Abstract
It has been widely described that immune activation, such as that induced by bacterial endotoxin (lipopolysaccharide, LPS) or by interleukin-1β (IL-1β) causes deficits in learning and memory. These studies have been performed in a limited number of paradigms and have often failed in their experimental design to account for features of sickness behaviour and have thus introduced potential confounding factors. As such, this literature provides an oversimplified view of the issues. A detailed reading of the literature reveals that rodents treated with LPS or IL-1β, whether systemically or centrally, do not reproducibly show clear impairments in spatial reference memory in the Morris Water Maze. Latency to find the platform is almost invariably increased, consistent with sickness and reduced locomotor speed in these animals. In studies where distance travelled or route to the platform have been examined there have been either very modest or no differences between treated groups, or stress-induced, thigmotaxic, strategies employed by the sick animals. This suggests that emotional and performance changes are more significant than cognitive impairments. There is better evidence for a deficit in contextual fear conditioning experiments induced by LPS and IL-1β and these effects are clearly dose dependent, with facilitation at low doses and impairment at higher doses. We propose that the field should be more cautious and more specific in its description of these cognitive effects and that new tasks be employed in these studies, that are not susceptible to confounding factors such as locomotor speed and elevated stress responses. Emerging data suggests that systemic insults induce more robust memory impairments in aged rodents or those with pre-exisiting neurodegenerative disease and these effects are consistent with the mild effects of infection on cognitive processes in young or healthy adults and the more severe effects, such as delirium, in the elderly and demented population.
Introduction
“What happens to the mouse that finally stops running the maze ? The doctors think he’s dumb, when he’s just disappointed”
Mark Eitzel, American Music Club
The concept that sickness behaviour has evolved as a coordinated set of behaviours driven by specific brain centres to conserve energy, support the fight against infection and mimimise spread of infection is now widely accepted. Since the demonstration that systemic events can alter CNS function many other CNS effects have been ascribed to this periphery-to-brain communication. Among these is the idea that systemic LPS, and/or pro-inflammatory cytokines have a deleterious effect on memory and learning. Though there are relatively few papers on this subject, employing few experimental paradigms, the idea that systemic inflammation impairs memory and learning appears to have become dogma in the psychoneuroimmunology field. However, in many cases the studies on which this reasoning is founded have failed to take account of the effects of LPS on stress, motivation, locomotor activity and speed of movement. All of these parameters are acutely impaired by LPS treatment (and to a lesser degree by IL-1β) and gradually recover over a period of approximately 24 hours. The effect of LPS on these parameters can confound tests of learning and memory. While it is undoubtedly true that LPS and IL-1β have deleterious effects in some paradigms, they are not necessarily amnesic effects. In this review, we will make a critical assessment of the current literature on the effects of infection, LPS and pro-inflammatory cytokines on learning and memory in rodents.
Early experiments
Most of what we know about immune stimulation and cognitive processes comes from studies in rodents. The first clear indications that immune stimulation had cognitive effects were in the mid-nineties when a number of groups reported effects of LPS, IL-1β or bacterial infection on learning and memory. In the first of these studies, Oitzl and colleagues (Oitzl et al., 1993) injected IL-1β intracerebroventricularly (i.c.v.), in rats and noted transient deficits in the Morris water maze (MWM), a spatial memory task. Animals injected with IL-1β acquired the task at the same rate as the control group, but 24 hours later did not recall the location of the hidden platform. This result suggested that IL-1β caused a deficit in recall or retrieval of the first day’s learning. In 1995, Aubert et al. (Aubert et al., 1995) suggested that immune challenge induced memory deficits were not limited to tests of spatial navigation, but also to nonspatial tasks. An autoshaping task was used in which lever presses in a Skinner operant box were rewarded with a food pellet. Rats were injected intraperitoneally (i.p.) with LPS at 250μg/kg before the second session. The LPS treated rats showed a long lasting increase, compared to controls, in latency (i.e. an increased time) to press the lever. The authors concluded that LPS had an effect on the formation of an association between the conditioned stimulus (presentation and pressing of the lever) and the unconditioned stimulus (presentation of the food reward). Further experiments examined treatment with IL-1β i.p. and also showed impaired learning relative to saline-injected controls.
Also in 1995, Gibertini et al. (Gibertini et al., 1995) studied the effect of a real infection with Legionella pneumophila. This paper showed a marked effect of either Legionella pneumophila or 100 ng IL-1β i.p. on learning in the MWM. In IL-1β-treated mice the latency to find the hidden platform in this maze did not decrease across 18 trials. Therefore, this result suggests that across this period of training IL-1β prevents learning about the location of the hidden platform. Similarly Legionella pneumophila-treated animals were slower to find the platform across this period. Administration of anti-IL-1β antibodies to infected animals in these experiments returned their latency on this task to near control levels. Together these data suggested that bacterial infection, bacterial endotoxin, or indeed a major pro-inflammatory mediator induced by bacterial infection, IL-1β, could induce impairments in different learning paradigms.
It is attractive to interpret these data as a failure in learning and memory, because long term potentiation, an electrophysiological correlate of many forms of learning, was also shown to be reduced by both LPS and IL-1β (Katsuki et al., 1990; Cunningham et al., 1996). Certainly this correlation between behavioural and electrophysiological data resulted in a strengthening in the association between immune stimulation and learning in the psychoneuroimmunology field. Since that time many researchers have used immune stimuli such as LPS and IL-1β to investigate their effects on learning and memory, but before examining these data we will consider the criteria that must be fulfilled to demonstrate a cognitive impairment.
What do we mean when we say cognitive impairment ?
In order to analyse the data suggesting that infection, LPS and/or IL-1β to impair cognitive processes we must examine the paradigms used and the types of learning and memory task that have been employed. In order to demonstrate that a learning or memory impairment has been induced by the treatment we must also ascertain that any impairment observed could not equally be explained by a lack of motivation, an increased stress or anxiety response or simply a decreased speed in responding due to locomotor activity decrements. It is well described that LPS and/or IL-1β induces locomotor hypoactivity (Hart, 1988; Kent et al., 1992; Kozak et al., 1994), decreased reward seeking activities (Yirmiya, 1996; Borowski et al., 1998), and conditioned taste aversion (Tazi et al., 1988) as well as inducing anorexia (Laye et al., 2000) and marked activation of the hypothalamic-pituitary-adrenal stress axis (Berkenbosch et al., 1987; Besedovsky et al., 1991). Thus we must examine the impact of all of these changes on the behaviour of the animal and control for these changes in the experimental design if we are to avoid possible confounding factors. If sickness behaviour respresents a reorganisation of priorities for the sick animal, we must attempt to assess how this reorganisation of priorities may alter behaviour in the paradigms used.
