Abstract
Across the rostrocaudal (RC) axis of the Xenopus tadpole optic tectum exists a developmental gradient. This gradient has served as a useful model to study many aspects of synapse and dendrite maturation. To compliment these studies, we characterized how the intrinsic excitability, the ease in which a neuron can fire action potentials, might also be changing across the same axis. Whole-cell recordings from tectal neurons at different points along the RC axis revealed a graded increase in intrinsic excitability: compared with neurons at the caudal end of the tectum, neurons at the rostral end fired more action potentials in response to current injection and expressed greater peak Na+ and K+ currents, the major intrinsic currents in these neurons that underlie the action potential. We also observed, along the same axis and in the same direction, a previously described increase in the amount of synaptic drive received by individual neurons (Wu GY, Malinow R, Cline HT. Science 274: 972–976, 1996). Thus as synaptic activity ramps up across the RC axis, so does intrinsic excitability. The reduction of overall circuit activity induced a compensatory scaling up of peak Na+ and K+ currents only in the caudal portion of the tectum, suggesting a region-specific, compensatory form of plasticity.
Keywords: development, instrinsic currents, optic tectum, region specific, Xenopus tadpole
at the caudal edge of the optic tectum of the Xenopus tadpole is a proliferative zone where progenitor cells give rise to tectal neurons. At a rate that is dependent on levels of visually driven activity, these newborn neurons migrate rostrally out of the proliferative zone and into the tectum proper, where they get incorporated into the functioning retinotectal circuit (Sharma and Cline 2010). As new members of the retinotectal circuit, they begin receiving direct synaptic input from retinal ganglion cells of the contralateral eye, the lateral line (via the brain stem), as well as other tectal neurons. This ongoing incorporation of newborn neurons from the proliferative zone creates a spatial developmental gradient, in which the most immature neurons are localized at the caudal end of the tectum, and the most mature are localized to the rostral end (Cline 2001; Lazar 1973; Wu et al. 1996). Many aspects of neural development have been described across this rostrocaudal (RC) axis: tectal neuron dendrites become increasingly complex and arborized (Wu et al. 1999), glutamatergic synapses gain α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Wu et al. 1996), AMPA receptors gain glutamate receptor 2 (GluR2) subunits (Aizenman et al. 2002), spontaneous synaptic frequency increases (Wu et al. 1996), and GABAergic transmission becomes increasingly hyperpolarizing (Khakhalin and Aizenman 2012). Thus across the RC axis, much is understood about the development of synapses and dendrites, factors determining the pattern and strength of synaptic input received by a tectal neuron. Here, we provide a description of how voltage-gated intrinsic currents involved in the generation of action potentials—namely, Na+ and K+ currents—are also changing across the same axis. The main findings of this study are as follows: that 1) across the RC axis, peak Na+ and K+ currents increase in a graded manner, such that tectal neurons in the most caudal portion of the tectum express relatively small peak currents, whereas more mature neurons at the most rostral end of the axis express the highest; 2) the position-dependent increase in intrinsic currents is correlated with the ability of the neurons to fire action potentials in response to a sustained current injection; 3) the amount of synaptic drive received by a given tectal neuron increases in the same direction; and 4) the chronic reduction of circuit activity to test the dependence of the gradient on activity induced upregulation of both Na+ and K+ currents only in neurons of the caudal portion of the tectum, suggesting a region-specific form of compensatory intrinsic plasticity.
MATERIALS AND METHODS
All animal protocols were approved by the University of Wyoming Institutional Animal Care and Use Committee. Wild-type Xenopus laevis tadpoles were raised in 10% Steinberg's solution at 23°C on a 12:12 light:dark cycle. Developmental stages were identified, according to Nieuwkoop and Faber (1994). Under our rearing conditions, tadpoles reach stages 44–46 at 7–10 days postfertilization (dpf) and stage 49 by ∼16 dpf. To reduce chronically action potential-dependent circuit activity, tadpoles were reared in Steinberg's solution containing 0.5 μM Na+ channel blocker TTX (Alomone Labs, Jerusalem, Israel), starting from approximately stage 45 for 7 days. TTX has been shown previously to cross the blood-brain barrier (Rajan and Cline 1998). Initially, we tried rearing the animals in 1 μM TTX, but that dose led to complete paralysis and death of all animals within 48 h. Therefore, we used one-half of that concentration (0.5 μM). Tadpoles that had been reared in this relatively low concentration of TTX for a maximum of 7 days appeared to develop properly and exhibited similar behaviors to control tadpoles. The TTX-containing solution was replaced every 48 h. Tadpoles were stage 49 at the time of recording. To block all excitatory synaptic events (action-potential dependent and independent), 30 μM AMPA receptor blocker 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX; Tocris, Bristol, UK) was added to the rearing solution, as described by Deeg and Aizenman (2011).
To record from neurons along the RC axis, the tadpole brain was dissected, as described by Pratt and Aizenman (2007) and Wu et al. (1996). In brief, tadpoles were anesthetized in 10% Steinberg's containing 0.01% tricaine methanesulfonate (MS-222) and transferred to a recording chamber containing HEPES-buffered extracellular saline (in mM: 115 NaCl, 2 KCl, 3 CaCl2, 3 MgCl2, 5 HEPES, and 10 glucose, pH 7.25; osmolarity 255 mosM). Next, the entire brain and brain stem were dissected out, filleted along the dorsal midline, and pinned to a submerged piece of Sylgard. To access tectal neurons for recording, the ventricular membrane covering the optic tectum was removed using a broken glass pipette.
Tectal cells were visualized using a Zeiss light microscope with a 60× water-immersion objective and a Hamamatsu infrared charge-coupled device camera. Whole-cell recordings were done using 8–12 MΩ glass micropipettes containing K+-gluconate internal saline (in mM: 100 K-gluconate, 8 KCl, 5 NaCl, 1.5 MgCl2, 20 HEPES, 10 EGTA, 2 ATP, and 0.3 GTP, pH 7.2; osmolarity 255 mosM) for mixed current recordings. To record Na+ and Ca2+ currents in the absence of K+ current, a Tris-based internal saline was used [in mM: 67 Tris PO4, 73 Tris OH, 20 tetraethylammonium (TEA)-Cl, 10 EGTA, 10 sucrose, 2 ATP, and 0.1 GTP]. The Tris-based saline effectively blocked all outward K+ currents expressed by tectal neurons. To compare neurons in different stages of maturation across the RC axis of the tectum, the neurons from which we recorded were imaged using a 10× objective and AxioVision software, and their position was mapped onto the RC axis. RC distance was measured as the relative distance between the caudal-lateral edge of the tectum of the dissected brain (corresponding to the caudal dorsomedial part of the intact tectum) and the anterior edge of the large, middle ventricle (corresponding, approximately, to the rostral ventromedial part of the intact tectum), with the maximal RC distance equal to 100% (Fig. 1A). The edge of the middle ventricle was chosen to represent the most rostral end of the RC axis, because it is well defined and therefore, readily identified; however, to avoid recording from nearby thalamic neurons that reside just rostral and ventral to the tectum (Eagelson and Harris 1990), no neurons with RC distances >80% were included in this study. Electrophysiological recordings were carried out using an Axon Instruments 700B Multipatch amplifier (Molecular Devices, Sunnyvale, CA), digitized at 10 kHz using a Digidata 1322A digitizer, and recorded using pCLAMP software. At least four tadpoles were used for each experimental group. To isolate active currents, leak current was subtracted in real time using the pCLAMP software. Resting membrane potential (RMP) was determined by switching out of voltage-clamp to “I = 0” mode and waiting an average of 5 s to get a stable RMP. Membrane potentials reported here were adjusted to compensate for a liquid-junction potential of a predicted 12 mV. Spike threshold was determined to be the voltage at which the linear phase of the spike becomes exponential (i.e., the voltage at which the slope of the initial depolarizing phase of the action potential abruptly becomes steeper). Neurons with series resistances >50 MΩ were not included in the data set, and the average series resistances for recordings from caudal and rostral neurons were 21.4 and 24.7 MΩ, respectively (Table 1). Recordings were analyzed using AxoGraph and IGOR software. All statistics, except for correlations, were carried out using InStat software, and significance was tested using an unpaired two-tailed t-test. Error bars represent the SE. Correlations between a neuron's maximum Na+ and K+ currents (Fig. 2C) and current densities (Fig. 2D) and also the correlation between the maximum number of spikes that a neuron could fire and its maximum peak Na+, peak transient K+, persistent K+ currents, and Na+:K+ ratio (Fig. 3, D–G) were quantified using a Spearman correlation test.
Fig. 1.
