Abstract
Different Leishmania species rely to different extents on abundant glycoconjugates, such as lipophosphoglycan (LPG) and related molecules, in mammalian infections. Previously, we showed that Leishmania major deletion mutants lacking the Golgi GDP-mannose transporter LPG2, which is required for assembly of the dominant phosphoglycan (PG) repeats of LPG, were unable to survive in macrophages. These lpg2− mutants, however, retained the ability to generate asymptomatic, persistent infections in mice. In contrast, Ilg and colleagues showed that Leishmania mexicana LPG2 mutants retained virulence for mice. Here we identified a partial revertant population of the L. major lpg2− mutants (designated lpg2−REV) that had regained the ability to replicate in macrophages and induce disease pathology through a compensatory change. Like the lpg2 parent, the lpg2−REV revertant was unable to synthesize LPG2-dependent PGs in the promastigote stage and thus remained highly attenuated in the ability to induce infection. However, after considerable delay lpg2−REV revertant-infected mice exhibited lesions, and amastigotes isolated from these lesions were able to replicate within macrophages despite the fact that they were unable to synthesize PGs. Thus, in some respects, the lpg2−REV amastigotes resemble L. mexicana amastigotes. Future studies of the gene(s) responsible may shed light on the mechanisms employed by L. major to survive in the absence of LPG2-dependent glycoconjugates and may also improve the potential of the lpg2− L. major line to serve as a live parasite vaccine by overcoming its tendency to revert toward virulence.
Infections by the protozoan parasite Leishmania can cause severe diseases in humans and animals, whose manifestations range from mild cutaneous to fatal visceral pathology. Leishmaniae are transmitted in the form of highly virulent metacyclic parasites, which develop inside the midgut of phlebotomine sand flies and are inoculated into the mammalian host by biting. Inside the acidified phagolysosome of host macrophages, leishmaniae differentiate into the aflagellate amastigote stage, which resists leishmanicidal activities and inhibits macrophage activation, processes that ultimately contribute to amastigote survival and replication and disease pathology (8). In most immunocompetent hosts, parasite growth and pathology are eventually controlled by adaptive immune mechanisms (2, 20), and the infection enters a chronic phase of parasite persistence in the absence of significant pathology. There are a number of host factors which are known to modulate parasite persistence and establish a delicate balance between gamma interferon-induced leishmanicidal NO and immunesuppressive interleukin-10 produced by CD25+ regulatory T cells (1, 2, 9, 25). In contrast, our knowledge concerning the identities and roles of parasite factors which function differentially in mediating persistence and in the acute pathogenic phase of infection is more limited.
Glycosylphosphatidyl inositol-anchored surface glycoconjugates, including lipophosphoglycan (LPG), glycoinositol phospholipids (GIPLs), and proteins, such as proteophosphoglycan (PPG) or gp63, form a dense glycocalyx in Leishmania. Many of these compounds are important determinants of parasite virulence, as shown by studies of purified molecules, as well as mutants defective in the synthesis of one or more of the glycoconjugates (11, 12, 14, 22-24, 28). In Leishmania major, LPG plays an important role in the establishment of infections in macrophages following metacyclic invasion, while both GIPLs and other alkylacyl phosphoglycerolipid moeities appear to have small roles in macrophage survival despite their abundance (12, 33). In contrast, parasite molecules that are dependent on the activity of the LPG2-encoded Golgi GDP-mannose transporter, such as LPG and other phosphoglycans (PGs) bearing the signature Gal-Man-P-based repeating unit, are required for both initial establishment and subsequent survival and replication of amastigotes within macrophages (24). It should be emphasized that the findings described above pertain to L. major and probably Leishmania donovani as well (M. Wilson and S. M. Beverley, unpublished data). Remarkably, studies of Leishmania mexicana have suggested that this species requires neither LPG, PGs, nor GIPLs for establishment or replication within macrophages (7, 10). This difference in reliance on highly abundant, structurally conserved molecules among Leishmania species was unanticipated and is not well understood (4, 29).
