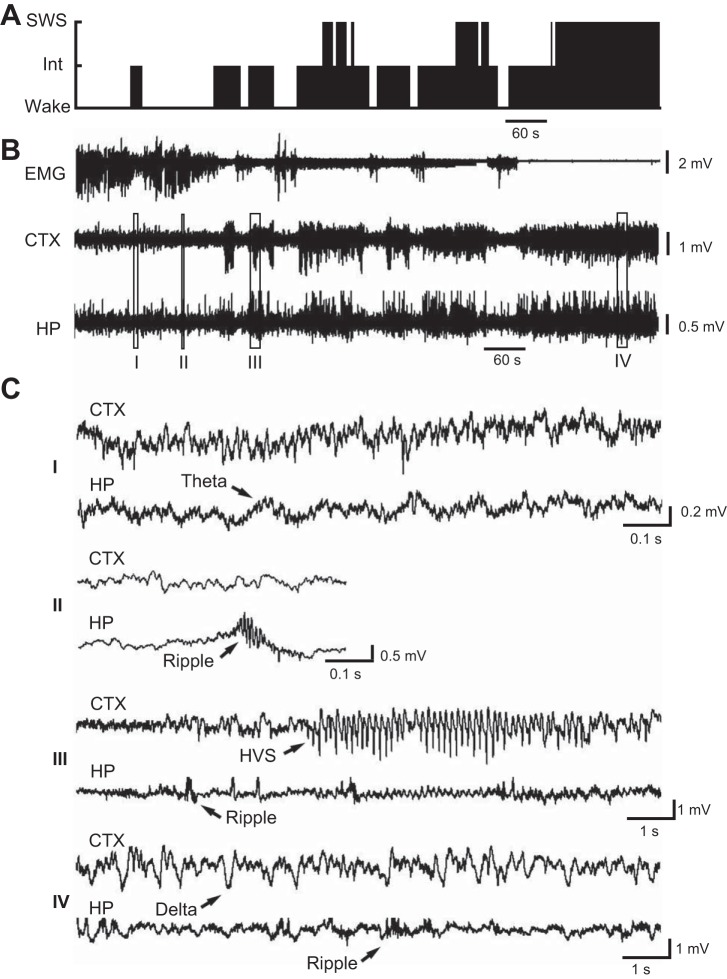
Fig. 2.
Hippocampal (HP) and cortical (CTX) LFPs during the transitional period from wakefulness to SWS. A: classified behavioral states during a 15-min period when a rat transitioned from wakefulness to sustained SWS. B: EMG, CTX LFP, and HP LFP during the same time period. C: expanded view of the CTX and HP LFPs at the boxed time points (I–IV) in B. Arrows highlight a theta wave, 3 ripple events (at various time scales), an HVS event, and a delta wave. Note the HVS and ripple events during the transition from wakefulness to SWS.
Identification of cortical HVS and hippocampal ripples.
HVS were identified on filtered cortical LFPs with a band pass of 6–12 Hz (Fig. 1C). For each filtered trace in a session, we computed its standard deviation (SD) and defined a “trough threshold” at −6 SD and a “start threshold” at −2.5 SD. A segment of the trace was defined as an HVS event if there were at least five troughs lower than the trough threshold with a maximum intertrough interval of 250 ms. The start and end times of the HVS event were determined as the time points when the segment crossed below the start threshold before the first trough and after the last trough (Fig. 1C). Neighboring events with gaps (between the end time of the last event and the start time of the next event) smaller than 0.5 s were combined into a single event. Hippocampal ripple events (Fig. 1C) were similarly defined on filtered hippocampal LFPs with a band pass of 100–250 Hz. Ripple events were identified using a trough threshold of −6 SD and a start threshold of −2.5 SD of the filtered hippocampal LFP traces. Neighboring events with gaps smaller than 30 ms were combined into a single event. Only those events with durations between 30 and 400 ms were included in the ripple analysis. The results reported here did not depend on these particular choices of HVS/ripple detection parameters but were robust to a range of parameter values tested.
Triggered averages of MUA and cross-correlations.
The steps to compute triggered MUA averages are shown in Fig. 1D. MUA spikes recorded from all of the tetrodes within either the hippocampal CA1 or primary visual cortex were combined and counted in 10-ms bins for a sleep session. The spike counts were smoothed using a 20-ms Gaussian kernel and then normalized by the maximum spikes per bin of the session. Therefore, at any given time bin, the MUA activity of a given sleep session in each area was between 0 and 1. The average MUA triggered by a series of time points (i.e., ripple-trough times, HVS trough times) was computed by stacking the MUA segments centered on these time points, which were aligned at time 0, and taking the averages (and the standard errors) for all of the MUAs across all of the sleep sessions for each given brain area (Fig. 1D). The modulation of MUA by the trigger time was quantified by modulation depth, defined as (max − min)/(max + min), where max and min were the maximum and minimum values of the average MUA within the trigger time range. Cross-correlograms of the normalized MUAs between CA1 and primary visual cortex were computed for each sleep session with a bin size of 10 ms and then averaged across all of the sessions. For computing the cross-correlation between hippocampal ripple power and cortical spindle power, cortical and hippocampal LFPs were filtered within spindle band (10–18 Hz) and ripple band, respectively. Power was computed for each 50-ms window as the mean square value of the window. Hippocampal ripple power and cortical spindle power were then cross-correlated during SWS and averaged across all of the sleep sessions as previously described (Siapas and Wilson 1998; Sirota et al. 2003).
RESULTS
LFPs and MUAs were recorded in the CA1 and primary visual cortex of 6 rats during 16 sleep sessions (1–4 sessions per rat). The mean duration of the sleep sessions was 118.9 ± 6.4 min. To understand how the hippocampal-cortical correlation during SWS was established during sleep onsets, we focused on the wakefulness-sleep transitional periods of these sessions (Fig. 2A), defined as the time window between the start of a session and the start of the first sustained SWS episode that lasted >60 s. The mean duration of the transitional period was 30.4 ± 4.6 min (n = 16).
Hippocampal and cortical LFP events during the transitional period.
