Abstract
MAEBL is a type 1 membrane protein that is implicated in the merozoite invasion of erythrocytes and sporozoite invasion of mosquito salivary glands. This apical organelle protein is structurally similar to the ebl erythrocyte binding proteins, such as EBA-175, except that the tandem ligand domains of MAEBL are similar to part of the extracellular domain of apical membrane antigen 1 and not the Duffy binding-like domain. Although midgut and salivary gland sporozoites are morphologically similar, salivary gland sporozoites undergo a period of new gene expression after infecting the salivary glands, display distinct phenotypic differences, and are more infectious for the mammalian host. The objectives of this project were to determine the molecular form of MAEBL in the infectious salivary gland sporozoites and whether the ligand has a role in the sporozoite development to exoerythrocytic stages in hepatocytes. We determined that MAEBL is newly expressed in salivary gland sporozoites and in a form distinct from what is present in the midgut sporozoites or present in erythrocytic stages. Both ligand domains (M1 and M2) were expressed as part of a full-length membrane form of MAEBL in the salivary gland sporozoites in contrast to the other stages that retain only the M2 ligand domain as part of the membrane form of the protein. Antisera developed against the cysteine-rich regions of the extracellular portion of MAEBL inhibited sporozoite development to exoerythrocytic forms in vitro. Together these data indicate that MAEBL has a role in this third developmental stage in the life cycle of the malaria parasite. Thus, MAEBL is another target for pre-erythrocytic-stage vaccine development against malaria parasites.
Malaria is one of the most serious human diseases, causing several million deaths and clinical illness in hundreds of millions of people every year. Infection is spread from person to person by the bite of a Plasmodium-infected anopheline mosquito. Sporozoites injected by the mosquito must infect and develop in a hepatocyte, which can then initiate the blood-stage infection that causes the severe pathogenesis of malaria. Molecules in the apical organelles and on the surface of malaria sporozoites play a role in sporozoite motility and target this infective stage to the liver. The completion of the Plasmodium falciparum genome has provided the basis for the identification of many proteins in sporozoites (5, 12), but very few of these proteins are characterized for their function in sporozoite infectivity and development in the liver. Proteomic and transcript analyses have identified a number of apical organelle and membrane-associated proteins expressed both in sporozoites and merozoites, with many belonging to molecular families conserved among the diverse species of Plasmodium (3, 5, 7, 19, 22-24).
The circumsporozoite protein (CSP) and to a lesser extent the thrombospondin-related anonymous protein (TRAP, or sporozoite surface protein 2 [SSP2]) have been the focus of most research on the sporozoite stages. CSP and TRAP are major sporozoite proteins that are functionally important for sporozoite development in the mosquito stage and are widely considered as important targets for vaccine development. However, both have obstacles for development as vaccines against the pre-erythrocytic stages of development. Polymorphism in the critical T-cell epitopes recognized by helper T cells and cytotoxic lymphocytes of CSP compromise its potential efficacy as a vaccine. While even though TRAP is essential for invasion of hepatocytes, antibodies against this transmembrane protein were shown not to inhibit sporozoite development into the exoerythrocytic stages. Therefore, it is important to identify additional sporozoite antigens that are potential targets for development as part of a multivalent pre-erythrocytic vaccine.
MAEBL was identified in Plasmodium yoelii and P. falciparum blood-stage parasites as a minor type 1 membrane protein with erythrocyte binding activity expressed in the apical organelles and on the surface of invasive merozoites, but it was later identified as an abundant protein expressed in sporozoites (4, 6, 8-11, 17). MAEBL is a paralogue of the products from the ebl family, similar except that its two extracellular ligand domains have identity to apical membrane antigen 1 and not the consensus Duffy binding-like ligand domains of other ebl products (10). The ebl family of erythrocyte binding proteins includes some of the best-characterized malarial ligands, such as the Plasmodium vivax Duffy binding protein and the P. falciparum erythrocyte binding protein EBA-175 (1). Exon structure, including the conserved position of splicing junctions within codons at the exon boundaries, is an important characteristic of ebl genes, and it is conserved with maebl (2). However, maebl evolved independently of ebl and ama1 in the ancestral Plasmodium genome prior to speciation (15). Interestingly, it appears that MAEBL may have an essential function in the midgut sporozoite invasion of the salivary glands but not in the merozoite invasion of erythrocytes (11 and unpublished data). Although morphologically similar to midgut sporozoites, salivary gland sporozoites are much more infectious for the mammalian host, have a unique gliding motility on a solid substrate, and can induce a strong protective immunity. These phenotypic differences appear to correspond with the expression of new proteins once sporozoites invade the salivary glands (14). Since MAEBL is differentially expressed in midgut and salivary gland sporozoites, we were interested to determine what form of MAEBL is expressed in infectious salivary gland sporozoites and whether MAEBL has an important role in sporozoite development in hepatocytes. We find that MAEBL is expressed in a different molecular form in salivary gland sporozoites compared to midgut sporozoites. Furthermore, we find that antibodies against MAEBL inhibit sporozoite development in hepatocytes, suggesting that MAEBL has an important role in the sporozoite infection of hepatocytes.