Significantly, the L. major lpg2− parasite maintained the ability to persist at the infection site in the absence of overt pathology for more than 2 years (24). This ruled out the possibility that LPG2-dependent factors (such as PGs) are required in the persistent, asymptomatic phase of the disease. To explain the fact that the lpg2− parasite is unable to survive in macrophages yet is able to survive indefinitely in animals, we suggested a model in which persistent parasites resided in another, presumably more hospitable compartment within the host, a compartment from which the parasite would be unable to induce disease through perturbation of macrophage function. A corollary of this safe haven model is that parasites that had recovered the ability to survive within macrophages would similarly recover the ability to induce disease pathology. Here we present evidence which supports this prediction and discuss its relevance to Leishmania virulence and the use of live parasite-based immunization strategies.
MATERIALS AND METHODS
Parasite strains and culture.
All parasites were derivatives of L. major strain LV39 clone 5 (Rho/SU/59/P) and were cultivated in M199 medium (13). The lpg2 line obtained by homologous gene replacement (ΔLPG2::HYG/ΔLPG2::HYG) has been described previously (24). P+ and P− derivatives of this line (Fig. 1A) were transfected with the construct pSNBR-LPG2 (B4831) and plated on semisolid M199 medium containing 20 μg of G418 per ml.
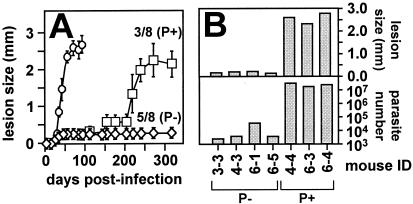
FIG. 1.
Emergence of the lpg2−REV compensatory partial revertant during mouse infection. (A) Lesion formation. A total of 106 WT or lpg2− metacyclic parasites were inoculated into groups of susceptible BALB/c mice, and the infections were monitored by comparing the thickness of the injected footpad to the thickness of the uninjected footpad with a vernier caliper. Inoculation of WT parasites resulted in infections that appeared within 25 days (○), and, as shown previously, five of eight mice infected with lpg2− parasites showed no lesion formation (P−) (⋄). In contrast, three of eight mice infected with lpg2− parasites in this experiment showed lesion formation beginning around day 125 (P+) (□); the parasites in these mice were designated lpg2−REV parasites. The bars indicate the standard deviations for WT parasites (n = 4), P− lpg2− parasites (n = 5), and P+ lpg2− parasites (n = 3). (B) Limiting dilution assay. Representative P− or P+ mice from the experiment shown in panel A were sacrificed on day 346 postinfection, and the numbers of parasites in the infected footpads were determined by limiting dilution. The lesion sizes (upper panel) and the parasite burdens (lower panel) of individual animals are indicated.
Monoclonal antibodies, lectins, immunofluorescence, and flow cytometry.
Monoclonal antibody WIC79.3 recognizes L. major Gal-substituted Gal-Man-P repeating units (6), and anti-trypanosomal tubulin antibody was provided by D. Russell (Cornell University, Ithaca, N.Y.). Flow cytometry with fluorescein-conjugated ricin agglutinin (Sigma, St. Louis, Mo.) was performed with live Leishmania. Parasites in the logarithmic growth phase (105 parasites/ml) were washed twice in cold phosphate-buffered saline and incubated with 5 μg fluorescein-conjugated ricin agglutinin per ml in phosphate-buffered saline for 10 min at 4°C. The analysis was performed with a Becton Dickinson FACS-Calibur system (excitation wavelength, 488 nm; detection wavelength, 520 nm). Cells were fixed with 4% paraformaldehyde for 5 min at room temperature, permeabilized by incubation with ice-cold 100% ethanol for 15 min at 4°C, and sequentially incubated with monoclonal anti-PG antibody WIC79.3 (27) and fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G. Parasites were counterstained with polyclonal anti-tubulin and Texas Red-conjugated anti-rabbit immunoglobulin G.
Mouse infection and limiting dilution assay.
Virulence was assessed by using 6- to 8-week-old female BALB/c mice (Charles River Laboratories Inc., Wilmington, Mass.) following subcutaneous inoculation of 106 metacyclic parasites into the hind footpads (26). The metacyclic parasites were isolated by density centrifugation as described previously (21). Infections were monitored by comparing the thickness of the injected footpad to the thickness of the uninjected footpad with a vernier caliper. Parasites in the infected tissue were enumerated by a limiting dilution assay (26).
Macrophage infection.