First, we characterized the nature of the cortical and hippocampal LFPs and EMG during the transitional periods. During these periods, the vigilance state of the brain switched among wakefulness, SWS, and intermediate states (Fig. 2A). The EMG amplitude gradually decreased while hippocampal and cortical LFPs started with low-amplitude fluctuations and then alternated between high- and low-amplitude episodes before eventually settled into continuous high-amplitude fluctuations (Fig. 2B). Expanding the view of the hippocampal low- and high-amplitude episodes during the transitional period revealed that theta oscillations (5–10 Hz), characteristic of an active hippocampus (Buzsáki 2002), were occasionally seen in the low-amplitude fluctuations, and the high-amplitude events were dominated by sharp-wave ripples (Fig. 2C). Because ripples were accompanied by the large-amplitude sharp waves (Ylinen et al. 1995) and our recording site was close to the CA1 pyramidal layer, where theta was relatively low (Buzsáki 2002), ripples were more visible than theta waves in our data (Fig. 2C). The high-amplitude cortical episodes were first dominated by HVS events, which were the largest events seen in cortical LFP throughout the recording session, with the characteristic repetitive spike waves lasting for seconds to tens of seconds (Fig. 2C). The HVS events later disappeared, and smaller delta waves dominated as the brain fell into sustained SWS (Fig. 2, B and C).
A total of 978 HVS events and 7,596 ripple events were identified within the transitional periods of all 16 sleep sessions. HVS events occurred in every session with a mean rate of 2.0 ± 0.3 events per minute (n = 16 sessions). The HVS events had a mean frequency of 8.57 ± 0.04 Hz and a mean duration of 3.52 ± 0.06 s. HVS were very rarely detected during later SWS with an incidence rate of 0.05 ± 0.03 events per minute (P = 1.3 × 10−6 compared with the transitional period, paired t-test). The ripple events during the transitional period occurred with a mean rate of 15.6 ± 1.5 events per minute (Fig. 3A), with a mean frequency of 176.5 ± 0.2 Hz and a mean duration of 71.9 ± 0.5 ms. Compared with the ripple events during later SWS, the ripples detected during the transitional period occurred with a lower rate (SWS rate: 39.8 ± 2.4 events per minute; P = 2.3 × 10−9, paired t-test; Fig. 3A), higher frequency (SWS frequency: 170.2 ± 0.2 Hz; P = 2.8 × 10−101, t-test; Fig. 3B), and similar duration (SWS duration: 70.9 ± 0.3 ms; P = 0.10, t-test; Fig. 3C). The results indicate that, during the transition from wakefulness to SWS, the major feature in the cortical LFP was the HVS event, which rarely occurred during SWS, whereas the major feature in the hippocampus was the ripple event, which occurred even more often during SWS. This suggests that during the transitional period the hippocampus progresses to SWS gradually, but the cortex enters SWS via dramatic appearance and disappearance of a new mode of oscillation (HVS).
Fig. 3.
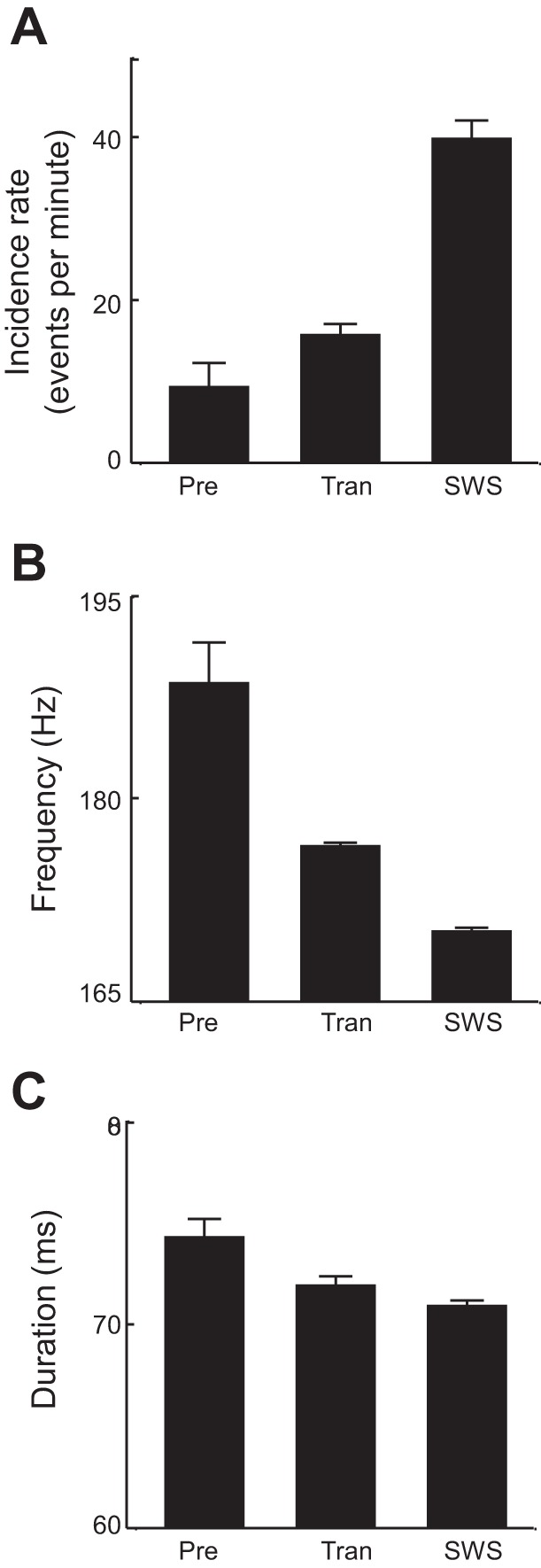
Quantification of hippocampal ripple events during different behavioral stages. Mean incidence rate (A), mean frequency (B), and mean duration (C) of ripple events are plotted for the 20-s period before the 1st detected HVS event (Pre), the entire transitional period (Tran), and SWS following the transitional period.
Hippocampus entered a synchronized state earlier than the cortex.
We asked whether cortical HVS events and hippocampal ripples occurred coincidentally during the transitional period. On average, the first hippocampal ripple was seen earlier than the first HVS event in a session (HVS mean lead time −61.3 ± 23 s; P = 0.018 compared with ripple mean lead time 0 s, t-test; n = 16 sessions), indicating that hippocampal ripples occurred earlier than cortical HVS events (Fig. 4). During the 20-s time period before the first detected cortical HVS, referred to as pre-HVS stage, hippocampal ripple events were detected at a rate of 9.3 ± 2.9 events per minute, which was significantly lower than the rate during the entire transitional period (P = 0.045, paired t-test) and the rate during the SWS following the transitional period (P = 1.5 × 10−8; Fig. 3A). The mean frequency of ripple events (188.5 ± 3.0 Hz) during this period was significantly higher than those during the entire transitional period (P = 0.0005, t-test) and during later SWS (P = 3.9 × 10−7), whereas the mean duration (74.3 ± 0.9 ms) was comparable across the three stages (P ≥ 0.63; Fig. 3, B and C).
