Abstract
Background
Routine HIV testing, called provider-initiated opt-out HIV testing and counseling (PITC), is recommended in African countries with high HIV prevalence. However, it is unknown whether PITC increases access to pediatric HIV care. In 2008 the Baylor International Pediatric AIDS Initiative implemented PITC (BIPAI-PITC) at a Malawian hospital. We sought to evaluate the influence of BIPAI-PITC, compared to non-routine HIV testing (NRT), on pediatric HIV care access.
Methods
Retrospective data from 7,077 pediatric inpatients were collected during sequential four-month periods of NRT and BIPAI-PITC. In-hospital and one-year outcomes for 337 HIV-infected and HIV-exposed uninfected inpatients not previously enrolled in HIV care were analyzed to assess the clinical influence of each testing strategy.
Results
During BIPAI-PITC, a greater proportion of all hospitalized children received HIV testing (81.0% vs 33.3%, p < 0.001), accessed inpatient HIV-trained care (7.5% vs 2.4%, p < 0.001), enrolled into an outpatient HIV clinic after discharge (3.2% vs 1.3%, p < 0.001), and initiated antiretroviral therapy (ART) following hospitalization (1.1% vs 0.6%, p = 0.010) compared to NRT. Additionally, BIPAI-PITC increased the proportion of hospitalized HIV-infected and HIV-exposed uninfected children receiving DNA PCR testing (73.5% vs 35.2%, p < 0.001), but did not improve outpatient enrollment or ART initiation of identified HIV-infected patients.
Conclusions
BIPAI-PITC increases access to inpatient and outpatient pediatric HIV care for hospitalized children, including DNA PCR testing and ART. Broader implementation of BIPAI-PITC or similar approaches, along with more pediatric HIV-trained clinicians and improved defaulter-tracking methods, would improve pediatric HIV service utilization globally.
Keywords: Pediatric HIV, Routine HIV Testing, Provider Initiated HIV Testing and Counseling, Early Infant Diagnosis, Africa South of the Sahara
INTRODUCTION
Although industrialized countries have nearly eliminated new HIV infections in children, new pediatric HIV infections and high HIV-related mortality continue in sub-Saharan Africa.1 In Malawi, one of the most severely affected countries, 12.7% of adults are HIV-infected and approximately 90,000 children are living with HIV.1, 2 Since 2004, more than 270,000 Malawians have started antiretroviral therapy (ART), although access for HIV-infected children has been disproportionately lower.3–5 Starting in 2007 Malawi adopted policies to improve ART access for children, including routine HIV testing (provider-initiated opt-out HIV testing and counseling [PITC]),6 DNA PCR testing for infant HIV diagnosis, and in late 2008, universal ART for HIV-infected infants under one year of age.7
While there is high pediatric HIV-related disease burden in African hospitals,8–10 most clinical interventions target outpatient HIV care. In 2007, the Baylor International Pediatric AIDS Initiative (BIPAI) began caring for hospitalized HIV-infected and HIV-exposed uninfected children, and in 2008, BIPAI implemented inpatient pediatric PITC (BIPAI-PITC) at Kamuzu Central Hospital (KCH) in Malawi.11 Program outcomes for this and similar initiatives established that in-hospital PITC is acceptable, feasible, and has the potential to increase pediatric HIV care enrollment.11–13
However, the influence of inpatient PITC on access to pediatric HIV services remains unknown. Our objective was to assess whether BIPAI-PITC, compared to non-routine HIV testing (NRT), improves access to pediatric HIV care. We also analyzed outcomes of patients identified by inpatient HIV testing to help assess whether BIPAI-PITC and similar routine testing approaches merit wider implementation.
MATERIALS AND METHODS
Study Setting
KCH is a busy, urban referral facility with more than 13,000 pediatric admissions annually and an 8.5% inpatient pediatric HIV-infection prevalence.11 In 2005, one year after the introduction of NRT at KCH,14 BIPAI opened an outpatient HIV clinic, the Baylor College of Medicine-Abbott Fund Clinical Centre of Excellence (COE), that currently treats more than 5,000 children.15
BIPAI-PITC has been described in detail11 and is compared with NRT in Figure 1. Briefly, BIPAI-PITC and NRT used the same number of HIV counselors and pediatric HIV-trained clinicians. BIPAI-PITC added four volunteer patient escorts and utilized a non-anonymous HIV testing register that included patient names and hospital bed locations to facilitate linkage between testing and care. Patient escorts were caregivers of HIV-infected children who displayed competent language skills. They coordinated patient flow and encouraged caregivers by sharing their own HIV experiences. Each escort received remuneration (2 USD/day) for food and transportation to work. During NRT, healthcare providers offered HIV testing based on the child’s clinical presentation or physical examination findings, while during BIPAI-PITC testing was routinely offered irrespective of these factors.11, 14
FIGURE 1.
