Abstract
It is widely accepted that inflammation plays some role in the progression of chronic neurodegenerative diseases such as Alzheimer’s disease but its precise role remains elusive. It has been known for many years that systemic inflammatory insults can signal to the brain to induce changes in CNS function, typically grouped under the syndrome of sickness behaviour. These changes are mediated via systemic and CNS cytokine and prostaglandin synthesis. When patients with dementia suffer similar systemic inflammatory insults, delirium is a frequent consequence. This profound and acute exacerbation of cognitive dysfunction is associated with poor prognosis: accelerating cognitive decline and shortening time to permanent institutionalization and death. Therefore a better understanding of how delirium occurs during dementia and how these episodes impact on existing neurodegeneration are now important priorities. The current review summarises the relationship between dementia, systemic inflammation and episodes of delirium and addresses the basic scientific approaches currently being pursued with respect to understanding acute cognitive dysfunction during aging and dementia. In addition, though there are limited studies on this subject, it is becoming increasingly clear that infections and other systemic inflammatory conditions do increase the risk of Alzheimer’s disease and accelerate the progression of established dementia. These data suggest that systemic inflammation is a major contributor to the progression of dementia and constitutes an important clinical target.
Keywords: animal model, delirium, dementia, systemic inflammation, infection, microglia, priming, Alzheimer’s disease
Introduction
It has been known for many years that inflammation has a role in the progression of Alzheimer’s disease (AD). Patients taking non-steroidal anti-inflammatory drugs for the amelioration of rheumatoid arthritis are significantly protected against the subsequent development of Alzheimer’s disease (1). These findings, first made more than 20 years ago, have been replicated many times but prospective studies using these and similar drugs have not had significant beneficial effects for patients (2, 3). Nonetheless, the original findings have launched an enormous field of research into the nature of the CNS inflammatory response during Alzheimer’s disease and this field continues apace. This research, unsurprisingly, has been firmly focused on the brain macrophage population, the microglia, since these cells clearly surround the amyloid plaques that become deposited in the brain.
However, one aspect of the RA studies that has received less attention is the fact that patients who were protected against development of AD, benefited from a therapy that targeted peripheral inflammation. Whether reducing peripheral inflammation contributed significantly to the protective effects observed has received remarkably little discussion. It is increasingly clear that major risk factors for the development of AD such as ageing, obesity, diabetes, hypertension and smoking (4) all comprise a significant increase in systemic inflammatory markers (5). While such systemic inflammatory activity is unlikely to provide the major explanation for the brain pathology associated with AD, there are good reasons to believe that systemic inflammation contributes to the progression and severity of this pathology.
Perhaps the strongest evidence for this exacerbation of dementia by systemic inflammation lies with the problem of delirium. Delirium is an acute and profound disturbance of thinking, memory, orientation, perception and emotion (6, 7). Dementia and aging are the biggest predisposing risk factors for suffering episodes of delirium and in this population systemic inflammation, caused by infection, injury or surgery, is one of the major triggers (8). In general, severe stimuli are required to precipitate delirium in healthy populations whereas much milder inflammatory stimuli can trigger a delirious episode in the elderly and demented (9).
It is now established that an episode of delirium is associated with a higher risk of long term cognitive impairment (10, 11), acceleration of dementia (12), and shortens time to permanent institutionalization and to death (13). Therefore, if infection superimposed on dementia predicts delirium and delirium during dementia predicts more rapid decline then this suggests that those insults that can cause delirium may directly exacerbate the underlying disease (Figure 1). In recent years we, and our collaborators, have used animal models to show that acute systemic inflammatory activation superimposed on existing progressive neurodegeneration can produce acute exacerbation of behavioural and cognitive function, resembling delirium, and can also induce acute neuronal death and accelerate the progression of disease. Similar findings have been made by other groups using various animal models of ageing and disease and this nascent field is beginning to provide evidence that this exacerbation of CNS disease by systemic inflammation is a significant phenomenon, and one that requires investigation in clinical populations with AD and other chronic neurodegenerative diseases.
Figure 1. Systemic inflammation, delirium and cognitive decline.
a) It is well established that systemic inflammation in those with dementia frequently leads to delirium. b) It is also established that delirium in demented patients tends to exacerbate disease progression. c) Thus insults that often trigger delirium may accelerate disease, in the presence or absence of the delirious episode. However, little research has addressed this scenario.
Systemic inflammation affects CNS function
It well known that systemic inflammation, induced by pathogen-associated molecular pattern stimulators of the toll-like receptors or by pro-inflammatory cytokines and indeed by surgery, can induce a spectrum of changes in CNS function including decreased locomotor activity, social engagement and feeding and also changes in the sleep-wake cycle. These changes may be collectively described as sickness behaviour and largely describe evolutionarily conserved behavioural and metabolic responses to infection, effected to conserve energy and minimize the spread of infection. This literature has been extensively reviewed elsewhere (14). There is also robust evidence that these inflammatory insults have a deleterious effect on the consolidation of new memories in contextual fear-conditioning experiments (see (15) for review) but these studies do not explain why relatively banal infections such as urinary tract infections in the elderly population produce the profound cognitive changes observed during episodes of delirium but have limited effects in younger, healthy populations.
