Abstract
Context:
Patterns of myocardial fluoro-2-deoxyglucose (FDG) uptake with respect to duration of fasting and dietary modifications.
Aim:
We observed the effect of duration of fasting and diet on the myocardial uptake pattern of F-18 FDG in patients routinely referred for oncological evaluation and no previous history of Coronary Artery Disease (CAD).
Settings and Design:
Prospective study.
Subjects and Methods:
A total of 153 patients (M: 81, F: 72; mean age: 47 ± 15 years; mean blood glucose level (mBG) 105 ± 23 mg/dl) were randomly divided in three groups. Group A: 4-6 h fasting; Group B: Overnight fasting (12–14 h); Group C: Low carbohydrate and fat rich diet for 2 days coupled with overnight fasting prior to the positron emission tomography (PET) scan. FDG uptake was classified as following: 1) homogeneous uptake, 2) heterogeneous uptake, and 3) ‘no uptake’ in the left ventricular (LV) myocardium. FDG PET study was performed as standard protocol for oncological conditions.
Statistical Analysis Used:
Descriptive statistics, Chi-square test or Fisher's exact test, and Spearman's rank correlation tests were applied.
Results:
We observed the ‘no uptake’ pattern in five (10%), 28 (55%), and 39 (77%), ‘heterogeneous’ pattern in 20 (39%), 14 (28%), and seven (14%), and ‘homogeneous’ pattern in 26 (51%), nine (18%), and five (10%) patients in Group A, B, and C, respectively. There was statistically significant difference of myocardial uptake pattern between group A and B (P < 0.0001), between group A and C (P < 0.0001), and between Group B and C (P = 0.023). The mBG was 102, 105, and 111 mg/dl in ‘no uptake’, heterogeneous, and homogeneous uptake pattern, respectively, (P = 0.103). Also, within each group the mBG was not related to the uptake pattern.
Conclusion:
Both restricted diet and duration of fasting play an important role in determining the pattern and suppression of myocardial F-18 FDG uptake. Overnight fasting and restricted diet together suppress myocardial FDG uptake more than overnight fasting alone, which suppresses uptake more than 4-h fasting.
Keywords: Fasting, fat-rich diet, fluoro-2-deoxyglucose, low carbohydrate, myocardial uptake, no uptake
INTRODUCTION
F-18-2-fluoro-2-deoxyglucose (F-18 FDG) is a glucose analog positron emission tomography (PET) radiotracer that is routinely used in cardiology for the assessment of myocardial viability in conjunction with perfusion tracer. F-18 FDG is transported within the myocytes and undergoes phosphorylation to FDG-6-phosphate where no further metabolism occurs.
In normal myocardium, oxidative metabolism is primary source of energy and utilizes free fatty acids, glucose, and lactate as substrates. In fasting state, free fatty acid becomes substrate of choice as the plasma insulin levels fall that results in lesser transport of glucose into the myocytes and an increase in the availability of free fatty acids secondary to increased lipolysis in peripheral adipose tissue. After feeding, plasma insulin levels rise, glucose becomes the primary substrate for oxidative metabolism as lipolysis within adipose tissue is inhibited, and arterial fatty acid content decreases.[1,2] Because FDG uptake reflects the glucose metabolism, then in fasting state there should be negligible FDG uptake in the myocardium. However, variable uptake of FDG is noted in the myocardium in fasting state.[3] Even in the post-glucose load protocol, if patient preparation is not adequate, a heterogeneous F-18 FDG uptake is noted in the myocardium making the interpretation of the scan difficult.
Therefore, we have designed this study to observe the variable patterns of myocardial FDG uptake in fasting state for a short interval of 4 h, longer duration of overnight fasting (>12 h), and overnight fasting with a low carbohydrate and fat-rich diet for 2 days prior to the study.
SUBJECTS AND METHODS
Study population
A total of 153 patients referred for FDG PET scan for routine oncological evaluation to our department were enrolled in the study. The patients were recruited after obtaining ethical clearance from ethics committee of the institute. Patients with any previous investigation suggestive of coronary artery disease, with past history of myocardial infarction, past history of revascularization, and uncontrolled diabetes were excluded from the study. Also patients with history of chemotherapy or radiotherapy within 8 weeks, age below 12 years, and mediastinal radiation were excluded. The recruited patients were randomized in blocks of three subjects in three groups.