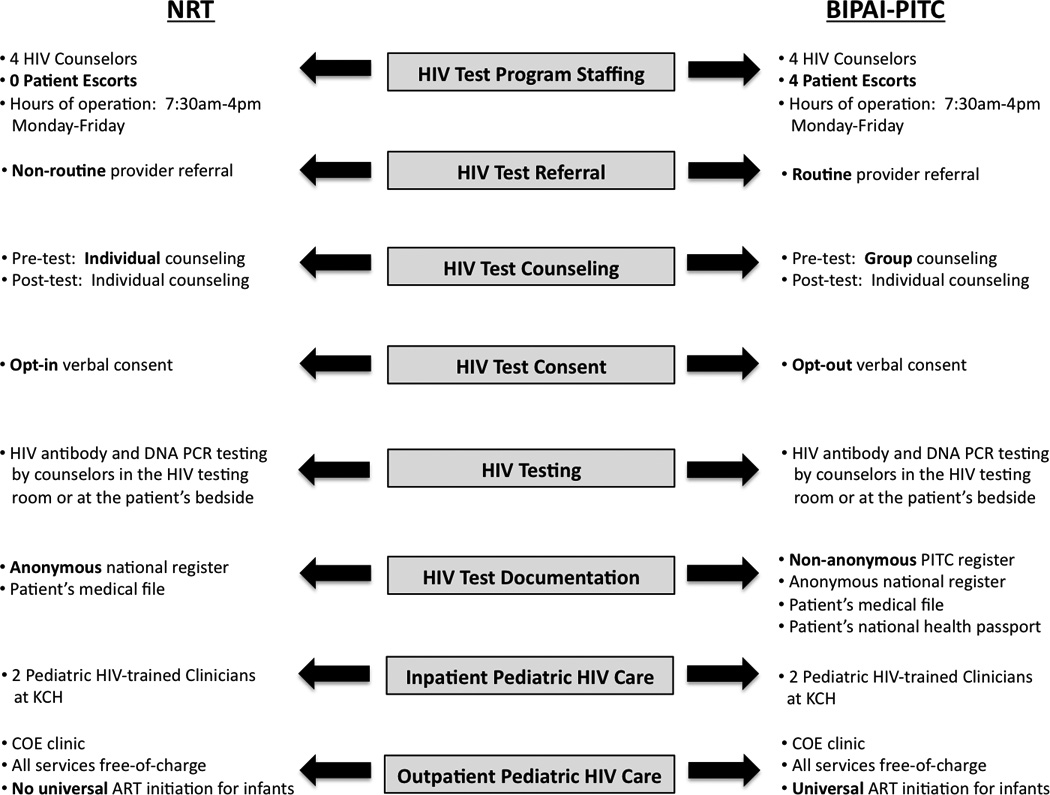
Comparison of NRT and BIPAI-PITC. BIPAI-PITC indicates Baylor International Pediatric AIDS Initiative System of Provider Initiated opt-out HIV Testing and Counseling; NRT, Non-Routine HIV Testing and Counseling; HIV, human immunodeficiency virus; PITC, Provider Initiated opt-out HIV Testing and Counseling; COE, Baylor College of Medicine-Abbott Fund Children’s Clinical Centre of Excellence; KCH, Kamuzu Central Hospital; ART, antiretroviral therapy.
Study Design
To compare the two HIV testing strategies while minimizing selection bias from seasonal admission patterns, we analyzed data from all hospitalized children at KCH from September 1 to December 31, 2007 (NRT), and from September 1 to December 31, 2008 (BIPAI-PITC). The entire pediatric inpatient population was included in the initial analysis because neither the HIV status of every child nor the total number of children eligible to access HIV care was known. To assess the influence of the HIV testing models on in-hospital and one-year patient outcomes, we further studied children who received BIPAI inpatient care but had not received HIV care before hospitalization (Figure 2).
FIGURE 2.
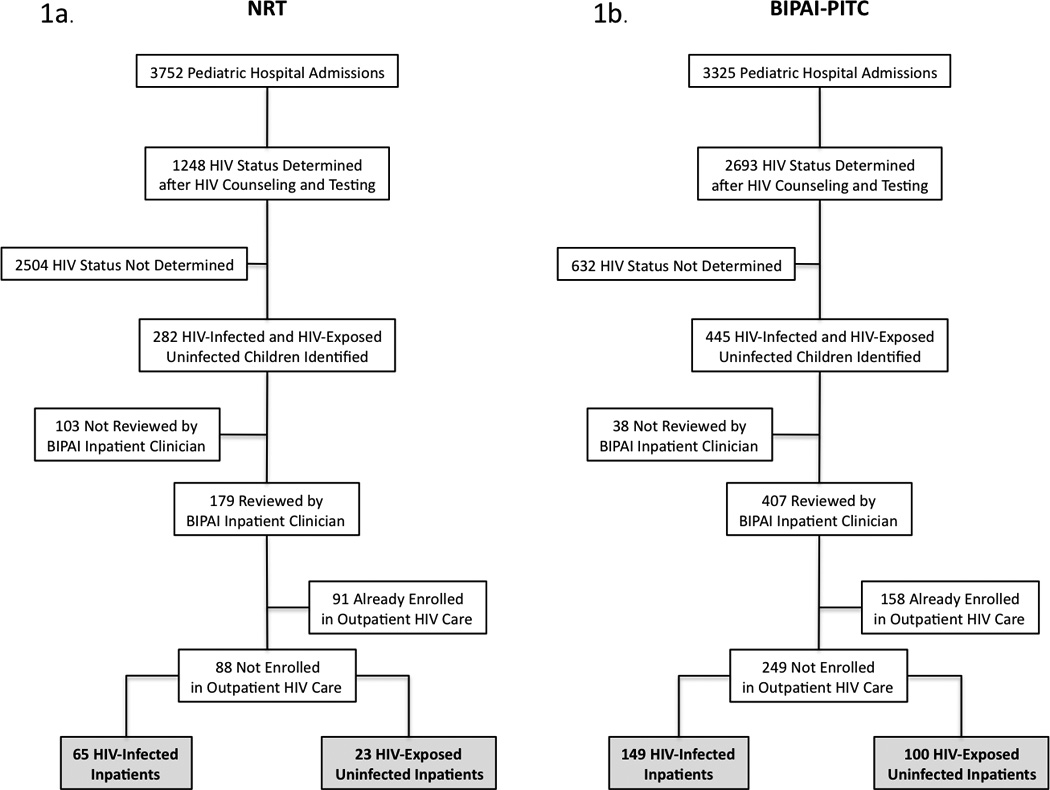
Cohort Schema 1a. NRT Program Cohort 1b. BIPAI-PITC Program Cohort. NRT indicates Non-Routine HIV Testing and Counseling; BIPAI-PITC, Baylor International Pediatric AIDS Initiative System of Provider Initiated opt-out HIV Testing and Counseling; HIV, human immunodeficiency virus; BIPAI, Baylor International Pediatric AIDS Initiative.