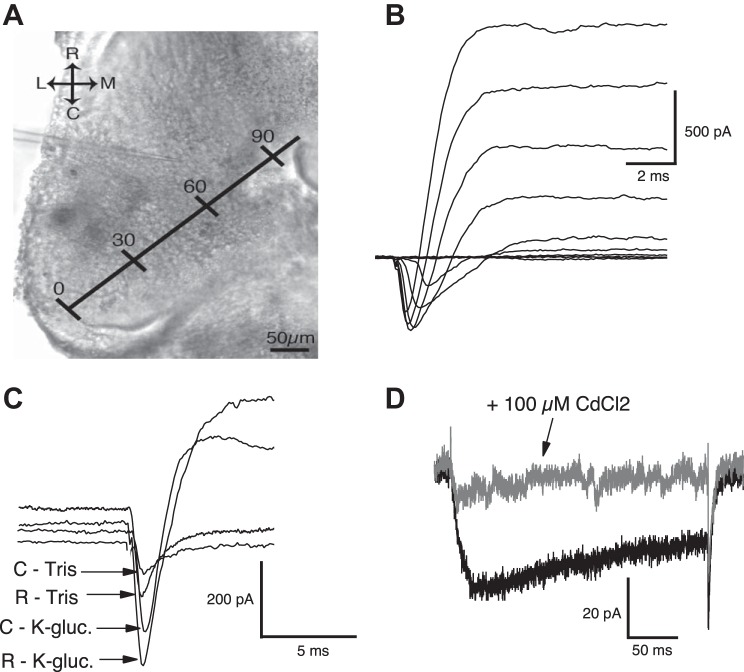
Quantifying maximum Na+ and K+ currents across the rostrocaudal (RC) axis of the tectum. A: image of a single optic tectum with plotted RC axis. The length of the RC axis is converted into a percentage, with 0% as the most caudal point and 100% the most rostral; 0–30%: “caudal,” 31–60%: “middle,” and 61–80%: “rostral.” R, rostral; C, caudal; M, medial; L, lateral. B: sample voltage-clamp recording of mixed currents from a single tectal neuron. Traces represent currents activated by stepping the neuron to increasingly more depolarized voltages. The inward (downward), inactivating portion of each trace reflects Na+ current, whereas the outward (upward) fraction of the trace represents K+ currents. C: to ensure that in these mixed current recordings, the Na+ and K+ currents are not interfering with each other, Na+ currents were isolated from outward K+ by recording with a Tris/tetraethylammonium (TEA) pipette to block all outward K+ current. Shown here are representative traces of Na+ currents in the absence (“Tris”; recorded using a Tris/TEA internal recording solution that blocks all K+ currents) and presence (“K-gluc.”; using a K+-gluconate internal solution, allowing for mixed current recording) of K+ currents from both caudal and rostral neurons. Notice that for both caudal and rostral neurons, the time at which the Na+ current peaks is quite similar in the absence and presence of K+ currents, suggesting the following: that 1) the peak Na+ current is not eclipsed by the K+ current and 2) the time at which the Na+ current peaks does not appear to be changing noticeably in neurons across the RC axis. Note: to facilitate visualizing the peak of each current, the baselines were offset along the y-axis. D: the blocking of all K+ currents (using Tris/TEA internal saline) and Na+ currents (by adding 1 μM TTX in the bath) reveals an inward current in both caudal and rostral neurons. This inward current was determined to be Ca2+ channel dependent, since it was blocked with the addition of the Ca2+ channel blocker cadmium chloride (CdCl2; 100 μM) to the recording bath.
Table 1.
Spike analysis and passive properties
| Parameter | Caudal | Rostral |
|---|---|---|
| AP threshold, mV | −19.76 ± 0.75 (n = 17) | −21.18 ± 0.83 (n = 20) |
| AP height, mV, | 19.57 ± 1.66 (n = 17) | 25.44 ± 2.23 (n = 20) |
| AP half-height width, ms | 4.47 ± 0.35 (n = 17) | 3.67 ± 0.33 (n = 20) |
| Resting membrane potential, mV | −45.82 ± 1.27 (n = 80) | −49.05 ± 0.05 (n = 94) |
| Input resistance, GΩ | 2.21 ± 0.13 (n = 91) | 1.93 ± 0.18 (n = 100) |
| Membrane capacitance, pF | 10.02 ± 0.34 (n = 91) | 12.3 ± 0.42 (n = 100) |
| Series resistance, MΩ | 21.38 ± 1.31 (n = 91) | 24.72 ± 1.41 (n = 100) |
AP, action potential.
Fig. 2.
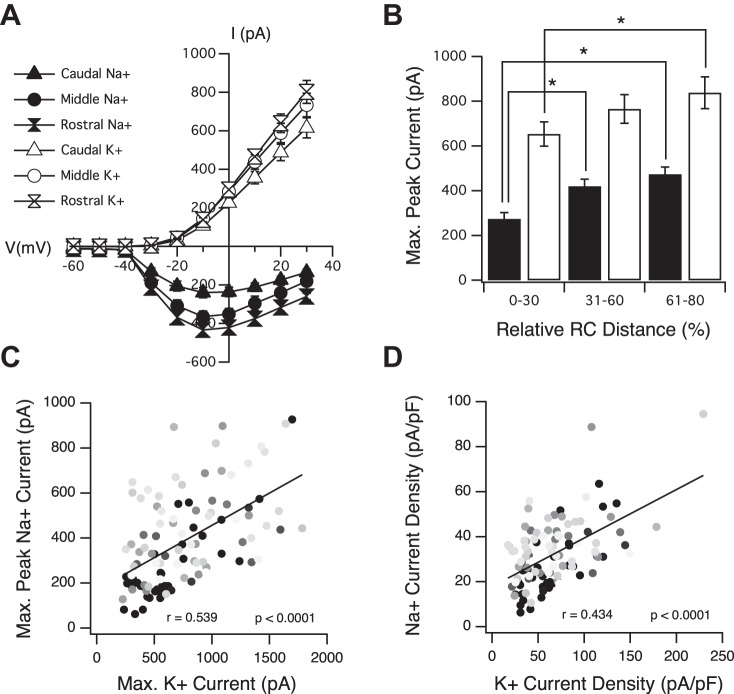
Peak Na+ and K+ currents increase along the RC axis of the optic tectum. A: averaged current-voltage (I-V) plots of tectal neurons from different segments of the RC axis. B: average maximum peak Na+ (black bars) and maximum persistent K+ (white bars) currents increase along the RC axis. *P < 0.05, t-test. C: maximum peak Na+ vs. maximum persistent K+ currents within individual neurons. Notice that the increase in these 2 currents is well correlated within individual neurons. Dark gray circles, most caudal (immature); light gray circles, most rostral (more mature). D: Na+ current density vs. K+ current density within individual neurons. A–D: n = 39 for caudal; n = 39 for middle; n = 34 for rostral.
Fig. 3.
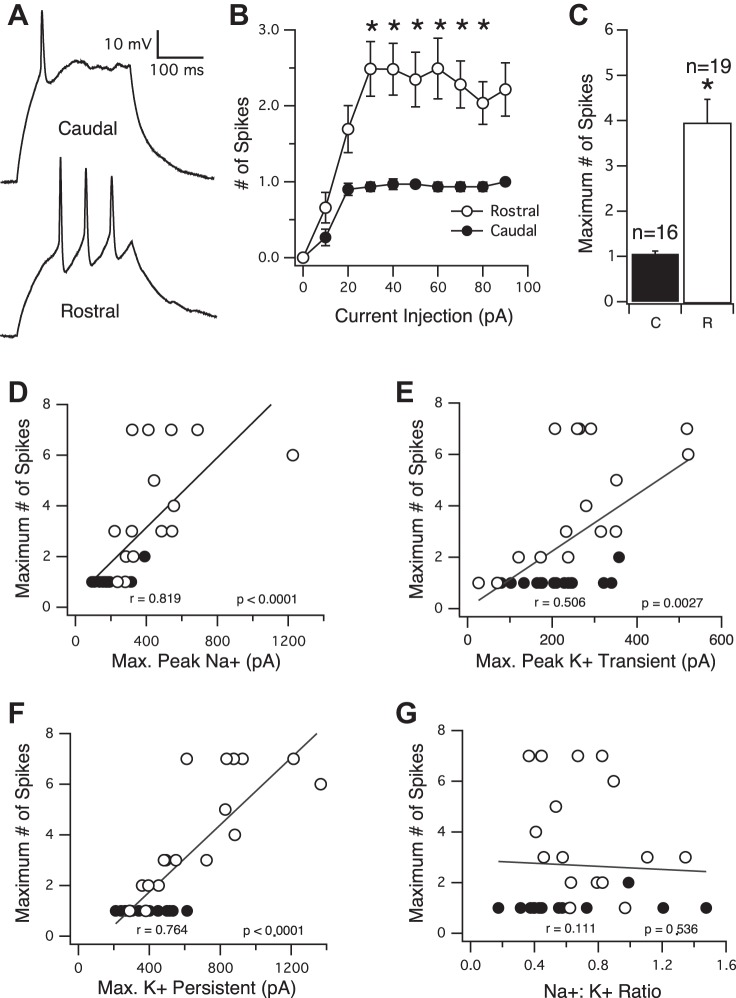
The number of spikes that a neuron can fire in response to a sustained injection of current also increases along the RC axis. A: sample current-clamp traces of action potentials recorded from a caudal (top) and rostral (bottom) neuron, evoked by injection of a square, 250 ms current pulse. B: averaged input-output curves showing the number of spikes evoked by injection of differing amounts of current (expressed in pA). Neurons from the rostral part of the tectum (white circles) fire significantly more action potentials than caudal neurons (black circles) at essentially every step of current injection. C: average maximum number of action potentials that a neuron fires in response to current injection. Rostral neurons fire significantly more action potentials than caudal neurons. *P < 0.0001. D–G: maximum number of spikes plotted as a function of peak Na+, transient K+, persistent K+ currents, and Na+:K+ ratio, respectively. Black circles, caudal; white circles, rostral.