Patients in each group were advised to follow instructions as part of preparation prior to PET scan. Group A patients were advised to fast for at least 4 h, and Group B patients were advised to fast overnight so as to have fasting period of at least 12 h (range 12-14 h). Group C patients in addition to fasting overnight were requested to follow low carbohydrate and fat-rich diet for period of 2 days before the scan. Group C patients were explained and a dietary chart of permitted and non-permitted foods was given based on dietary habits, and all the patients who complied and followed the diet chart were considered. We did not follow a fixed standard diet so as to have a better patient compliance. Also, it was not logistically possible to provide a special diet to each patient in the department before the FDG injection. These patients were requested to avoid carbohydrate-rich diet like rice, potato, and sweet potatoes and fruits like grape, banana, and litchi and sweets like chocolate, honey, and jam. Depending upon dietary habits, they were asked to consume protein-rich diet like pulses, cottage cheese, green vegetables, and egg and high fat-rich diet like butter, cheese, chicken, meat, and fried food.
Before the FDG study, we confirmed compliance of each patient for the instructions given. Blood glucose level was checked for each patient using glucometer. Ten-15 mCi (370-555 MBq) of F-18 FDG was injected into the patient through an intravenous catheter. A whole body PET scan was acquired 40-60 min after injection on dedicated PET-computed tomography (CT) scanner (Biograph 2, Siemens, Erlangen, Germany). In the PET-CT system, CT acquisition was performed on spiral dual slice CT with a Kv of 130, mAs of 60, slice thickness of 4 mm, and a pitch of 1. Image was acquired using a matrix of 512 × 512 pixels and pixel size of 1 mm. After CT, three-dimensional (3D) PET acquisition was done for 2-3 min per bed position. PET data were acquired using matrix of 128 × 128 pixels with a slice thickness of 1.5 mm. CT-based attenuation correction of the emission images was used. PET images were reconstructed by iterative method ordered subset expectation maximization (OSEM; two iterations and eight subsets). After CT acquisition, PET acquisition of the same axial range was done with the patient in the same position. After completion of PET acquisition, the reconstructed attenuation-corrected PET images, CT images, and fused images of matching pairs of PET and CT images were available for review in axial, coronal, and sagittal planes, as well as in maximum intensity projections (MIP), and 3D cine mode. Attenuation-corrected PET images focussing the heart were reoriented from the transaxial slices to generate the conventional cardiac short axis and vertical and horizontal long axis. Two nuclear medicine physicians reported the PET studies that were blinded to patient group.
Image interpretation
All the studies were viewed in MIP and in 3D cine mode for initial assessment of cardiac and extracardiac FDG uptake. Patients with abnormal FDG uptake in mediastinum were excluded from further analysis. F-18 FDG PET cardiac images were analyzed for myocardial uptake in conventional cardiac format as short axis vertical and horizontal long axis. To observe the variable pattern of FDG uptake in the left ventricular (LV) myocardium, we classified the uptake as follows: 1) homogeneous uptake in the entire LV myocardium, 2) heterogeneous uptake in the LV myocardium. This regional uptake was assigned to conventional anatomical walls of the LV myocardium based on 20 segment model using Emory Cardiac Toolbox (ECTbox; Emory University, Atlanta, Georgia (GA), United States of America (USA)). The regions were identified irrespective of the intensity of the uptake, provided they could be delineated separately from the blood pool activity in the LV cavity and the surrounding background. 3) No uptake or negligible uptake in the LV myocardium.
Statistical analysis
Descriptive statistics was used for reporting the demographics of patient data. Findings of the three groups of uptake patterns were compared with Spearman's rank correlation test. Chi-square test or Fisher's exact test was applied to assess relationship between categorical variables within group. P < 0.05 was taken as significant. The statistical package used in the study was International Business Machines Corporation (IBM) Statistical Package for the Social Sciences (SPSS) Statistics 19.0.0.