MATERIALS AND METHODS
Parasites and DNA material.
The salivary glands and midgut were dissected from infected Anopheles stephensi and gently ground in phosphate-buffered saline to release P. yoelii sporozoites. After the removal of tissue fragments by centrifugation at 18 × g for 3 min, sporozoites were collected from the supernatant by centrifugation at 5,000 × g for 3 min.
Anti-MAEBL sera.
Antiserum against the P. falciparum MAEBL CT1 was prepared by using a linear keyhole lympet hemocyanin-conjugated peptide (CHWFKNDHRKVV) injected in rabbits with complete Freund's adjuvant by intraperitoneal injection, followed by incomplete Freund's adjuvant at 2, 4, and 10 weeks. Rabbit antisera were produced against the deduced C terminus of P. yoelii yoelii MAEBL as previously described (18). Polyclonal antisera to the cysteine-rich regions of P. yoelii yoelii and P. falciparum MAEBL were prepared prior to the glutathione transferase fusion proteins of each domain (4, 18).
Western immunoblotting and indirect immunofluorescence assay.
Approximately 105 sporozoites per lane were resolved by electrophoresis on 4 to 12% Bis-Tris NuPAGE Gels (Invitrogen) in 1× morpholineethanesulfonic acid sample buffer (Invitrogen) under reducing conditions. Specific proteins transferred onto Nitrocellulose Extra blotting membrane (Sartorius AG) were detected by polyclonal sera raised against the different regions of MAEBL and horseradish peroxidase-linked secondary antibodies (Bio-Rad) and by enhanced chemiluminescence (Pierce). The dilutions of the primary sera were 1:500 to 1:800. Serum reactivity was tested by an immunofluorescence assay using methanol-fixed midgut or salivary gland sporozoites, previously air dried on microscope slides, or malaria liver-stage parasites as previously described (20).
Inhibition of sporozoite development.
Rabbit antisera (at a 1:100 dilution) were tested in triplicate for inhibition of sporozoite penetration and development in primary hepatocyte cultures as previously described (13). Sporozoites were added to cultures with immune serum (prepared against M1, M2, and C-Cys) or with preimmune serum as a negative control at a concentration in serum of 1:100. The cultures were incubated for 3 h and washed, and fresh medium was added. After 24 h the medium was changed once and incubated another 24 h. The schizonts in each culture well were stained with anti-PfHSP70 antibodies to count hepatic schizonts (20). Inhibition was determined by counting the number of hepatic schizonts in the test cultures relative the number in cultures with preimmune serum.