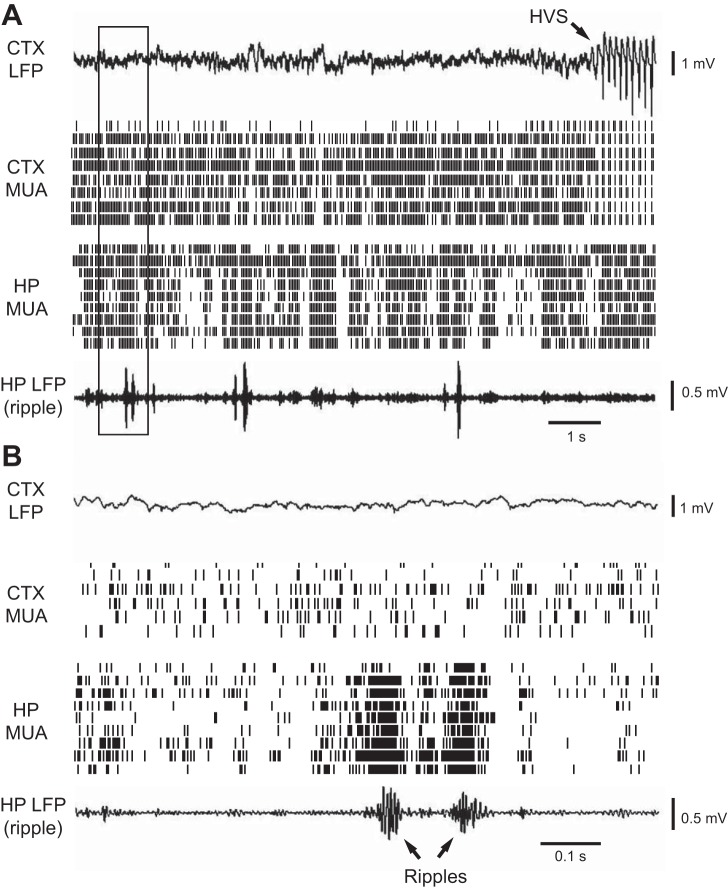
Fig. 4.
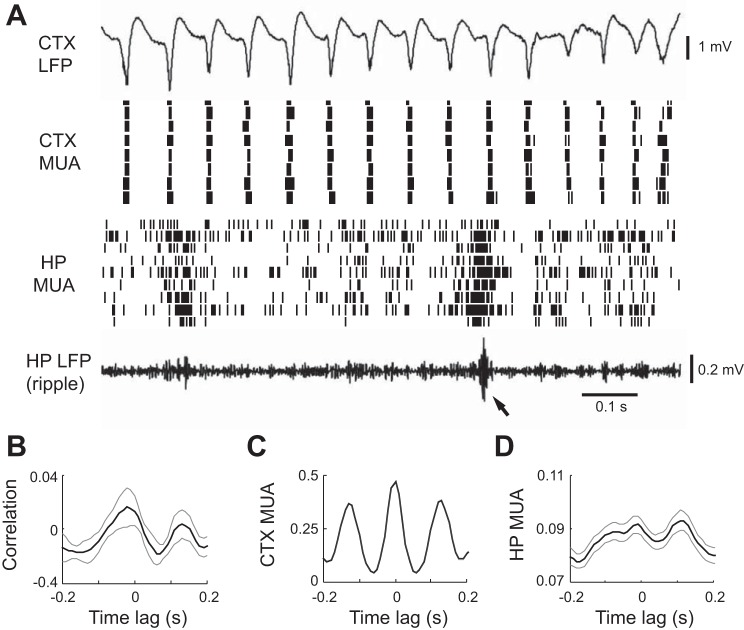
HP ripples occurred earlier than CTX HVS events. A: CTX LFP, CTX MUA, HP MUA, and HP LFP (filtered within the ripple band) in an 11-s time window of a sleep session before the 1st HVS event. Arrow: onset of an HVS event. B: expanded view of the boxed area in A. Arrows: ripple events. Note the repetitive incidence of HP ripples and the synchronized activity of HP MUAs when ripples occurred. Also, note the low CTX LFP amplitude (compared with Fig. 2B) and continuous, nonpatterned nature of CTX MUA activity.
At this pre-HVS stage, cortical LFPs were relatively flat with small-amplitude fluctuations. At the same time, cortical MUA showed a tonic, unstructured firing pattern (Fig. 4A). No obvious synchronization was seen among MUAs recorded across multiple cortical sites. In contrast, hippocampal MUA dramatically increased firing within individual ripple events and MUA across multiple sites within CA1 burst together in a synchronous fashion (Fig. 4B). We quantified the extent of cortical and hippocampal MUAs interaction during the pre-HVS stage by computing the cross-correlation between the cortical and hippocampal MUA. The mean correlogram, averaged over all of the sessions, did not show a significant peak (P = 1, ANOVA; Fig. 5A). Next, we computed the average cortical and hippocampal MUA triggered by the trough times of the detected ripple events. As expected, the average hippocampal MUA showed a significant peak at 0 ms (modulation depth 0.64; P = 1.2 × 10−81; Fig. 5B), indicating a high synchronization of hippocampal neuronal activity within ripples. In contrast, the average cortical MUA was flat without a significant peak (modulation depth 0.087; P = 1; Fig. 5C). The results show that, although ripple events and hippocampal neuronal synchronization had already occurred at this pre-HVS stage, ripples elicited no response in the cortex, which remained in a desynchronized state.
Fig. 5.
Absence of interaction in HP and CTX MUAs during the pre-HVS stage. A: average cross-correlogram between CTX and HP MUAs of all sleep sessions during the 20-s window before the 1st detected HVS event. Bin size: 10 ms. Note the absence of a peak. Thin gray lines: standard errors. B and C: HP (B) and CTX (C) MUA averages triggered by ripple trough times. Thin gray lines: standard errors. Note the peak in the average HP MUA but not the average CTX MUA.
HVS attenuated the incidence of hippocampal ripples and reduced hippocampal neuronal activities.