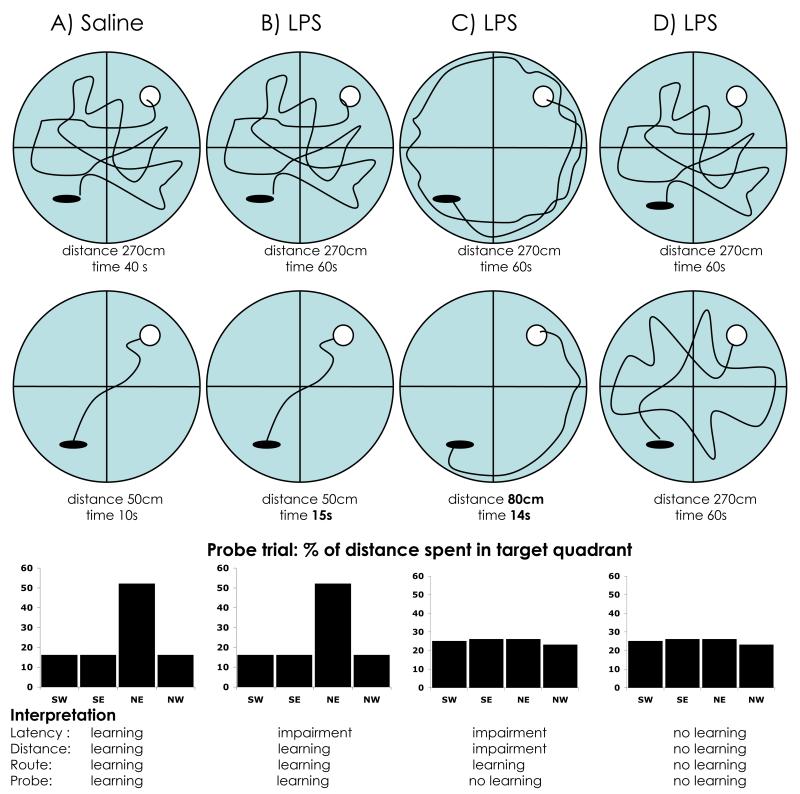
Perhaps the best example of reorganisation of priorities after an immune challenge is in the Morris Water Maze. In the MWM task a decrease in the time taken to find a hidden platform or a decrease in path length to the platform can indicate an improvement in spatial learning. Given the impact the immune stimulation may have on speed of locomotion, an increase in the time taken could also simply indicate slower movement. This issue can be addressed to by assessing speed of movement and integrating these data with latency data to estimate whether animals have travelled further in locating the exit. In addition, one can assess whether the treated and control animals show differences in the time to solve the maze when the platform is clearly visible. These approaches have been used by a number of authors to facilitate at least a partial delineation of performance and cognitive deficits (Yirmiya et al., 2002; Sparkman et al., 2005b). However, even this is insufficient to detect some apparent deficits that are not necessarily cognitive. For example, animals could learn to use a searching strategy that is not reliant on the extramaze spatial cues, such as swimming a certain distance from the edge of the pool. One possible way of testing whether animals are learning the location of the platform relative to the extramaze cues is to perform a probe test at the end of training, or at a point in training where the experimentor predicts that a clear impairment will be apparent in some subjects. In this test the platform is removed and the percentage of time spent exploring the quadrant of the maze that previously contained the platform can be measured. Learning strategies that are not reliant on the extramaze cues may lead to equal exploration in all four qudrants of the pool, whereas allocentric learning will result in a preference for the training quadrant. Hippocampal lesioned animals fail to use the extramaze spatial cues to navigate to a hidden platform location (Morris et al., 1982). However, hippocampal lesioned animals can show normal performance on non-spatial learning such as swimming to a visible platform (Morris et al., 1982). Therefore, whereas a deficit in allocentric spatial cue learning may indicate impared hippocampal function, a reduction in learning that is not dependent on allocentric cues may be due to non-hippocampal dependent effects. If an animal does pursue a thigmotaxic strategy (i.e. remaining close to the walls of the maze) in order to minimise the stressful conditions of immune stimulation combined with forced swimming, this will result in different learning rather than simply impaired learning.
In addition to the well-described changes that may affect performance in behavioral tasks, we must also take account of ‘state-dependent’ changes that might occur in immune challenged animals. In a typical learning experience a number of contextual stimuli may be present at the time of the presentation of the relevant cues. Contextual cues typically refer to external stimuli, but it is possible for the internal state of the animal to be a contextual cue. Therefore, learning episodes may be ‘state-dependent’ in that internal state cues may also form part of the association with the outcome. It is well known that in humans the context or state in which learning occurs plays an important role in subsequently retrieving the memories. For example, if learning occurs whilst intoxicated with alcohol, subsequent retrieval is poor if tested when sober, but this decrement does not occur if the subject is once again intoxicated (Goodwin et al., 1969). Similarly, with animals the administration of drugs can lead to state-dependent effects on learning (Overton, 1964). Also, as in case of electroconvulsive shocks (Thompson and Grossman, 1972), it is possible for stimuli that are presumed to have an amnesic effect to simply form part of the context or state in which learning occurs. Thus, many stimuli may appear to have an effect on learning, but they may simply form part of the context of learning, which may help the retrieval of memory, and in their absence, retrieval may be impaired. For it to be possible to conclude that a manipulation, such as LPS, has an effect on learning, the manipulation must be present at both the training and the test of learning to ensure that changes in the state cannot be used to explain any observed effects on learning. In many studies, animals have been injected with LPS or IL-1β and trained on certain tasks, and then asked to demonstrate retention of the prior learning in the absence of these internal contexts. Therefore, the task is contextually different in training and testing, and this is another possible confounding factor in cognitive experiments with immune stimuli. Throughout this review, when identifying possible state-dependent effects we aim to suggest that while an amnesic effect may have occurred (i.e. the consequence for the sick animal may be a failure to remember) it is possible that this reflects a failure to retrieve the memory due to it’s state-dependency rather than an inability to encode the stimuli in the first instance (Miller and Springer, 1973).
Finally, a number of authors who have reported effects of LPS on memory and learning have employed severe dosing strategies and it is our opinion that these studies are not particularly informative. Hauss-Wegryzniak et al. showed that when given at i.c.v. at 0.25 microgram per hour for 28 days LPS impaired latency in the MWM (Hauss-Wegrzyniak et al., 1998). Tanaka and colleagues injected 20 micrograms of LPS into the hippocampal CA1, on 5 consecutive days (Tanaka et al., 2006) and found similar impairments. The former study, and others by the same group, causes marked neuronal death, while the latter study appears to cause synaptic loss or damage as measured by MK801 binding. These types of study, which cause neuropathological deficits rather than pharmacological effects, will not be considered further in this review.
A second look at the early studies
With the previously discussed points in mind, it is informative to once again examine the early studies of LPS and IL-1β effects on learning and memory. As stated above, injection of IL-1β i.c.v., 1 hour before training did not affect learning of the location of the hidden platform on day 1 of training, but on the first trial of day 2 the treated animals were impaired compared to controls (Oitzl et al., 1993). However, they quickly learned the location of the platform such that by trial 2 on day 2, performance was once again indistinguishable from controls. The first potential concern is that the only measure provided in the study is the latency to locate the platform. These authors did use videotracking and path analysis software, which became available in the early nineties, but have presented only latency in this study. Latency will be dscussed further below. The main concern in this study is that state-dependent effects have not been controlled for, and thus, on day 2 the task is contextually different from day 1, because the animals are no longer experiencing the IL-1β-induced sickness they experienced during learning. This may explain why there is a difference in latency on the first trial of day 2 that very quickly resolves upon habituation to the new (IL-1β-free) context. If IL-1β is given directly before training, these animals are not impaired on day 2. The authors conclude that these animals have achieved both the acquisition and its consolidation before IL-1β effects on the process take hold. The trials in this control experiment were massed such that all three trials on day 1 would have been completed before 1 hour had elapsed since IL-1β treatment. Thus from a ‘state-dependent’ point of view, these animals learned largely in the absence of IL-1β effects and were then tested one day later in the absence of IL-1β and showed normal performance in both situations. What is clear is that the presence of IL-1β-induced sickness for the first few hours after learning the location of the platform has no negative impact on their recollection of that location the following day.