RESULTS AND DISCUSSION
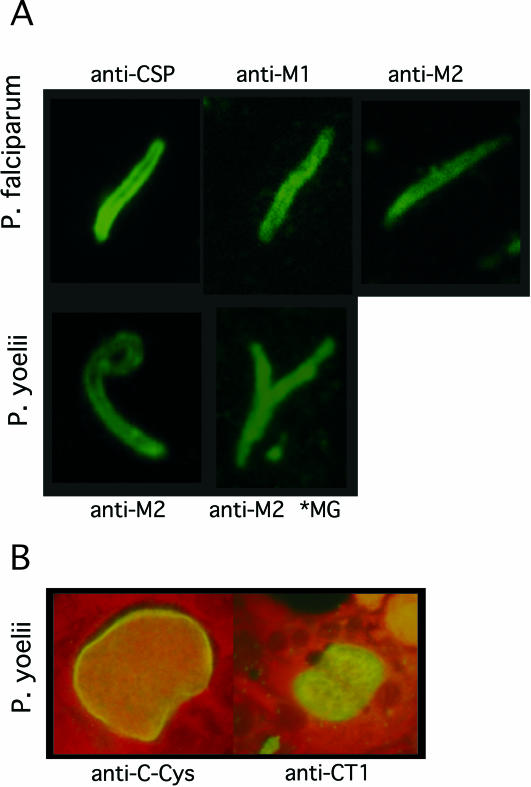
We used a panel of anti-MAEBL sera, prepared against the three extracellular cysteine-rich domains (M1, M2, and C-CYS) and the C terminus of the cytoplasmic domain (CT1) (Fig. 1) to determine the MAEBL expression patterns in sporozoites. Serum reactivity was tested by immunofluorescence on air-dried and methanol-fixed midgut or salivary gland sporozoites, dissected from the salivary glands and midgut of infected A. stephensi mosquitoes. MAEBL was shown to localize to internal and surface structures of P. falciparum and P. yoelii salivary gland sporozoites, confirming the expression of MAEBL products in sporozoites (Fig. 2A). Importantly, antisera to the M1 ligand domain and the cytoplasmic terminus CT1 were reactive to salivary gland sporozoites (Fig. 2A and data not shown), indicating that expression of the full-length transmembrane form of MAEBL occurred in the infectious sporozoites. Immunolocalization for most of the anti-MAEBL sera showed a pattern at the apical end and on the surface of sporozoites similar to that observed for CSP. This pattern for MAEBL is also similar to that of TRAP, which only completely redistributes to the sporozoite apical end after injection by the mosquito (16). The MAEBL immunofluorescence patterns appeared to be significantly more intense in midgut sporozoites than in salivary gland sporozoites (Fig. 2A). The anti-MAEBL sera were also tested on methanol-fixed malaria liver-stage parasites as previously described (20). All anti-MAEBL but no control sera were reactive with P. yoelii liver-stage parasites (Fig. 2B and data not shown).
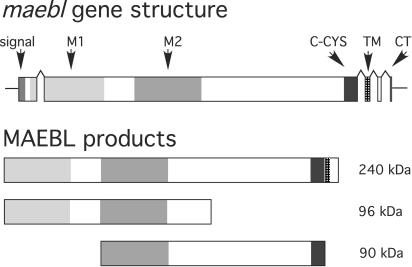
FIG. 1.
Diagram of the maebl gene structure and predicted protein products expressed in sporozoites. The upper figure depicts the coding sequence in five exons (shaded boxes), which are conserved among all maebl genes characterized. The open reading frame in exon 1 begins with a signal peptide (signal) targeting the MAEBL product to the secretory pathways and is immediately followed by two cysteine-rich ligand domains (M1 and M2). These tandem domains both have similarity to domains 1 and 2 of apical membrane antigen 1 and are highly conserved among Plasmodium species. The M1 domain is present at the N terminus of the full-length MAEBL protein identified as expressed in salivary gland sporozoites; but in erythrocyte-stage development, this domain was determined to be lost, and the mature MAEBL protein in merozoites contained only the M2 domain. A third cysteine-rich domain, C-CYS, which is homologous to the C-CYS domain of ebl products, immediately precedes the transmembrane domain (TM) and a short cytoplasmic (CT) domain at the carboxyl terminus.
FIG. 2.
(A) Immunofluorescence localization of MAEBL in sporozoites. Immunolocalization of MAEBL in salivary gland sporozoites and midgut sporozoites (*MG) by using antisera to specific regions of MAEBL cysteine-rich ligand domains M1 and M2. The anti-CSP localization pattern for P. falciparum sporozoites was used as a control and reference. Control antisera were not reactive by immunofluorescence assay to sporozoites (data not shown). (B) Immunofluorescence localization of MAEBL in P. yoelii exoerythrocytic forms with anti-C-CYS (left) and anti-CT1 (right) sera, with Evans blue used as a counterstain in both images.