RESULTS
Demographic profile
Among 153 patients in our study, 81 (53%) were male, whereas remaining 72 (47%) were female. Mean age of the patients included in the study was 47 ± 15 years (median age: 48 years, range: 16-84 years). Mean blood glucose level (mBG) was 105 ± 23 mg/dl (median: 102 mg/dl, range: 62-200 mg/dl). Among 153 patients, 30 (20%) were smokers, 26 (17%) had diabetes well controlled by medication, 20 (13%) had controlled hypertension, and 62 (41%) patients had history of chemotherapy. All the three groups were matching for confounding factors like sex, age, diabetes, smoking, hypertension, and chemotherapy [Table 1].
Table 1.
Matching of confounding variables in three groups

There was statistically significant difference in the mBG between group A and B (P = 0.0001) and between group A and C (P = 0.0001), but the difference in mBG between group B and C was statistically insignificant (P = 0.425). Groupwise mBG distribution is depicted in Figure 1. The mBG of patients in Group A was 117 ± 21, Group B was 101 ± 17, and Group C was 98 ± 26 mg/dl.
Figure 1.
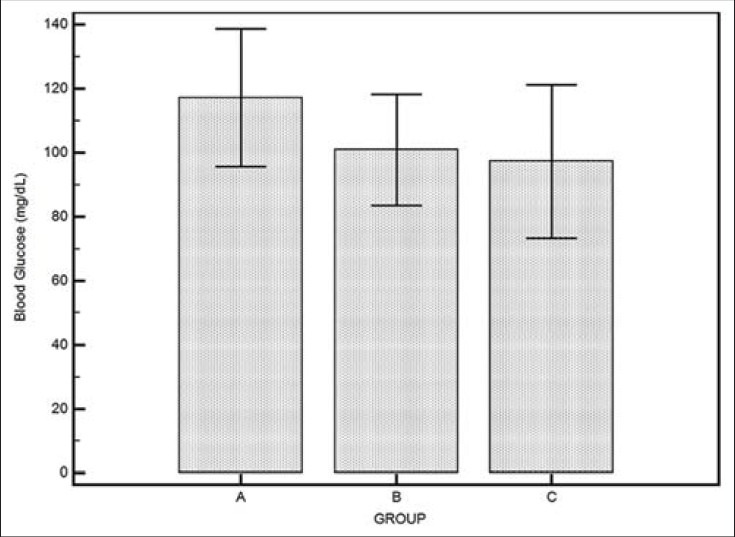
Bar plot with error bars showing mean ± SD of blood glucose level in three groups. SD = Standard deviation
Distribution of myocardial FDG uptake pattern
We analyzed all the 153 studies to observe the pattern of FDG uptake in the myocardium classified as homogenous, heterogeneous, and ‘no uptake’ pattern.
We observed the ‘no uptake’ pattern in five (10%), 28 (55%), and 39 (77%), ‘heterogeneous’ pattern of FDG uptake in 20 (39%), 14 (28%), and seven (14%), and ‘homogeneous’ pattern in 26 (51%), nine (18%), and five (10%) patients in Group A, B, and C, respectively [Figure 2].
Figure 2.
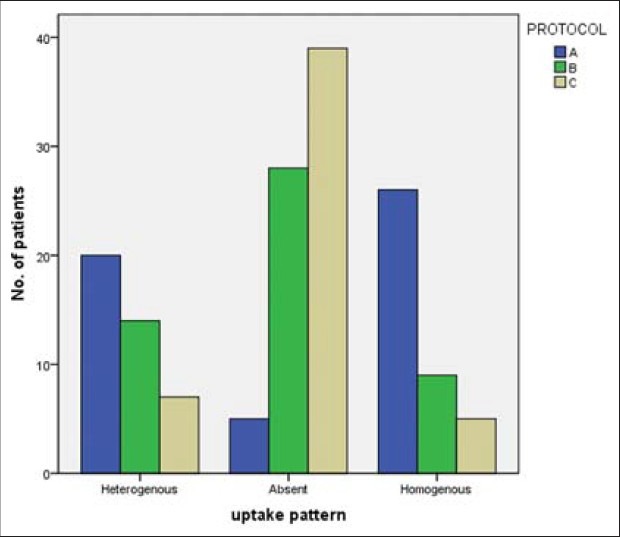
Myocardial uptake pattern distribution between the three groups
On overall groupwise comparison between Group A, Group B, and Group C, there was statistically significant difference of myocardial uptake pattern between the three groups (P < 0.0001). There was also significant difference of myocardial uptake pattern between group A and B (P < 0.0001), between group A and C (P < 0.0001), and between Group B and C (P = 0.023).