Children classified as HIV-infected were either DNA PCR-positive (if younger than 18 months of age), antibody-positive (if older than 18 months of age), or produced valid HIV-infected test results. HIV-exposed infants were either breastfeeding from their HIV-infected mother within six weeks of a negative DNA PCR test, or born to an HIV-infected mother but without a test result. Alternatively, their HIV-exposed status was known but they were ineligible for another DNA PCR due to a pending test. If an HIV-exposed infant tested DNA PCR-positive during hospitalization, the child’s status was re-classified as HIV-infected. Additionally, if the inpatient DNA PCR result was negative and they had last breastfed outside the six-week window period, then the patient was re-identified as HIV-uninfected and excluded from the outcomes analysis. Only the first hospitalization was analyzed for patients admitted more than once during the study period, irrespective of the child’s HIV-status.
Two BIPAI clinicians reviewed inpatients on a daily basis, assigning a World Health Organization (WHO) clinical stage to each HIV-infected child and WHO Integrated Management of Childhood Illness (IMCI)-based diagnoses to every child upon hospital discharge.16 Because this study took place at a resource-limited hospital, laboratory tests were inconsistently available for most conditions. At the time of hospital discharge, BIPAI clinicians offered HIV-infected and HIV-exposed patients referral to the COE clinic. Patient outcomes were re-evaluated one year after hospital discharge, and patients who did not return to the COE clinic for three months were considered lost to follow-up. One-year outcome data was obtained for subjects who attended the COE clinic after hospital discharge.
Statistical methods
Continuous, normally-distributed variables were described by mean and standard deviation, and compared by student’s t test. Non-normally distributed continuous variables were described with median and interquartile range, and compared by Mann-Whitney U test. Categorical variables were analyzed using Pearson’s chi square test, and when more than two variable categories were present, a significant global test (alpha = 0.05) was followed by post-hoc pairwise chi square tests, using Bonferroni’s correction to adjust alpha for multiple variable levels. A comparison was considered significant only when the p value was smaller than the corrected alpha. All analyses were performed using SPSS software (version 17.0, SPSS Inc., Chicago, IL).
Ethical Approval
The Malawi National Health Sciences Research Committee and Baylor College of Medicine institutional review board approved this study. Nationally certified HIV counselors obtained verbal consent for HIV testing from caregivers according to national guidelines.17 Consent was waived for study participation because data was collected retrospectively from patient medical files.
RESULTS
Children admitted to the pediatric ward during both periods of study were similar with respect to age and gender. Sepsis, gastroenteritis, and severe anemia were more frequently diagnosed in all children hospitalized during BIPAI-PITC (p < 0.001), irrespective of HIV status, while malaria comprised the most common diagnosis in either group (Table 1). There was a trend towards increased mortality in all patients admitted to KCH during BIPAI-PITC (p = 0.080).
TABLE 1.
Overview of All Hospitalized Children during NRT and BIPAI-PITC
| NRT Sep–Dec 2007 (n = 3752) |
BIPAI-PITC Sep–Dec 2008 (n = 3325) |
P | |
|---|---|---|---|
| Age in Months, median (IQR) | 20.4 (8.7—44.6) | 18.8 (9.1—41.6) | 0.168 |
| Females, n (%) | 1664 (44.3) | 1498 (45.1) | 0.565 |
| Final Diagnoses, n (%) | |||
| Severe Acute Malnutrition | 141 (3.8) | 142 (4.3) | 0.275 |
| Severe/Very Severe Pneumonia | 709 (18.9) | 635 (19.1) | 0.832 |
| Gastroenteritis | 368 (9.8) | 680 (20.5) | <0.001 |
| Sepsis | 555 (14.8) | 688 (20.7) | <0.001 |
| Malaria | 1853 (49.4) | 1620 (48.7) | 0.584 |
| Bacterial Meningitis | 180 (4.8) | 187 (5.6) | 0.120 |
| Severe Anemia | 228 (6.1) | 295 (8.9) | <0.001 |
| Hospital Outcome of Death, n (%) | 237 (6.3) | 245 (7.4) | 0.080 |
NRT indicates Non-Routine HIV Testing; BIPAI-PITC, Baylor International Pediatric AIDS Initiative System of Provider Initiated opt-out HIV Testing and Counseling; IQR, interquartile range.