Starch-elicited peritoneal exudate macrophages (PEM) from BALB/c mice were infected as described previously (22). Metacyclic parasites were opsonized by 30 min of incubation in Dulbecco modified Eagle medium containing 4% C5-deficient mouse serum (16) and were allowed to invade macrophages in Dulbecco modified Eagle medium containing 0.7% bovine serum albumin for 2 h at 33°C at multiplicities of infection of 10 and 3 parasites per PEM for wild-type (WT) and lpg2 parasites, respectively. Amastigotes were isolated from progressive lesions by tissue homogenization and differential centrifugation, and PEM were infected for 2 h at 33°C at a ratio of three parasites per PEM. Intracellular growth was determined by nuclear staining and fluorescence microscopy as described previously (22).
RESULTS
Emergence of an lpg2− parasite population showing late pathology.
In previous studies performed in three different laboratories, it was found that in most infections of susceptible BALB/c mice with the lpg2− null mutant, parasites were able to persist indefinitely without pathology (24, 30). In these experiments, footpad inoculation was used to infect groups of female BALB/c mice with 106 WT or lpg2− metacyclic parasites that were isolated by Ficoll density centrifugation (21). WT metacyclic parasites elicited lesions that appeared within 25 days (Fig. 1A), while in most experiments lpg2− metacyclic parasites failed to induce pathology, in agreement with previous findings (24). However, in two experiments involving inoculation with higher numbers of parasites, we observed that a few mice developed lesions, which developed late (after 150 to 200 days) but progressed rapidly thereafter. For example, in the experiment shown in Fig. 1A, three of eight of the lpg2− parasite-infected mice showed this same late-lesion profile (P+ mice), while the remaining mice showed no pathology, as observed previously (24). The numbers of parasites were determined by a limiting dilution assay (26) on day 346 postinfection, and the results showed that, as expected, infected mice that did not exhibit overt pathology (mice 3-3, 4-3, 6-1, and 6-5) contained a low level of persistent parasites (∼103 parasites) (Fig. 1B). In contrast, in mice with the late-lesion pathology (mice 4-4, 6-3, and 6-4), the numbers of parasites were nearly 10,000-fold higher (Fig. 1B).
The timing of lesion appearance differed from the timing observed previously for LPG-deficient lpg1− L. major, which requires LPG for establishment of a macrophage infection but not for replication as amastigotes (22). Prompted by the supposition that this finding might reflect a new genetic difference between the late-lesion lpg2− parasites and the canonical persistent lpg2− parasites, we recovered parasites separately from mice which never exhibited lesion formation (designated P−) and mice which had lesions (designated P+) (Fig. 1A).
Late-pathology lpg2− P+ parasites do not make PGs in the promastigote form.
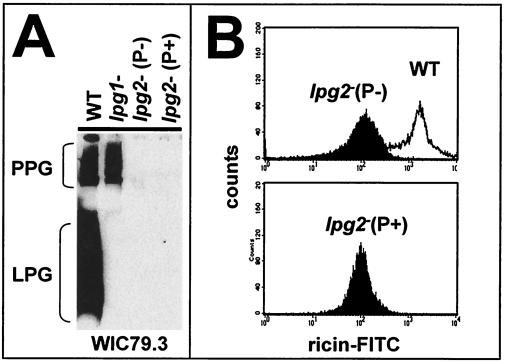
Following recovery, the two populations of parasites were allowed to differentiate into promastigotes in vitro, and PG expression was analyzed by examining reactivity with anti-PG antibody WIC79.3 in a Western blot analysis (Fig. 2A) and by flow cytometry with the β-Gal-binding lectin ricin agglutinin (Fig. 2B). WT promastigotes expressed an abundance of LPG and PPG, as shown by Western blotting, while the lpg1− mutant (defective in LPG core synthesis) showed the expected synthesis of PPG but did not express LPG (Fig. 2A). Importantly, the lpg2− P− and P+ populations maintained the total PG-deficient phenotype expected for the lpg2− promastigotes, showing that they were neither WT contaminants nor second-site revertants that exhibited restored promastigote PG synthesis. It should be emphasized that the LPG2 coding region had been removed completely from the lpg2− line by homologous gene replacement (24), and thus reconstitution of LPG2 itself was impossible. Interestingly, inspection of the available L. major genome sequence data revealed the presence of several members of the nucleotide sugar transporter family whose functions are unknown, in addition to the anticipated UDP-Gal transporters (A. Capul and S. M. Beverley, unpublished data). However, activation of an alternative GDP-Man transport activity in P+ lpg2− promastigotes was ruled out by these data.