A total of 41 patients showed heterogeneous pattern, of which 20 (39%) were noted in Group A, 14 (28%) in Group B, and seven (14%) in Group C. We could identify seven different patterns involving the ventricular walls [Table 2]. The most common regions that showed myocardial FDG uptake were lateral wall (90%), followed by base of the heart (88%), inferior wall (56%), and distal anteroapical region (46%).
Table 2.
Regional distribution of heterogeneous uptake pattern

The mBGs were calculated and analyzed for effect of blood glucose on myocardial uptake pattern. We observed that in ‘no uptake’ pattern the mBG was lowest 102 ± 25 mg/dl and it was highest in ‘homogeneous’ uptake pattern 111 ± 24 mg/dl and 105 ± 17 mg/dl in heterogenous pattern. However, there was no statistically significant difference in the mBG in between the three patterns (P = 0.103) [Figure 3].
Figure 3.

Bar plot with error bars showing mean ± SD of blood glucose level with respect to uptake pattern. SD = standard deviation
We observed the effect of mBG on myocardial uptake pattern in each group. Within each group the mBG was not related to the uptake pattern. Groupwise mBG and myocardial FDG uptake pattern distribution results are shown below Table 3.
Table 3.
mBG and myocardial FDG uptake pattern distribution in three groups

We observed and analyzed the myocardial uptake pattern in patients with controlled diabetes. A total of 26 diabetics were present in the study population. Overall, no significant difference was noted between the uptake pattern in the diabetics and the nondiabetic patients (P = 0.475).
We also analyzed the effect of chemotherapy on the myocardial uptake pattern. There was no significant relation between the uptake pattern noted between the patients who received chemotherapy (n = 61) and those who need not receive any chemotherapy (P = 0.747).
DISCUSSION
Unlike a perfusion tracer that is solely flow-dependent and any heterogeneity reflects abnormal myocardial perfusion, FDG uptake is dependent on many factors and produces a variable uptake in myocardium depending on the substrate available for oxidative metabolism and the hormonal status. PET scans routinely performed for oncological evaluation have shown a variable spectrum of FDG uptake in myocardium even in fasting state. This variable uptake poses difficulty in interpretation when suppression of FDG uptake in normal myocardium is desired, e.g., in detection of ischemic myocardium,[4,5,6,7] in oncological evaluation of thorax and upper abdominal area,[8] and when a low myocardial uptake is required in plaque imaging of the coronary vessels.[9] Even in the post-glucose load studies for assessment of viability, often a heterogeneous F-18 FDG uptake is noted in the myocardium making the interpretation difficult. Therefore, we designed this study to observe the influence of duration of fasting and dietary modifications on myocardial FDG uptake.
In our study, we evaluated a total of 153 patients who were divided into three groups, Group A: Patients with 4-h fasting (range 4-6 h), Group B: Patients with 12-h overnight fasting (range 12-14 h), and Group C: Patients with overnight fasting and on low carbohydrate and fat-rich diet for 2 days prior to the study.
To observe the variable pattern of FDG uptake in the LV myocardium, we visually classified the uptake into homogeneous uptake, heterogeneous uptake, and ‘no uptake’ in the LV myocardium. Some authors have compared the myocardial FDG uptake with background or other organs like liver by visually grading the uptake or using standardized uptake value (SUV).[10,11] In our study, we did not take into consideration myocardial SUV values or compared the ratio of myocardial uptake with background or other organs. These parameters routinely are not taken into consideration when reporting cardiac study. The focus of our study was to observe and identify the pattern of FDG myocardial uptake in relation to duration of fasting and diet so as to bePnefit the interpretation of cardiac PET studies in day to day practice. Although all the studies were viewed in the 3D cine format, the final analysis of all the studies was done in conventional cardiac format as short axis and vertical and horizontal long axis, like any routine cardiac study.