RESULTS
All experiments were carried out using developmental stage 49 (16–24 dpf) tadpoles unless noted otherwise, and recordings were obtained using an isolated brain preparation (see materials and methods). The relationship between a neuron's intrinsic currents and its location in the tectum was directly assessed by quantifying peak currents as a function of neuron position along the RC axis. In this preparation, the RC axis was defined as beginning at the most caudal-lateral point of the tectum, projecting medially and rostrally, and ending at the anterior edge of the large middle ventricle, a well-defined and obvious landmark (Fig. 1A). A neuron's location along this axis, referred to as its “RC distance,” was expressed as its distance from the most caudal-lateral point. RC distances were normalized across tadpoles by converting this distance into a percentage, with “0%” as the most caudal point of the axis and “100%” representing the most rostral point. Neurons that had RC distances between 0% and 30% were designated as “caudal,” 31–60% as “middle,” and 61–80% as “rostral.” We considered this axis as consisting of true tectal neurons, since the neurons across the entire axis were observed to receive direct monosynaptic input from the retinal ganglion cells in the eye. To avoid the possibility of recording from nontectal neurons (i.e., thalamic neurons), this study does not include any neurons with RC distances >80%.
Peak Na+ and K+ currents increase along the RC axis of the optic tectum.
To quantify peak Na+ and K+ currents, neurons were held at −60 mV in a whole-cell voltage-clamp configuration, stepped to increasingly more depolarized potentials for a duration of 250 ms, and the resulting voltage-dependent Na+ and K+ currents were recorded (Fig. 1B). In these tectal neurons, the inward, inactivating current was previously shown pharmacologically to be due to Na+ ions moving into the neuron through Na+ channels and the outward current, consisting of a transient and persistent component, K+ ions moving out through their respective channels (Aizenman et al. 2003). To confirm this for neurons across the entire RC axis and to determine if these opposing currents are separated sufficiently in time, as to not eclipse one another, Na+ currents were isolated from outward K+ currents by using a Tris-based solution in the recording pipette (see materials and methods). This effectively blocked all of the K+ currents and showed that blocking K+ currents did not alter the time (latency) at which the amplitude of the Na+ currents peaked. Figure 1C shows sample traces in the presence and absence of the outward K+ currents [peak latency (in ms) of Na+ currents recorded in K+-gluconate internal saline: caudal 2.05 ± 0.16, n = 11, and rostral 1.73 ± 0.17, n = 13; peak latency of Na+ currents recorded in Tris-based internal saline: caudal 2.45 ± 0.54, n = 11, and rostral 2.21 ± 0.23, n = 14; data not shown]. This suggests that when not blocked, the outward K+ currents are not masking, at least, the peak in Na+ currents in caudal or rostral neurons (Fig. 1C) and also shows that the inward Na+ current peaks before the outward K+ current in both caudal and rostral neurons. We also observed that neurons across the entire RC axis express a voltage-gated inward Ca2+ current, which was isolated by blocking all K+ and Na+ currents by using a combination of Tris/TEA internal and 2 μM TTX in the external recording solution, respectively. The perfusion of 100 μM cadmium chloride, a Ca2+ channel blocker, into the bath completely blocked this inward current, indicating that this is a Ca2+ channel-dependent current (Fig. 1D).
Having established that the peak inward Na+ and outward K+ currents expressed by a neuron can be resolved when recorded as a “mixed” current, voltage curves were generated by plotting the peak inward and outward current amplitudes for Na+ and persistent K+, respectively, as a function of the magnitude of the voltage step. We observed a graded increase in both Na+ and K+ currents across the tectum in the caudal to rostral direction (Fig. 2A). At most voltage steps, neurons in the rostral portion of the tectum (RC distance = 61–80%) expressed greater peak Na+ and K+ currents compared with those expressed by neurons in the caudal portion (RC distance = 0–30%). However, due to the voltage-clamp error that is inherent with imperfect series resistances and the variable morphology of the neurons across this axis, here, we focus mainly on maximum peak Na+ currents—the greatest peak Na+ current expressed by a neuron, regardless of which voltage step by which this current was elicited—and maximum K+ currents, which occur on the last (most depolarized) voltage step. Note that since the K+ current is not in the form of a peak, the greatest K+ current is reported only as maximum rather than maximum peak.
The absolute maximum peak Na+ current expressed by a neuron increased in a graded manner across the RC axis (Fig. 2B). Average maximum peak Na+ current (in pA) for neurons with RC distances between 0% and 30% (or caudal) = −273.6 ± 28.0, n = 39; 31% and 60% (or middle) = −419.4 ± 31.8, n = 39; and 61% and 80% (or rostral) = −473.53 ± 31.9, n = 34. Increase in peak currents observed between the caudal and middle and caudal and rostral groups was significant (P = 0.001 and P < 0.0001, respectively). To a slightly lesser extent, maximum K+ currents also increased along the axis in a graded fashion (Fig. 2B). Average maximum K+ currents (in pA) for neurons with RC distances between 0% and 30% = 652.8 ± 54.0, n = 39; 31% and 60% = 764.9 ± 63.9, n = 39; and 61% and 80% = 838 ± 71, n = 34. For K+ currents, only the increase between caudal and rostral neurons was statistically significant (P = 0.036). Maximum Ca2+ currents (Fig. 1D) appeared relatively small and constant across the tectum. Average maximum Ca2+ current (in pA) for caudal neurons = −54.53 ± 8.93, n = 9, and for rostral neurons = −47.63 ± 5.6, n = 12 (P = 0.5005; data not shown).
Along the entire axis, a neuron's maximum peak Na+ amplitude was found to be strongly correlated with its maximum K+ amplitude. In other words, if a tectal neuron displayed a relatively high Na+ amplitude, it also displayed a relatively high K+ amplitude (Fig. 2C), suggesting that within an individual neuron, the development of these two currents likely shares a common regulatory mechanism(s) or that one current may be regulating the other. The expression of currents as current densities (Fig. 2D) to normalize for cell size indicates that the increase in Na+ currents is the result of an actual increase in the density of Na+ channels in a given amount of plasma membrane. Na+ current density (in pA/pF) for caudal neurons = −25.9 ± 21.14, n = 39, and for rostral neurons = −34.53 ± 1.73, n = 34 (P = 0.003). The K+ current densities, however, appeared strikingly constant across the tectum, suggesting that the observed increase in maximum K+ current is most likely the result of an increase in cell-surface area. K+ current density (in pA/pF) for caudal neurons = 64.3 ± 5.09, n = 39, and for rostral neurons = 63.4 ± 5.26, n = 34 (P = 0.9017). Hence, the plotting of the Na+ and K+ current densities expressed by individual neurons (Fig. 2D) reveals a slightly less correlated relationship between these two ions, since Na+ current densities are increasing, whereas K+ current densities are more constant. The same pattern of development for these currents has been described for mouse cortical neurons: Na+ current densities expressed by neurons of the cortical plate increase, whereas K+ current densities are constant, because the increase in the K+ current matches the increase in capacitance (Picken Bahrey and Moody 2003), as we observe here. In addition, we noticed that the peak Na+:K+ ratio (calculated by dividing a neuron's maximum peak Na+ current by its maximum persistent K+ current) steadily increased across the RC axis, indicating that even though both types of intrinsic currents are growing in tandem as the neuron matures, the increase in Na+ currents is greater than the increase in K+ currents. Average peak Na+:K+ ratio for caudal neurons = 0.43 ± 0.03, n = 39, and for rostral neurons = 0.67 ± 0.07, n = 34 (P = 0.0009; data not shown).
Compared with neurons in the caudal portion of the tectum, neurons in the rostral portion fire more action potentials in response to current injection.
