Abstract
Background
Initially described in 1926, spinal dural arteriovenous fistula remains diagnostically challenging. Presenting symptoms are often common complaints in general practice or the emergency department, such as leg weakness or numbness. One of the less recognized early features is that of sphincteric disturbance.
Objectives
To elucidate the underappreciated early feature of urinary sphincter disturbance in spinal arteriovenous fistula.
Case Report
We report on 2 patients with early urinary symptoms that mimicked obstructive uropathy, both of whom sought medical attention on several occasions before the diagnosis was reached. The clinical and imaging findings of spinal dural fistula are discussed.
Conclusion
Atypical presentations of bladder dysfunction in addition to other neurologic complaints in the appropriate patient population should prompt spinal imaging to exclude a spinal dural fistula. Timely diagnosis and treatment can prevent the progression of spinal cord edema to infarction with paraparesis, and permanent bladder and bowel dysfunction.
Keywords: Spinal dural arteriovenous fistula, prostate hyperplasia, obstructive uropathy, myelopathy, flow void
Introduction
Spinal dural arteriovenous fistula (SDAVF) remains a diagnostic challenge 82 years after its first description by Foix and Alajouanine (1). The presenting symptoms simulate a wide variety of diagnoses, and the radiological findings are often subtle (2). Among the underappreciated early features are sphincteric difficulties. We report 2 patients with early urinary symptoms that mimicked obstructive uropathy, both of whom sought medical attention on several occasions before the correct diagnosis was reached.
Case Reports
Patient 1
A 67-year-old man presented to the Emergency Department (ED) with urinary retention. He had been well until 1 month before admission, when he complained of urinary hesitancy and frequency without dysuria. Tamsolusin was prescribed for benign prostatic hypertrophy but did not improve his symptoms. Over the following weeks, he sought medical attention repeatedly, but no conclusive diagnosis or treatment was reached. During that month, he was also troubled by left leg pain radiating from hip to knee, followed by right hip and posterior thigh tightness radiating down to the ankle. The pain and increasing difficulty walking were attributed to a previous lumbar disc surgery. He was treated with non-steroidal anti-inflammatory drugs for presumed osteoarthritis.
On examination, there was increased tone and mild symmetrical weakness (4/5 Medical Research Council scale) of both legs. Patellar and Achilles reflexes were brisk and there was an extensor plantar response on the right. Light touch and joint position sensations were preserved. Pinprick was diminished in the legs, with a sensory level at T7. His gait was narrow-based and antalgic. Rectal tone was normal.
Magnetic resonance imaging (MRI) scan of the spine showed an abnormal central T2 signal extending from T6 to the conus. There was no cord compression or abnormal vasculature. Cerebrospinal fluid (CSF) examination showed protein 99 mg/dL, 183 red blood cells (RBC)/mm3, 12 white blood cells (WBC)/mm3, without xanthochromia. A spinal angiogram revealed a dural fistula supplied by bilateral L4 segmental arteries that were successfully occluded by embolization.
At 6 months follow-up, the patient's walking had improved and the constipation resolved, but he was unable to void spontaneously and continued to self-catheterize several times per day.
Patient 2
A 75-year-old man with prostate cancer, treated by prostatectomy and radiotherapy several years earlier, began experiencing numbness and tingling in the ankles 2 years before presentation to the ED. Metastatic disease to the spine was suspected, but lumbar spine MRI study showed only multilevel foraminal and lumbar spinal canal stenoses. Several months later, the sensory symptoms had progressed to the thighs, and he complained of leg weakness and loss of leg muscle bulk, but no cause was identified despite a thorough neuropathy workup.
Over the same time period, mild urinary incontinence, which had begun after the prostatectomy, had worsened severely, to the extent that he required hourly change of incontinence pads. There was no dysuria or constipation. Despite recurrent visits to several physicians, the symptoms were attributed to worsening of the post-surgical effects of the prostatectomy and after-effects of radiation therapy.
On examination, he had proximal (3/5) greater than distal (4/5) leg weakness. Rectal tone was normal. Reflexes were absent at the knees and ankles, and the toes were flexor. Vibration sense was preserved above the ankles. Pinprick and light touch were diminished in both lower extremities, up to the proximal thighs, without a sensory level. He was unable to walk without assistance.
CSF examination revealed protein 128 mg/dL, glucose 50 mg/dL, RBC 2, and WBC 1 without xanthochromia.