During BIPAI-PITC, 2693 hospitalized children accepted HIV counseling and were either HIV tested or produced valid HIV test results, representing 81% of all admissions, an increase from the 33.3% of hospital admissions that accepted HIV counseling with NRT (p < 0.001, Table 2). No differences were found with respect to age, gender, WHO clinical stage, CD4-positive cell count percentage, in-hospital mortality, COE clinic enrollment, or ART initiation between the two identified HIV-infected cohorts (Table 3). In the BIPAI-PITC cohort, severe acute malnutrition was only half as prevalent as in the NRT cohort (24.2% vs 43.1%, p = 0.005), while sepsis (40.3% vs 23.1%, p = 0.015) and malaria (24.2% vs 12.3%, p = 0.048) were nearly twice as prevalent. Although a smaller proportion of hospitalized HIV-exposed infants were diagnosed with bacterial meningitis during BIPAI-PITC (2.0% vs 13.0%, p = 0.045), there were no differences with respect to other baseline characteristics or clinical outcomes (Table 4).
TABLE 2.
HIV Status and Pediatric HIV Care Received by All Hospitalized Children
| NRT September–December 2007 (n = 3752) | BIPAI-PITC September–December 2008 (n = 3325) | P | |
|---|---|---|---|
| HIV status determined,* n (%) | 1248 (33.3) | 2693 (81.0) | <0.001 |
| HIV status,† n/N (%) | <0.001 | ||
| HIV-infected | 168/1248 (13.5) | 236/2693 (8.8) | <0.001 |
| HIV-exposed uninfected | 114/1248 (9.1) | 209/2693 (7.8) | 0.144 |
| HIV-uninfected | 966/1248 (77.4) | 2248/2693 (83.5) | <0.001 |
| Received in-hospital HIV care,‡ n (%) | 88 (2.4) | 249 (7.5) | <0.001 |
| HIV status of all children receiving in-hospital HIV care,§ n/N (%) | 0.019 | ||
| HIV-infected | 65/88 (73.9) | 149/249 (59.8) | |
| HIV-exposed uninfected | 23/88 (26.1) | 100/249 (40.2) | |
| DNA PCR tests performed,¶ n/N (%) | 31/88 (35.2) | 183/249 (73.5) | <0.001 |
| Pediatric HIV clinic enrollment,‡‖ n (%) | 48 (1.3) | 106 (3.2) | <0.001 |
| HIV status of all HIV clinic enrollees, n/N (%) | 0.018 | ||
| HIV-infected | 42/48 (87.5) | 74/106 (69.8) | |
| HIV-exposed uninfected | 6/48 (87.5) | 32/106 (30.2) | |
| Initiated antiretroviral therapy, n (%) | 21 (0.6) | 37 (1.1) | 0.010 |
1248 (100.0%) NRT and 2757 (82.9%) BIPAI-PITC patients were HIV counseled, with 0 (0.0%) NRT and 64 (2.3%) BIPAI-PITC patients declining subsequent HIV testing.
1167 (93.5%) NRT and 2243 (83.3%) BIPAI-PITC patients were classified based on HIV test results obtained during their hospital admission and 81 (6.5%) NRT and 450 (16.7%) BIPAI-PITC patients were classified based on valid HIV test results obtained prior to their hospital admission.
Patients were not enrolled in outpatient HIV care at the time of hospitalization.
Seventy (79.5%) NRT and 108 (43.4%) BIPAI-PITC patients were classified based on HIV test results obtained during their hospital admission and 18 (20.5%) NRT and 141 (56.6%) BIPAI-PITC patients were classified based on valid HIV test results obtained prior to their hospital admission.
Twenty-one (67.7%) NRT and 81 (44.3%) BIPAI-PITC patients tested HIV DNA PCR positive, and 10 (32.3%) NRT and 102 (55.7%) BIPAI-PITC patients tested HIV DNA PCR negative.
Patients received outpatient HIV care at the Baylor College of Medicine-Abbott, Fund Children's Clinical Centre of Excellence.
NRT indicates nonroutine HIV testing; BIPAI-PITC, Baylor International Pediatric AIDS Initiative system of provider-initiated opt-out HIV testing and counseling; HIV, human immunodeficiency virus; IQR, interquartile range.
TABLE 3.