During the last decade, an emerging literature has begun to attempt to explain this phenomenon of exaggerated CNS effects of systemic inflammation in elderly people and patients with dementia. We have studied the ME7 model of prion disease for many years since it displays robust progressive amyloidosis, microgliosis, synaptic loss, neurodegeneration and progressive affective, cognitive and neurological changes (16-18). As such it represents an excellent model system with which to study the natural history of progression of neurodegenerative disease and to examine the impact of environmental influences and insults that are perhaps generic to all chronic neurodegenerative diseases. In 2002 we showed that animals with prior neurodegenerative disease displayed exaggerated sickness behaviour and CNS inflammatory responses to systemic challenge with bacterial LPS, as a mimic of systemic inflammation (19). Other groups replicated aspects of these findings in aged rodents (20, 21). The explanation for this exaggerated CNS inflammatory response appears to lie with the ‘priming’ of microglial cells, first demonstrated in the ME7 model of prion disease (22). That is to say, microglial cells are primed by some aspect(s) of neurodegeneration and aging, such that they now show more robust pro-inflammatory responses to subsequent stimulation with bacterial LPS (21, 22), bacterial infection (20), double stranded RNA (23) and perhaps other inflammatory stimuli. Furthermore it is clear that this priming phenomenon is not specific to prion disease; exaggerated CNS inflammatory responses to systemic inflammatory stimulation have now been demonstrated in several other models of neurodegeneration. These include models of Alzheimer’s disease (24-26), Parkinson’s disease (27, 28), brain ischemia (29), experimental autoimmune encephalitis (30) and Wallerian degeneration (31).
Acute cognitive changes in the presence of prior risk factors: delirium during dementia
One of the first applications of this concept of microglial priming or hyper-reactivity of the aged or diseased brain to subsequent stimulation was in the demonstration that systemic infection with E. coli leads to more severe cognitive impairments in the aged compared to the young rodent (20). These cognitive impairments were chiefly focused on consolidation of memory in contextual fear-conditioning experiments. In this task animals are exposed to a context, which they explore for some time, and are then exposed to an auditory cue (a tone) and a foot-shock and are then returned to their home cage. The consolidation of the memory of the context in which they received the shock is impaired by systemic infection induced 4 days prior to, or immediately after, conditioning, as measured by decreased freezing on subsequent exposure to the same context. The authors concluded that some aspect of memory consolidation is affected by systemic inflammation, particularly in the aged rodents (20).
The contextual fear-conditioning task has also recently been used to demonstrate post-operative cognitive dysfunction, a complication that, at least in the immediate post-operative period, shows considerable overlap with delirium. In these experiments the tibia of rodents was fractured under general anaesthesia, to mimic traumatic surgery, immediately after exposure to the context and the delivery of a foot shock. Once again a failure to consolidate the memory of the context in which the shock was delivered was observed and this impairment was found to be dependent on both TNF-α (32) and IL-1β (33). These experiments confirm and extend previous observations that CNS IL-1β can impair consolidation of contextual memory in rodents (34). However the applicability of these data to delirium and post-operative cognitive decline are limited by the fact that the long-term decline observed in post-operative patients, that the authors attempt to model, is not similar to the cognitive impairment actually observed in the rodent studies. The latter is not cognitive decline, but failure to consolidate this memory in the first place. Furthermore, the above rodent studies of POCD have been performed in young healthy animals and this population is certainly much less susceptible to post-operative cognitive decline in the clinic (35). While it is clear that pro-inflammatory molecules such as IL-6 and IL-8 are associated with post-operative delirium in the clinic, these associations are much weaker than that between prior-cognitive impairment and subsequent delirium (36). The contextual fear-conditioning task is the best-characterized paradigm in which systemic inflammation has robust effects in healthy young animals (see (15) for review) and thus appears be particularly sensitive to inflammatory stimulation. Both memory consolidation and long term potentiation (LTP; a paradigm for how memory may be consolidated at a molecular level) require new transcriptional and translational events and require hours to be achieved. In addition there are significant caveats in using the freezing response in contextual fear conditioning to measure learning in animals that may have an issue with maintaining the freezing response, as in the case of tibial fracture (37). All of these factors limit its utility in capturing the acute onset and transient cognitive deficits observed during delirium and the long-term decline sometimes observed in post-operative cognitive dysfunction and post-delirium. Having said that, it is nonetheless significant that older animals are more sensitive than young rodents to memory consolidation deficits induced by LPS in this paradigm and similar LPS-induced Impairments in active avoidance of the context in which a similar foot shock was experienced are also accentuated by aging (38).