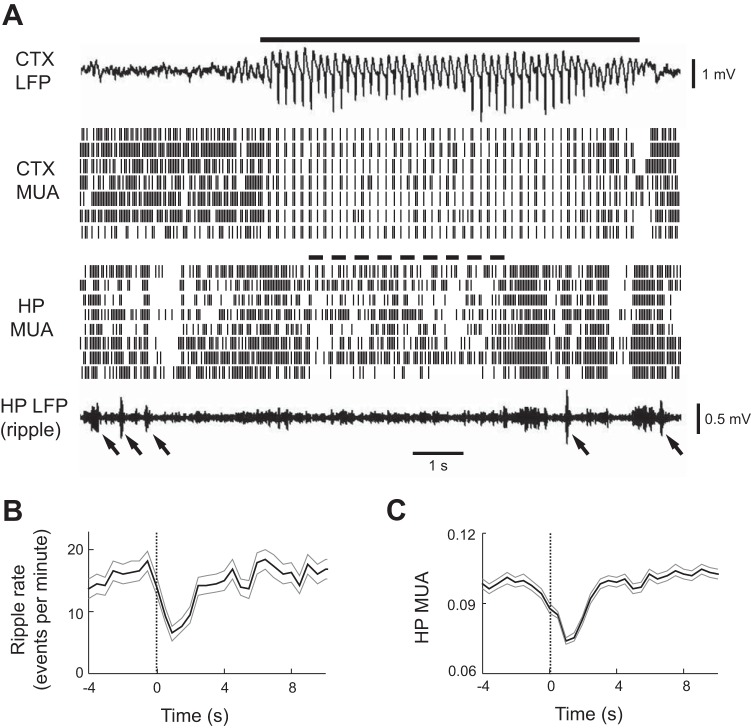
We next examined whether the emergence of cortical HVS impact hippocampal MUA and ripple incidence. HVS arose rapidly from low-amplitude LFP activity (Fig. 6A). Interestingly, hippocampal ripples were rarely seen shortly after the HVS onset, and hippocampal MUA was also reduced during this time, although both recovered a few seconds later (Fig. 6A). We quantified this observation by computing the average number of ripple events and the average hippocampal MUA, triggered by the HVS event onset times (Fig. 6, B and C). The ripple incidence rate and hippocampal MUA decreased at the HVS onset and were lowest at 1 s after the HVS onset compared with those during a baseline period, which was defined as a window between 4 and 1 s before the HVS onset. Specifically, the ripple incidence was not different from the baseline (15.8 ± 0.8 events per minute) at 0.5 s before the HVS onset (18.1 ± 1.6 events per minute; P = 0.10, paired-t test), was slightly reduced at the sleep onset (13.7 ± 1.4 events per minute; P = 0.28), and then significantly reduced at 1 s after the HVS onset (6.4 ± 1.0 events per minute; P = 6.0 × 10−13, paired-t test), rebounding back to baseline 5 s after the HVS onset (14.0 ± 1.4 events per minute; P = 0.34; Fig. 6B). The mean hippocampal MUA was similar with the baseline (0.098 ± 0.001) at 0.5 s before the HVS onset (0.094 ± 0.002; P = 0.07), significantly reduced at the sleep onset (0.088 ± 0.002; P = 2.7 × 10−6), and further reduced at 1 s after the HVS onset (0.074 ± 0.002; P = 1.3 × 10−27), rebounding back to baseline 5 s after the HVS onset (0.096 ± 0.002; P = 0.31; Fig. 6C). These data suggest that the onset of cortical HVS events accompany a rather surprising change in the hippocampal network, a reduction in both hippocampal ripple incidence and MUA activities.
Fig. 6.
HP ripple incidence and MUA were reduced during CTX HVS events. A: CTX LFP, CTX MUA, HP MUA, and HP LFP (filtered within ripple band) are shown in a 12-s time window. Solid line: HVS event. Dashed line: reduced HP MUA. Arrows: ripple events. Note the reduced HP MUA and the absence of ripples during a time window (dashed line) after the HVS onset. Also note the rebound HP MUA and ripples at the end of the HVS event. B and C: average ripple incidence rate (B) and average HP MUA (C) triggered by HVS event onset times. Thin gray lines: standard errors.
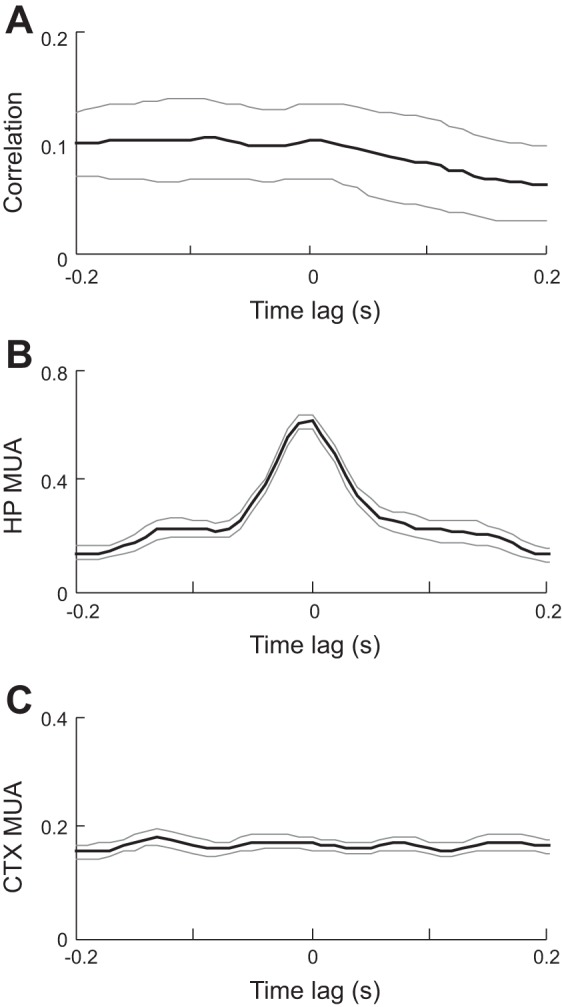
Weak correlation between cortical and hippocampal MUAs during HVS.