In our study, we observed the ‘no uptake’ pattern in 10, 55, and 77%, ‘heterogeneous’ pattern of FDG uptake in 39, 28, and 14%, and ‘homogeneous’ pattern in 51, 18, and 10% patients in Group A, B, and C, respectively. These results showed that Group C protocol of overnight fasting and restricted diet (2 days of low carbohydrate and fat-rich diet) before F-18 FDG administration suppresses myocardial F-18 FDG uptake more than Group B that suppresses the uptake more than Group A. Our results suggest that myocardial uptake suppression is better with combination of controlled diet and fasting than fasting alone.
Several authors have studied the role of diet and fasting on myocardial FDG uptake.[10,12,13,14] De Groot et al.,[12] had concluded that age, fasting period, and blood glucose levels did not influence physiological uptake of FDG in myocardium. This study suggests that carbohydrate-restricted fat-rich diet may be more effective than fasting to suppress myocardial FDG uptake. Balink et al.,[10] in their study described the effect of a 1-day fat-allowed carbohydrate-restricted diet on myocardial F-18 FDG uptake in fasting protocol for PET oncology study. They found that after a carbohydrate-restricted diet, 68% of patients had a homogeneously low myocardial uptake of F-18 FDG. Therefore, we designed the 2-day protocol of carbohydrate-restricted fat-rich diet in order to achieve better myocardial FDG suppression and also compare the findings with prolonged fasting period. In our study, myocardial suppression was higher (77%) in patients who fasted overnight and were put on a controlled diet for 2 days.
In a recent study, Harisankar et al.,[13] evaluated the feasibility to consistently suppress myocardial FDG uptake with a low carbohydrate high fat protein permitted (LCHFPP) diet. In their study, they compared 50 patients (>12-h fasting) and 60 patients on special LCHFPP diet, which the patients were advised to consume on previous night and also at 4 h before the test. After exclusion of noncompliant patients in LCHFPP group, 84% of subjects demonstrated significant FDG suppression. The authors observed better myocardial FDG uptake suppression using LCHFPP diet compared with prolonged (>12 h) fasting protocol.
We analyzed the relation of blood glucose level on the myocardial uptake pattern. The mBG was lowest in ‘no uptake’ pattern calculated to be 102 ± 25 mg/dl, which was comparable with 111 ± 24 mg/dl in homogeneous pattern. Also within each group, no relationship could be established between mBG and the uptake pattern. These results suggest that mBG does not determine the uptake of FDG in the myocardium. Other factors like insulin level, free fatty acid concentration, glucagon level, and influx rate constants of FDG may also play role in myocardial FDG uptake as suggested by Choi et al.[15] Similarly, de Groot et al.,[12] in their study on patients who underwent repeated PET scans for oncological evaluation suggested that the variable uptake in the myocardium is not influenced by the blood glucose level.
We further evaluated the heterogeneous uptake pattern in relation to the regions in the ventricular wall. The regions were identified irrespective of the intensity of the uptake, provided they could be delineated separately from the blood pool activity in the LV cavity and the surrounding background. A total of 41 patients showed heterogeneous pattern, of which 20 (39%) were noted in Group A, 14 (28%) in Group B, and least seven (14%) in Group C. We could identify seven different patterns involving the ventricular walls. Region wise the most common regions that showed myocardial FDG uptake were lateral wall (90%), followed by base of the heart (88%), inferior wall (56%), and distal anteroapical region (46%). The regions with negligible or absent FDG uptake were septum, mid anterior wall, and inferoapical region. Similar findings of regional heterogeneity of FDG uptake in the myocardium have been described in previous studies.[2,15,16,17,18] Gropler et al.,[2] reported that myocardial accumulation of FDG in the septum and anterior wall averaged 80% of that in the lateral and posterior walls in the subjects with regional uptake. The regional heterogeneity of FDG uptake could be attributed to preferential substrate utilization by different regions of the myocardium in the fasting state. Hicks et al.,[17] studied normal variation in regional myocardial substrate metabolism and quantitatively evaluated myocardial oxidative metabolism with carbon-11 acetate and glucose metabolism using F-18 FDG. They observed that the glucose utilization was 13% lower in the septum compared with the lateral wall. This could not be explained by decreased metabolic demand because C-11 clearance constants were marginally higher in the septum than in the lateral wall in both studies. The authors suggested that relatively decreased septal glucose utilization could reflect regional variation in substrate use and possible preferential free fatty acid utilization by the septum.