FIG. 2.
Characterization of P+ and P− lpg2− promastigotes. (A) Western blot analysis. Promastigotes from WT, lpg1−, and lpg2− P+ and P− populations were subjected to Western blot analysis with the anti-PG antibody WIC79.3 (6). The positions of WT PPG and LPG are shown. (B) PG flow cytometry. A total of 105 promastigotes from a logarithmic culture were incubated with ricin-fluorescein isothiocyanate (FITC) at 4°C prior to analysis. (Upper panel) WT and lpg2− P− parasites; (lower panel) lpg2− P+ parasites. The parental lpg2− population was indistinguishable from the P− and P+ populations (data not shown).
P− and P+ lpg2− parasites breed true.
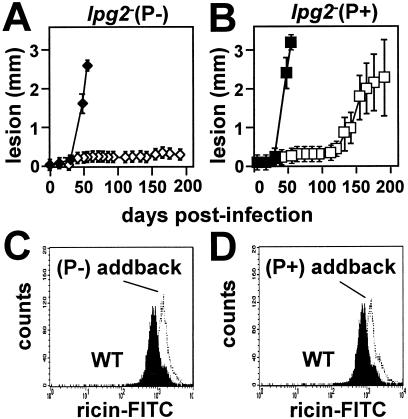
When 106 metacyclic parasites were inoculated into mice, the P− lpg2− parasites yielded persistent infections similar to those seen previously; as in previous studies, no infected mouse went on to develop lesions or pathology over a 1-year period (Fig. 3A and data not shown). Notably, restoration of LPG2 expression restored PG synthesis to WT levels (Fig. 3C) and lesion formation to control levels (Fig. 3A). These results demonstrated that the P− lpg2− parasites were not generally and irreversibly avirulent, a potential concern since loss of virulence occurs sporadically during long-term culture or genetic manipulation of Leishmania in vitro (5). Thus, the P− lpg2− parasites appear to be identical to the original lpg2− population described previously (24).
FIG. 3.
P+ lpg2− population breeds true. (A and B) Mouse infections. A total of 106 purified WT (⧫ and ▪), lpg2− (⋄), or lpg2− P+ (□) metacyclic parasites were inoculated into the footpads of BALB/c mice, and lesion formation was monitored. Each point indicates the average for three mice inoculated with WT parasites or five mice inoculated with lpg2− or lpg2− P+ parasites; the error bars indicate standard deviations. (C and D) Restoration of LPG2 expression fully restores surface LPG expression in P+ and P− lpg2− lines. LPG expression in promastigotes of WT parasites (solid profile) and the lpg2− (P−)/+ LPG2 (panel C, open profile) or lpg2− (P+)/+ LPG2 addback lines was assessed by flow cytometry with fluorescent ricin as described in Materials and Methods. As shown previously, the dominant PG detected by this assay is LPG (22). FITC, fluorescein isothiocyanate.
In contrast, the P+ lpg2− parasites produced lesions in every infected mouse, again with a delay of ∼125 days (Fig. 3B). This showed that the late-lesion or P+ phenotype was a stable trait, and we designated this population lpg2−REV. As in the lpg2− parental line, reexpression of LPG2 in the lpg2−REV population restored LPG and PG synthesis (Fig. 3D).
lpg2−REV parasites survive in macrophages in the amastigote form but not in the promastigote form.