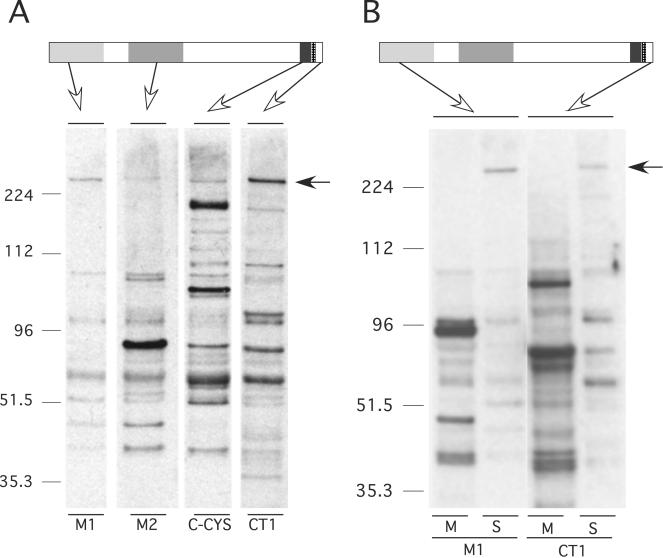
We were particularly interested to determine the molecular form of MAEBL present in the salivary gland sporozoites, since this is the infective stage injected into the blood of a new host and a possible target for immune attack. Unexpectedly, we detected the full-length membrane form of MAEBL in the P. yoelii salivary gland sporozoite as a 240-kDa protein reactive with all anti-MAEBL sera (anti-M1, anti-M2, anti-C-CYS, and anti-CT1) (Fig. 3A). Further analysis of P. yoelii compared MAEBL expression in midgut sporozoites to its expression in salivary gland sporozoites (Fig. 3B). There appeared to be significantly more protein product reactive with the anti-MAEBL sera in the midgut sporozoites than in the equivalent number of salivary gland sporozoites. However, the intact 240-kDa full-length MAEBL product was not detected in these midgut sporozoite preparations. Instead, two major products were detected in salivary gland and midgut sporozoites at 96 and 90 kDa, which appeared to represent amino and carboxyl fragments of posttranslationally processed MAEBL (Fig. 1 and 3B). These data clearly show that the full-length membrane form of MAEBL is newly expressed in sporozoites after they invade the salivary glands, demonstrating that the salivary gland sporozoites are actively synthesizing molecules for the next stage of invasion. In addition, most of the processed forms of MAEBL in midgut sporozoites were lost after invading the salivary glands, which is consistent with its predicted important role in the invasion of the salivary glands (11).
FIG. 3.
(A) Western blot analysis of MAEBL expression in sporozoites. A putative full-length MAEBL product of 240 kDa was detected in salivary gland sporozoites by using antibodies against the extracellular M1, M2, and C-CYS regions and the cytoplasmic CT1. A schematic diagram of the MAEBL protein structure identifies the reactivity for each antiserum. (B) Western blot analysis of MAEBL expression in midgut (M) and salivary gland (S) sporozoites. Equal amounts of salivary gland and midgut sporozoites were probed with the same antibodies to M1 and CT1 as described in the legend of Fig. 2. The antisera to M2 and the C-CYS domains also reacted to this full-length 240-kDa MAEBL product (data not shown).
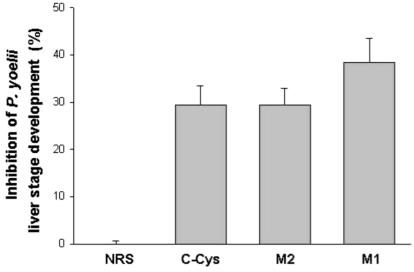
The active expression of MAEBL in salivary gland sporozoites and its presence on the surface of the sporozoite stages that are invasive for the vertebrate host suggested that MAEBL has a role in the invasion of hepatocytes. Therefore, rabbit antisera (1:100 dilution) were tested for the inhibition of sporozoite penetration and development in primary hepatocyte cultures as previously described (13). Antiserum to the M1 domain had the highest level of inhibition, reducing the number of developing liver-stage parasites by greater than 42% (Fig. 4). Inhibition with antisera to both M1 and M2 domains suggests that both ligand domains are functionally important for the sporozoite invasion of hepatocytes.
FIG. 4.
Inhibition of sporozoite invasion and development in hepatocyte cultures. Antibodies to the C-CYS, M2, and M1 regions of MAEBL inhibited P. yoelii sporozoite invasion and development in hepatocyte cultures compared to development in nonimmune rabbit serum (NRS). Inhibition is indicated as a percentage compared to the control experiment. Results are expressed as means ± standard deviation. There were 105 ± 9.5 schizonts in the control well without antibodies and 112 ± 1 in the well containing the normal rabbit serum.
Previous studies have indicated an essential role for MAEBL for the sporozoite invasion of salivary glands (11, 21). Consistent with this role during the invasion of salivary glands, we demonstrate that several MAEBL products were very abundant in midgut sporozoites and then disappeared from sporozoites after infection of the salivary glands. Once inside the salivary glands, P. yoelii sporozoites expressed the full-length MAEBL, suggesting that this form of MAEBL is important for the sporozoite invasion of hepatocytes. The inhibition of P. yoelii developing liver-stage parasites by both anit-M1 and anti-M2 sera supports this conclusion. It is interesting that infective salivary gland sporozoites have both M1 and M2 ligand domains retained in a full-length type 1 membrane form of MAEBL. This is a major functional difference compared to erythrocytic stages, in which the M2 domain appears to be the principal ligand and M1 is posttranslationally deleted (6, 18).