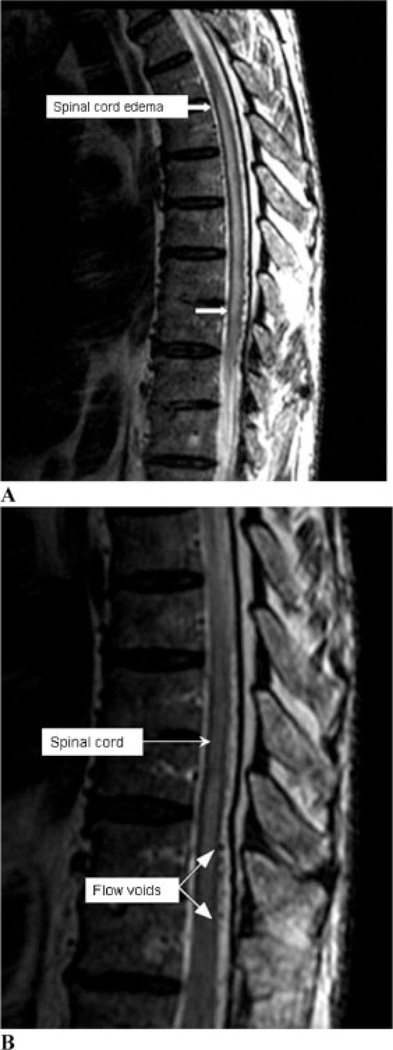
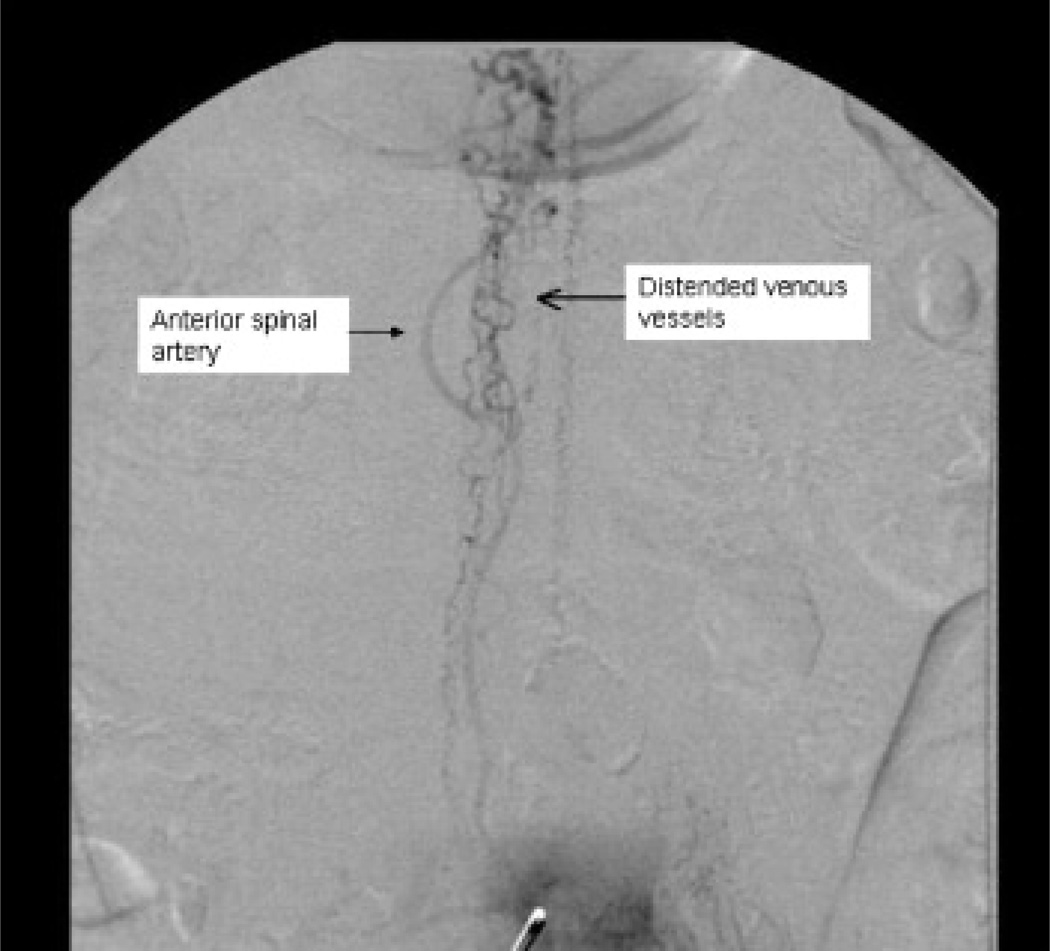
MRI study of the spine showed a central cord signal abnormality from T4/5 to the conus, with flow voids along the dorsal and ventral surface of the thoracic cord (Figure 1). An angiogram confirmed a dural fistula supplied by a radicular branch of the left L3 segmental artery (Figure 2), which was embolized.
Figure 1.
Magnetic resonance image of the spine. (A) T2- weighted image showing a hyperintense signal (best visible in area between white arrows), indicating spinal cord edema. (B) T2-weighted image showing hypointense flow voids (black round dots at the posterior aspect of the cord, best visible in area between white arrows), indicating high flow through venules due to the spinal dural fistula.