In the other early MWM spatial learning and memory study (Gibertini et al., 1995), the authors also used latency to find the platform as the principal measure of learning, but did address the potential confounds of swim speed and search strategy with limited video data of path lengths. These data show that despite latencies that did not appear to decrease across the period of training, the path length for IL-1β treated animals did decrease (~620 cm -> 390 cm) suggesting that searching for the hidden platform became more efficient over training. So, if latency does not improve, but path length does decrease with successive trials, it suggests that IL-1β-treated animals are learning the task to a certain degree. This suggests that a thorough analysis of distance travelled and route taken would be more informative for sick animals than a simple measure of time taken. It is possible that LPS or IL-1β, both of which are known to activate the HPA axis and raise hippocampal corticosterone at the doses used in these studies, may simply induce a stressed/anxious behavioral pattern that is different from saline-treated controls. It is certainly true that stressors such as LPS, IL-1β and indeed the temperature of the water may all have different effects on the behavioral strategy of an animal placed in a water maze (see (Joels et al., 2006) for review). A full discussion of the interactions of stress with immune stimulation is beyond the scope of this review, but it is common for stressed animals to pursue thigmotaxic strategies to avoid spending time in open, anxiogenic, areas of mazes (Treit and Fundytus, 1988; Simon et al., 1994) and thus the route taken by animals could clarify whether there is a failure to learn or whether they are using a stress-minimizing strategy. Subsequent experiments in mice by Gibertini emphasize this point (Gibertini, 1998). Using path length as the major behavioural measure, young female mice were treated with IL-1β i.p. (100 or 1000 ng) and trained in the MWM by 2 different protocols. Across training (3 days x 5 trials per day) IL-1β treated animals found the platform with the same path length as control animals. However, probe trials, in which the animals were allowed to explore the maze with the platform absent, revealed that at the end of training control mice were searching predominantly in the training quadrant, but IL-1β-treated animals were not. Therefore, although the two groups did not differ in their efficiency in locating the platform, only the control group had learnt the location of the platform in relation to the extramaze spatial cues. As might be predicted from animals now experiencing heightened stress with respect to the saline controls, IL-1β-treated mice showed a trend toward thigmoataxis (remaining close to the wall of the maze) in searching for the platform. Furthermore, when used at higher doses, IL-1β actually facilitated learning, consistently producing shorter path lengths to find the platform despite inducing profound sickness behaviour. One final interesting and novel result that emerged from this study was that when tested in cold water (18oC) IL-1β at 100 ng did not induce any impairment in learning the location of the hidden platform, while at 23oC the IL-1β treated animals were impaired. Therefore, IL-1β may impair motivation to learn the task, but not actually the ability to learn the task.
The other early paradigm in which LPS/IL-1β was shown to induce cognitive impairments was autoshaping (Aubert et al., 1995). Rats were placed in an operant box, and after a time a lever was presented. This lever could be pressed, which would prompt its retraction, and after a 2 second delay food was presented. If the lever was not pressed by the rat, it would nonetheless be retracted after 15 seconds and food was presented regardless. Upon successive exposures control rats quickly learn to associate the lever press with the food reward and the latency to press the lever thus decreases. When LPS was injected after a single training session in this apparatus the latency to press the lever remained very high for many successive trials and remained considerably higher than controls for 12 days (when the experiment was terminated). If LPS was given after animals had fully made the association, the latency was not affected, suggesting that LPS did not merely retard responses. The authors conclude that LPS impaired acquisition of the lever and food reward association, but did not impair subsequent retrieval of the association once acquired. Similar experiments with IL-1β injection early in training also induced increased latency but, unlike with LPS, these animals eventually learned the association to the same extent as controls, but showed higher latencies for approximately 4 days. In the discussion the authors quite rightly point out the possibility that the LPS-treated, and to a lesser degree, the IL-1β treated animals, may develop place aversion for the novel environment (the operant box) in which they have experienced the episode of sickness and that this place aversion will provoke a certain degree of caution in the apparatus. Both place (Mormede et al., 2003) and taste aversion (Tazi et al., 1988) occur upon IL-1β and LPS treatment, probably through the induction of corticotropin releasing factor (Cador et al., 1992) and both would be predicted to increase latency in this paradigm. The most compelling evidence that this is indeed what is being observed in the Aubert studies is that the latency does not return to control levels throughout the 12 days of testing after LPS treatment. If learning the association of the stimulus with the food reinforcer were blocked by LPS/IL-1β one would predict that the learning is simply delayed by 24 hours because effects of the immune stimulation should have resolved by this time (Bluthe et al., 1992). That this effect lasted for 12 days with LPS and 4 days with IL-1β suggests that the most salient association made by these sick animals is between the context and the sickness they experienced there, rather than that between the lever and the reward. These are factors that could cause immune-challenged animals to appear to have impaired learning when in fact normal learning could have been masked by sickness association learning. Furthermore, one might argue that LPS-induced taste-aversion is clear evidence that learning does occur during sickness. Thus the observed impairment is more likely to be an emotional rather than a cognitive response to sickness.
Thus, while all of these studies report effects of immune stimulation on learning and memory it is conceivable that all the findings can be explained by place or taste aversion, state-dependent effects, or alternative search strategies induced by stress. This does not prove that immune stimulation does not have cognitive effects, but it does emphasize the need to address these possibilities in the design of subsequent experiments.
Contextual fear conditioning & two-way active avoidance
Within a few years of the aforementioned three studies, a number of other laboratories continued investigations of immune stimulation and learning and memory, and among these next experiments was the use of contextual fear conditioning as a measure of learning. Impairment of rats in this paradigm was demonstrated (Pugh et al., 1998) when LPS was injected directly after conditioning. In this task a distinction could be made between contextual fear conditioning, which is dependent on the hippocampus, and auditory fear conditioning which occurs independent of the hippocampus (Kim and Fanselow, 1992). Rats were placed into context A and received two shocks that were paired with the conditioned stimulus (CS), a tone (1st shock after 120s, 2nd shock after 240s, then removed after 270s). 24 hours later mice were placed into context A for 5 minutes and the amount of freezing was measured. Mice were then placed into Context B (distinct from context A). After a 180s period (pre-CS period) the tone was presented for 180s (CS-period). Thus the freezing response to the context and to the tone can be assessed separately. LPS was given immediately after the conditioning phase, and, therefore, could potentially interfere with the subsequent process of memory consolidation. LPS doses of 0.125 mg/kg (but not 0.5 mg/kg) impaired contextual fear conditioning (i.e. they froze less), but not auditory-cue fear conditioning in these juvenile rats. In adult rats, in the same study, LPS had similar effects, but only at higher doses: increased freezing was seen at 1 mg/kg but not at 0.5 mg/kg or at 2 mg/kg.
There are a number of advantages in the paradigm used by these authors. Due to freezing being the ‘learned’ response and, because LPS-treated animals freeze less (i.e. move more) than controls in this paradigm, it is unlikely that decreased locomotor activity, a possible confound in many other paradigms, could be mistaken for impairment in this task. Both groups show freezing levels in context B, during the pre-CS period, that are substantially less than in Context A. Therefore, this suggests that freezing levels in Context A are due to learning the association between the context and shock.