Since voltage-gated Na+ and K+ currents underlie the action potential and since these two currents are increasing in neurons along the RC axis, we tested the ability of tectal neurons at different points along the RC axis to generate action potentials (Fig. 3A). For these experiments, we switched to recording in current-clamp mode. From a baseline potential of −60 mV (maintained by a small amount of direct current), neurons were injected with 250 ms square pulses of current of varying magnitudes and the number of action potentials elicited by each current injection counted. To be counted as an action potential, the height of the spike (the exponential phase of the action potential) had to be no less than one-half the height of the preceding spike, and its width at one-half its maximal height (“half-height width”) had to be no more than 3× the width of the first spike in the train. Compared with neurons residing in the caudal portion of the axis, neurons in the rostral portion were found to fire more action potentials in response to most of the current injections tested. The full current vs. spike curve is shown in Fig. 3B. In addition, the overall maximum number of spikes that each neuron could fire, regardless of which current step by which these spikes were elicited, was found to be significantly greater in the rostral portion of the RC axis (Fig. 3C). Average maximum number of spikes for caudal neurons = 1.06 ± 0.06, n = 16, and average maximum number of spikes for rostral neurons = 3.95 ± 0.52, n = 19 (P < 0.0001). Spike analysis, focusing on the first spike in the train, revealed that rostral neuron spikes had a lower threshold (see materials and methods), an increased height, and a more narrow spike half-height width compared with spikes generated by neurons in the caudal portion of the tectum (Table 1). Although these changes in the spike form are not statistically significant, they are consistent with an increase in the Na+ current. Across the axis, we also observed a graded decrease in both RMP and input resistance, which together, are indicative of an increase in a voltage-independent K+ leak current. Finally, compared with neurons in the caudal portion of the tectum, we observed a modest, yet significant, increase in membrane capacitance in rostral neurons (Table 1; caudal = 10.03 ± 0.34, n = 91; rostral = 12.31 ± 0.42, n = 100; P < 0.0001), which is likely a reflection of overall neuronal growth.
To investigate further the relationship between a neuron's peak intrinsic currents and its ability to fire action potentials, we plotted the maximum number of action potentials that a neuron fired as a function of the following: 1) its maximum peak Na+ current, 2) its maximum peak transient K+ current, and 3) its maximum persistent K+ current (Fig. 3, D–F, respectively). We found all of these intrinsic currents to be well correlated with the number of action potentials that the neuron could fire. Peak Na+:K+ ratio, on the other hand, was observed to be a very poor indicator of the number of action potentials, as there was essentially no correlation between this ratio and the number of action potentials (Fig. 3G). This suggests that the absolute magnitude of intrinsic currents is what determines a neuron's “spikiness” and that even though the Na+:K+ ratio is indeed increasing across the RC axis, it is not linked to the increase in the ability of the neuron to fire action potentials. These data support the concept that for a neuron to fire repetitively in response to sustained depolarization, the activation of a certain number of Na+ channels is necessary for the exponential depolarization, or upswing, phase of the spike but also that a certain magnitude of the K+ current is required to repolarize the neuron sufficiently to relieve the inactivation of Na+ channels to allow for the proceeding upswing phase of the next action potential (Bean 2007).
Presence of RC axis maturation gradient earlier in development.
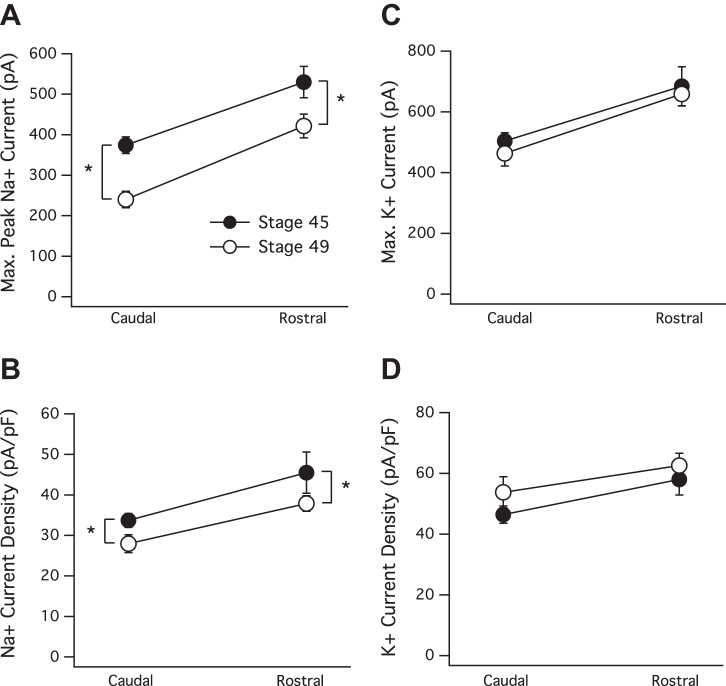
We next tested whether the maturation gradient of Na+ and K+ currents along the RC axis was present at stage 45 (7–10 dpf). This stage is characterized by high rates of synapse formation and loss (Cline et al. 1997) and a transient peak in tectal neuron intrinsic excitability (Pratt and Aizenman 2007) and is known to be an especially dynamic stage of retinotectal development. Similar to that observed at stage 49, neurons in the rostral portion of the stage 45 tectum expressed significantly greater peak Na+ and K+ currents compared with neurons in the caudal portion (Fig. 4A). Average maximum peak Na+ current (in pA) for stage 45 caudal neurons = −374.3 ± 20.5, n = 29, and stage 45 rostral = −541.6 ± 37.6, n = 29, P = 0.0003; stage 49 caudal = −240.2 ± 20.5, n = 37, and stage 49 rostral = −421.6 ± 29.5, n = 58, P < 0.0001. In Fig. 4C, average maximum K+ current (in pA) for stage 45 caudal neurons = 503.8 ± 27.1, n = 29, and stage 45 rostral = 684.1 ± 64, n = 29, P = 0.012; stage 49 caudal = 462.9 ± 41.1, n = 37, and stage 49 rostral = 659 ± 39.5, n = 58, P = 0.0013. To test how much of the increase in currents could be attributed to an increase in membrane surface area (cell capacitance), current densities were calculated. At both developmental stages, we observed a significant increase in Na+ current densities across the tectum (Fig. 4B). Na+ current density (in pA/pF) for stage 45 caudal neurons = −33.69 ± 1.7, n = 26, and stage 45 rostral = −45.5 ± 2.83, n = 26, P = 0.0008; stage 49 caudal = −27.98 ± 2.19, n = 35, and stage 49 rostral = −37.85 ± 1.91, n = 57, P = 0.0013. However, the gradient of K+ current densities did not appear to increase significantly across either axes (Fig. 4D). K+ current density (in pA/pF) for stage 45 caudal neurons = 46.4 ± 2.8, n = 26, and stage 45 rostral = 57.99 ± 5.1, n = 26, P = 0.052; stage 49 caudal = 53.75 ± 5.09, n = 35, and stage 49 rostral = 62.56 ± 4.07, n = 57, P = 0.182. Thus here again, we observed that increases in the Na+ current density are significant, and the increase in K+ is not, because the increase in the K+ current matches the increase in cell capacitance. Figure 4A clearly shows, however, that the two gradients are not identical: by stage 49, the entire gradient in maximum peak Na+ currents has shifted down to lower values. This is consistent with previous work that describes a drop in intrinsic excitability in tectal neurons in the middle third of the tectum between these same two developmental stages (Pratt and Aizenman 2007). The drop in Na+ currents between the two stages was significant at both the rostral and caudal ends of the axis (P = 0.0017 and P < 0.0001, respectively), and as would be predicted by their maximum peak Na+ currents, neurons in the rostral and caudal portion of a stage 45 tectum fired more action potentials in response to current injection compared with the corresponding neurons in a stage 49 tectum. This difference in spiking ability between these two stages was statistically significant between caudal neurons but not between rostral neurons. Caudal stage 49 = 1.06 ± 0.06, n = 16, and stage 45 = 4.88 ± 0.86, n = 8, P < 0.0001; rostral stage 49 = 3.95 ± 0.52, n = 19, and stage 45 = 5.88 ± 1.17, n = 8, P = 0.092 (data not shown). Overall, the comparison of the graded increase in Na+ and K+ currents at developmental stages 45 and 49 suggests that this gradient, although appearing to shift across developmental time, is not transient and probably persists for as long as new neurons are incorporated into the tectum.
Fig. 4.
The presence of the gradient at stage 45. A: maximum peak Na+ currents, as well as peak Na+ current density (B) increase significantly along the RC axis in both stage 45 (black circles) and stage 49 (white circles) tadpoles. Also notice that both rostral and caudal peak Na+ current and current density are significantly higher in the stage 45 tectum, indicated by the asterisks (*). In other words, the entire gradient is shifted to higher values at stage 45, but the slope of the gradient appears constant. C: maximum persistent K+ current and K+ current density (D) increase similarly along the RC axis at both stages. The increase observed in maximum K+ currents expressed by rostral neurons is significant, but the slight increase in K+ current density is not significant across either axis, because the increase in the K+ current is matched closely with the increasing cell capacitance.