Figure 2.
Spinal angiogram (conventional digital subtraction angiography) showing arteriovenous fistula (large vessel _ anterior spinal artery; left side: retrograde filling of “corkscrew”-shaped vessels, which are small but distended venous vessels).
At 6 months, his leg strength had improved, and he was able to walk independently, but severe incontinence persisted.
Discussion
SDAVF accounts for up to 70% of the spinal arteriovenous malformations (3). Its incidence is believed to be 0.5 to 1 per 100,000 and rises with age. At diagnosis, more than two-thirds of patients are above the age of 60 years (2). For unknown reasons, males outnumber females at rates of 5M:1F.
The slowly progressive course of the disease and the insidious onset of symptoms, which can be attributed to a number of other, perhaps even co-existent, pathologies, often delay the diagnosis by a year or more, with one report citing more than 20 years from onset to time of diagnosis (4 and 5). Around 1% of patients, in whom the fistula extends intracranially, present more dramatically with subarachnoid hemorrhage (2).
Although the exact pathophysiology remains unknown, it is believed to be an acquired disease. SDAVF is a vascular malformation in which an abnormal group of vessels in the dura surrounding the spinal cord is fed by dural branches of radicular arteries. These, in turn, drain into an “arterialized” vein and lead to retrograde flow into the coronal venous plexus of the spinal cord. The imbalance of arterial inflow to venous outflow leads to elevated pressure within the subarachnoid venous plexus and edema of the cord. Although initially reversible, over time, progressive clinical symptoms of myelopathy are caused by chronic venous hypertension of the cord associated with venous engorgement culminating in cord edema, ischemia and, ultimately, necrosis. A recent case report describes this pathophysiology in action: acute paraplegia during singing in a professional opera singer—most likely caused by intermittent sharp increases in venous pressure due to respiratory changes—is shown to be due to a SDAVF (6).
The fistula between a radicular artery and its corresponding radicular vein tends to be located intra-durally at or in the nerve root sleeve. Any artery supplying the dura may be involved, but SDAVFs are predominantly located in the lower portion of the thoracic and upper part of the lumbar spinal cord (2 and 7). The lesions tend to be single, and only rarely are two fistulae reported in the same patient (8).
Infection, syringomeylia, trauma, and surgery are a minority of so-far described risk factors (2). In contrast to intracranial dural arteriovenous fistula, prothrombotic conditions have not been shown to be related to SDAVF (9). Although patient 1 had previous lumbar disc surgery, a causative relationship to the SDAVF seems rather unlikely without any reported damage to the dura or its blood supply during the procedure.
The typical age of onset and male predominance of spinal dural fistula makes this a condition that is likely to occur in the same population that experiences prostatic urinary symptoms (2). As a presenting symptom, one often encounters lower back or radicular pain, suggesting spinal degenerative disease (10). Once the disease has progressed to the fully established syndrome of paraparesis, most patients have bladder and bowel dysfunction and erectile dysfunction. However, when sphincteric symptoms and urinary retention are early features, they are liable to be attributed to prostatic hypertrophy.
Both patients described here had significant early bladder symptoms, thought to be due to prostate hyperplasia in the first patient and worsening of pre-existing urinary symptoms in the second patient. Particularly in the latter, the deterioration in urinary function was difficult to appreciate given previous surgery and baseline incontinence. It is rather unfortunate that urinary dysfunction is less likely to recover than are the other features of myelopathy (2 and 11).
The diagnosis, once suspected on the basis of a progressive, unusual history, is confirmed with imaging. MRI of the spine shows high signal in the cord on T2-weighted imaging, indicative of cord edema and ischemia. Interestingly, irrespective of the location of the fistula, the MRI T2 signal changes mostly involve the lower cord, thought to be due to orthostasis.
MRI will also show so-called flow voids of abnormal veins in the adjacent subarachnoid space (12). Other diagnoses, which are commonly associated with urinary dysfunction, such as spinal cord compression or tethered cord syndrome, are readily ruled out by spinal cord MRI.
Spinal angiography allows diagnostic confirmation and offers the possibility of treatment by embolization of the feeding vessels. Our patients were both treated with embolization. Studies have shown surgery to be more effective than embolization in curing SDAVF, but surgeons require a precise site and level of the fistula to approach it correctly. Given the paucity of data regarding the use of magnetic resonance angiography (MRA) and other non-invasive angiographic assessments, such as multi-detector computed tomographic angiography (MDCTA), to establish these reliably, this field is awaiting further studies, though MRA and, more recently, MDCTA do look promising, and MRA is more readily available (12 and 13).
In many centers, the current approach to diagnosis would initially be to obtain an MRI/A for suspected SDAVF and to proceed with angiography if indicated.