Baseline Characteristics and Outcomes of HIV-infected Hospitalized Children*
| NRT September–December 2007 (n = 65) | BIPAI-PITC September–December 2008 (n = 149) | P | |
|---|---|---|---|
| Age in months, media (IQR) | 21.4 (8.0–38.2) | 15.7 (7.7–32.7) | 0.309 |
| Females, n (%) | 26 (40.0) | 77 (51.7) | 0.116 |
| Hospital WHO Stage, n (%) | 0.651 | ||
| Stage I | 7 (10.8) | 22 (14.8) | |
| Stage II | 10 (15.4) | 15 (10.1) | |
| Stage III | 33 (50.8) | 77 (51.7) | |
| Stage IV | 15 (23.1) | 35 (23.5) | |
| Hospital CD4-Positive cell count percentage obtained, n (<5 y/o) | 28 | 46 | |
| Mean (SD) | 19.9 (8.9) | 22.3 (9.4) | 0.260 |
| CPT initiation in hospital,† n/N(%) | 60/61 (98.4) | 139/141 (98.6) | 0.905 |
| ART-eligible in hospital,‡ n (%) | 60 (92.3) | 128 (85.9) | 0.187 |
| Final hospital diagnoses, n (%) | |||
| Severe acute malnutrition | 28 (43.1) | 36 (24.2) | 0.005 |
| Severe/very severe pneumonia | 27 (41.5) | 63 (42.3) | 0.919 |
| Gastroenteritis | 18 (27.7) | 38 (25.5) | 0.738 |
| Sepsis | 15 (23.1) | 60 (40.3) | 0.015 |
| Malaria | 8 (12.3) | 36 (24.2) | 0.048 |
| Pulmonary tuberculosis | 8 (12.3) | 15 (10.1) | 0.626 |
| In-hospital outcome of death, n (%) | 11 (16.9) | 22 (14.8) | 0.688 |
| Enrollment of referred hospital patients into COE clinic,§ n/N (%) | 42/54 (77.8) | 74/110 (67.3) | 0.165 |
| COE clinic, enrollees initiated on ART, n/N (%) | 21/39 (53.9) | 37/67 (55.2) | 0.891 |
| Duration from HIV test to ART initiation in months, mean (SD) | 4.7 (4.3) | 4.0 (4.9) | 0.563 |
| One year outcome of COE clinic enrollees (Total), n/N (%) | 0.610 | ||
| Alive and in care | 20/42 (47.6) | 27/74 (36.5) | |
| Dead | 8/42 (19.0) | 15/74 (20.3) | |
| Lost to follow-up | 7/42 (16.7) | 13/74 (17.6) | |
| Transferred out | 7/42 (16.7) | 19/74 (25.7) |
Patients were not enrolled in outpatient HIV care at the time of hospitalization.
Sixty-one NRT and 141 BIPAI-PITC patients were eligible for CPT initiation. 3 NRT and 6 BIPAI-PITC patients were already on treatment-dose cotrimoxazole; 1 NRT and 2 BIPAI-PITC patients were too young to initiate CPT.
ART eligibility for NRT patients was determined by WHO and CD4 criteria only. ART eligibility for BIPAI-PITC patients was determined by WHO, CD4, and age-based criteria. Note that CD4 was available for only 38 (58.5%) NRT children and 56 (3.7.6%) BIPAI-PITC children.
Fifty-four (100.0%) NRT and 110 (86.6%) BIPAI-PITC patients were referred to the COE clinic at hospital discharge.
NRT indicates nonroutine HIV testing; BIPAI-PITC, Baylor for International Pediatric AIDS Initiative system of provider-initiated opt-out HIV testing and counseling; HIV, human immunodeficiency virus; IQR, interquartile range; WHO, World Health Organization; SD, standard deviation; CPT, cotrimoxazole prophylactic therapy; ART, antiretroviral therapy; COE, Baylor College of Medicine-Abbott Fund Children's Centre of Excellence.
TABLE 4.
Baseline Characteristics and Outcomes of HIV-exposed Uninfected Hospitalized Children*
| NRT September–December 2007 (n = 23) |
BIPAI-PITC September–December 2008 (n = 100) | P | |
|---|---|---|---|
| Age in months, median (IQR) | 6.4 (3.8–9.8) | 7.7 (2.5–12.2) | 0.881 |
| Females, n (%) | 10 (43.5) | 47 (47.0) | 0.760 |
| CPT initiation in hospital, n/N (%)† | 22/22 (100.0) | 81/85 (95.3) | 0.579 |
| Final hospital diagnoses, n (%) | |||
| Severe acute malnutrition | 5 (21.7) | 16 (16.0) | 0.542 |
| Severe/very severe pneumonia | 6 (26.1) | 25 (25.0) | 0.914 |
| Gastroenteritis | 10 (43.5) | 38 (38.0) | 0.627 |
| Sepsis | 7 (30.4) | 42 (42.0) | 0.307 |
| Malaria | 3 (13.0) | 21 (21.0) | 0.561 |
| Bacterial meningitis | 3 (13.0) | 2 (2.0) | 0.045 |
| In-hospital outcome of death, n (%) | 5 (21.7) | 12 (12.0) | 0.311 |
| Enrollment of referred hospital patients into COE, n/N (%) | 6/18 (33.3) | 32/69 (46.4) | 0.320 |
| Final HIV status of COE clinic enrollees, n/N (%) | 1.000 | ||
| HIV-exposed uninfected | 1/6 (16.7) | 6/32 (18.8) | |
| HIV-uninfected | 5/6 (83.3) | 26/32 (81..3) | |
| One year outcome of COE clinic enrollees, n/N (%) | 0.664 | ||
| Lost to follow-up | 1/6 (16.7) | 3/32 (9.4) | |
| Transferred out | 0/6 (0.0) | 3/32 (9.4) | |
| Dischareed from clinic/HIV-uninfected | 5/6 (83.3) | 26/32 (81.3) |
Ten NRT patients and 63 BIPAI-PITC patients had negative DNA PCR test results obtained during hospitalization. Patients were not enrolled in outpatient HIV care at the time of hospitalization.
Twenty-two NRT and 85 BIPAI-PITC patients were eligible for CPT initiation. 1 NRT patient and 12 BIPAI-PITC patients were too young to initiate CPT; 3 BIPAI-PITC patients were already on proscription-dose cotrimoxazole.