Excitatory synaptic drive increases along the RC axis.
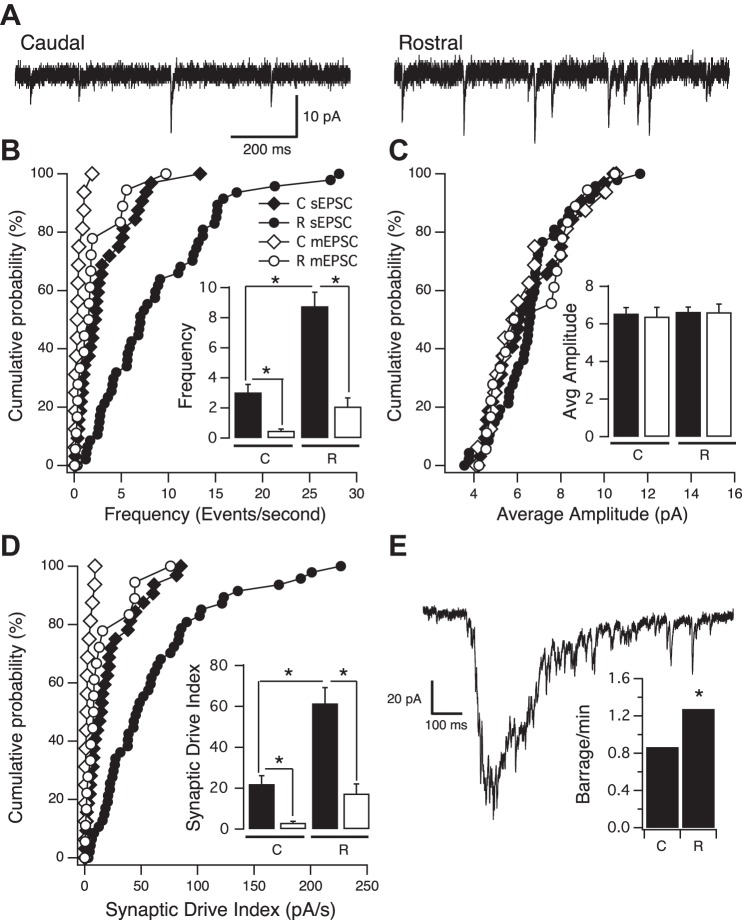
Next, we tested how the graded increase in intrinsic excitability along the RC axis relates to the increase in excitatory synaptic amplitudes and frequencies reported previously along the same axis. To isolate excitatory synapses, inhibitory (GABAergic) transmission was blocked by adding picrotoxin to the external recording solution and recording spontaneous excitatory postsynaptic currents (sEPSCs) with the neuron voltage clamped at −60 mV (Fig. 5A). We found a significant increase in the frequency of sEPSCs along the RC axis (Fig. 5B; caudal = 3.04 ± 0.53 Hz, n = 33; rostral = 8.77 ± 0.93 Hz, n = 48; P < 0.0001), but no significant difference in the average sEPSC amplitude was observed, at least when neurons are binned the way we have done so here (Fig. 5, A and C; caudal = 6.55 ± 0.32 pA, n = 33; rostral = 6.65 ± 0.25 pA, n = 48; P = 0.8124). The overall amount of synaptic drive received by an individual neuron, estimated by multiplying each neuron's average sEPSC amplitude by its sEPSC frequency, increased significantly along the RC axis (Fig. 5D; caudal = 21.98 ± 4.08 pA/Hz, n = 33; rostral = 61.53 ± 7.71 pA/Hz, n = 48; P < 0.0001). sEPSCs, as we record here, consist of a mixture of action potential-dependent synaptic currents (“EPSCs”), as well as currents produced by the random, action potential-independent release of single presynaptic vesicles [miniature EPSCs (“mEPSCs”)]. Therefore, an increase in the frequency of sEPCS could be due to a general increase in intrinsic excitability in the rostral portion of the tectum, an increase in the number of synapses received by any one neuron, and/or an increase in the probability of presynaptic vesicle release; the latter is an unlikely possibility, since it has been reported that probability of presynaptic vesicle release is consistent across the RC axis (Wu et al. 1996). To determine if the increase in sEPSCs, displayed by neurons in the rostral portion of the tectum, is the result of increased action-potential firing or an increase in the number of synapses, we recorded excitatory synaptic events in the presence of 0.5 μM TTX to block all action-potential firing while sparing the action potential-independent events (mEPSCs). We observed that compared with the frequency of sEPSCs, the frequency of mEPSCs was noticeably lower for neurons in both the rostral and caudal portions of the axis (Fig. 5B; caudal mEPSC frequency = 0.48 ± 0.12, n = 17; rostral mEPSC frequency = 2.09 ± 0.58, n = 19). This indicates that across the RC axis, the frequency of sEPSCs is determined most by action potential-dependent synaptic events, supporting our finding of increased levels of intrinsic excitability in the rostral portion of the tectum. The average mEPSC frequency displayed by neurons at the rostral end of the RC axis remained significantly greater than the frequency displayed by neurons at the caudal end (P = 0.0135). This suggests that compared with a caudal neuron, an individual rostral neuron expresses a greater number of functional synapses, consistent with an increase in complexity of tectal neuron dendritic arbors in the rostral part of the tectum (Wu et al. 1999). Hence, the observed increase in sEPSC frequency, displayed by neurons in the rostral portion of the tectum, is most likely due to a combination of increased intrinsic excitability (i.e., increased likelihood of action-potential firing and thus increased frequency of EPSCs) combined with an increase in the number of synapses expressed by these more mature neurons. That the average amplitude of mEPSCs was essentially equal to the average sEPSC amplitude for both caudal and rostral neurons suggests that the majority of presynaptic axonal inputs synapses onto their postsynaptic tectal targets on an average of one time and that this pattern of connectivity is consistent across the tectum (Fig. 5C).
Fig. 5.
Increase in excitatory synaptic drive along the RC axis. A: sample voltage-clamp traces of spontaneous excitatory postsynaptic currents (sEPSCs) from caudal and rostral neurons. B: cumulative probability distribution of sEPSC (black symbols) and synaptic events in the presence of TTX [miniature EPSC (mEPSC); white symbols] frequencies for individual caudal and rostral neurons. Each point on the graph represents the frequency of an individual neuron. Inset: bar graph of the same data showing average sEPSC (black bars) and mEPSC (white bars) frequency, increasing significantly along the RC axis. Notice that the addition of acute TTX (0.5 μM) to the bath results in a significant reduction in the number of synaptic events in both caudal and rostral neurons and that the mEPSC frequency in rostral neurons is significantly higher than in caudal neurons. C: cumulative probability distribution of sEPSC (black symbols) and mEPSC (white symbols) amplitude for caudal and rostral neurons. Each point on the graph represents the average synaptic current amplitude expressed by an individual neuron. The distribution of average amplitudes is similar across all groups. Inset: bar graph of the same data showing that average sEPSC (black bars) and mEPSC (white bars) amplitude does not change significantly. D: cumulative probability distribution of synaptic drive index of caudal and rostral neurons recorded in the absence (black symbols) and presence (white symbols) of TTX in the bath. Each point on the graph represents average synaptic drive index calculated for a given neuron. For each neuron, synaptic drive index was calculated by multiplying its sEPSC frequency (events/s) by its average sEPSC amplitude (pA). Inset: bar graph of the same data showing average excitatory synaptic drive increasing along the RC axis. Notice that adding acute TTX to the bath significantly reduces synaptic drive in both caudal and rostral neurons; however, synaptic drive in the rostral portion is still significantly higher than in the caudal portion. A–D: caudal, n = 33; rostral, n = 48; caudal with TTX, n = 17; rostral with TTX, n = 19. E: sample trace of a synaptic barrage. Average frequency of synaptic barrages increases significantly along the RC axis (caudal, n = 42; rostral, n = 55). *P < 0.05, t-test.
In addition, we observed a significant increase in the frequency of spontaneous synaptic barrages along the RC axis (Fig. 5E; caudal = 0.87 ± 0.14 barrages/min, n = 42; rostral = 1.27 ± 0.11 barrages/min, n = 55; P = 0.0269). These barrages have been reported previously in the same isolated brain preparation as used in this study (Pratt and Aizenman 2007) and in vivo (Zhang et al. 2000) and are thought to reflect overall circuit connectivity within the tectum. The barrages are action-potential dependent, as we did not observe a single one when recording in the presence of TTX. That rostral neurons showed a higher frequency of these barrages suggests a more complex and active tectal-tectal circuitry in the rostral portion of the tectum.