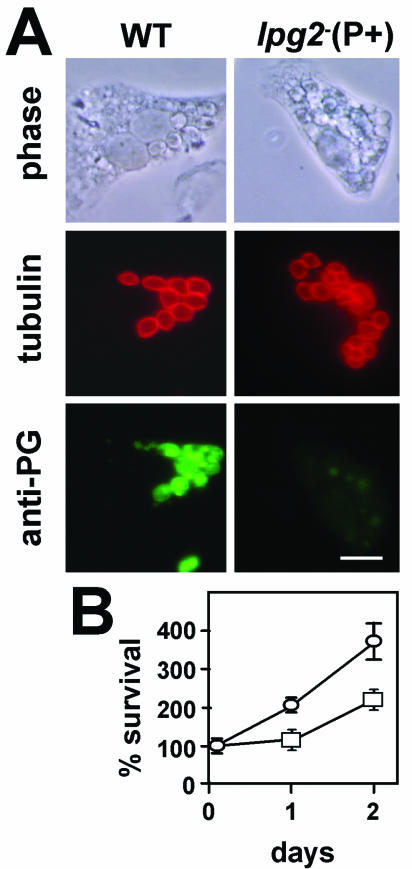
Infection of mouse macrophages with lpg2− (P−) or lpg2−REV (P+) metacyclic parasites showed that neither survived, as observed previously (24; data not shown). While lpg2− (P−) amastigotes could not be obtained, we were able to recover amastigotes from mouse lesions obtained with lpg2−REV parasites (obtained around day 150). The lpg2−REV amastigotes were capable of surviving and replicating well in macrophages, although the growth rate was about twofold lower than that of WT amastigotes (Fig. 4B). The size of the lpg2−REV parasitophorous vacuole was similar to that seen in other L. major infections, and the vacuole was not extended like that seen in L. mexicana infections (Fig. 4A).
FIG. 4.
lpg2−REV parasites replicate in macrophages but do not synthesize PGs. (A) Immunofluorescence analysis of amastigote PGs. PEM were infected with WT and lpg2−REV amastigote parasites and stained 2 days postinfection for reactivity with tubulin or PGs as described in Materials and Methods. Bar = 10 μm. (B) Macrophage replication. Murine macrophages were infected with WT (○) or lpg2−REV (□) amastigotes as described in Materials and Methods, and the numbers of parasites were determined and normalized to the initial infection obtained at 2 h (day zero). The values are averages and standard deviations for a representative experiment performed in triplicate.
lpg2−REV parasites do not make PGs.
As described above for promastigotes, it was possible that the lpg2−REV amastigotes had recovered the ability to synthesize PGs through activation of an amastigote-specific pathway that restored Golgi GDP-Man uptake. In WT amastigotes, PGs predominantly localized to the parasite cytoplasm and accumulated in a vesicular compartment, most likely the flagellar pocket, as expected for secreted PGs (Fig. 4A, left panels). However, immunofluorescence analysis of the infected macrophages with anti-PG antisera showed that the lpg2−REV amastigotes failed to synthesize detectable levels of PGs, and only background fluorescence was evident (Fig. 4A, right panels). These data eliminated the possibility that there was activation of an alternative amastigote Golgi GDP-Man transport activity in this line.
DISCUSSION
In previous studies it was shown that LPG was important for the ability of L. major to establish infections in macrophages, while LPG2-dependent molecules, such as LPG and related PGs, were required for replication of parasites within macrophages in the amastigote form and subsequent pathology (24). The lpg2− parasites used in these studies failed to induce any pathology in mice, although they were able to persist indefinitely in the absence of overt lesion formation. In this study we characterized a variant of the lpg2 line designated the lpg2−REV line, which is able to reproducibly produce pathology in mice, albeit after a considerable delay (Fig. 1A). Notably, neither promastigote nor amastigote forms of the lpg2−REV parasite regained the ability to synthesize PGs, and thus this mutant did not arise through activation of an alternative Golgi GDP-Man transporter activity.
In our experience the delayed-lesion phenotype manifested by the lpg2−REV parasite is characteristic of mutants with mutations that affect the ability of Leishmania to establish infections in macrophages (e.g., to enter, survive, and differentiate) but not the ability to replicate in the amastigote form. This may be due to defects in macrophage survival (as observed previously with LPG-deficient LPG1 null mutants or ether phospholipid-deficient ADS1 null mutants [22, 33]) or to differentiation to form the infectious metacyclic stage (as observed in sphingolipid synthetic mutants [32; unpublished data]). Consistent with this notion, lpg2−REV parasites were poorly infective in macrophages in the promastigote form, reflecting the absence of PGs, such as LPG. However, in striking contrast to the amastigote form of the lpg2− parent, lpg2−REV amastigotes showed good survival and replication in macrophages (Fig. 4B). Thus, these parasites in some manner acquired a compensatory mutation that enables amastigote replication in the absence of PGs or perhaps other molecules dependent on the activity of LPG2.