Ideally animal models of infection- or surgery-induced delirium should combine relevant risk factors with relevant acute triggers and should assess the impact of this combination on tasks with face validity for the delirium as observed clinically. Dementia and aging are the major risk factors for delirium and peripheral infections and sterile inflammation induced by insults such as injury and surgery are major triggers for delirium in these populations. Among the core symptoms are inattention and short term and working memory deficits. In addition, to address delirium, impairments should be of acute onset and reversible (the majority of delirium is transient, though 20% of cases still show deficits at 6 months (39)). Experiments in spatial and working memory have demonstrated impairments on a serial reversal version of a radial arm maze in aged mice but not in younger mice (40). This task requires animals to quickly ignore hidden platform locations learned on the previous day in order to locate the maze exit on the current day’s testing and may be thought of as relying on working memory. Systemic LPS (330 μg/kg) induced impairments in performance in this task associated with exaggerated CNS transcription of IL-1β, IL-6 and TNF-α in aged compared to young mice.
With a particular focus on dementia, mice with prior neurodegenerative disease made more errors in learning a reference memory Y-maze task under the influence of systemic LPS (100μg/kg) than normal animals challenged with LPS but could clearly remember the location of the exit if learned prior to systemic inflammation (41). This suggests difficulties in forming new memories when under the influence of systemic inflammatory stimulation. To attempt to specifically address the acute onset and transient changes in orientation, working memory and attention that are observed in delirium during early stages of dementia we have developed a novel water-adapted T–maze alternation task. In this maze animals must retain, for just 30 seconds, a recollection of the arm that they have just visited in order to now exit the maze via the opposite arm. Animals in the early stages of neurodegenerative disease can learn and maintain good alternation performance on this task, as can normal animals treated with LPS (100 and 200 μg/kg), but ME7 animals treated systemically with LPS (100μg/kg) show acute onset and transient working memory deficits (42). It would appear that prior pathology, in the latter case synaptic loss and microglial priming in the hippocampus, is necessary for the expression of robust working memory deficits after systemic stimulation. Current studies suggest that these deficits are ameliorated by inhibition of COX-1 mediated prostagtlandin E2 (PGE2) production (Skelly et al., submitted) and this is consistent with the idea that PGE2 produced by COX-1 can produce hippocampal-dependent cognitive deficits in response to robust IL-1β expression (43).
Although most amyloid transgenic models of AD do not show robust neurodegeneration or synaptic loss they do show some cognitive deficits and these are associated with increased concentrations of low order amyloid beta oligomers (44). There has been very little work examining the impact of systemic inflammation on acute measures of cognitive function in these animals. However, treatment of normal mice for 7 consecutive days with systemic LPS (250 μg/kg) can actually induce increased Aβ1-42 generation and deposition of amyloid plaque material via increased beta and gamma secretase activities (45). These data are consistent with earlier studies that suggested that systemic LPS altered APP processing in C57 mice (25, 46). Cognitive changes in the water maze and passive avoidance tasks, induced by LPS, were partially prevented by the cycloxygenase inhibitor sulindac (45).
Collectively these data show that systemic LPS or sterile systemic inflammation can induce impairments in memory consolidation in normal animals, but these deficits are exaggerated in aged animals. Furthermore, working memory, which is intact in the normal animal upon treatment with systemic LPS is acutely and transiently impaired when similar challenges are made in animals with prior age-related or neurodegenerative pathology. It remains likely that pro-inflammatory cytokines such as IL-1β and TNF-α as well as prostaglandins have a role but their involvement in CNS and/or systemic compartments, and across multiple cognitive domains has not yet been elucidated. It also should be noted that glucocorticoids may also play some role in acute cognitive deficits but this area is beyond the scope of this review and has been reviewed elsewhere (8).
Long term cognitive decline/acceleration of dementia
The mechanisms by which delirium during dementia occurs are critical to unravel since it is clear that these episodes are associated with more rapid long-term functional and cognitive decline (see (13) for meta-analysis). AD patients suffering delirious episodes show accelerated cognitive decline (12) but it is unclear from those studies whether delirium itself, or the triggers that induce delirium, are responsible for altering disease trajectory.
It is important to distinguish between severe systemic inflammation, during sepsis, leading to delirium in critical care patients and that seen during milder infections that are sufficient to cause delirium in the aged and demented population. It is known that systemic inflammation during sepsis has extremely negative consequences for the brain. In a rodent model large doses of LPS (10 mg/kg) induce hippocampal and cortical neuronal loss, increased microglial activation, decreased regional blood flow and loss of cholinergic innervation. This level of inflammatory stimulation leads to cognitive impairment long after the systemic event (47, 48). LPS-induced long-term cognitive impairments were shown to be dependent on inducible nitric oxide synthase (49). Similarly in humans brain lesions can visualized by MRI after septic shock (50) and microglial activation has also been reported in post-mortem brains after sepsis (51). Examining the impact of milder infections on disease progression requires a more subtle approach and considerably lower doses of LPS.