It is possible that LPS may have a state-dependent effect on consolidation. This would imply that the context-shock association is consolidated in the context of (LPS induced) sickness and that sickness would become a necessary cue for successful retrieval of the memory. Indeed it has been shown that an electroconvulsive shock (ECS) given after training can disrupt consolidation of learning, but if an ECS is given prior to testing the memory is rescued (Thompson and Grossman, 1972). Therefore, it is possible for amnesic stimuli to cause a state-dependent retrieval deficit rather than impair consolidation of a memory. However, consideration of the experiments by Pugh and colleagues (1998) reveals that a state-dependent account is unlikely to be an adequate explanation of the data. Firstly, only the context fear conditioning is impaired and not the cue fear conditioning. This would suggest that LPS selectively impairs consolidation of hippocampal-dependent memory, but not hippocampal-independent memory. Secondly, Pugh et al., (1998) demonstrate that pre-exposure to the context 24 hours prior to training eliminates the LPS induced deficit on contextual fear conditioning. This suggests that LPS impairs the ability to form a representation of the context, but if animals are given prior opportunity to form a representation LPS has no effect. It is possible that prior exposure to the context might reduce the ability of LPS to cause state-dependent effects on context learning (possibly through the process of latent inhibition). However, if this was the case then it would be expected that pre-exposure to the context should also retard, rather than facilitate, context-shock association learning.
The contextual fear conditioning experiments of Pugh and colleagues provide evidence that consolidation of contextual memory is impaired by LPS and has been repeated in many studies by this group. This paradigm has subsequently been used by others, with largely similar results for LPS (Thomson and Sutherland, 2005). These authors replicate the work of Pugh et al., matching LPS dose on the basis of endotoxin units rather than pharmacological mg per kg matching. They observe an effect of LPS on hippocampal-dependent memory consolidation (freezing to context, but not to tone) at one dose (2μg/kg), but not at double this dose, as previously reported. This was not replicated by injecting IL-1β systemically, even at a very wide range of doses: (100ng, 2000ng, 4000ng, 100 μg). Thus while Pugh et al., showed that systemic administration of IL-1 receptor antagonist blocked LPS effects on contextual fear conditioning, suggesting that systemic IL-1β was the key mediator, Thomson & Sutherland suggest that systemic IL-1β is not sufficient to cause the same consolidation deficit.
There are studies suggesting impairments induced by LPS in passive avoidance experiments (Jain et al., 2002), but the doses of LPS used by these authors were extremely high (50 μg/mouse i.p). More recently another paradigm, two-way active avoidance, has been used to show LPS-induced cognitive deficits in rodents (Sparkman et al., 2005a). In this paradigm, animals are placed in a shuttlebox with 2 compartments of equal size. After a period of habituation, a light comes on that precedes a footshock by 5 seconds. If the animal crosses to the other compartment both the light and the shock go off. The animal should thus cross when the light appears to avoid shock or cross after the shock begins to escape the shock and consequently learn the association between the light and shock. Using active two-way avoidance these authors have shown that LPS at 250μg/kg, 4 hours before the first testing session, decreased the number of avoidance responses with respect to saline controls on days 2, 3 and 4, with no effect on days 1 and 5. LPS treated animals were considerably less active in the inter-trial interval on training day 1 and yet still made the same amount of avoidance responses (i.e. cued movement to avoid being shocked). Thus the LPS-treated mice were nearly four times more likely to make a response in the presence of the light than they were in the absence of the light, indicating that they had acquired the task to some extent during this first training session. In addition, LPS given 4 hours before training could influence cognition on the first day of training, but its effects would be effectively absent 24 hours later. Therefore, any deficit seen 24 hours later could be attributable to state-dependent effects. It is also of note that pro-inflammatory cytokines can induce hyperalgesia (Maier et al., 1993) and thus, heightened pain responses to shock on day 1 may have some role to play in possible state-dependent effects (i.e. shock may be less aversive on day 2 and subsequent days since the effects of LPS have now passed). Thus, the effects of LPS in this active avoidance paradigm are very modest, and may also be state-dependent. This seems a less likely explanation for the effects reported by Pugh et al., (1998) despite the similarity in the aversive stimuli used in these paradigms. However, the tasks are also different in that animals use both the auditory cue and the context to predict the shock in fear conditioning experiments, while in active avoidance the context is a poor predictor of shock, because it can be associated with either the shock itself or the avoidance of shock. Thus learning is likely governed by the light stimulus and thus, modest or absent aversive conditioning to the light stimulus (Sparkman et al., 2005a) could be similar to the lack of effect of LPS on auditory cue conditioning shown by Pugh (Pugh et al., 1998). It has also been argued that the interoceptive sensations of sickness are likely to increase the load on working memory and thus compromise the ability of subjects to coordinate nociceptive stimuli with diffuse environmental stimuli (contexts), while auditory cues may be more salient (Dantzer et al., 2008). This could possibly explain why contextual fear conditioning is more easily disrupted than cued fear conditioning or active avoidance.
An earlier paper examined sickness-induced alterations in performance and motivation as potential confounds in LPS experiments, both in operant boxes and mazes (Gahtan and Overmier, 2001). These authors used a delayed matching to sample task in which rats must press a lever under a light when it is illuminated or under the other unlit light after a tone in order to receive a food reward. The accuracy of animals injected with saline and 125 and 250 μg/kg LPS were not significantly different. The response latency was not significantly longer if they made the correct response, but was longer when choosing incorrectly. They made less reponses overall (though this was not statistically significant) and got less rewards in the whole session, which was due to cessation of responding. Thus their motivation to respond decreased, but their accuracy did not. Similarly, in an appetitive Y-maze reference memory task rats showed the same rate of learning. There was a significant decrease in the number of trials completed, and a trend towards a longer latency in the LPS group, but importantly there was no difference in the numer of trial blocks in which they acquired the task. All of this is consistent with a decreased speed of movement and a decreased motivation, but no change in the accuracy of responding.