Taken together, the intrinsic and synaptic data show a positive correlation among a neuron's RC distance, the amount of synaptic drive it is receiving, its peak Na+ and K+ currents, and its ability to fire action potentials.
Chronically dampening circuit activity scales up intrinsic channels in a region-specific manner.
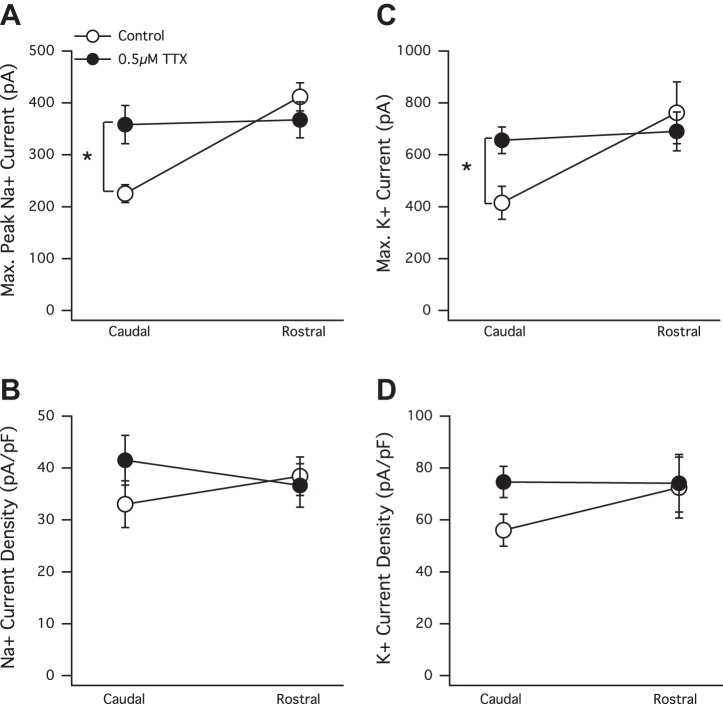
There are many examples demonstrating that changes in the pattern or level of activity experienced by a developing neuron can shape its intrinsic currents (Aizenman et al. 2003; Desai et al. 1999b; Turrigiano et al. 1994). Therefore, to explore how overall activity levels may affect the gradient of voltage-gated intrinsic currents that exists across the RC axis, we chronically reduced overall circuit activity by raising tadpoles for many days in a low concentration (0.5 μM) of TTX (a Na+ channel blocker). We found that raising tadpoles for 7 days in this low concentration of TTX induced a significant increase in both peak Na+ and K+ currents in tectal neurons in the caudal portion of the axis (Fig. 6, A and C). Average caudal control maximum peak Na+ currents = −225.3 ± 17 pA, n = 8; average maximum K+ = 414.9 ± 63.5, n = 8; average caudal TTX maximum peak Na+ currents = −358.1 ± 36.7 pA, n = 14, P = 0.0165; and average maximum K+ = 692.2 ± 52.5, n = 14, P = 0.009. However, in the more mature neurons in the rostral portion, there was not a significant increase. Average rostral control maximum peak Na+ = −411.7 ± 27.1, n = 8; average maximum K+ = 762 ± 119.5, n = 8; average rostral TTX maximum peak Na+ = −367.4 ± 34.5, n = 16, P = 0.4112; and average maximum K+ = 690.4 ± 74.6, n = 16, P = 0.6015. The scaling up of only the caudal end of the axis flattened the gradient (Fig. 6, A and C).
Fig. 6.
The chronic dampening of overall circuit activity induces what appears to be a region-specific plasticity in peak Na+ and K+ currents in stage 49 tadpoles. A: average maximum peak Na+ currents significantly increase in caudal but not in rostral neurons in response to chronic TTX treatment (7 days). B: Na+ current density also increases in only the TTX-treated caudal neurons, but this increase is not statistically significant. C: average maximum persistent K+ currents significantly increase in caudal but not rostral neurons in response to chronic TTX treatment. *P < 0.05, t-test. D: insignificant increase in peak K+ current density only in caudal neurons of the TTX-treated group. Note that even though both peak (maximum) Na+ and K+ currents are significantly higher in TTX-treated caudal neurons, the average current density expressed by TTX-treated caudal neurons is not significantly different than that expressed by non-TTX-treated controls. This is because the cell capacitances were slightly higher in the TTX-treated neurons. TTX-exposed rostral neurons did not express an increase in intrinsic currents and also did not display an increase in cell capacitance.
Chronic exposure to TTX also elicited an increase in both Na+ and K+ current densities in neurons in the caudal portion of the tectum, although this increase was not statistically significant because of a modest increase in cell capacitance displayed by the TTX-exposed caudal neurons (Fig. 6, B and D). Average control caudal Na+ current density = −33.04 ± 4.51, n = 8; average TTX caudal Na+ current density = −41.5 ± 4.78, n = 14, P = 0.2545; average control caudal K+ current density = 56.03 ± 6.152, n = 8; and average TTX caudal K+ current density = 74.6 ± 6.04, n = 14, P = 0.0589. How could a general decrease in overall circuit activity appear to induce a significant increase in intrinsic currents in neurons in the caudal portion of the tectum but not in the rostral portion? One possibility is that the frequency of mEPSCs, displayed by rostral neurons in the presence of TTX (Fig. 5, B and D), is sufficient to prevent triggering the compensatory increase in Na+ and K+ currents. In other words, the compensatory type of intrinsic plasticity displayed by caudal neurons may require synaptic drive to be depressed beyond a certain “critical minimum” threshold. To test this, we added the AMPA receptor blocker NBQX (30 μM) to the rearing solution containing 0.5 μM TTX. This concentration of NBQX blocks all mEPSCs in these tectal neurons. We found that the chronic blockade of both action potential-dependent and -independent events still failed to elicit any significant change in peak Na+ or K+ currents in rostral neurons. Average control rostral maximum peak Na+ current (in pA) = −332.83 ± 28.45, n = 13; average TTX + NBQX rostral maximum peak Na+ current = −299.5 ± 47.33, n = 13; average control rostral maximum K+ current = 507.99 ± 36.99, n = 13; and average TTX + NBQX rostral maximum K+ current = 533.11 ± 57.97, n = 13 (data not shown). The results of this “total block” experiment show that the decrease of synaptic frequency received by rostral neurons below a putative critical minimum does not trigger a response in their peak intrinsic currents.
In summary, we observed that the chronical reducing of overall circuit activity (action potentials and synaptic drive) induced a robust increase in intrinsic currents, specifically in neurons of the caudal portion of the tectum, but induced no noticeable response in neurons of the rostral region. The total blockade experiment (TTX + NBQX) rules out the possibility that a certain, higher frequency of mEPSCs received by rostral neurons is preventing the activation of this type of intrinsic plasticity and instead suggests an innate, region-specific difference between neurons in the caudal region of the tectum and those localized to the more rostral region.
DISCUSSION
The understanding of how voltage-gated intrinsic currents are regulated over development is essential, because these currents, along with the pattern and type of synaptic input, largely determine the way in which a neuron functions as part of a circuit (Nelson and Turrigiano 2008; Schulz 2006; Zhang and Linden 2003). The natural maturation of voltage-gated intrinsic currents has been described in a variety of neuron types, including grasshopper sensory neurons (Goodman and Spitzer 1981), chick nucleus laminaris neurons (Gao and Lu 2008), ferret dorsal lateral geniculate nucleus neurons (Ramoa and McCormick 1994), tadpole spinal (Desarmenien et al. 1993; O'Dowd et al. 1988; Spitzer and Lamborghini 1976; Sun and Dale 1998) and tectal (Pratt and Aizenman 2007) neurons, Drosophila embryonic central neurons (Baines and Bate 1998), and mouse cortical (Picken Bahrey and Moody 2003) and spinal cord (MacDermott and Westbrook 1986) neurons.