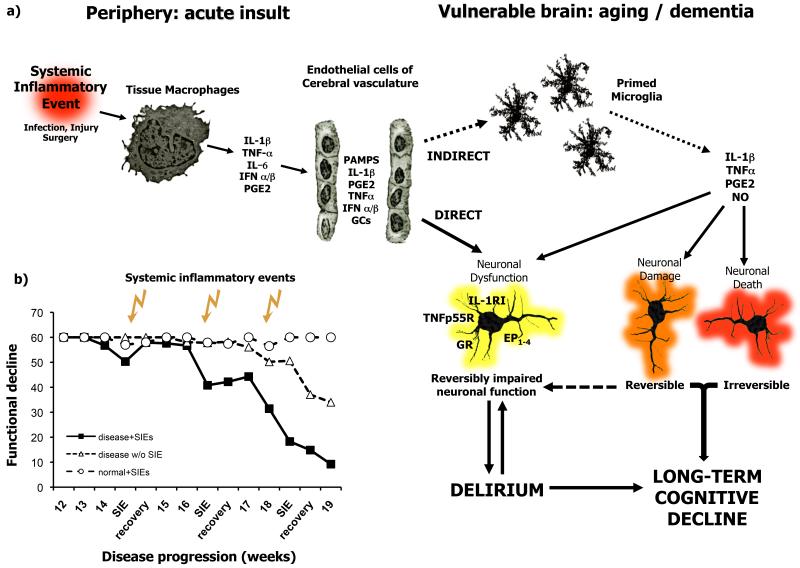
There are some studies in chronic disease in which systemic LPS has produced exacerbations of existing disease: Nguyen et al., made repeated challenges with LPS (1 mg/kg every 2 weeks for a 3 month period) to induce markedly more severe axonal pathology and progression of disease in the G93ASOD1 mouse model of ALS (52). In the triple transgenic model of AD, LPS treatment increased the severity of Tau tangle pathology when administered at 500 μg/kg twice weekly for 6 weeks (53). LPS-induced Tau hyperphosphorylation could be blocked by inhibiting the activity of cyclin-dependent kinase 5 (cdk5). In the ME7 model of prion disease, a single challenge with LPS (500 μg/kg i.p., from Salmonella equine abortus) induced acutely increased neuronal apoptosis in ME7 animals but not in NBH controls. (22). This same dose, when administered just once at 15 weeks post-inoculation with disease, was sufficient to cause acute and reversible neurological changes in ME7 but not normal animals. However, even after full recovery from acute deficits, the LPS-treated ME7 animals developed progressive and irreversible neurological impairments earlier and with greater severity than ME7 animals not treated with LPS (41). In the 6-OHDA model of Parkinson’s disease, it was demonstrated that adenovirally-mediated systemic expression of IL-1β significantly exacerbated neuronal loss in the substantia nigra and exacerbated motor symptoms when this expression was insufficient to induce any neuropathology in normal animals (27). The inhibition of iNOS partially protected against IL-1β-induced neurotoxicity in the substantia nigra in these animals. Using another model of Parkinson’s disease, induced by intra-nigral injection of LPS, Villaran et al showed that ulcerative colitis could also significantly exacerbate dopaminergic neuronal loss (28). Interestingly, infection with the Gram-positive bacterium Streptococcus pneumoniae did not exacerbate features of disease in a number of disease models (54). Although these studies are confounded to some degree by the administration of antibiotics to all infected animals within 24 hours of infection it may be of interest that Gram-positive bacteria, lacking LPS, may constitute a less significant insult to the diseased brain. Moreover, disease exacerbating effects have not been observed only with LPS: the acute and longitudinal exacerbation of chronic neurodegenerative disease has been demonstrated after systemic challenge with the double-stranded RNA poly I:C (23).These animals showed exaggerated CNS IL-1β and also type I interferon (IFN α/β) responses to this mimic of a systemic viral infection. Among the specific type I interferon-responsive genes robustly elevated was RNA-dependent protein kinase (PKR). Increased activity of this kinase has been shown both to induce apoptosis (55) and to impair LTP and consolidation of memory (56). It is of particular interest to the idea of multiple systemic inflammatory insults triggering delirium but also contributing in a cumulative way to disease progression that poly I:C was administered three times, two weeks apart, in the latter study. Each successive challenge produced acute deficits, but these deficits were progressively more severe and less reversible as the challenges were superimposed on disease at more progressed stages (23) (Figure 2). This appears to mimic the fluctuating and variable rate of decline seen in Alzheimer’s disease patients (57) in the manner that we had previously proposed that this model would (58).