Next, we asked whether the cortex and hippocampus interacted at fine time scales within HVS events. As expected, cortical MUA across multiple recording sites were highly synchronized and burst-fired in a periodic fashion within HVS events (Fig. 7A). At the same time, hippocampal MUA displayed only a loosely structured firing pattern with an occasional rapid increase and decrease in activity, often associated with ripple events (Fig. 7A). We computed the cross-correlations between cortical and hippocampal MUA within HVS events. The average cross-correlogram showed a significant modulation of correlation by the lag time (P = 0.028, ANOVA) with a small peak (0.016) at −20 ms and a small trough (−0.019) at 70 ms (another peak appeared at 130 ms due to the periodic nature of HVS events; Fig. 7B). The peak correlation was not significantly different from 0 (P = 0.30, t-test), whereas the trough (anti)correlation was (P = 0.01), suggesting that hippocampal activity tends to shut down 70 ms after the cortical activity peaks. Because the cortical activity also shuts down around the same time (given the HVS period of 130 ms; see below and Fig. 7C), the results suggest that the activity in the two brain regions during HVS tends to cofluctuate. We also computed the average cortical and hippocampal MUA triggered by HVS event trough times. As expected from the periodic firing pattern, the average cortical MUA showed prominent, periodic peaks with a dominant peak at 0 ms and a peak interval of 130 ms (modulation depth 0.84; P = 0, ANOVA; Fig. 7C). Additionally, the average hippocampal MUA displayed a small yet significant modulation by the lag time from HVS troughs (modulation depth 0.12; P = 0.017, ANOVA) with positive peaks occurring at −10 and 110 ms (Fig. 7D). In short, these triggered average results suggest a small tendency for the hippocampal and cortical MUA to cofluctuate.
Fig. 7.
HP and CTX MUAs were weakly correlated within HVS events. A: CTX LFP, CTX MUA, HP MUA, and HP LFP (filtered within ripple band) in a 2-s time window within an HVS event. Arrow: ripple event. B: average cross-correlogram between CTX and HP MUAs within HVS events. C and D: average CTX MUA (C) and average HP MUA (D) triggered by the HVS trough times. Thin gray lines: standard errors.
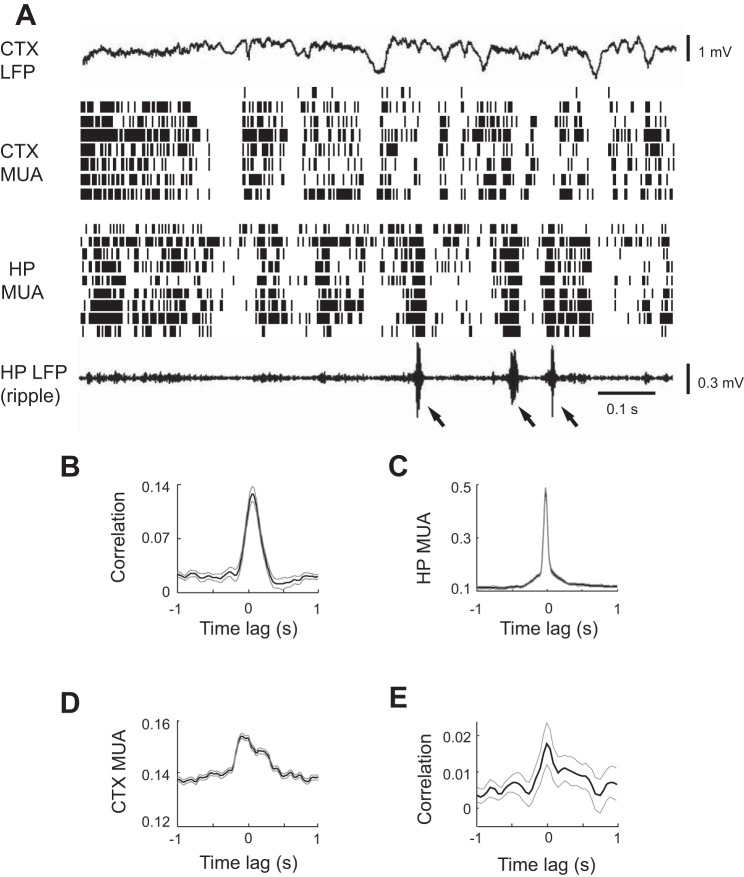
Strong correlation between cortical and hippocampal MUAs during SWS.
Transitional periods were followed by sustained SWS. To confirm that the cortical and hippocampal activities in our data are correlated during SWS, we examined cortical and hippocampal MUA patterns during the SWS following the transitional period. At this stage of sleep, cortical HVS events transformed to the well-known delta waves (Steriade et al. 1993), and cortical MUA displayed a synchronized pattern with alternating active and inactive periods (Fig. 8A). In the meantime, hippocampal MUA also showed alternating active and inactive time periods, and the active periods were often accompanied by ripple events in the hippocampal LFPs (Fig. 8A). These observations were consistent with previous studies (Battaglia et al. 2004; Ji and Wilson 2007). Accordingly, we computed the cross-correlation between cortical and hippocampal MUA during the SWS following the transitional period for each sleep session (Fig. 8B). Given that the delta waves and the corresponding cortical neuronal active periods occurred at a slower time scale (∼1 s) than individual ripple events and HVS cycles (∼100 ms), we computed the cross-correlation with a maximum time lag of ±1 s. The resulting mean cross-correlogram (Fig. 8B) showed a significant modulation of correlation by time lag (P = 1.3 × 10−33, ANOVA) with a prominent peak (peak correlation 0.13, P = 1.1 × 10−9 compared with 0, t-test) at 80 ms, indicating that, on average, cortical MUA tend to lead hippocampal MUA by ∼80 ms. We then computed the ripple trough-triggered averages of hippocampal (Fig. 8C) and cortical (Fig. 8D) MUAs. The average hippocampal MUA showed a large peak at 0 ms (modulation depth 0.61; P = 0, ANOVA). The average cortical MUA showed a small but significant peak (modulation depth 0.062; P = 0) at −60 ms, indicating that ripples tend to appear 60 ms after the peak cortical MUA. To confirm previous reports on the relationship between hippocampal ripples and cortical spindles (10–18 Hz) during SWS power (Siapas and Wilson 1998; Sirota et al. 2003), we calculated the cross-correlogram between hippocampal ripple power and spindle power in the visual cortex. The mean cross-correlogram revealed a significant correlation (P = 0.008, ANOVA) at peak time 0 ms (peak correlation 0.018, P = 0.007 compared with 0, t-test; Fig. 8E). In general, our results agree with the findings in Sirota et al. (2003) but differ slightly from Siapas and Wilson (1998), which reports that hippocampal ripples and MUA lead prefrontal cortical spindles and MUA by <1 s. The discrepancy between our findings and those of Siapas and Wilson (1998) may be explained by the different cortical areas (prefrontal cortex vs. visual cortex) studied. The relatively small effect of the ripple modulation of cortical spindles and MUA are probably related to the fact that ripple events were much shorter than the cortical spindles and cortical/hippocampal active periods (Ji and Wilson 2007), and thus ripple events could occur either before or after the peak of cortical MUA or spindle power. Nevertheless, the results are consistent with previous results that cortical and hippocampal neuronal activities are significantly correlated during SWS (Isomura et al. 2006; Ji and Wilson 2007; Sirota et al. 2003).