The creation of a different combination of binding domains due to posttranslational processing represents a possible mechanism by which a malaria parasite is able to use a single gene locus to create multiple ligands capable of recognizing different receptors. The expression of distinct molecular forms of MAEBL in different stages of parasite development is analogous to the tissue-specific expression of proteins in more complex organisms. Little is known about the posttranslational processing of malaria proteins in Plasmodium species, although many proteins are known to undergo extensive, functionally important processing. The expression of the full-length membrane form of MAEBL is consistent with other data that sporozoites undergo a transformation after invading the salivary gland as preparation to infect the vertebrate host. The inhibition of sporozoite development into the exoerythrocytic form identifies MAEBL as another target in a multivalent pre-erythrocytic-stage vaccine.
Acknowledgments
This work was supported by the National Institutes of Health (grant R01 AI33656) and a Burroughs Wellcome Fund Travel Grant (J.H.A.). Travel (P.R.P. and L.R.) was funded by The Royal Society's Joint Project under European Science Exchange Programme and by The British Council and French Ministry of Foreign Affairs Alliance: Franco-British Partnership Programme. T.V. held a fellowship from MRT. This work was supported in part by Grants-in-Aid for Scientific Research 13576007 (M.T.) from the Ministry of Education, Science, Sports and Culture, Japan.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Adams, J. H., O. Kaneko, P. L. Blair, and D. S. Peterson. 2001. An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 17:297-299. [DOI] [PubMed] [Google Scholar]
- 2.Adams, J. H., B. K. L. Sim, S. A. Dolan, X. Fang, D. C. Kaslow, and L. H. Miller. 1992. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. USA 89:7085-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnwell, J. W., M. R. Galinski, S. G. DeSimone, F. Perler, and P. Ingravallo. 1999. Plasmodium vivax, P. cynomolgi, and P. knowlesi: identification of homologue proteins associated with the surface of merozoites. Exp. Parasitol. 91:238-249. [DOI] [PubMed] [Google Scholar]
- 4.Blair, P. L., S. H. Kappe, J. E. Maciel, B. Balu, and J. H. Adams. 2002. Plasmodium falciparum MAEBL is a unique member of the ebl family. Mol. Biochem. Parasitol. 122:35-44. [DOI] [PubMed] [Google Scholar]
- 5.Florens, L., M. P. Washburn, J. D. Raine, R. M. Anthony, M. Grainger, J. D. Haynes, J. K. Moch, N. Muster, J. B. Sacci, D. L. Tabb, A. A. Witney, D. Wolters, Y. Wu, M. J. Gardner, A. A. Holder, R. E. Sinden, J. R. Yates, and D. J. Carucci. 2002. A proteomic view of the Plasmodium falciparum life cycle. Nature 419:520-526. [DOI] [PubMed] [Google Scholar]
- 6.Ghai, M., S. Dutta, T. Hall, D. Freilich, and C. F. Ockenhouse. 2002. Identification, expression, and functional characterization of MAEBL, a sporozoite and asexual blood stage chimeric erythrocyte-binding protein of Plasmodium falciparum. Mol. Biochem. Parasitol. 123:35-45. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko, O., T. Tsuboi, I. T. Ling, S. Howell, M. Shirano, M. Tachibana, Y. M. Cao, A. A. Holder, and M. Torii. 2001. The high molecular mass rhoptry protein, RhopH1, is encoded by members of the clag multigene family in Plasmodium falciparum and Plasmodium yoelii. Mol. Biochem. Parasitol. 118:223-231. [DOI] [PubMed] [Google Scholar]
- 8.Kappe, S. H., M. J. Gardner, S. M. Brown, J. Ross, K. Matuschewski, J. M. Ribeiro, J. H. Adams, J. Quackenbush, J. Cho, D. J. Carucci, S. L. Hoffman, and V. Nussenzweig. 2001. Exploring the transcriptome of the malaria sporozoite stage. Proc. Natl. Acad. Sci. USA 98:9895-9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kappe, S. H. I., G. P. Curley, A. R. Noe, J. P. Dalton, and J. H. Adams. 1997. Erythrocyte binding protein homologues of rodent malaria parasites. Mol. Biochem. Parasitol. 89:137-148. [DOI] [PubMed] [Google Scholar]