Figure 2. Systemic inflammatory events may trigger delirium and contribute to pathological burden.
a) Systemic inflammatory events trigger the release of inflammatory mediators by tissue macrophages and brain vascular endothelial cells. These mediators may impact on neuronal function directly, or via the activation of microglial cells that have become primed by neurodegenerative disease or aging. Inflammatory mediators may cause reversible disruption of neuronal function, perhaps resulting in delirium. These mediators may also induce acute neuronal synaptic or dendritic damage that may be reversible and contribute to delirium or may be irreversible and contribute to long-term cognitive decline. These inflammatory mediators can also bring about neuronal death acutely and these changes are obviously irreversible and contribute to the accumulating damage and neuropathological burden. Thus acute and long-term cognitive effects are likely to occur by both overlapping and distinct mechanisms. b) Successive systemic inflammatory insults induce acute dysfunction, which is progressively less reversible each time, but also contribute to the progression of permanent disability (adapted from Field et al., 2010 (ref 23)). Abbreviations: IL-1β: interleukin 1β; IL-1RI: interleukin 1 receptor type I; TNF-α: tumour necrosis factor α; TNFp55: TNF p55 receptor; IL-6: interleukin 6; GCs: glucocorticoids; GR: glucocorticoid receptor; NO: nitric oxide; PGE2: prostaglandin E2, EP1-4: prostaglandin receptors 1-4; PAMPs: pathogen associated molecular patterns; IFNα/β, interferons a and b; SIEs: systemic inflammatory events.
Relevance in human disease
These animal studies beg the question: does infection have a significant role in the progression of AD. Several studies have examined the association of specific infectious agents with Alzheimer’s disease. These include HSV-1, Chlamydia pneumonia and spirochetes (See (59) for review) but these avenues have not progressed significantly due to a lack of consistent observations. Nonetheless, a review of general practitioner databases revealed that two or more infections over a 4 year period increased the risk of AD by 2-fold (60). Similarly, in a study in elderly patients, MMSE scores decreased with increasing viral burden, with herpes virus and cytomegalovirus burden being of particular risk (61). Other studies by the same authors showed that general ill health was significantly associated with cognitive decline (62, 63). Similarly there is evidence that periodontitis, caused chiefly by infection with the gram-negative Porphyromonas Gingivalis, is a significant risk factor for Alzheimer’s disease (64). It has also been shown that treating infection can reduce the risk of development of Alzheimer’s disease and that vaccination against a number of common diseases can protect against subsequent development of AD (65). Antibiotic treatment of mild to moderate AD patients with doxycycline and rifampin was also associated with slower decline in the treated group (66). Interestingly this did not appear to be associated with effects on Chlamydia pneumonia, the rationale for the study.
Based on the ME7 animal studies discussed above we and our clinical collaborators examined the contribution of systemic infection to disease progression in 85 AD patients and showed that elevated serum IL-1β was associated with increased cognitive decline across a two month period (67). In a cohort of 275 AD patients across 6 months, it was found that elevated serum TNF-α was highly significantly correlated with accelerated cognitive decline. Indeed those patients with low serum TNF-α showed almost no decline across this period. As hypothesized those with elevated serum TNF-α or with reported infection showed greater decline, but those with both elevated TNF-α and systemic inflammatory conditions showed the greatest decline in this cohort (68). Interestingly, many of those with elevated serum TNF-α did not suffer acute systemic inflammatory events and this TNF-α may arise from a number of other chronic co-morbidities such as obesity, atherosclerosis, diabetes and smoking. Significantly, those patients meeting criteria for delirium were excluded from the analysis of cognitive decline, indicating that inflammation-associated exacerbation of cognitive decline occurred even in the absence of a delirium. Consistent with this are findings that even patients with altered mental status not fulfilling criteria for delirium (often classified as subsyndromal delirium) show evidence of poor outcomes and long term cognitive impairment and this occurs in both the geriatric (69) and critical care settings (70). Further studies with the AD cohort discussed above show that these patients do show an exaggerated sickness behaviour response to systemic inflammation, as originally demonstrated in the ME7 model, and now show some symptoms more typical of delirium than of sickness behaviour per se (Holmes et al., in press). Thus, while systemic inflammation leads to an adaptive sickness behaviour response in the healthy brain it produces an exaggerated sickness behaviour response in the demented population and this syndrome may now comprise symptomatology not normally associated with these adaptive sickness responses. In more extreme cases, this can present as delirium. It is possible that the delirium syndrome may impact on hydration, nourishment, sleep quality and quantity and anxiety, which could influence an underlying condition, but it is apparent that even in the absence of delirium, these systemic inflammatory events are associated with increased pathological burden in individuals with prior pathology. This does not demonstrate causality but It is plausible that mediators such as IL-1β, TNF-α, NO and PGE2 impact on neuronal function via neuronal receptors and downstream effects on neurotransmitter release and action, but may also set in motion neurotoxic effects, dependent on the same or different inflammatory mediators, that will ultimately increase pathological burden. Evidence from animal model studies, and possible mechanisms contributing to acute and lasting cognitive dysfunction are illustrated in Figure 2.