Fig. 8.
HP and CTX MUAs were strongly correlated during the SWS after the transitional period. A: CTX LFP, CTX MUA, HP MUA, and HP LFP (filtered within ripple band) in a 4-s time window. Arrow: ripple event. B: average cross-correlogram between CTX and HP MUAs within SWS. C and D: average CTX MUA (C) and HP MUA (D) triggered by the troughs of ripple events. E: average cross-correlogram between HP ripple power and cortical spindle power during SWS. Thin gray lines: standard errors.
DISCUSSION
To understand how the cortical-hippocampal correlation is established during SWS, we have examined LFPs and MUA in the hippocampus and primary visual cortex as rats transitioned from wakefulness to SWS. On the basis of our analyses on hippocampal ripples, cortical HVS, and their associated MUAs, the major findings are as follows. First, owing to the incidence of ripples, it would appear that the hippocampus enters a synchronized state earlier than the cortex. Second, the cortex subsequently enters a synchronized state as HVS events start to occur. Interestingly, the HVS events are initially accompanied with a reduction in hippocampal ripple events and MUA, both of which later rebound back to the pre-HVS levels. Third, within HVS events, there is a weak correlation between cortical and hippocampal MUA, which evolves to a strong correlation during later SWS.
These findings point to a two-step process for the hippocampus and cortex to become correlated during SWS. The first step results in synchronization within each brain region, i.e., with MUA becoming synchronized intrahippocampally and intracortically. The synchronization is manifested as either ripple events within the hippocampus or HVS events within the cortex. Our data reveal that the hippocampus enters a synchronized state earlier than the cortex. A possible explanation for this is because the hippocampus is positioned much further away from the external sensory input than the visual cortex. It is believed that brain areas further away from the sensory pathway are more susceptible to producing internally driven states that are not necessarily associated with the sensory world (Buzsáki 2013). Therefore, when the brain starts to disengage from the external input at the beginning of the wakefulness-to-sleep transition, the hippocampus becomes internally driven earlier than the sensory cortex. The internal drive may originate in CA3, an area with extensive recurrent connections that is known to initiate the ripple events in CA1 (Csicsvari et al. 2000). This explanation is also consistent with our finding that, while the hippocampus starts to synchronize at the pre-HVS stage, the cortex appears to remain unchanged, with MUA and LFP displaying normal wakeful state characteristics. As the wakefulness-sleep transition progresses, the cortex enters a highly synchronized state with the incidence of HVS events. Previous works have shown that cortical HVS events likely originate within the cortex itself and lead to the entrainment of the entire corticothalamic network (Kandel and Buzsáki 1997; McCormick and Contreras 2001; Pinault et al. 2001). Therefore, our results are consistent with the idea that activities within the cortex and the hippocampus are synchronized by separate, local circuits within each area and that the synchronization arises at different time points because the two areas sit at different operational distances from the sensory world.
The second step in the coordination process is the linking of the two intraregionally synchronized areas into a larger interregional synchronous system. Our results provide clues about how this may be achieved. The data presented here demonstrate that cortical HVS events are accompanied by a temporary reduction in hippocampal ripples and MUA. The reduction in ripples suggests that the internally driven predominant hippocampal activities are suppressed at or soon after the HVS onset. Temporary suppression of ripples may be reflective of a reshuffling process that breaks the autonomy of hippocampal activity and forces it to engage with the cortex. After this reshuffling process, when ripple and hippocampal MUA are restored to original levels, hippocampal activities may be biased by the cortical input. Indeed, our data show a correlation between cortical and hippocampal MUA within HVS events. However, this correlation is weak, presumably because a large proportion of the hippocampal network is still functioning autonomously. On the other hand, the fact that the hippocampal and cortical activity within HVS events tends to cofluctuate within a fine time scale (∼20 ms) is consistent with the idea that the weak hippocampal-cortical correlation seen here may be produced by a direct hippocampal response to synchronized firing in the cortex during HVS events. The visual cortex and hippocampus are connected through a multisynaptic pathway via the temporal cortex, the postrhinal/perirhinal cortices, and the entorhinal cortex (Furtak et al. 2007; Lavenex and Amaral 2000; Miller and Vogt 1984; Vaudano et al. 1991). Assuming a minimum of four synapses in the pathway and a ∼4-ms delay per synapse (Canning et al. 2000; Liu and Bilkey 1997), 20 ms is sufficient for a hippocampal response to visual cortical activities (Vastola 1982). An alternative explanation is that the correlation is due to a broad neuromodulatory effect taking place at the HVS event onset. For example, changes in modulatory neurotransmitters, such as increase in norepinephrine and decrease in acetylcholine, could promote HVS events and, at the same time, reduce hippocampal activity. In this way, the increase in cortical synchronization together with the decrease in internal hippocampal activity could gradually drive the coordinated activity between the cortex and hippocampus.
The firing of cortical neurons is highly synchronized during HVS events. Given the extensive forward and backward connections between the cortex and hippocampus (Rolls 2000), it is puzzling that there is only a weak correlation between cortical and hippocampal MUA within HVS events. Previous studies that have examined the issue produced conflicting results. An earlier study showed that single-unit activities in the two areas do not show obvious correlations (Kandel et al. 1996). However, later studies based on LFPs suggest that cortical HVS may impact hippocampal activities and vice versa. For example, LFPs at different hippocampal sites have been found to increase their correlations when cortical HVS occur (Velazquez et al. 2007). Increasing GABAergic inhibition in the hippocampus is found to reduce the incidence of HVS (Tolmacheva and van Luijtelaar 2007). Our data show a weak but significant correlation, likely because MUA is more sensitive than single-unit activity when detecting correlations. Nevertheless, there is a clear reduction in hippocampal MUA and ripples at HVS onset, indicating an interaction between the cortex and hippocampus when HVS occur, which may explain the previously found interactions at the LFP level.