Patients often undergo lumbar puncture to exclude alternative diagnoses. Up to threequarters of patients present with increased CSF protein without pleocytosis, indicative of blood-brain-barrier breakdown. Both patients presented here underwent lumbar puncture, with the most notable finding being elevated protein in the absence of pleocytosis. In the cases we present, the saltatory progression of symptoms and elevated CSF protein gave hints to the nature of the vascular lesion. MRI and angiography established the diagnosis.
Conclusion
Although rare, these cases are presented to increase awareness that dural fistula may cause atypical presentations of bladder dysfunction, especially when presenting with other unexplained or unsuspected neurological symptoms or examination findings. Timely diagnosis and treatment can prevent the progression of spinal cord edema to infarction with paraparesis, and permanent bladder and bowel dysfunction.
Acknowledgements
This work was supported by the NIH/NINDS: R01 NS067139 (Ning).
References
- 1.Foix C, Alajouanine T. Subacute necrotic myelitis, slowly progressive central myelitis with vascular hyperplasia, and slowly ascending, increasingly flaccid amyotrophic paraplegia accompanied by albuminocytologic dissociation. Rev Neurol (Paris) 1926;33:1–42. [Google Scholar]
- 2.Koch C. Spinal dural arteriovenous fistula. Curr Opin Neurol. 2006;19:69–75. doi: 10.1097/01.wco.0000200547.22292.11. [DOI] [PubMed] [Google Scholar]
- 3.Gilbertson JR, Miller GM, Goldman MS, Marsh WR. Spinal dural arteriovenous fistulas: MR and myelographic findings. AJNR Am J Neuroradiol. 1995;16:2049–2057. [PMC free article] [PubMed] [Google Scholar]
- 4.Jellema K, Canta LR, Tijssen CC, et al. Spinal dural arteriovenous fistulas: clinical features in 80 patients. J Neurol Neurosurg Psychiatry. 2003;74:1438–1440. doi: 10.1136/jnnp.74.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dijk JM, TerBrugge KG, Willinsky RA, et al. Multidisciplinary management of spinal dural arteriovenous fistulas: clinical presentation and long-term follow-up in 49 patients. Stroke. 2002;33:1578–1583. doi: 10.1161/01.str.0000018009.83713.06. [DOI] [PubMed] [Google Scholar]
- 6.Khurana VG, Perez-Terzic CM, Petersen RC, Krauss WE. Singing paraplegia: a distinctive manifestation of a spinal dural arteriovenous fistula. Neurology. 2002;58:1279–1281. doi: 10.1212/wnl.58.8.1279. [DOI] [PubMed] [Google Scholar]
- 7.Hassler W, Thron A, Grote EH. Hemodynamics of spinal dural arteriovenous fistulas. An intraoperative study. J Neurosurg. 1989;70:360–370. doi: 10.3171/jns.1989.70.3.0360. [DOI] [PubMed] [Google Scholar]
- 8.Krings T, Mull M, Reinges MH, Thron A. Double spinal dural arteriovenous fistulas: case report and review of the literature. Neuroradiology. 2004;46:238–242. doi: 10.1007/s00234-003-1147-1. [DOI] [PubMed] [Google Scholar]
- 9.Jellema K, Tijssen CC, Fijnheer R, et al. Spinal dural arteriovenous fistulas are not associated with prothrombotic factors. Stroke. 2004;35:2069–2071. doi: 10.1161/01.STR.0000135766.22285.dc. [DOI] [PubMed] [Google Scholar]
- 10.Sleiman M, Leclerc X, Lejeune JP, et al. Clinical aspects and treatment of dural spinal arteriovenous fistulas with perimdedullary venous drainage. 10 cases [French] Neurochirurgie. 1999;45:276–285. [PubMed] [Google Scholar]
- 11.Jellema K, Tijssen CC, van Gijn J. Spinal dural arteriovenous fistulas: a congestive myelpathy that initially mimics a peripheral nerve disorder. Brain. 2006;129:3150–3164. doi: 10.1093/brain/awl220. [DOI] [PubMed] [Google Scholar]
- 12.Zampakis P, Santosh C, Taylor W, Teasdale E. The role of noninvasive computed tomography in patients with suspected dural fistulas with spinal drainage. Neurosurgery. 2006;58:686–694. doi: 10.1227/01.NEU00199163.10539.56. [DOI] [PubMed] [Google Scholar]
- 13.Saraf-Lavi E, Bowen BC, Quencer RM, et al. Detection of spinal dural arteriovenous fistulae with MR imaging and contrast-enhanced MR angiography: sensitivity, specificity and prediction of vertebral level. AJNR Am N Neuroradiol. 2002;22:858–867. [PMC free article] [PubMed] [Google Scholar]