- 10.Kappe, S. H. I., A. R. Noe, T. S. Fraser, P. L. Blair, and J. H. Adams. 1998. A family of chimeric erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. USA 95:1230-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kariu, T., M. Yuda, K. Yano, and Y. Chinzei. 2002. MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J. Exp. Med. 195:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Roch, K. G., Y. Zhou, P. L. Blair, M. Grainger, J. K. Moch, J. D. Haynes, P. De La Vega, A. A. Holder, S. Batalov, D. J. Carucci, and E. A. Winzeler. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301:1503-1508. [DOI] [PubMed] [Google Scholar]
- 13.Marussig, M., L. Renia, A. Motard, F. Miltgen, P. Petour, V. Chauhan, G. Corradin, and D. Mazier. 1997. Linear and multiple antigen peptides containing defined T and B epitopes of the Plasmodium yoelii circumsporozoite protein: antibody-mediated protection and boosting by sporozoite infection. Int. Immunol. 9:1817-1824. [DOI] [PubMed] [Google Scholar]
- 14.Matuschewski, K., J. Ross, S. M. Brown, K. Kaiser, V. Nussenzweig, and S. H. Kappe. 2002. Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J. Biol. Chem. [DOI] [PubMed]
- 15.Michon, P., J. R. Stevens, O. Kaneko, and J. H. Adams. 2002. Evolutionary relationships of conserved cysteine-rich motifs in adhesive molecules of malaria parasites. Mol. Biol. Evol. 19:1128-1142. [DOI] [PubMed] [Google Scholar]
- 16.Mota, M. M., J. C. Hafalla, and A. Rodriguez. 2002. Migration through host cells activates Plasmodium sporozoites for infection. Nat. Med. 8:1318-1322. [DOI] [PubMed] [Google Scholar]
- 17.Noe, A. R., and J. H. Adams. 1998. Plasmodium yoelii YM MAEBL protein is coexpressed and colocalizes with rhoptry proteins. Mol. Biochem. Parasitol. 96:27-35. [DOI] [PubMed] [Google Scholar]
- 18.Noe, A. R., D. J. Fishkind, and J. H. Adams. 2000. Spatial and temporal dynamics of the secretory pathway during differentiation of the Plasmodium yoelii schizont. Mol. Biochem. Parasitol. 108:169-185. [DOI] [PubMed] [Google Scholar]
- 19.Rayner, J. C., M. R. Galinski, P. Ingravallo, and J. W. Barnwell. 2000. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc. Natl. Acad. Sci. USA 97:9648-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renia, L., D. Mattei, J. Goma, S. Pied, P. Dubois, F. Miltgen, A. Nussler, H. Matile, F. Menegaux, M. Gentilini, et al. 1990. A malaria heat-shock-like determinant expressed on the infected hepatocyte surface is the target of antibody-dependent cell-mediated cytotoxic mechanisms by nonparenchymal liver cells. Eur. J. Immunol 20:1445-1449. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan, P., E. G. Abraham, A. K. Ghosh, J. G. Valenzuela, J. M. C. Ribeiro, G. Dimopoulos, F. C. Kafatos, J. H. Adams, H. Fujioka, and M. Jacobs-Lorena. Analysis of the Plasmodium and Anopheles transcriptomes during oocyst differentiation. J. Biol. Chem. 279:5581-5587. [DOI] [PMC free article] [PubMed]
- 22.Tchavtchitch, M., K. Fischer, R. Huestis, and A. Saul. 2001. The sequence of a 200-kb portion of a Plasmodium vivax chromosome reveals a high degree of conservation with Plasmodium falciparum chromosome 3. Mol. Biochem. Parasitol. 118:211-222. [DOI] [PubMed] [Google Scholar]
- 23.Thompson, J. K., T. Triglia, M. B. Reed, and A. F. Cowman. 2001. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol. Microbiol. 41:47-58. [DOI] [PubMed] [Google Scholar]
- 24.Vargas-Serrato, E., J. W. Barnwell, P. Ingravallo, F. B. Perler, and M. R. Galinski. 2002. Merozoite surface protein-9 of Plasmodium vivax and related simian malaria parasites is orthologous to p101/ABRA of P. falciparum. Mol. Biochem. Parasitol. 120:41-52. [DOI] [PubMed] [Google Scholar]