NRT indicates nonroutine HIV testing; BIPAI-PITC, Baylor International Pediatric AIDS Initiative system of provider initiated opt-out HIV testing and counseling; HIV, human immunodeficiency virus; IQR, interquartile range; CPT, cotrimoxazole prophylactic therapy; COE, Baylor College of Medicine-Abbott Fund Children's Centre of Excellence; SD, standard deviation.
DISCUSSION
HIV-infected African children continue to be denied ART due to inadequate and inaccessible HIV services. Our results show that with a relatively modest investment of resources, BIPAI-PITC not only increased HIV testing rates, but also increased access to inpatient pediatric HIV clinicians, outpatient pediatric HIV care, DNA PCR, and ART. Furthermore, increasing access to inpatient and outpatient pediatric HIV care for hospitalized HIV-exposed infants during BIPAI-PITC is particularly important in this era of new PMTCT strategies such as extended post-natal antiretroviral prophylaxis during breastfeeding.18–22
BIPAI-PITC, compared to NRT, better utilized skilled healthcare personnel in a setting with inadequate numbers of healthcare providers. During BIPAI-PITC, the total number of HIV-infected and HIV-exposed patients identified by inpatient HIV testing increased by 163, and those receiving inpatient and outpatient pediatric HIV care increased by 161 and 58, respectively, compared to NRT. These results were achieved with the same staffing levels of HIV counselors and pediatric HIV-trained clinicians, aided by four unskilled volunteer patient escorts during BIPAI-PITC.
Given that early ART slows disease progression and reduces mortality among HIV-infected children,23–27 the higher proportion of hospitalized children accessing ART during BIPAI-PITC achieved a key program objective. Along with the incorporation of recently-revised WHO guidelines that expand ART eligibility for children,28 wider implementation of similar inpatient PITC strategies could narrow the gap between children in need of ART and PMTCT and those who have access to it.
BIPAI-PITC also identified HIV-infected patients with a different set of clinical presentations. In particular, HIV-infected children during BIPAI-PITC had a higher proportion of malaria and a lower proportion of severe acute malnutrition, despite these diagnoses being equally represented in both periods at KCH. These data suggest that NRT misses many HIV-infected children hospitalized with malaria. One explanation is that these patients may lack the physical stigmata typical of HIV infection, including marasmus. A non-routine HIV testing system that relies solely on clinical judgment to refer patients for HIV testing is therefore more likely to miss HIV-infected patients with non-specific febrile syndromes.
However, BIPAI-PITC fell short of its ART potential. More than 85% of all identified HIV-infected patients were ART-eligible during hospitalization, but none were started on ART in the hospital, and only half of those enrolling in outpatient care initiated ART within one year of hospital discharge. Furthermore, although BIPAI-PITC improved DNA PCR access, more than 70% of ART-eligible HIV-infected infants below 18 months of age who followed-up at the COE clinic failed to start ART within one year of enrollment despite the broadening of ART eligibility guidelines during BIPAI-PITC. Routine in-hospital ART initiation for eligible children is one potential means of addressing these shortcomings, but the feasibility of this strategy needs further evaluation.
Additionally, we observed high in-hospital and outpatient mortality in the HIV-infected and HIV-exposed cohorts regardless of the testing approach, confirming reports from comparable settings.8–10 This high mortality could reflect the severity of disease observed in HIV-infected children at the time of testing. Specifically, children in both cohorts presented with advanced WHO clinical stages, low mean CD4-positive cell count percentages, severe acute malnutrition, severe forms of pneumonia, and sepsis, all of which are highly predictive of in-hospital mortality in HIV-infected and HIV-exposed children (Preidis GA, McCollum ED et al unpublished data). Case management guidelines of HIV-infected children with these diagnoses, while based upon the best available evidence, are in need of further examination to refine best practices in resource-limited inpatient and outpatient settings.29–31
Although BIPAI-PITC increased access to pediatric HIV care for all hospitalized children, we did not find that a higher proportion of identified HIV-infected and HIV-exposed patients received in-hospital or outpatient HIV care during BIPAI PITC, compared to NRT. These observations highlight the challenges associated with resource-limited health systems, rather than inefficiencies of access to care specific to BIPAI-PITC, for two reasons. Firstly, BIPAI inpatient clinicians nearly tripled their workload to meet the greater inpatient clinical demands of BIPAI-PITC but were still unable to review every hospitalized patient. While training additional KCH clinicians in HIV could further increase the proportion of HIV-infected patients receiving in-hospital HIV care, high government-mandated training fees along with poor retention of hospital staff would have inflated program costs beyond what was available at KCH. Secondly, the high outpatient attrition rate during BIPAI-PITC, while similar to NRT and other reports,32–34 suggests that the hospital’s current strategy to reduce the number of patients lost-to-follow-up, which utilizes vehicles to visit defaulted patients, needs re-evaluation. For instance, mechanisms of tracking defaulted patients could be made more successful and less costly with greater utilization of community volunteers or mobile phone technology.35
There are limitations to this study. Evaluating overall access to pediatric HIV care with the general pediatric inpatient population as a common denominator is limited by the inclusion of HIV-uninfected children. However, this cohort was the most appropriate to assess HIV care access, given that each testing period includes comparable proportions of unidentified HIV-infected patients, based upon stable national HIV prevalence data.36 Undiagnosed HIV-infected children were also likely to distort the in-hospital outcomes of the HIV-exposed group, given that 37% of HIV-exposed uninfected children did not have a negative inpatient DNA PCR test result to confirm their current hospital HIV status (data not shown). Although a potential confounder, this situation accurately reflects the typical clinical environment encountered by healthcare providers working in government facilities in resource-constrained countries.