Malaise in the water maze
Many of the studies investigating cognitive effects of LPS and IL-1β in rats and mice have employed the Morris Water Maze or variants of it, and these studies have provided some contradictory claims (see table 1). Deterioration of spatial learning in mice treated with LPS at 400-800 μg/kg was reported by Arai et al. (Arai et al., 2001) using a small, and relatively simple water maze. Despite recording the route taken by all mice with a computerized tracking system, this report, unfortunately, only presents data for latency rather than distance swum as the measure of memory and learning. As we stated at the beginning of this review, the probe trial is a strong indicator of the appropriate use of spatial cues to learn the location of the platform and in this study a probe trial on day 3 reveals equivalent learning despite 2 days of treatment with LPS. Since the deficits are reported in the first 2 days, an earlier probe trial would have been more useful, but certainly at 3 days LPS has not impacted on spatial learning, which is dependent on the hippocampus (Morris et al., 1982). Also in 2001, LPS was reported to impair spatial learning in rats (Shaw et al., 2001), but close examination of these data reveal some interesting points. Firstly, the animals are trained from a fixed start location, and thus solving the maze may have little hippocampal involvement. Secondly, the impairments induced by LPS were a modest increase in latency on day 4 (i.e. three days after LPS challenge) and a modest decrease in the ‘percentage of time spent on direct route’ to the platform. This novel measure assessed whether the animals are travelling directly towards the visuospatial location of the platform. This impairment is also only observed from day 4 onwards. However, the data from this novel measure were not supported by the cumulative distance data presented (i.e. LPS-treated animals did not travel longer distances than controls) and this may suggest that alternative search strategies were pursued by LPS-treated animals. Thirdly, both latency and distance travelled were decreased relative to controls on the day of treatment with LPS, so if there were any early effect on learning it would appear to be opposite to that predicted. Indeed saline-treated, daily LPS and single LPS all appeared different on day one, further complicating analysis. Fourthly, despite the claim that from day 4 onwards the LPS-treated animals were impaired, the probe trial in this study indicates equivalent learning by the LPS and control groups. Thus it is difficult to see any basis for the conclusions in this study. This study also uses the unusual approach of treating animals for 5 consecutive days with LPS. The rationale for doing this is not clear and no attempt is made to monitor the effects of this treatment on inflammatory mediators. Repeated challenges with LPS induce early hypersensitivity followed by tolerance (Greer and Rietschel, 1978) and this is manifest in the marked decrease in swim speed on day 2 of LPS treatment and its gradual increase to normal speed by day 5. Complicating the inflammatory milieu can only further complicate attempts to understand the influence of LPS on learning and memory. Although the analysis of these data is complicated by the effects of hypersensitivity and tolerance to LPS, the observation that consecutive treatments with LPS caused no memory deficits, while a single challenge with LPS reportedly did, is further evidence that the reported LPS effects on memory may be state-dependent. Other recent studies with rats report learning impairments induced by i.c.v IL-1β, but these animals pursued a non-spatial thigmotaxic strategy (Song and Horrobin, 2004) and parallel experiments show 7 fold increases in serum concentrations of the stress hormone corticosterone (Song and Horrobin, 2004; Song et al., 2004). Thigmotaxis is a typical response to anxiety in rats and mice (Treit and Fundytus, 1988; Simon et al., 1994) and clearly, in studies inducing stress responses, is a confounding factor. The least stressful strategy to escape the water in this case is likely to involve avoiding the anxiogenic central areas of the maze, but this strategy may be inappropriately interpreted as a failure to learn (see figure 1).
Table 1.
Summary of Morris Water Maze findings after LPS or IL-1β challenges. All data refer to hidden platform versions of the MWM and all papers are fully referenced and discussed in the main text. N.D.: not determined in this study. No effect: LPS/IL-1β did not impair animals on this measure. Arrows indicate increase (↑) or decrease (↓). In probe trials (in which the hidden platform has been removed): + no preference (the data is shown, or described in text, and animals spend only 25% of distance travelled in target quadrant).
| Reference | Species | Treatment | Dose | Route of Admin | Timing (before testing) | Latency to find platform | Distance (path length) | Route taken | Probe or retention (r) trial |
|---|---|---|---|---|---|---|---|---|---|
| Oitzl (1993) | Rat | IL-1β | 100 ng | i.c.v. | 60 min | ↑(day 2, trial 1) | N.D. | N.D. | N.D. |
| Rat | IL-6 | 100 ng | i.c.v. | 60 min | No effect | N.D. | N.D. | N.D. | |
| Rat | IL-1β | 100 ng | i.c.v. | 0 min | No effect | N.D. | N.D. | N.D. | |
| Gibertini(1995) | Mouse | Legionella | 8 × 106 | i.p. | 24 hours | ↑ | ↑ | N.D. | no+preference |
| Mouse | IL-1β | 100 ng | i.p. | 2 hours | ↑ | no effect | N.D. | N.D. | |
|
Gibertini (1998) spaced protocol |
Mouse | IL-1β | 100 ng | i.p. | 2 hours | N.D. | no effect | ↑ thigmotaxis | no preference |
| spaced protocol | Mouse | IL-1β | 1000 ng | i.p. | 2 hours | N.D. | ↓ | N.D. | N.D. |
| massed protocol | Mouse | IL-1β | 100 ng | i.p. | 2 hours | 23°C↑ 18°C No effect |
N.D. | N.D. | N.D. |
| Arai (2001) | Mouse | LPS | 400-800 μg/kg | i.p. | 6 hours | ↑ | N.D. | N.D. | no* impairment |
|
Shaw (2001) Fixed start location |
Rat | LPS | 100μg/kg | i.p. | 4 hours | no effect | ↓(day 1-3) | path analyses suggest deficit | no^ difference |
| Yirmiya (2002) | Rat | IL-1β | 10 ng | i.c.v. | immediately after training | no effect | N.D. | N.D. | N.D. |
| Rat | IL-1 ra | 100 ng | i.c.v. | immediately after training | ↑ | N.D. | N.D. | N.D. | |
| Song (2004) | Rat | IL-1β | 15 ng | i.c.v. | immediately after training | ↑ | N.D. | ↑ thigmotaxis | no preference |
| Sparkman (2005c) | Mouse | LPS | 250μg/kg | i.p. | 4 hours | ↑ | no effect d1 modest ↑d3 | N.D | No (r) impairment |
| Sparkman (2005a) | Mouse | LPS | 250μg/kg | i.p. | 4 hours | ↑ | no effect | thigmotaxis shown | No (r) impairment |
| Thomson (2006) | Rat | IL-1β | 2μg/kg | i.p. | 1 and 24 hours | no effect | N.D. | N.D. | no impairment |
no impairment (the data is shown and treated animals show a clear preference for the target quadrant).
no difference - the limited probe data shows no difference between treated and control groups. Probe trials have been performed at variable times post-training as discussed in the text. Retention trials (r) are performed, like probe trials, at the end of training but retain the hidden platform.
Figure 1.
Theoretical illustrations of Morris Water Maze strategies. When latency is the only measure of performance in the MWM, the improvement between trials 1 and 2 (directly below) would be interpreted as successful learning in A with impairments in B and C, and no learning whatsoever in D. If distance is considered, B now shows equivalent learning to A, C appears somewhat impaired, and D shows no evidence of learning. If the route is examined it is obvious that A and B are using spatial cues to move directly towards the platform while neither C nor D use these spatial cues. Nonetheless, C shows clear learning by a non-spatial strategy (thigmotaxis). Examination of the probe trial data that would have resulted from the removal of the hidden platform, reveals that only A and B know where the platform is relative to spatial cues while C and D are indistinguishable in they both visit all quadrants equally. Only examination of all four of these parameters give the full picture of how the animals behave in the maze. Most studies have used only latency. Those using distance, route and/or probe trials have shown evidence for strategies B and C. No studies, to our knowledge, have shown evidence for D (see table 1 for details).
Aspects of the experimental design of the Shaw study were repeated in mice by Sparkman et al. (Sparkman et al., 2005b), among which is the treatment of animals for 5 consecutive days with LPS. This point aside, the authors show clearly that whereas LPS induced prolonged latency to find the hidden platform in the MWM, the distance travelled by LPS and saline treated mice was equivalent. Further experiments by these authors (Sparkman et al., 2005c) focused on potential confounding factors. LPS (250μg/kg) on day 1, LPS everyday or saline everyday do not show any differences on day 1 or 2, despite a profound depression of swim speed. Thus, LPS does not affect learning either on the day of treatment or on the following day, but the slower movement of these animals would have suggested decreased learning had latency been the main measure. To their credit, these authors have gone to some lengths, using comparisons of latency, distance and swim speed measures as well as visible platform versions of the maze in order to show that performance deficits are considerably more prominent than learning deficits. Nonetheless, they claim that there is some evidence for learning decrements on later days in these experiments. These effects are very modest and retention trials performed 3 days after the end of the 5-day learning period show no difference between LPS- and saline-treated groups. In the same study, rats were trained on a visible platform version of the MWM (Sparkman et al., 2005a). At the end of training the platform was removed and probe task was performed to examine the amount of time spent exploring the different quadrants of the maze. Therefore, although the extra-maze cues were not necessary for learning the position of the visible platform, only extra-maze cue learning would result in a preference for the quadrant in which the platform had previously been located. LPS treated rats did not differ from controls in their preference for the training quadrant. Therefore, both groups were, to some degree, using the extra-maze spatial cues to learn the task.