The following hypothetical scenario accounts for the emergence and properties of the lpg2−REV parasites (Fig. 5A) (24). As shown previously, in a normal infection with metacyclic promastigotes most lpg2− parasites were phagocytosed by macrophages and destroyed, while a small proportion of the parasites escaped, possibly by residing in a cell type other than macrophages that lacked leishmanicidal potential (3, 22). In this presumptive safe haven the lpg2− parasites were able to persist indefinitely (>700 days) at low levels (∼1,000 parasites). Little is known about the parasites during this stage, including their differentiation state or whether they replicate. One possibility is that the persistent parasites slowly turn over and constantly leave their safe haven, where they are then challenged by host macrophages. This possibility is consistent with the role of negative host factors, such as NO, which mediate parasite killing by macrophages, thereby keeping persistent Leishmania infections in check (25). For the lpg2− parasites, entry into macrophages would lead to destruction until the emergence of variant cells that had acquired the ability to survive and replicate in macrophages, such as lpg2−REV cells, which ultimately would lead to disease pathology. Since the lpg2−REV parasites did not regain the ability to synthesize LPG or PGs, they remained attenuated in the ability to establish macrophage infections initially in the promastigote form, as observed here. This model makes several testable predictions regarding the nature of the host cell phenotype, its leishmanicidal potential, and the interaction with the host immune system. However, validating these predictions requires identification of the persistent lpg2− parasite population, a challenging task which is currently under way.
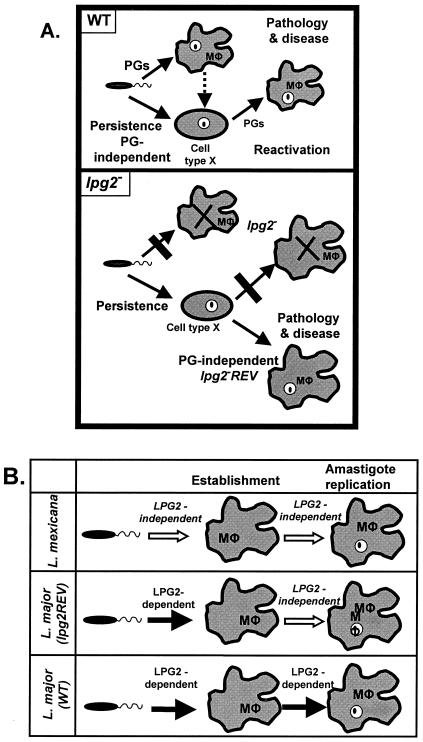
FIG. 5.
Summary of comparisons of lpg2−REV L. major. In both panels, the known role of LPG2 is in transport of GDP-mannose into the parasite Golgi apparatus, where it is utilized for the synthesis of PGs of various forms. (A) Proposed working model for pathology and persistence of WT parasites (upper panel) and lpg2− or lpg2−REV L. major parasites (lower panel) in mouse infections. See Discussion for more information. This figure is modified from that presented by Spáth et al. (24). (B) L. major lpg2−−REV parasites have a PG dependence phenotype intermediate between those of WT L. mexicana and L. major. See Discussion for more information. Mφ, macrophage.
The emergence of the lpg2−REV phenotype is relevant to the development of live vaccines in which the lpg2− parasite is used (30). To date, numerous protocols in which recombinant leishmanial antigens or heat-killed Leishmania have been used have failed to induce long-lasting immunity (15, 18, 19). In contrast, long-term protection is achieved following recovery from a natural infection, suggesting that parasite persistence is a prerequisite for sterile immunity (2, 31). lpg2− parasites fulfill the major requirements for a live vaccine candidate (i.e., persistent infection in the absence of disease), and vaccination studies with BALB/c mice and lpg2− parasites indeed induced dramatic protection against virulent challenge in the absence of a strong Th1 response (30). An interesting question for future study is the contribution of the host immune response to the delayed emergence of lpg2−REV parasite infections and whether this may provide some further perspective on the nature of the immune response to lpg2− parasites. Regardless, it is very clear the lpg2−REV phenotype compromises the potential utility of the lpg2− line as a vaccine candidate, and thus preventing its occurrence is important.