In summary, we found that transitioning from NRT to BIPAI-PITC led to improvements in hospitalized children’s access to HIV testing, DNA PCR, inpatient and outpatient pediatric HIV care, and ART, without increasing the number of HIV counselors or pediatric HIV-trained clinicians. Where resources allow, even greater improvements in access to pediatric HIV care may be realized by also increasing the number of HIV-trained clinicians and utilizing improved defaulter-tracking methods. Thus, the expansion of BIPAI-PITC or similar systems of inpatient PITC would improve utilization of global pediatric HIV services.
Acknowledgements
We would like to thank the children and caregivers whom we serve at the Pediatric Department of Kamuzu Central Hospital and the Baylor College of Medicine-Abbott Fund Children’s Clinical Centre of Excellence, as well as acknowledge the following individuals for their important contributions to this work: Ms. Charity Kanga, Mr. Andrea Tembo, Ms. Effie Phanga, Mr. Paul Kalionde, Ms. Gertrude Chaponda, Ms. Lonely Kalinde, Mr. Gift Chidathawa, Ms. Christina Ngozo, Ms. Gladys Mwenechanya, Ms. Lennie Nkhata, Dr. Mark M. Kabue, PhD, Mr. Emmanuel B.M. Singogo, the Baylor International Pediatric AIDS Initiative Pediatric AIDS Corps physicians, the Baylor College of Medicine-Abbott Fund Children’s Clinical Centre of Excellence clinical and administrative staff, and the healthcare providers and administration at Kamuzu Central Hospital.
Financial disclosure: The Baylor International Pediatric AIDS Initiative, which receives primary funding from Bristol Myers Squibb, Abbott Fund, Texas Children’s Hospital, United Nations Children’s Fund, and the Malawi Ministry of Health, provided program support. This work was also sponsored in part by the National Institutes of Health (R24 TW007988) through the Fogarty International Center and International Clinical Research Fellows Program at Vanderbilt University and the University of North Carolina Center for AIDS Research (5 P30-AI50410). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. AIDS epidemic update December 2009. Geneva, Switzerland: WHO; 2009. [Accessed August 16, 2010]. Available at: http://data.unaids.org:80/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf. [Google Scholar]
- 2.United Nations Children's Fund. Children and AIDS: Third Stocktaking Report. New York, NY: UNICEF; 2008. 2008. [Accessed August 16, 2010]. Available at: http://www.unicef.org/publications. [Google Scholar]
- 3.Ministry of Health Malawi: HIV Unit. Malawi ART Programme Report for Quarter 1 2009. Lilongwe: Malawi; 2009. [Google Scholar]
- 4.The Malawi Paediatric Antiretroviral Treatment Group. Antiretroviral therapy for children in the routine setting in Malawi. Trans R Soc Trop Med Hyg. 2007;101:511–516. doi: 10.1016/j.trstmh.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Makombe S, Libamba E, Mhango E, et al. Who is accessing antiretroviral therapy during national scale-up in Malawi? Trans R Soc Trop Med Hyg. 2006;100:975–979. doi: 10.1016/j.trstmh.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Guidance on Provider-Initiated HIV Testing and Counselling in Health Facilities. Geneva, Switzerland: WHO; 2007. [Accessed August 16, 2010]. Available at: http://whqlibdoc.who.int/publications/2007/9789241595568_eng.pdf. [Google Scholar]
- 7.World Health Organization. Report of the WHO Technical Reference Group, Paediatric HIV/ART Care Guideline Group Meeting. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 8.Rogerson SR, Gladstone M, Callaghan M, et al. HIV infection among paediatric in-patients in Blantyre, Malawi. Trans R Soc Trop Med Hyg. 2004;98:544–552. doi: 10.1016/j.trstmh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Thurstans S, Kerac M, Maleta K, Banda T, Nesbitt A. HIV prevalence in severely malnourished children admitted to nutrition rehabilitation units in Malawi: Geographical and seasonal variations a cross-sectional study. BMC Pediatr. 2008;8:22. doi: 10.1186/1471-2431-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinkhumba J, Tomkins A, Banda T, Mkangama C, Fergusson P. The impact of HIV on mortality during in-patient rehabilitation of severely malnourished children in Malawi. Trans R Soc Trop Med Hyg. 2008;102:639–644. doi: 10.1016/j.trstmh.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 11.McCollum ED, Preidis GA, Kabue MM, et al. Task Shifting Routine Inpatient Pediatric HIV Testing Improves Program Outcomes in Urban Malawi: A Retrospective Observational Study. PLos ONE. 2010;5:3. doi: 10.1371/journal.pone.0009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kankasa C, Carter RJ, Briggs N, et al. Routine Offering of HIV Testing to Hospitalized Pediatric Patients at University Teaching Hospital, Lusaka, Zambia: Acceptability and Feasibility. J Acquir Immune Defic Syndr. 2009;51:202–208. doi: 10.1097/qai.0b013e31819c173f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanyenze RK, Nawavvu C, Ouma J, Namale A, Colebunders R, Kamya MR. Provider-initiated HIV testing for paediatric inpatients and their caretakers is feasible and acceptable. Trop Med Int Health. 2010;15:113–119. doi: 10.1111/j.1365-3156.2009.02417.x. [DOI] [PubMed] [Google Scholar]