In our study, we studied the effect of diabetes on the myocardial FDG uptake. On overall analysis, we found no relation between uptake pattern and presence of diabetes. Previous studies have assessed the role of diabetes on myocardial FDG uptake and suggested that in diabetics, myocardium shows preference for fatty acid as substrate compared with glucose.[19,20,21] But the same tendency was not noted in our study population. However, due to small numbers of diabetic patients in our study population and still lesser number in each group, a definite conclusion cannot be made.
In our study, we also assessed whether chemotherapy can affect the myocardial uptake pattern. Overall, we found no relation between uptake patterns in patients who received chemotherapy when compared with patients who had not received chemotherapy.
In summary, the results of our study show that Group C protocol of overnight fasting and restricted diet (low carbohydrate and fat-rich diet) for 2 days before F-18 FDG study suppresses myocardial F-18 FDG uptake more than Group B protocol of overnight fasting alone that suppresses uptake more than Group A protocol of 4-h fasting. Blood glucose, diabetes, and chemotherapy do not influence myocardial FDG uptake. Therefore, our results suggest that both restricted diet and duration of fasting play an important role in suppression of myocardial FDG uptake.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Camici P, Ferrannini E, Opie LH. Myocardial metabolism in ischemic heart disease: Basic principles and application to imaging by positron emission tomography. Prog Cardiovasc Dis. 1989;32:217–38. doi: 10.1016/0033-0620(89)90027-3. [DOI] [PubMed] [Google Scholar]
- 2.Gropler RJ, Siegel BA, Lee KJ, Moerlein SM, Perry DJ, Bergmann SR, et al. Nonuniformity in myocardial accumulation of fluorine-18-fluorodeoxyglucose in normal fasted humans. J Nucl Med. 1990;31:1749–56. [PubMed] [Google Scholar]
- 3.Fukuchi K, Ohta H, Matsumura K, Ishida Y. Benign variations and incidental abnormalities of myocardial FDG uptake in the fasting state as encountered during routine oncology positron emission tomography studies. Br J Radiol. 2007;80:3–11. doi: 10.1259/bjr/92105597. [DOI] [PubMed] [Google Scholar]
- 4.He ZX, Shi RF, Wu YJ, Tian YQ, Liu XJ, Wang SW, et al. Direct imaging of exercise-induced myocardial ischemia with fluorine-18-labeled deoxyglucose and Tc-99m-sestamibi in coronary artery disease. Circulation. 2003;108:1208–13. doi: 10.1161/01.CIR.0000088784.25089.D9. [DOI] [PubMed] [Google Scholar]
- 5.Jain D, McNulty PH. Exercise-induced myocardial ischemia: Can this be imaged with F-18-fluorodeoxyglucose? J Nucl Cardiol. 2000;7:286–8. doi: 10.1016/s1071-3581(00)70020-1. [DOI] [PubMed] [Google Scholar]
- 6.Camici P, Araujo LI, Spinks T, Lammertsma AA, Kaski JC, Shea MJ, et al. Increased uptake of 18F-fluorodeoxyglucose in postischemic myocardium of patients with exercise-induced angina. Circulation. 1986;74:81–8. doi: 10.1161/01.cir.74.1.81. [DOI] [PubMed] [Google Scholar]
- 7.Arrighi JA. F-18 fluorodeoxyglucose imaging in myocardial ischemia: Beyond myocardial viability. J Nucl Cardiol. 2001;8:417–20. doi: 10.1067/mnc.2001.115646. [DOI] [PubMed] [Google Scholar]