Why do anti-inflammatory treatments not help?
It has been shown in several studies that NSAID use can protect against subsequent development of AD (72) but these drugs do not appear to be effective when administered to patients with established AD or even as a primary preventative strategy for those at high risk (3). While it is possible that these drugs simply target the wrong inflammatory mediators, being primarily directed at inhibition of prostaglandin synthesis, there is intriguing recent evidence that there may indeed be some protection offered by these drugs. Patients already on the cusp of impairment appeared to worsen upon treatment with the cyclooxygenase inhibitor naproxen but those who were not yet impaired showed significant protection at two year follow up (73). The timing and consequences of inhibiting brain cyclooxygenases may be crucial since both isoforms of this enzyme are constitutively expressed and have roles in normal brain function, but inhibiting PGE2 production while the brain still has significant cognitive reserve appears to be beneficial. Returning to the very beginning of the field of inflammation in AD, it is of considerable interest that treatment of rheumatoid arthritis patients with anti-TNFα antibodies has recently been reported to offer very significant protection against the subsequent development of AD (74). Thus it seems increasingly likely that pro-inflammatory factors induced during RA and other inflammatory conditions, including prostaglandins and TNF-α accelerate disease progression and this is certainly consistent with our recent demonstration that elevated systemic TNF-α is associated with more rapid cognitive decline (68). Therefore, blocking these systemic mediators, whether in RA or some other condition is likely to block their negative consequences on the brain and slow progression of AD. Furthermore these data suggest that if we wish to assess the ability of anti-inflammatory drugs to slow or prevent AD, we must do so in populations that do not enforce strict exclusion criteria that exclude those in the population with significant co-morbidity, who it would appear are most likely to demonstrate the importance of systemic inflammation in disease progression.
Conclusion
It is possible that delirium that resolves within days or weeks and long-term cognitive decline are two different manifestations of the same insult but they may occur by distinct mechanisms or overlapping ones as discussed above. However, based on the growing basic and clinical literature, it seems clear that systemic inflammation has negative consequences for brain function, both acutely and in the long term. Growing recognition that amyloid-β alone cannot account for progression of dementia across the population (75) increasingly implicates systemic inflammatory conditions as contributors to disease progression and recent animal model advances are well placed to begin to delineate at least some of the multifactorial influences on disease progression in the real world, where co-morbidity is a feature or aging for most people. In the context of this Biochemical Society Transactions issue on useful models with which to model the progression of dementia, it is significant that models of chronic neurodegeneration, such as the ME7 model of prion disease, which are not reliant on genetic manipulation of amyloid-β levels, have made predictions (41) about the influence of systemic inflammation on disease progression that have successfully translated into the human population (68).
Acknowledgements
The financial support of the Wellcome Trust is gratefully acknowledged. Thanks are due to Celine Bourdon and Edel Hennessy for assistance with the figures in this review.
References
- 1.McGeer PL, McGeer E, Rogers J, Sibley J. Anti-inflammatory drugs and Alzheimer disease. Lancet. 1990;335:1037. doi: 10.1016/0140-6736(90)91101-f. [DOI] [PubMed] [Google Scholar]
- 2.Aisen PS. The potential of anti-inflammatory drugs for the treatment of Alzheimer’s disease. The Lancet Neurology. 2002;1:279–284. doi: 10.1016/s1474-4422(02)00133-3. [DOI] [PubMed] [Google Scholar]
- 3.Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. A summary risk score for the prediction of Alzheimer disease in elderly persons. Arch Neurol. 2010;67:835–841. doi: 10.1001/archneurol.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatry Association, A. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. American Psychiatry Association; Washington, DC: 1994. [Google Scholar]
- 7.World Health Organisation, W. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research; WHO (Geneva): 1992. [Google Scholar]
- 8.MacLullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65:229–238. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. Jama. 1996;275:852–857. [PubMed] [Google Scholar]