This study has investigated the interaction between the rat hippocampus and the visual cortex at the transition between wakefulness and SWS. It is known that hippocampal neurons in rats are place cells that fire at specific locations of an environment (O'Keefe and Dostrovsky 1971) and that visual cues are critical to the firing locations of the place cells (Knierim 2002; Muller and Kubie 1987). Therefore, if spatial memories are to be consolidated among many cortical areas, as proposed by “index theory” (Teyler and Rudy 2007), the visual cortex should be an important area for consolidating the visual components of spatial memories. Indeed, our previous study shows that the neuronal firing patterns associated with spatial trajectories are coordinately replayed in the visual cortex and hippocampus during SWS, in particular through the correlated visual cortical and hippocampal activities (Ji and Wilson 2007). Here, we show that this coordination during SWS is established through a synchronization and then correlation mechanism. The present study adds further evidence for an active communication between the sensory cortex and hippocampus, possibly involved in memory consolidation. Given that HVS across many cortical areas are highly synchronized (Steriade and Amzica 1994; Vergnes et al. 1990), it is reasonable to believe that the nature of hippocampal-visual cortical interactions presented here can be extended to other cortical areas, especially those sensory cortices with a similar synaptic distance to the hippocampus as the visual cortex.
HVS has been considered a form of epileptic activity and has been used as a model for human absence seizure (McCormick and Contreras 2001; Noebels and Sidman 1979; Panayiotopoulos 2008; Shaw 2004; Vergnes et al. 1990). However, it is unclear whether HVS are actually pathological. It is possible that epilepsy involves the neural circuits underlying normal sleep behavior, and many seemingly pathological activities may be an erroneous rendition of normal sleep oscillations (Beenhakker and Huguenard 2009). HVS is commonly seen at sleep onsets in many rat strains (Jandó et al. 1995). In our data, recorded from naturally behaving Long-Evans rats, it occurred in all sleep sessions. The widespread nature of HVS points to the possibility that it serves a normal function. Indeed, there is evidence that rats with ongoing HVS can react to incoming sensory stimuli with active behavioral responses (Wiest and Nicolelis 2003). It has been proposed that HVS may be a rat analog of the human alpha and mu rhythms (Nicolelis and Fanselow 2002), which have a similar frequency (∼10 Hz) as HVS and occur when subjects are at rest with eyes closed (Hari and Salmelin 1997; Pfurtscheller 1992). As we proposed above, the powerful synchronization during HVS events and the associated changes in hippocampal ripples and MUAs may be a blunt, rapid, reshuffling process to switch the brain into a synchronized mode and establish the cortical-hippocampal correlation during later SWS. Although it is too early to speculate the relevance of our finding to human sleep, it underscores the importance of probing HVS not only as a model for absence seizure, but also a form of oscillation serving normal brain functions.
GRANTS
This work was supported by a Whitehall Foundation Grant and a National Eye Institute Grant (EY-020676) to D. Ji.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.C.H. and D.J. conception and design of research; D.C.H. performed experiments; D.C.H. and D.J. analyzed data; D.C.H. and D.J. interpreted results of experiments; D.C.H. and D.J. prepared figures; D.C.H. and D.J. drafted manuscript; D.C.H. and D.J. edited and revised manuscript; D.C.H. and D.J. approved final version of manuscript.
REFERENCES
- Amzica F, Steriade M. Disconnection of intracortical synaptic linkages disrupts synchronization of a slow oscillation. J Neurosci 15: 4658–4677, 1995a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (< 1 Hz) cortical oscillation. J Neurophysiol 73: 20–38, 1995b [DOI] [PubMed] [Google Scholar]
- Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron 61: 454–466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem 11: 697–704, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron 62: 612–632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci 15: 1439–1444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Cognitive neuroscience: time, space and memory. Nature 497: 568–569, 2013 [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res 7, Suppl 1: 17–23, 1998 [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron 33: 325–340, 2002 [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science 256: 1025–1027, 1992 [DOI] [PubMed] [Google Scholar]
- Canning KJ, Wu K, Peloquin P, Kloosterman F, Leung LS. Physiology of the entorhinal and perirhinal projections to the hippocampus studied by current source density analysis. Ann NY Acad Sci 911: 55–72, 2000 [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A, Buzsáki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron 28: 585–594, 2000 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci 11: 114–126, 2010 [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20: 1–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science 318: 1147–1150, 2007 [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci 6: 119–130, 2005 [DOI] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus 17: 709–722, 2007 [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Lin SC, Ribeiro S, Soares ES, Pantoja J, Nicolelis MA. Global forebrain dynamics predict rat behavioral states and their transitions. J Neurosci 24: 11137–11147, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour TP, Fang J, Guan Z, Subramanian T. Manual rat sleep classification in principal component space. Neurosci Lett 469: 97–101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12: 1222–1223, 2009 [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell 147: 678–689, 2011 [DOI] [PubMed] [Google Scholar]
- Gottesmann C. The transition from slow-wave sleep to paradoxical sleep: evolving facts and concepts of the neurophysiological processes underlying the intermediate stage of sleep. Neurosci Biobehav Rev 20: 367–387, 1996 [DOI] [PubMed] [Google Scholar]
- Hahn TT, Sakmann B, Mehta MR. Differential responses of hippocampal subfields to cortical up-down states. Proc Natl Acad Sci USA 104: 5169–5174, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TT, Sakmann B, Mehta MR. Phase-locking of hippocampal interneurons' membrane potential to neocortical up-down states. Nat Neurosci 9: 1359–1361, 2006 [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci 20: 44–49, 1997 [DOI] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science 297: 2070–2073, 2002 [DOI] [PubMed] [Google Scholar]
- Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsáki G. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron 52: 871–882, 2006 [DOI] [PubMed] [Google Scholar]
- Jandó G, Carpi D, Kandel A, Urioste R, Horvath Z, Pierre E, Vadi D, Vadasz C, Buzsáki G. Spike-and-wave epilepsy in rats: sex differences and inheritance of physiological traits. Neuroscience 64: 301–317, 1995 [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10: 100–107, 2007 [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Firing rate dynamics in the hippocampus induced by trajectory learning. J Neurosci 28: 4679–4689, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel A, Bragin A, Carpi D, Buzsáki G. Lack of hippocampal involvement in a rat model of petit mal epilepsy. Epilepsy Res 23: 123–127, 1996 [DOI] [PubMed] [Google Scholar]