We have also performed an aversively motivated form of reference memory in a Y maze filled to a depth of 2cm with water (paddling Y maze) and also found no effect of LPS (500μg/kg) in normal mice despite clear sickness and slower responding (Cunningham et al, submitted). A failure to find any effect of i.p. IL-1β (sufficient to induce anorexia leading to a 15g reduction in body weight) on learning, consolidation or retrieval of memory, in rats, was also reported by Thomson and Sutherland (Thomson and Sutherland, 2006). Using three versions of the MWM they show no effect of systemically administered IL-1β on acquisition, consolidation or retention of spatial learning. Thus, on a number of reference memory tasks, LPS and IL-1β have only very subtle effects, if any at all, on reliable measures of learning i.e. distance travelled and accuracy of responding, as opposed to simple latency to find hidden platforms or exits. A summary of findings in the hidden platorm version of the MWM is shown in table 1 and an illustration of potential confounds is shown in figure 1.
More difficult spatial tasks have revealed LPS-induced decrements. A ‘spatial working memory’ version of the MWM has been used by Sparkman et al. (2006). This working memory task involves the hidden platform being moved on successive days, such that the animals have to constantly shift from the previous day’s learned location to find the new platform location (Sparkman et al., 2006). On day 9 animals were injected with LPS or saline and the distance travelled to the platform measured across three trials. The animals treated with LPS travelled considerably further than those treated with saline. What is different about this task from the standard reference memory MWM task is that animals have to learn something new that renders what they have previously learnt irrelevant. Thus, if mice have to learn a new location every day, the first trial of the day is as informative about their learning as is the last trial of the day. It is possible that perseverative responding or indeed superior learning of the previous training location could contribute to the increased distance travelled by LPS-treated animals. Therefore, details of the within-session learning on the LPS treatment day would be extremely useful in ascertaining the nature of the deficit.
The authors go on to show that these learning decrements are not present in IL-6-/-mice. This is consistent with previous studies by (Balschun et al., 2004) that suggest that IL-6 has a role in blocking long term potentiation: i.c.v. infusion of anti-IL-6 antibodies increased LTP and improved performance on an aversively motivated Y maze. Since the induction of LTP by tetanic stimulation appears to induce brain IL-6 expression it is postulated that the blocking antibodies are interfering with the function of physiologically, rather than pathologically, relevant IL-6. The authors of this study suggest that IL-6 may be involved in limiting the storage of some types of information. Thus, it may be significant that IL-6 was necessary for LPS-induced impairments in which the suppression of recently learned information is necessary for solving the new problem (Sparkman et al., 2006) when it may have no role in a more straightforward reference memory version of the MWM (Oitzl et al., 1993). A clearer knowledge of search patterns (i.e. the extent to which animals search in the previously rewarded location) on the first trial of each session in the Sparkman studies would be helpful to clarify some of these issues.
Working memory deficits were also reported in a radial arm maze after i.c.v. administration of IL-1β (Song et al., 2004). The effects are described as targeting memory retrieval. However, the recently formed memories of the locations of the food-baited arms have been learnt in the absence of IL-1β and are tested in its presence and thus, this could be accounted for by state-dependent effects. The authors also report that pellets were eaten equally by saline and IL-1β treated animals in the training phase, but no mention is made of consumption in the test phase. There is evidence that animals develop taste aversion upon i.c.v. IL-1β infusion (Janz et al., 1991) and the aversion develops after pairing of IL-1β and food pellets. The possibility that taste aversion occurs is strengthened by evidence that reducing stress, by infusion of the glucocorticoid receptor antagonist RU486, blocks the impairment. It remains possible that these animals have a working memory deficit, but the study lacks the controls to state this with confidence.
IL-1β and the facilitation of learning
It is apparent that some of the early reports of deleterious effects of IL-1β or LPS on learning and memory are likely to be accounted for by inappropriate measures or confounding factors. A recent body of work from the laboratory of Raz Yirmiya may help to explain some of the remaining contradictions in the literature. This work started with the injection of 10 ng IL-1β into rats (icv) without any impairment of accuracy in the MWM (Yirmiya et al., 2002). In parallel expts, IL-1 receptor antagonist was found to lengthen latency. Animals were trained for 12 trials and then immediately infused with IL-1β or IL-1 ra, so the drugs are not present during learning, but are during “consolidation”. So, IL-1β injected after learning, albeit at a lower dose than that used by Oitzl et al., does not affect retention, while IL-1ra appears to impair it. While it is unfortunate that these authors presented latency instead of distance travelled, IL-1 ra does not induce stress responses or locomotor hypoactivity and, therefore, these confounding factors are not a major worry in these experiments. Likewise, though state-dependent effects could conceiveably interfere with the consolidation period and thus account for the impairments observed, how IL-1 ra might alter contexts in a manner that might influence learning and memory is not intuitive. Administration of IL-1 ra to normal animals also impaired performance on a passive avoidance task (i.e. decreased latencies to cross into the goal area). These authors proposed that physiological levels of IL-1β were actually necessary for learning. This work was expanded to show that the IL-1 receptor type I (IL-1 RI) knockout mouse showed a longer path length to reach the MWM hidden platform across three days of testing than did wild type controls (Avital et al., 2003). The probe trial in these studies showed that less learning had occurred in the knockouts than in the controls. In contextual fear conditioning experiments, the IL-1 RI knockout also showed diminished freezing responses to contextual, but not to auditory cues and the knockouts were also unable to sustain LTP. Together these studies make a strong case that IL-1β can actually facilitate memory. Many authors have previously found differential effects of LPS and/or IL-1β at different doses and have speculated on a U-shaped curve which may govern the effects of immune stimulation on learning and memory, but few have actually provided enough data to construct such a curve. In 2007 Goshen et al., (Goshen et al., 2007) presented evidence that i.c.v. injection of 1 ng of IL-1β actually increased freezing in contextual fear conditioning while 10 ng IL-1 impaired freezing. In addition, icv injection of 100μg of IL-1ra also impaired freezing. This establishes a conceptual framework whereby very low, ‘physiological’ levels of IL-1β facilitate learning and blocking these levels can cause impairments, but pathological levels of IL-1β may cause deficits. These IL-1β induced deficits are induced by i.c.v injection and testing on the fear conditioning task that appears among the most robust across the many studies performed in the area of memory and learning during immune stimulation.