- 10.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 11.MacLullich AM, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21:30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- 12.Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, Yang FM, Kiely DK, Inouye SK. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 14.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham C, Sanderson DJ. Malaise in the water maze: Untangling the effects of LPS and IL-1beta on learning and memory. Brain Behav Immun. 2008;22:1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betmouni S, Deacon RMJ, Rawlins JNP, Perry VH. Behavioural consequences of prion disease targeted to the hippocampus in a mouse model of scrapie. Psychobiology. 1999;27(1):63–71. [Google Scholar]
- 17.Betmouni S, Perry VH, Gordon JL. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience. 1996;74:1–5. doi: 10.1016/0306-4522(96)00212-6. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham C, Deacon R, Wells H, Boche D, Waters S, Diniz CP, Scott H, Rawlins JN, Perry VH. Synaptic changes characterize early behavioural changes in the ME7 model of murine prion disease. Eur. J. Neurosci. 2003;17:2147–2155. doi: 10.1046/j.1460-9568.2003.02662.x. [DOI] [PubMed] [Google Scholar]
- 19.Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 20.Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Field R, Campion S, Warren C, Murray C, Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav Immun. 2010;24:996–1007. doi: 10.1016/j.bbi.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Chan SL, Mattson MP. Adverse effect of a Presenilin-1 mutation in microglia results in enhanced nitric oxide and inflammatory cytokine responses to immune challenge in the brain. MeuroMolecular Medicine. 2002;2:29–45. doi: 10.1385/NMM:2:1:29. [DOI] [PubMed] [Google Scholar]
- 25.Sheng JG, Bora SH, Xu G, Borchelt DR, Price DL, Koliatsos VE. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol Dis. 2003;14:133–145. doi: 10.1016/s0969-9961(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 26.Sly LM, Krzesicki RF, Brashler JR, Buhl AE, McKinley DD, Carter DB, Chin JE. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res Bull. 2001;56:581–588. doi: 10.1016/s0361-9230(01)00730-4. [DOI] [PubMed] [Google Scholar]
- 27.Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain. 2008 doi: 10.1093/brain/awn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villaran RF, Espinosa-Oliva AM, Sarmiento M, De Pablos RM, Arguelles S, Delgado-Cortes MJ, Sobrino V, Van Rooijen N, Venero JL, Herrera AJ, Cano J, Machado A. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson‘s disease. J Neurochem. 2010;114:1687–1700. doi: 10.1111/j.1471-4159.2010.06879.x. [DOI] [PubMed] [Google Scholar]
- 29.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serres S, Anthony DC, Jiang Y, Broom KA, Campbell SJ, Tyler DJ, van Kasteren SI, Davis BG, Sibson NR. Systemic inflammatory response reactivates immune-mediated lesions in rat brain. J Neurosci. 2009;29:4820–4828. doi: 10.1523/JNEUROSCI.0406-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palin K, Cunningham C, Forse P, Perry VH, Platt N. Systemic inflammation switches the inflammatory cytokine profile in CNS Wallerian degeneration. Neurobiol Dis. 2008;30:19–29. doi: 10.1016/j.nbd.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 35.Krenk L, Rasmussen LS, Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand. 2010;54:951–956. doi: 10.1111/j.1399-6576.2010.02268.x. [DOI] [PubMed] [Google Scholar]
- 36.van Munster BC, Bisschop PH, Zwinderman AH, Korevaar JC, Endert E, Wiersinga WJ, Oosten HE, Goslings JC, Rooij SE. Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn. 2010;74:18–23. doi: 10.1016/j.bandc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- 38.Kohman RA, Tarr AJ, Byler SL, Boehm GW. Age increases vulnerability to bacterial endotoxin-induced behavioral decrements. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 39.Cole MG. Persistent delirium in older hospital patients. Curr Opin Psychiatry. 2010;23:1661–1666. doi: 10.1097/YCO.0b013e32833861f6. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, Cunningham C. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging (in press) 2010 doi: 10.1016/j.neurobiolaging.2010.04.002. doi:10.1016/j.neurobiolaging.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matousek SB, Hein AM, Shaftel SS, Olschowka JA, Kyrkanides S, O’Banion MK. Cyclooxygenase-1 mediates prostaglandin E(2) elevation and contextual memory impairment in a model of sustained hippocampal interleukin-1beta expression. J Neurochem. 2010;114:247–258. doi: 10.1111/j.1471-4159.2010.06759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashe KH, Zahs KR. Probing the biology of Alzheimer’s disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brugg B, Dubreuil YL, Huber G, Wollman EE, Delhaye-Bouchaud N, Mariani J. Inflammatory processes induce beta-amyloid precursor protein changes in mouse brain. Proc Natl Acad Sci U S A. 1995;92:3032–3035. doi: 10.1073/pnas.92.7.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, Heneka MT. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204:733–740. doi: 10.1016/j.expneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Semmler A, Hermann S, Mormann F, Weberpals M, Paxian SA, Okulla T, Schafers M, Kummer MP, Klockgether T, Heneka MT. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5:38. doi: 10.1186/1742-2094-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weberpals M, Hermes M, Hermann S, Kummer MP, Terwel D, Semmler A, Berger M, Schafers M, Heneka MT. NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. J Neurosci. 2009;29:14177–14184. doi: 10.1523/JNEUROSCI.3238-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharshar T, Carlier R, Bernard F, Guidoux C, Brouland JP, Nardi O, de la Grandmaison GL, Aboab J, Gray F, Menon D, Annane D. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806. doi: 10.1007/s00134-007-0598-y. [DOI] [PubMed] [Google Scholar]