In contrast to L. major, L. mexicana does not require PGs for virulence or amastigote replication, implying that there are PG-independent mechanisms of macrophage survival (7, 10). In a recent review the possibility that different Leishmania species placed different levels of emphasis during evolution on the use of surface glycoconjugates for intracellular survival was discussed (29). The differences among the species are summarized in Fig. 5B, which shows that L. major requires LPG2-dependent glycoconjugates, such as LPG and/or PGs, for establishment of macrophage infections by promastigotes, as well as for survival and induction of pathology by amastigotes, while L. mexicana does not. Interestingly, the L. major lpg2−REV line exhibits intermediate behavior; it is deficient in the ability to establish macrophage infections without PGs, like L. major, but it retains the ability to survive and replicate in the amastigote form without PGs, like L. mexicana. It is tempting to speculate that the gene(s) or pathway(s) altered in the L. major lpg2−REV line is related to the pathways that enable L. mexicana to replicate in macrophages in the absence of PGs. Some interesting glycoconjugates recently implicated in amastigote virulence in L. mexicana are the β1-2 mannans, which are also found in L. major (17; unpublished data). However, the cytoplasmic location of mannans makes it unlikely that their synthesis is LPG2 dependent (17).
It may be possible to exploit the differences between the L. major lpg2− and lpg2−REV lines identified in this study in genetic strategies to identify the relevant genes responsible. Thus, the lpg2−REV line provides a unique tool that may facilitate studies to gain important insight into mechanisms of vaccination and virulence.
Acknowledgments
We thank A. Capul and K. Zhang for reading the manuscript and P. Scott and J. Uzonna for thoughtful discussions about the nature of the immune response to the lpg2− and lpg2−REV parasites.
This work was supported by NIH grant AI31078 (to S.J.T. and S.M.B.), by the Deutscher Akademischer Austauschdienst (DAAD), and by the Human Frontiers Science Program (G.F.S.).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Belkaid, Y., B. Butcher, and D. L. Sacks. 1998. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 28:1389-1400. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan, C., N. Donhauser, R. Doring, M. Rollinghoff, A. Diefenbach, and M. G. Rittig. 2000. Fibroblasts as host cells in latent leishmaniosis. J. Exp Med. 191:2121-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colmenares, M., S. Kar, K. Goldsmith-Pestana, and D. McMahon-Pratt. 2002. Mechanisms of pathogenesis: differences amongst Leishmania species. Trans. R. Soc. Trop. Med. Hyg. 96(Suppl. 1):S3-S7. [DOI] [PubMed] [Google Scholar]
- 5.Cruz, A. K., R. Titus, and S. M. Beverley. 1993. Plasticity in chromosome number and testing of essential genes in Leishmania by targeting. Proc. Natl. Acad. Sci. USA 90:1599-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Ibarra, A. A., J. G. Howard, and D. Snary. 1982. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology 85:523-531. [DOI] [PubMed] [Google Scholar]
- 7.Garami, A., A. Mehlert, and T. Ilg. 2001. Glycosylation defects and virulence phenotypes of Leishmania mexicana phosphomannomutase and dolicholphosphate-mannose synthase gene deletion mutants. Mol. Cell. Biol. 21:8168-8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handman, E., and D. V. Bullen. 2002. Interaction of Leishmania with the host macrophage. Trends Parasitol. 18:332-334. [DOI] [PubMed] [Google Scholar]
- 9.Holaday, B. J., M. M. Pompeu, S. Jeronimo, M. J. Texeira, A. Sousa Ade, A. W. Vasconcelos, R. D. Pearson, J. S. Abrams, and R. M. Locksley. 1993. Potential role for interleukin-10 in the immunosuppression associated with kala azar. J. Clin. Investig. 92:2626-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilg, T., M. Demar, and D. Harbecke. 2001. Phosphoglycan repeat-deficient Leishmania mexicana parasites remain infectious to macrophages and mice. J. Biol. Chem. 276:4988-4997. [DOI] [PubMed] [Google Scholar]
- 11.Ilg, T., E. Handman, and Y. D. Stierhof. 1999. Proteophosphoglycans from Leishmania promastigotes and amastigotes. Biochem. Soc. Trans. 27:518-525. [DOI] [PubMed] [Google Scholar]