- Kandel A, Buzsáki G. Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neurosci 17: 6783–6797, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ. Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci 22: 6254–6264, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus 10: 420–430, 2000 [DOI] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36: 1183–1194, 2002 [DOI] [PubMed] [Google Scholar]
- Liu P, Bilkey DK. Current source density analysis of the potential evoked in hippocampus by perirhinal cortex stimulation. Hippocampus 7: 389–396, 1997 [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102: 419–457, 1995 [DOI] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol 63: 815–846, 2001 [DOI] [PubMed] [Google Scholar]
- Miller MW, Vogt BA. Direct connections of rat visual cortex with sensory, motor, and association cortices. J Comp Neurol 226: 184–202, 1984 [DOI] [PubMed] [Google Scholar]
- Mölle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol 96: 62–70, 2006 [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7: 1951–1968, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nádasdy Z, Hirase H, Czurkó A, Csicsvari J, Buzsáki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci 19: 9497–9507, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Baccala LA, Lin RC, Chapin JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science 268: 1353–1358, 1995 [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Fanselow EE. Thalamocortical optimization of tactile processing according to behavioral state. Nat Neurosci 5: 517–523, 2002 [DOI] [PubMed] [Google Scholar]
- Nielsen TA, Stenstrom P. What are the memory sources of dreaming? Nature 437: 1286–1289, 2005 [DOI] [PubMed] [Google Scholar]
- Noebels JL, Sidman RL. Inherited epilepsy: spike-wave and focal motor seizures in the mutant mouse tottering. Science 204: 1334–1336, 1979 [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34: 171–175, 1971 [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos C. Controversial topics in epilepsy. Epilepsia 49: 1277–1293, 2008. 18638280 [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. San Diego, CA: Academic Press, 2007 [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA 100: 13638–13643, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci 12: 919–926, 2009 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol 83: 62–69, 1992 [DOI] [PubMed] [Google Scholar]
- Pinault D, Vergnes M, Marescaux C. Medium-voltage 5–9-Hz oscillations give rise to spike-and-wave discharges in a genetic model of absence epilepsy: in vivo dual extracellular recording of thalamic relay and reticular neurons. Neuroscience 105: 181–201, 2001 [DOI] [PubMed] [Google Scholar]
- Qin YL, McNaughton BL, Skaggs WE, Barnes CA. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Philos Trans R Soc Lond B Biol Sci 352: 1525–1533, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315: 1426–1429, 2007 [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Gervasoni D, Soares ES, Zhou Y, Lin SC, Pantoja J, Lavine M, Nicolelis MA. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol 2: E24, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Guilpin C, Limoge A. Automated sleep staging systems in rats. J Neurosci Methods 88: 111–122, 1999 [DOI] [PubMed] [Google Scholar]
- Rolls ET. Hippocampo-cortical and cortico-cortical backprojections. Hippocampus 10: 380–388, 2000 [DOI] [PubMed] [Google Scholar]
- Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci 25: 9398–9405, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science 326: 1079, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S, Yamamori T, Sakurai Y. 7–12 Hz cortical oscillations: behavioral context and dynamics of prefrontal neuronal ensembles. Neuroscience 134: 1099–1111, 2005 [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034, 2000 [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131: 1–11, 2005 [DOI] [PubMed] [Google Scholar]
- Shaw FZ. Is spontaneous high-voltage rhythmic spike discharge in Long Evans rats an absence-like seizure activity? J Neurophysiol 91: 63–77, 2004 [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21: 1123–1128, 1998 [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA 100: 2065–2069, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271: 1870–1873, 1996 [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Dynamic coupling among neocortical neurons during evoked and spontaneous spike-wave seizure activity. J Neurophysiol 72: 2051–2069, 1994 [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Slow sleep oscillation, rhythmic K-complexes, and their paroxysmal developments. J Sleep Res 7, Suppl 1: 30–35, 1998 [DOI] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85: 1969–1985, 2001 [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature 437: 1272–1278, 2005 [DOI] [PubMed] [Google Scholar]
- Stickgold R, Malia A, Maguire D, Roddenberry D, O'Connor M. Replaying the game: hypnagogic images in normals and amnesics. Science 290: 350–353, 2000 [DOI] [PubMed] [Google Scholar]
- Sullivan D, Mizuseki K, Sorgi A, Buzsáki G. Comparison of sleep spindles and theta oscillations in the hippocampus. J Neurosci 34: 662–674, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, Rudy JW. The hippocampal indexing theory and episodic memory: updating the index. Hippocampus 17: 1158–1169, 2007 [DOI] [PubMed] [Google Scholar]
- Tolmacheva EA, van Luijtelaar G. Absence seizures are reduced by the enhancement of GABA-ergic inhibition in the hippocampus in WAG/Rij rats. Neurosci Lett 416: 17–21, 2007 [DOI] [PubMed] [Google Scholar]
- Vastola EF. Electrical signs of an oligosynaptic visual projection to the rat hippocampus. Brain Behav Evol 20: 1–18, 1982 [DOI] [PubMed] [Google Scholar]
- Vaudano E, Legg CR, Glickstein M. Afferent and efferent connections of temporal association cortex in the rat: a horseradish peroxidase study. Eur J Neurosci 3: 317–330, 1991 [DOI] [PubMed] [Google Scholar]
- Velazquez JL, Huo JZ, Dominguez LG, Leshchenko Y, Snead OC., 3rd Typical versus atypical absence seizures: network mechanisms of the spread of paroxysms. Epilepsia 48: 1585–1593, 2007 [DOI] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Depaulis A. Mapping of spontaneous spike and wave discharges in Wistar rats with genetic generalized non-convulsive epilepsy. Brain Res 523: 87–91, 1990 [DOI] [PubMed] [Google Scholar]
- Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations [corrected]. J Neurosci 26: 5665–5672, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron 61: 587–596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest MC, Nicolelis MA. Behavioral detection of tactile stimuli during 7–12 Hz cortical oscillations in awake rats. Nat Neurosci 6: 913–914, 2003 [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science 265: 676–679, 1994 [DOI] [PubMed] [Google Scholar]
- Wolansky T, Clement EA, Peters SR, Palczak MA, Dickson CT. Hippocampal slow oscillation: a novel EEG state and its coordination with ongoing neocortical activity. J Neurosci 26: 6213–6229, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 15: 30–46, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]