Dissociations in the reported impairments
While the experiments of the Rudy (Pugh et al., 1998; Barrientos et al., 2002) and Yirmiya groups provide evidence for the ability of LPS and IL-1β at certain concentrations to impair hippocampal-dependent consolidation of contextual fear conditioning, in the light of the current discussion we must conclude that LPS in general does not robustly and reproducibly impair hippocampal-dependent spatial learning. While it is possible that electric shock could be perceived as a more salient cue to motivate a sick animal, it is notable that in the studies of Yirmiya et al 10 ng of IL-1β i.c.v impaired hippocampal-dependent contextual fear conditioning (Goshen et al., 2007), but did not affect spatial learning in the MWM (Yirmiya et al., 2002). It has been shown that spatial learning and contextual fear conditioning do not necessary rely on the same neural substrates (Richmond et al., 1999). Also, it has been demonstrated that the ventral hippocampus is not necessary for the acquisition of spatial learning (Moser et al., 1993; Bannerman et al., 1999; Richmond et al., 1999), but lesions of the ventral hippocampus are sufficient to cause retrograde amnesia for a previously learnt spatial task (Moser and Moser, 1998). Thus, the function of the hippocampus, and dissociated regions within the hippocampus, may be more sensitive to post-learning manipulations than to manipulations that are imposed on acquisition and the subsequent test of learning. In the case of consolidation of contextual fear conditioning, it is possible that when a normal animal is exposed to the training conditions a larger proportion of the hippocampus is engaged in the consolidation process than may be necessary for the encoding of contextual fear association. This may have the consequence that previously acquired learning may be more vulnerable to the effects of an immune challenge.
Conclusion
There are indeed effects of LPS and IL-1β on learning and memory but these effects are not straightforward. It appears that some doses can impair contextual fear conditioning while others can facilitate it. In spatial tasks such as the MWM, latency is almost invariably increased, but distance travelled is often not affected and when it is, thigmotaxic learning strategies, specific to treated animals, have been recorded. In some cases where memory appears impaired after recovery from sickness, state-dependent changes may explain these deficits. On balance it seems reasonable to conclude that in the MWM, LPS and IL-1β do not reproducibly impair learning, but can promote a different, less anxiogenic strategy of solving the maze. Thus the question arises: If the data generated in the MWM is difficult to interpret and to disentangle cognitive, emotional and locomotor impairments, why does it remain a widely used test of hippocampal impairments in rodents experiencing sickness behaviour or stress? It is essential that distance and route travelled, as well as probe trial information, be supplied if we are to correctly interpret what animals are doing in this maze when sick. Recent studies adopting this approach have revealed that performance deficits are more marked than cognitive deficits (Sparkman et al., 2005b). We have recently developed a number of new tasks that can examine reference or working memory in animals exhibiting sickness behaviour. These tasks include a paddling (i.e. non-swim) version of the Y maze (Cunningham et al., submitted). This and other variants, by focussing on correct and incorrect responses rather than time or even distance travelled, ask whether there are any differences in the accuracy of animals treated with LPS or IL-1β, and we propose that these tasks may be of utility in seperating some of these confounding factors. It should also be stressed that designing paradigms to examine the effects of LPS or pro-inflammatory cytokines on learning and memory is challenging. In order to exclude state-dependent effects the immune stimulator should ideally be present during training and testing. In essence this requires paradigms that can be learned in a single session, such as fear conditioning experiments or more simple spatial learning paradigms. Even if this is achieved it then becomes difficult to delineate effects on acquisition, consolidation and retrieval of learning. However, with appropriate paradigms and appropriate controls a certain amount of clarity could be achieved.
Relevance for human health & disease
There is an existing literature on cognitive effects of infection and of experimental endotoxin treatment in humans. Although a full discussion of this literature is beyond the scope of this review, it is useful to make a brief comparison between this and the rodent literature. Studies of bacterial endotoxin injected into healthy volunteers suggests that at 0.8 ng/kg there were no effects on attention or executive functions, but, verbal and non-verbal memory were significantly decreased (Reichenberg et al., 2001). Further studies with 0.8 ng/kg LPS actually showed an improvement in working memory, but an impairment in declarative memory (Cohen et al., 2003). Subsequent studies with 0.2 ng/kg LPS produced no significant effects on working memory, executive function or attention (Krabbe et al., 2005). That these effects are relatively subtle and are dose-dependent is reflected in the variability of data in studies of the common cold, influenza and other upper respiratory tract infections (Bucks et al., 2008). In their studies of cognition, mood and emotional processing in upper respiratory tract infection, they report no decrements in accuracy of episodic memory or quality of working memory, but a major decrease in speed of cognitive processing, particularly in retrieval of memory. They propose that the preservation of accuracy of memory may occur at the expense of speed (Bucks et al., 2008). Likewise Capuron et al., have shown that high dose IFN-α immunotherapy causes psychomotor slowing, with deficits becoming more marked as the attentional demands of the tasks increase (Capuron et al., 2001). These studies make for an interesting comparison with LPS in rodents. Most human studies show increased anxiety and depressed mood after infection or LPS or cytokine treatment and the Bucks study shows that this is elevated in aged patients with respect to younger ones. There is already very clear evidence that systemic infections have more profound outcomes in elderly and demented patients than in younger healthy controls. This is best illustrated by the frequent occurrence of episodes of delirium in this population. Delirium is an acute disturbance of cognition, attention and consciousness that is induced by, among other things, systemic inflammatory events such as infection, injury or surgery (Brown and Boyle, 2002). The interaction between prior brain ageing or neurodegeneration and superimposed systemic inflammation would appear to be a key factor in induction of these episodes (Voyer et al., 2006).
Consistent with this, some of the paradigms described in the main review have now provided evidence that ageing is a risk factor for exaggerated repsonses to systemic LPS, infection and/or IL-1β. In 2006 Barrientos et al showed that infection with E. Coli had no effect on freezing in contextual fear conditioning experiments with 3 month old rats, but impaired freezing in 24 month old rats (Barrientos et al., 2006). Acquisition in the MWM was also not affected by E. Coli in young or aged animals, but a probe trial 48 hours later suggested less memory of the location in the aged animals infected with E. Coli than in controls. Age-related decrements in two-way active avoidance were also reported after LPS challenge i.p. (250μg/kg) in mice (Kohman et al., 2007). However the major effects in this study were observed using the strategy of challenging everyday with LPS, which as previously stated causes considerable difficulties in interpreting the results. The effects of a single LPS challenge on aged animals does not appear to be different from this effect in younger animals, although the aged animals perform worse than the younger ones per se. A radial arm variant of the matching-to-place version of the MWM has also been used to show that LPS (330 μg/kg), injected i.p., impaired learning of the new location of this platform. Aged mice were impaired with respect to young controls, when compared on distance travelled across the 8 day acquisition period. Upon injection with LPS there was no effect on distance travelled in young animals, but aged mice clearly showed longer distances to find the hidden platform (Chen et al., 2008). It should be added that all of the potential confounding factors discussed in the main review are even more relevant in ageing or neurodegenerative states since these animals show exaggerated sickness behaviour responses to systemic inflammatory activation (Combrinck et al., 2002; Godbout et al., 2005). Accuracy, i.e. arms entered, as well as information about perseverance at the prior location of the platform in this paradigm should give important information about the strategy pursued by aged LPS-treated animals. We have recently found that performance in a reference memory Y maze was not impaired by LPS in normal animals, but induced increased incorrect arm entries in animals with neurodegenerative disease (Cunningham et al., submitted). These diseased animals were not impaired when injected with saline and thus, this impairment is specific to the combination of early neurodegeneration and peripheral immune stimulation. There is a growing literature on microglial priming as a potential explanation of how normally banal systemic infections can have major effects on brain function in the aged (Godbout and Johnson, 2006) and demented population (Cunningham et al., 2005; Perry et al., 2007) and the above memory and learning studies suggest possible inroads into study of the acute cognitive impairments in such situations.
Acknowledgements
The financial support of the Wellcome Trust and the BBSRC is gratefully acknowledged.