- 12.Joshi, P. B., B. L. Kelly, S. Kamhawi, D. L. Sacks, and W. R. McMaster. 2002. Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol. Biochem. Parasitol. 120:33-40. [DOI] [PubMed] [Google Scholar]
- 13.Kapler, G. M., C. M. Coburn, and S. M. Beverley. 1990. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol. Cell. Biol. 10:1084-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McConville, M. J., T. A. Collidge, M. A. Ferguson, and P. Schneider. 1993. The glycoinositol phospholipids of Leishmania mexicana promastigotes. Evidence for the presence of three distinct pathways of glycolipid biosynthesis. J. Biol. Chem. 268:15595-15604. [PubMed] [Google Scholar]
- 15.Modabber, F. 1995. Vaccines against leishmaniasis. Ann. Trop. Med. Parasitol. 89(Suppl. 1):83-88. [DOI] [PubMed] [Google Scholar]
- 16.Racoosin, E. L., and S. M. Beverley. 1997. Leishmania major: promastigotes induce expression of a subset of chemokine genes in murine macrophages. Exp. Parasitol. 85:283-295. [DOI] [PubMed] [Google Scholar]
- 17.Ralton, J. E., T. Naderer, H. L. Piraino, T. A. Bashtannyk, J. M. Callaghan, and M. J. McConville. 2003. Evidence that intracellular beta1-2 mannan is a virulence factor in Leishmania parasites. J. Biol. Chem. 278:40757-40763. [DOI] [PubMed] [Google Scholar]
- 18.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 19.Satti, I. N., H. Y. Osman, N. S. Daifalla, S. A. Younis, E. A. Khalil, E. E. Zijlstra, A. M. El Hassan, and H. W. Ghalib. 2001. Immunogenicity and safety of autoclaved Leishmania major plus BCG vaccine in healthy Sudanese volunteers. Vaccine 19:2100-2106. [DOI] [PubMed] [Google Scholar]
- 20.Scott, P. 2003. Development and regulation of cell-mediated immunity in experimental leishmaniasis. Immunol. Res. 27:489-498. [DOI] [PubMed] [Google Scholar]
- 21.Späth, G. F., and S. M. Beverley. 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol. 99:97-103. [DOI] [PubMed] [Google Scholar]
- 22.Späth, G. F., L. Epstein, B. Leader, S. M. Singer, H. A. Avila, S. J. Turco, and S. M. Beverley. 2000. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. USA 97:9258-9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Späth, G. F., L. A. Garraway, S. J. Turco, and S. M. Beverley. 2003. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc. Natl. Acad. Sci. USA 100:9536-9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Späth, G. F., L. F. Lye, H. Segawa, D. L. Sacks, S. J. Turco, and S. M. Beverley. 2003. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science 301:1241-1243. [DOI] [PubMed] [Google Scholar]
- 25.Stenger, S., N. Donhauser, H. Thuring, M. Rollinghoff, and C. Bogdan. 1996. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 183:1501-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titus, R. G., M. Marchand, T. Boon, and J. A. Louis. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545-555. [DOI] [PubMed] [Google Scholar]
- 27.Tolson, D. L., S. J. Turco, R. P. Beecroft, and T. W. Pearson. 1989. The immunochemical structure and surface arrangement of Leishmania donovani lipophosphoglycan determined using monoclonal antibodies. Mol. Biochem. Parasitol. 35:109-118. [DOI] [PubMed] [Google Scholar]
- 28.Turco, S. J., and A. Descoteaux. 1992. The lipophosphoglycan of Leishmania parasites. Annu. Rev. Microbiol. 46:65-94. [DOI] [PubMed] [Google Scholar]
- 29.Turco, S. J., G. F. Späth, and S. M. Beverley. 2001. Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species. Trends Parasitol. 17:223-226. [DOI] [PubMed] [Google Scholar]
- 30.Uzonna, J., G. Späth, S. M. Beverley, and P. Scott. 2004. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J. Immunol. 172:3793-3797. [DOI] [PubMed] [Google Scholar]
- 31.Uzonna, J. E., G. Wei, D. Yurkowski, and P. Bretscher. 2001. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J. Immunol. 167:6967-6974. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, K., M. Showalter, J. Revollo, F. F. Hsu, J. Turk, and S. M. Beverley. 2003. Sphingolipids are essential for differentiation but not growth in Leishmania. EMBO J. 22:6016-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zufferey, R., S. Allen, T. Barron, D. R. Sullivan, P. W. Denny, I. C. Almeida, D. F. Smith, S. J. Turco, M. A. Ferguson, and S. M. Beverley. 2003. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. J. Biol. Chem. 278:44708-44718. [DOI] [PubMed] [Google Scholar]