In the optic tectum of the Xenopus tadpole, incorporation of newborn neurons from the proliferative zone into the functioning tectum creates an organized spatial gradient of maturation, in which neurons at the caudal end of the axis are relatively immature compared with neurons residing at the rostral end. Although many aspects of synapse and dendrite maturation have been described along this axis, a description about how intrinsic currents may be changing was missing. Here, we describe a spatial gradient of intrinsic excitability that parallels the gradient of synaptic maturation. Immature tectal neurons in the caudal third of the tectum display relatively small peak Na+ and K+ currents and are able to fire, on average, a maximum of one action potential in response to the injection of various amounts of current. In contrast, more mature neurons in the rostral third of the tectum display increased peak Na+ and K+ currents and can fire significantly more action potentials compared with neurons in the caudal region. We focus predominantly on the maximum currents expressed by a tectal neuron, due to inherent errors associated with voltage clamp. In some respects, tectal neurons are amenable for voltage-clamp recordings. First, compared with Na+ and K+ currents expressed by mammalian neurons, those of tadpole tectal neurons are slower and smaller, promoting the accurate detection of relative changes in peak currents. Another characteristic that renders these neurons especially amenable for voltage-clamp recordings is that they are relatively small (average soma diameters are in the range of 5–8 μm), and these small neurons are theoretically easier to voltage clamp than large ones. On the other hand, the recording of small neurons requires using a pipette with a relatively small tip (10–11 MΩ pipette resistance). As the size of the pipette decreases, series resistance increases and thus compromises temporal control of voltage, and always a concern when carrying out voltage-clamp recordings from neurons is the inability to clamp the voltage in dendrites and axons (i.e., space-clamp error). We cannot rule out that the differing morphologies of the neurons across the tectum could create differences in spatial voltage control: more complex dendritic arbors, characteristic of the neurons in the rostral portion of the tectum and also a probable increase in distance from the soma to the site of action-potential generation, would theoretically result in poorer control of voltage compared with recording from the morphologically simpler caudal neurons. This theoretical decrease in voltage-clamp control across the tectum cannot account for the findings here, however, since we observe an increase in peak currents and the ability of neurons to fire action potentials in rostral neurons compared with caudal neurons.
This program of development that we observed across the RC axis, in which both intrinsic excitability and levels of synaptic drive are increasing, is similar to what has been described to take place in neurons in the middle third of tectum between developmental stages 42—just after retinal ganglion cell axons have reached the tectum (Dingwell et al. 2000) and have begun forming immature synapses onto the tectal neurons—and 45. During this time in development, both synaptic drive and intrinsic excitability tended to increase (Pratt and Aizenman 2007). The joint increase in intrinsic excitability and synaptic drive that we describe here across the RC axis is in contrast, however, to the program of development described to take place between stages 45 and 49. During this slightly later time in development, the increase of synaptic drive triggers a homeostatic decrease in peak Na+ and K+ currents and the ability of the neurons to fire action potentials (Pratt and Aizenman 2007). Together, these studies suggest that perhaps a relatively immature, newly integrated caudal neuron is probably most similar to a stage 42 (midtectum) neuron that is also just beginning to receive synaptic input, before (perhaps) homeostatic mechanisms have come into play. Exactly what it is in a given developing neuron that determines whether to grow or homeostase remains unknown.
To determine whether the gradient of currents observed at developmental stage 49 is present earlier in development, the same set of whole-cell recordings was carried out across the stage 45 tectum. At this highly dynamic developmental stage, midtectum neurons are reported to express a transient peak in intrinsic excitability, which plummets steeply by stage 49 (Pratt and Aizenman 2007). Across the stage 45 tectum, we observed a gradient of maximum peak Na+ and K+ currents that displayed a similar slope to that of stage 49 (i.e., the gradients of peak currents were of equal steepness); however, in accordance with the previously described transient peak in intrinsic excitability, the entire stage 45 gradient appeared shifted to significantly higher values for maximum peak Na+ currents (and current density). This suggests that this graded increase in intrinsic currents across the tectum, although shifting to different levels across developmental time, most likely exists for as long as new neurons are incorporated from the proliferative zone, which is reported to be the case throughout all stages of tadpole development (Sharma and Cline 2010; Straznicky and Gaze 1972).
Relationships between different intrinsic currents within individual neurons are not always apparent when individual parameters (such as maximum peak Na+ current or maximum K+ current) are reported separately as averages (Marder 2011). Therefore, we tested the correlation between several parameters within single neurons. Taking this approach, we found that the graded increase in Na+ and K+ currents across the RC axis strongly correlated within individual neurons. The high degree of synchronicity observed between these two currents during a period of development in which the expression of each is dynamic suggests that they may share a common coregulator, a hypothesis originating from studies in stomatogastric ganglion neurons of the crab that describe how certain sets of intrinsic channels get modulated together (Khorkova and Golowasch 2007; Schulz et al. 2006). An alternative interpretation to account for this correlation is that one current is regulating the other, such as that described for the development of Na+ currents in tadpole spinal neurons: when a spinal neuron is immature, the depolarizing portion of its action potential is driven by the influx of Ca2+, which is gradually replaced by a faster Na+ current (Spitzer and Lamborghini 1976). The transition to Na+ is dependent on the presence of the delayed rectifier K+ current, because if the K+ rectifier is prevented from being translated, then the action potential remains to be driven by Ca2+ (Ribera and Spitzer 1989). Thus in this case, development of the Na+ current is dependent on the K+ current.
In addition to correlations between Na+ and K+ currents, strong correlations were also identified within individual neurons, between maximum Na+ and K+ (transient and persistent) currents, and the ability to fire action potentials in response to sustained current injection. Our data indicate that the maximum peak Na+ current was the most correlated (the highest “r” value) with the ability to fire action potentials repetitively, followed closely by maximum persistent K+ and maximum peak transient K+ currents, in that order. Although all of these individual currents appear to be well correlated with the number of action potentials, they are not perfect. To test whether a certain ratio of Na+:K+ currents could be even more tightly linked to the ability to fire action potentials, the Na+:K+ ratio for an individual neuron was plotted vs. the maximum number of action potentials. This plot clearly indicates that in a given neuron, a particular ratio of these two currents is not important for firing action potentials. In other words, the neurons displaying the most extreme ratios (highest and lowest) do not appear to fire an especially high or low number of action potentials. Instead, these neurons appear to fire more action potentials when they express a combination of relatively big Na+ and K+ currents.
In other cases, increases in the outward K+ current in response to increases in the inward Na+ current have been interpreted as a compensatory response that works to regulate action-potential firing in both Drosophila neurons (Baines et al. 2001) and embryonic Xenopus skeletal muscle (Lindsell and Moody 1994). Consistent with this, in intrinsic homeostasis, a decrease in the outward K+ current paired with an increase in the Na+ current has been interpreted as enhancing the ability of a neuron to fire action potentials (Desai et al. 1999b). In these cases, a decrease in K+ is associated with increased excitability and vice versa. Our data, however, suggest that the bigger the K+ current, the more action potentials the neuron can fire in response to a sustained current injection. So, at least the ability to fire repetitively appears to be enhanced in tectal neurons expressing relatively big K+ currents.
It has been well established that the way a neuron functions as part of a circuit is shaped by the complement of intrinsic currents it expresses (Bean 2007; Connors et al. 1982; Marder 2011; Sim et al. 2013; Turrigiano et al. 1996). In turn, alterations in the amount or pattern of circuit activity (spontaneous or sensory driven) received by an individual neuron can shape the expression of its intrinsic currents (Aizenman et al. 2003; Aizenman and Linden 2000; Baines et al. 2001; Cudmore et al. 2010; Desai et al. 1999b; Driscoll et al. 2013; Turrigiano et al. 1994). That neural circuit function shapes, and is shaped by, the compliment of intrinsic currents expressed by the neurons that comprise the circuit is an intriguing concept, because it poses one way in which sensory-driven synaptic input could custom tune neurons to be most responsive to that particular input. To explore how circuit activity, in general, may shape the developmental gradient of intrinsic currents across the tectum, we chronically reduced overall circuit activity by raising tadpoles in 0.5 μM TTX for 6–8 days. This manipulation led to what appears to be a compensatory scaling up of both peak Na+ and K+ currents in the neurons located in the caudal portion of the tectum. In contrast, no change in intrinsic currents of neurons located in the rostral portion was observed, even when all synaptic activity (action-potential dependent and independent) was blocked. What, at the molecular level, could account for this region-specific form of compensatory intrinsic plasticity? There are several possible candidate signaling systems: 1) the brain-derived neurotrophic factor is known to be expressed in the Xenopus tadpole optic tectum at this time in development (Cohen-Cory et al. 1996), and this molecule has been shown to play a role in intrinsic homeostatic plasticity in rat visual cortex (Desai et al. 1999a); 2) calcium/calmodulin-dependent kinase II, expressed in a low-caudal, high-rostral pattern across the RC axis, is associated with the maturation of synapses and stabilization of tectal dendrite outgrowth along the RC axis (Wu and Cline 1998); and 3) the EphA/ephrin-A signaling pathway, the enduring spatial gradient of ephrin-A expression across the tectum (high caudal, low rostral), is necessary for guiding retinal ganglion cell axons to their proper topographically correct targets [reviewed in McLaughlin et al. (2003)], and this gradient is reported to exist up through the late stages of Xenopus tadpole metamorphosis (Higenell et al. 2012), suggesting the possibility that this signaling system could serve other roles beyond axon guidance. Further supporting the possibility that EphA/ephrin-A signaling could underlie this region-specific, intrinsic plasticity is a study from the Mu-Ming Poo lab (Lim et al. 2010). This comprehensive study describes a region-specific plasticity of tectal neuron-receptive fields that is mediated by the opposing gradients of ephrin-B and Wnt, which exist along the dorsal-ventral axis of the tectum. Importantly, this study demonstrates the multifunctional capability of cell-signaling systems throughout development. Experiments in our lab are under way to test the roles of these molecular signaling systems in the regulation and plasticity of intrinsic currents and to define further what aspects of the gradient are developmental and what aspects are region specific.