- 8.Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: Physiologic and benign variants. Radiographics. 1999;19:61–77. doi: 10.1148/radiographics.19.1.g99ja0761. [DOI] [PubMed] [Google Scholar]
- 9.Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–11. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 10.Balink H, Hut E, Pol T, Flokstra FJ, Roef M. Suppression of 18F-FDG myocardial uptake using a fat-allowed, carbohydrate-restricted diet. J Nucl Med Technol. 2011;39:185–9. doi: 10.2967/jnmt.110.076489. [DOI] [PubMed] [Google Scholar]
- 11.Inglese E, Leva L, Matheoud R, Sacchetti G, Secco C, Gandolfo P, et al. Spatial and temporal heterogeneity of regional myocardial uptake in patients without heart disease under fasting conditions on repeated whole-body 18F-FDG PET/CT. J Nucl Med. 2007;48:1662–9. doi: 10.2967/jnumed.107.041574. [DOI] [PubMed] [Google Scholar]
- 12.de Groot M, Meeuwis AP, Kok PJ, Corstens FH, Oyen WJ. Influence of blood glucose level, age and fasting period on non-pathological FDG uptake in heart and gut. Eur J Nucl Med Mol Imaging. 2005;32:98–101. doi: 10.1007/s00259-004-1670-2. [DOI] [PubMed] [Google Scholar]
- 13.Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011;18:926–36. doi: 10.1007/s12350-011-9422-8. [DOI] [PubMed] [Google Scholar]
- 14.Lum D, Wandell S, Ko J, Coel M. 1. Positron emission tomography of thoracic malignancies. reduction of myocardial fluorodeoxyglucose uptake artifacts with a carbohydrate restricted diet. Clin Positron Imaging. 2000;3:155. doi: 10.1016/s1095-0397(00)00067-4. [DOI] [PubMed] [Google Scholar]
- 15.Choi Y, Brunken RC, Hawkins RA, Huang SC, Buxton DB, Hoh CK, et al. Factors affecting myocardial 2-[F-18]fluoro-2-deoxy-D-glucose uptake in positron emission tomography studies of normal humans. Eur J Nucl Med. 1993;20:308–18. doi: 10.1007/BF00169806. [DOI] [PubMed] [Google Scholar]
- 16.Tamaki N, Yonekura Y, Kawamoto M, Magata Y, Sasayama S, Takahashi N, et al. Simple quantification of regional myocardial uptake of fluorine-18-deoxyglucose in the fasting condition. J Nucl Med. 1991;32:2152–7. [PubMed] [Google Scholar]
- 17.Hicks RJ, Herman WH, Kalff V, Molina E, Wolfe ER, Hutchins G, et al. Quantitative evaluation of regional substrate metabolism in the human heart by positron emission tomography. J Am Coll Cardiol. 1991;18:101–11. doi: 10.1016/s0735-1097(10)80225-6. [DOI] [PubMed] [Google Scholar]
- 18.Maurer AH, Burshteyn M, Adler LP, Steiner RM. How to differentiate benign versus malignant cardiac and paracardiac 18F FDG uptake at oncologic PET/CT. Radiographics. 2011;31:1287–305. doi: 10.1148/rg.315115003. [DOI] [PubMed] [Google Scholar]
- 19.McGill JB, Peterson LR, Herrero P, Saeed IM, Recklein C, Coggan AR, et al. Potentiation of abnormalities in myocardial metabolism with the development of diabetes in women with obesity and insulin resistance. J Nucl Cardiol. 2011;18:421–9. doi: 10.1007/s12350-011-9362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson LR, Herrero P, McGill J, Schechtman KB, Kisrieva-Ware Z, Lesniak D, et al. Fatty acids and insulin modulate myocardial substrate metabolism in humans with type 1 diabetes. Diabetes. 2008;57:32–40. doi: 10.2337/db07-1199. [DOI] [PubMed] [Google Scholar]
- 21.Ohtake T, Yokoyama I, Watanabe T, Momose T, Serezawa T, Nishikawa J, et al. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med. 1995;36:456–63. [PubMed] [Google Scholar]