- 51.Lemstra AW, Groen in’t Woud JC, Hoozemans JJ, van Haastert ES, Rozemuller AJ, Eikelenboom P, van Gool WA. Microglia activation in sepsis: a case-control study. J Neuroinflammation. 2007;4:4. doi: 10.1186/1742-2094-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen MD, D’Aigle T, Gowing G, Julien JP, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebert S, Goos M, Rollwagen L, Baake D, Zech WD, Esselmann H, Wiltfang J, Mollenhauer B, Schliebs R, Gerber J, Nau R. Recurrent systemic infections with Streptococcus pneumoniae do not aggravate the course of experimental neurodegenerative diseases. J Neurosci Res. 2010;88:1124–1136. doi: 10.1002/jnr.22270. [DOI] [PubMed] [Google Scholar]
- 55.Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, Barber GN. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. Embo J. 1998;17:6888–6902. doi: 10.1093/emboj/17.23.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang Z, Belforte JE, Lu Y, Yabe Y, Pickel J, Smith CB, Je HS, Lu B, Nakazawa K. eIF2alpha Phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation. J Neurosci. 2010;30:2582–2594. doi: 10.1523/JNEUROSCI.3971-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holmes C, Lovestone S. Long-term cognitive and functional decline in late onset Alzheimer’s disease: therapeutic implications. Age Ageing. 2003;32:200–204. doi: 10.1093/ageing/32.2.200. [DOI] [PubMed] [Google Scholar]
- 58.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 59.Holmes C, Cotterell D. Role of infection in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs. 2009;23:993–1002. doi: 10.2165/11310910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 60.Dunn N, Mullee M, Perry VH, Holmes C. Association between Dementia and Infectious Disease: Evidence from a Case-Control Study. Alzheimer Dis Assoc Disord. 2005;19:91–94. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- 61.Strandberg TE, Pitkala KH, Linnavuori KH, Tilvis RS. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke. 2003;34:2126–2131. doi: 10.1161/01.STR.0000086754.32238.DA. [DOI] [PubMed] [Google Scholar]
- 62.Strandberg TE, Pitkala KH, Linnavuori K, Tilvis RS. Cognitive impairment and infectious burden in the elderly. Arch Gerontol Geriatr Suppl. 2004:419–423. doi: 10.1016/j.archger.2004.04.053. [DOI] [PubMed] [Google Scholar]
- 63.Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- 64.Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, Nehorayoff A, Glodzik L, Brys M, de Leon MJ. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol. 2009;216:92–97. doi: 10.1016/j.jneuroim.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verreault R, Laurin D, Lindsay J, De Serres G. Past exposure to vaccines and subsequent risk of Alzheimer’s disease. CMAJ. 2001;165:1495–1498. [PMC free article] [PubMed] [Google Scholar]
- 66.Loeb MB, Molloy D, Smieja M, Standish T, Goldsmith CH, Mahony J, Smith S, Borrie M, Decoteau E, Davidson W, McDougall A, Gnarpe J, O’Donnell M, Chernesky M. A Randomized, Controlled Trial of Doxycycline and Rifampin for Patients with Alzheimer’s Disease. J Am Geriatr Soc. 2004;52:381–387. doi: 10.1111/j.1532-5415.2004.52109.x. [DOI] [PubMed] [Google Scholar]
- 67.Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH. Systemic inflammation and disease progression in Alzheimer’s disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cole M, McCusker J, Dendukuri N, Han L. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760. doi: 10.1046/j.1365-2389.2003.51255.x. [DOI] [PubMed] [Google Scholar]
- 70.Jones C, Griffiths RD, Slater T, Benjamin KS, Wilson S. Significant cognitive dysfunction in non-delirious patients identified during and persisting following critical illness. Intensive Care Med. 2006;32:923–926. doi: 10.1007/s00134-006-0112-y. [DOI] [PubMed] [Google Scholar]
- 71.Holmes C, Cunningham C, Zotova E, Culliford D, Perry VH. Pro-inflammatory cytokines, sickness behaviour and Alzheimer’s disease. Neurology. 2011 doi: 10.1212/WNL.0b013e318225ae07. in the press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laino C. In Follow-up Analysis of Clinical Trial, NSAIDs Seem to Preserve Cognitive Function in Patients with Healthy Brains. Neurology Today. 2009 [Google Scholar]
- 74.Chou RC. Anti-TNF therapies for rheumatoid arthritis could reduce Alzheimer’s risk. Amercican College of Rheumatology; 2010. [Google Scholar]
- 75.MRCCFAS Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]