In the tadpole tectum, the birth of new neurons and their incorporation into the functioning tectum are ongoing throughout all stages of Xenopus tadpole development (Sharma and Cline 2010; Straznicky and Gaze 1972). This means that there is a persistent flux of immature neurons into the tectum that we propose can be functionally tuned, or shaped, by visually driven activity. At the same time and in the same tadpole, there is a more mature pool of tectal neurons already tuned by visual experience, which no longer responds to extreme changes in activity levels. What may be a benefit for an optic tectum to be comprised of both a functionally stable (rostral) and more plastic (caudal) region? During development, a sensory circuit is required to process sensory input simultaneously while being shaped by the same input (Turrigiano and Nelson 2004). Perhaps during development, the combination of a stable and plastic region allows for the circuit to function effectively, as newer-born caudal neurons are shaped by their inputs.
GRANTS
Support for this work was funded by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (Grant P30-GM-32128).
DISCLOSURES
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
Author contributions: A.S.H. and K.G.P. conception and design of research; A.S.H. and K.G.P. performed experiments; A.S.H. and K.G.P. analyzed data; A.S.H. and K.G.P. interpreted results of experiments; A.S.H. and K.G.P. prepared figures; A.S.H. and K.G.P. drafted manuscript; A.S.H. and K.G.P. edited and revised manuscript; A.S.H. and K.G.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Diem Thu Pham for help with statistical analysis.
REFERENCES
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron 39: 831–842, 2003 [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci 3: 109–111, 2000 [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Muñoz-Elías G, Cline HT. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron 34: 623–634, 2002 [DOI] [PubMed] [Google Scholar]
- Baines RA, Bate M. Electrophysiological development of central neurons in the Drosophila embryo. J Neurosci 18: 4673–4683, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci 21: 1523–1531, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007 [DOI] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol 11: 118–126, 2001 [DOI] [PubMed] [Google Scholar]
- Cline HT, Wu GY, Malinow R. In vivo development of neuronal structure and function. Cold Spring Harb Symp Quant Biol 61: 95–104, 1997 [PubMed] [Google Scholar]
- Cohen-Cory S, Escandon E, Fraser SE. The cellular patterns of BDNF and trkB expression suggest multiple roles for BDNF during Xenopus visual system development. Dev Biol 179: 102–115, 1996 [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ, Prince DA. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol 48: 1302–1320, 1982 [DOI] [PubMed] [Google Scholar]
- Cudmore RH, Fronzaroli-Molinieres L, Giraud P, Debanne D. Spike-time precision and network synchrony are controlled by the homeostatic regulation of the D-type potassium current. J Neurosci 30: 12885–12895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg KE, Aizenman CD. Sensory modality-specific homeostatic plasticity in the developing optic tectum. Nat Neurosci 14: 548–550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. BDNF regulates the intrinsic excitability of cortical neurons. Learn Mem 6: 284–291, 1999a [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2: 515–520, 1999b [DOI] [PubMed] [Google Scholar]
- Desarmenien MG, Clendening B, Spitzer NC. In vivo development of voltage-dependent ionic currents in embryonic Xenopus spinal neurons. J Neurosci 13: 2575–2581, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwell KS, Holt CE, Harris WA. The multiple decisions made by growth cones of RGCs as they navigate from the retina to the tectum of Xenopus embryos. J Neurobiol 44: 246–259, 2000 [PubMed] [Google Scholar]
- Driscoll HE, Muraro NI, He M, Baines RA. Pumilio-2 regulates translation of Nav1.6 to mediate homeostasis of membrane excitability. J Neurosci 33: 9644–9654, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagelson GW, Harris WA. Mapping of the presumptive brain regions in the neural plate of Xenopus laevis. J Neurobiol 21: 427–440, 1990 [DOI] [PubMed] [Google Scholar]
- Gao H, Lu Y. Early development of intrinsic and synaptic properties of chicken nucleus laminaris neurons. Neuroscience 153: 131–143, 2008 [DOI] [PubMed] [Google Scholar]
- Goodman CS, Spitzer NC. The development of electrical properties of identified neurons in grasshopper embryos. J Physiol 313: 385–403, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higenell V, Han SM, Feldheim DA, Scalia F, Ruthazer ES. Expression patterns of Ephs and ephrins throughout retinotectal development in Xenopus laevis. Dev Neurobiol 72: 547–563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakhalin AS, Aizenman CD. GABAergic transmission and chloride equilibrium potential are not modulated by pyruvate in the developing optic tectum of Xenopus laevis tadpoles. PLoS One 7: e34446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorkova O, Golowasch J. Neuromodulators, not activity, control the coordinated expression of ionic currents. J Neurosci 27: 8709–8718, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar G. The development of the optic tectum in Xenopus laevis: a Golgi study. J Anat 116: 347–355, 1973 [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Cho SJ, Sumbre G, Poo MM. Region-specific contribution of ephrin-B and Wnt signaling to receptive field plasticity in developing optic tectum. Neuron 65: 899–911, 2010 [DOI] [PubMed] [Google Scholar]
- Lindsell P, Moody WJ. Na+ channel mis-expression accelerates K+ channel development in embryonic Xenopus laevis skeletal muscle. J Physiol 480: 405–410, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott AB, Westbrook GL. Early development of voltage-dependent sodium currents in cultured mouse spinal cord neurons. Dev Biol 113: 317–326, 1986 [DOI] [PubMed] [Google Scholar]
- Marder E. Variability, compensation, and modulation in neurons and circuits. Proc Natl Acad Sci USA 108: 15542–15548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, O'Leary DD. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol 13: 57–69, 2003 [DOI] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron 60: 477–482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin). New York: Garland, 1994 [Google Scholar]
- O'Dowd DK, Ribera AB, Spitzer NC. Development of voltage-dependent calcium, sodium, and potassium currents in Xenopus spinal neurons. J Neurosci 8: 792–805, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picken Bahery HL, Moody WJ. Early development of voltage-gated ion currents and firing properties in neurons of the mouse cerebral cortex. J Neurophysiol 89: 1761–1773, 2003 [DOI] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci 27: 8268–8277, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci 18: 7836–7846, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa AS, McCormick DA. Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. J Neurosci 14: 2089–2097, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera AB, Spitzer NC. A critical period of transcription required for differentiation of the action potential of spinal neurons. Neuron 2: 1055–1062, 1989 [DOI] [PubMed] [Google Scholar]
- Schulz DJ. Plasticity and stability in neuronal output via changes in intrinsic excitability: it's what's inside that counts. Proc Natl Acad Sci USA 104: 13187–13191, 2006 [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, Marder E. Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci 9: 356–362, 2006 [DOI] [PubMed] [Google Scholar]
- Sharma P, Cline HT. Visual activity regulates neural progenitor cells in developing xenopus CNS through musashi1. Neuron 68: 442–455, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S, Antolin S, Lin CW, Lin Y, Lois C. Increased cell-intrinsic excitability induces synaptic changes in new neurons in the adult dentate gyrus that require Npas4. J Neurosci 33: 7928–7940, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC, Lamborghini JE. The development of the action potential mechanism of amphibian neurons isolated in cell culture. Proc Natl Acad Sci USA 73: 1641–1645, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straznicky K, Gaze RM. The development of the tectum in Xenopus laevis: an autoradiographic study. J Embryol Exp Morphol 28: 87–115, 1972 [PubMed] [Google Scholar]
- Sun QQ, Dale N. Developmental changes in expression of ion currents accompany maturation of locomotor pattern in frog tadpoles. J Physiol 15: 257–264, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science 264: 974–977, 1994 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Marder E, Abbott LF. Cellular short-term memory from a slow potassium conductance. J Neurophysiol 75: 963–966, 1996 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107, 2004 [DOI] [PubMed] [Google Scholar]
- Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science 279: 222–226, 1998 [DOI] [PubMed] [Google Scholar]
- Wu GY, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science 274: 972–976, 1996 [DOI] [PubMed] [Google Scholar]
- Wu GY, Zou DJ, Rajan I, Cline H. Dendritic dynamics in vivo change during neuronal maturation. J Neurosci 19: 4472–4483, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Poo M. Visual input induces LTP of developing retinotectal synapses. Nat Neurosci 3: 708–715, 2000 [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci 4: 885–900, 2003 [DOI] [PubMed] [Google Scholar]