- 14.Weigel R, Kamthunzi P, Mwansambo C, Phiri S, Kazembe PN. Effect of provider-initiated testing and counselling and integration of ART services on access to HIV diagnosis and treatment for children in Lilongwe, Malawi: a pre-post comparison. BMC Pediatr. 2009;9:80. doi: 10.1186/1471-2431-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabue MM, Chitsulo C, Kazembe P, et al. A paediatric HIV care and treatment programme in Malawi. Malawi Med J. 2008;20:19–22. doi: 10.4314/mmj.v20i1.10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Technical updates of the guidelines on the Integrated Management of Childhood Illness (IMCI): evidence and recommendations for further adaptations. Geneva, Switzerland: WHO; 2005. [Accessed August 16, 2010]. Available at: http://whqlibdoc.who.int/publications/2005/9241593482.pdf. [Google Scholar]
- 17.Ministry of Health Malawi: HIV Unit. Guidelines for Paediatric HIV Testing and Counseling. Lilongwe: Malawi; 2008. [Google Scholar]
- 18.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or Infant Antiretroviral Drugs to Reduce HIV-1 Transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro RL, Ogwu A, Kitch D, et al. Antiretroviral Regimens in Pregnancy and Breast-Feeding in Botswana. N Engl J Med. 2010 Jun 17;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended Antiretroviral Prophylaxis to Reduce Breast-Milk HIV-1 Transmission. N Engl J Med. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 21.Kilewo C, Karlsson K, Massawe A, et al. Prevention of Mother-to-Child Transmission of HIV-1 Through Breast-Feeding by Treating Infants Prophylactically With Lamivudine in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2008;48:315–323. doi: 10.1097/QAI.0b013e31816e395c. [DOI] [PubMed] [Google Scholar]
- 22.Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–313. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 23.Berk DR, Falkovitz-Halpern MS, Hill DW, et al. Temporal Trends in Early Clinical Manifestations of Perinatal HIV Infection in a Population-Based Cohort. JAMA. 2005;293:2221–2231. doi: 10.1001/jama.293.18.2221. [DOI] [PubMed] [Google Scholar]
- 24.Violari A, Cotton MF, Gibb DM, et al. Early Antiretroviral Therapy and Mortality among HIV-Infected Infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luzuriaga K, McManus M, Mofenson LM, Britto P, Graham B, Sullivan JL. A trial of three antiretroviral regimens in HIV-1 infected children. N Engl J Med. 2004;350:2471–2480. doi: 10.1056/NEJMoa032706. [DOI] [PubMed] [Google Scholar]
- 26.Chiappini E, Galli L, Tovo PA, et al. Virologic, immunologic, and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS. 2006;20:207–215. doi: 10.1097/01.aids.0000200529.64113.3e. [DOI] [PubMed] [Google Scholar]
- 27.Faye A, Le Chenadec J, Dollfus C, et al. Early versus deferred antiretroviral multidrug therapy in infants infected with HIV type 1. Clin Infect Dis. 2004;39:1692–1698. doi: 10.1086/425739. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Antiretroviral Therapy For HIV Infection In Infants and Children: Towards Universal Access. Geneva, Switzerland: WHO; 2010. [Accessed August 16, 2010]. Available at: http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. [PubMed] [Google Scholar]
- 29.World Health Organization. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. Geneva, Switzerland: WHO; 2005. [Accessed August 16, 2010]. Available at: http://whqlibdoc.who.int/publications/2005/9241546700.pdf. [Google Scholar]
- 30.Heikens GT, Bunn J, Amadi B, et al. Case Management of HIV-infected severely malnourished children: challenges in the area of highest prevalence. Lancet. 2008;371:1305–1307. doi: 10.1016/S0140-6736(08)60565-6. [DOI] [PubMed] [Google Scholar]
- 31.Enarson P, Gie R, Enarson D, Mwansambo C, Graham SM. Impact of HIV on standard case management for severe pneumonia in children. Expert Rev Respir Med. 2010;2:211–220. doi: 10.1586/ers.10.14. [DOI] [PubMed] [Google Scholar]
- 32.Braitstein P, Katshcke A, Shen C, et al. Retention of HIV-infected and HIV-exposed children in a comprehensive HIV clinical care programme in Western Kenya. Trop Med Int Health. 2010;7:833–841. doi: 10.1111/j.1365-3156.2010.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The KIDS-ART-LINC Collaboration. Low Risk of Death, but Substantial Program Attrition, in Pediatric HIV Treatment Cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49:523–531. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 34.Rosen S, Fox MP, Gill CJ. Patient Retention in Antiretroviral Therapy Programs in Sub-Saharan Africa: A Systematic Review. PLoS Med. 2007;4:10. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 36.Bello GA, Chipeta J, Aberle-Grasse J. Assessment of trends in biological and behavioural surveillance data: is there any evidence of declining HIV prevalence or incidence in Malawi? Sex Transm Infect. 2006;82:i9–i13. doi: 10.1136/sti.2005.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]