References
- Arai K, Matsuki N, Ikegaya Y, Nishiyama N. Deterioration of spatial learning performances in lipopolysaccharide-treated mice. Jpn J Pharmacol. 2001;87:195–201. doi: 10.1254/jjp.87.195. [DOI] [PubMed] [Google Scholar]
- Aubert A, Vega C, Dantzer R, Goodall G. Pyrogens specifically disrupt the acquisition of a task involving cognitive processing in the rat. Brain Behav Immun. 1995;9:129–148. doi: 10.1006/brbi.1995.1013. [DOI] [PubMed] [Google Scholar]
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. Faseb J. 2004;18:1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A, Klusman I, Furukawa H, Monge Arditi G, Kabiersch A. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J Steroid Biochem Mol Biol. 1991;40:613–618. doi: 10.1016/0960-0760(91)90284-c. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Crestani F, Kelley KW, Dantzer R. Mechanisms of the behavioral effects of interleukin 1. Role of prostaglandins and CRF. Ann N Y Acad Sci. 1992;650:268–275. doi: 10.1111/j.1749-6632.1992.tb49135.x. [DOI] [PubMed] [Google Scholar]
- Borowski T, Kokkinidis L, Merali Z, Anisman H. Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport. 1998;9:3797–3802. doi: 10.1097/00001756-199812010-00006. [DOI] [PubMed] [Google Scholar]
- Brown TM, Boyle MF. Delirium. BMJ. 2002;325:644–647. doi: 10.1136/bmj.325.7365.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucks RS, Gidron Y, Harris P, Teeling J, Wesnes KA, Perry VH. Selective effects of upper respiratory tract infection on cognition, mood and emotion processing: a prospective study. Brain Behav Immun. 2008;22:399–407. doi: 10.1016/j.bbi.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Cador M, Ahmed SH, Koob GF, Le Moal M, Stinus L. Corticotropin-releasing factor induces a place aversion independent of its neuroendocrine role. Brain Res. 1992;597:304–309. doi: 10.1016/0006-8993(92)91487-y. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med. 2001;63:376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen O, Reichenberg A, Perry C, Ginzberg D, Pollmacher T, Soreq H, Yirmiya R. Endotoxin-induced changes in human working and declarative memory associate with cleavage of plasma “readthrough” acetylcholinesterase. J Mol Neurosci. 2003;21:199–212. doi: 10.1385/jmn:21:3:199. [DOI] [PubMed] [Google Scholar]
- Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahtan E, Overmier JB. Performance more than working memory disrupted by acute systemic inflammation in rats in appetitive tasks. Physiol Behav. 2001;73:201–210. doi: 10.1016/s0031-9384(01)00471-1. [DOI] [PubMed] [Google Scholar]
- Gibertini M. Cytokines and cognitive behavior. Neuroimmunomodulation. 1998;5:160–165. doi: 10.1159/000026332. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin. 2006;24:521–538. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Goodwin DW, Powell B, Bremer D, Hoine H, Stern J. Alcohol and recall: state-dependent effects in man. Science. 1969;163:1358–1360. doi: 10.1126/science.163.3873.1358. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Greer GG, Rietschel ET. Lipid A-induced tolerance and hyperreactivity to hypothermia in mice. Infect Immun. 1978;19:357–368. doi: 10.1128/iai.19.2.357-368.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Jain NK, Patil CS, Kulkarni SK, Singh A. Modulatory role of cyclooxygenase inhibitors in aging- and scopolamine or lipopolysaccharide-induced cognitive dysfunction in mice. Behav Brain Res. 2002;133:369–376. doi: 10.1016/s0166-4328(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Janz LJ, Brown R, Zuo L, Falk J, Greenberg AH, Dyck DG. Conditioned taste aversion but not adrenal activity develops to ICV administration of interleukin-1 in rats. Physiol Behav. 1991;49:691–694. doi: 10.1016/0031-9384(91)90303-6. [DOI] [PubMed] [Google Scholar]
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992;89:9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Tarr AJ, Byler SL, Boehm GW. Age increases vulnerability to bacterial endotoxin-induced behavioral decrements. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Kozak W, Conn CA, Kluger MJ. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am J Physiol. 1994;266:R125–135. doi: 10.1152/ajpregu.1994.266.1.R125. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Reichenberg A, Yirmiya R, Smed A, Pedersen BK, Bruunsgaard H. Low-dose endotoxemia and human neuropsychological functions. Brain Behav Immun. 2005;19:453–460. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Laye S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, Parnet P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–98. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- Maier SF, Wiertelak EP, Martin D, Watkins LR. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Res. 1993;623:321–324. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- Miller RR, Springer AD. Amnesia, consolidation, and retrieval. Psychol Rev. 1973;80:69–79. doi: 10.1037/h0033897. [DOI] [PubMed] [Google Scholar]
- Mormede C, Castanon N, Medina C, Dantzer R. Conditioned place aversion with interleukin-1beta in mice is not associated with activation of the cytokine network. Brain Behav Immun. 2003;17:110–120. doi: 10.1016/s0889-1591(02)00054-5. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Distributed encoding and retrieval of spatial memory in the hippocampus. J Neurosci. 1998;18:7535–7542. doi: 10.1523/JNEUROSCI.18-18-07535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, van Oers H, Schobitz B, de Kloet ER. Interleukin-1 beta, but not interleukin-6, impairs spatial navigation learning. Brain Res. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- Overton DA. State-Dependent or “Dissociated” Learning Produced with Pentobarbital. J Comp Physiol Psychol. 1964;57:3–12. doi: 10.1037/h0048023. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O’Mara SM. Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behav Brain Res. 2001;124:47–54. doi: 10.1016/s0166-4328(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Song C, Horrobin D. Omega-3 fatty acid ethyl-eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1beta administration. J Lipid Res. 2004;45:1112–1121. doi: 10.1194/jlr.M300526-JLR200. [DOI] [PubMed] [Google Scholar]
- Song C, Phillips AG, Leonard BE, Horrobin DF. Ethyl eicosapentaenoic acid ingestion prevents corticosterone-mediated memory impairment induced by central administration of interleukin-1beta in rats. Mol Psychiatry. 2004;9:630–638. doi: 10.1038/sj.mp.4001462. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Kohman RA, Garcia AK, Boehm GW. Peripheral lipopolysaccharide administration impairs two-way active avoidance conditioning in C57BL/6J mice. Physiol Behav. 2005a;85:278–288. doi: 10.1016/j.physbeh.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Kohman RA, Scott VJ, Boehm GW. Bacterial endotoxin-induced behavioral alterations in two variations of the Morris water maze. Physiol Behav. 2005b;86:244–251. doi: 10.1016/j.physbeh.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Martin LA, Calvert WS, Boehm GW. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav Brain Res. 2005c;159:145–151. doi: 10.1016/j.bbr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Ide M, Shibutani T, Ohtaki H, Numazawa S, Shioda S, Yoshida T. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J Neurosci Res. 2006;83:557–566. doi: 10.1002/jnr.20752. [DOI] [PubMed] [Google Scholar]
- Tazi A, Dantzer R, Crestani F, Le Moal M. Interleukin-1 induces conditioned taste aversion in rats: a possible explanation for its pituitary-adrenal stimulating activity. Brain Res. 1988;473:369–371. doi: 10.1016/0006-8993(88)90868-2. [DOI] [PubMed] [Google Scholar]
- Thompson CI, Grossman LB. Loss and recovery of long-term memories after ECS in rats: evidence for state-dependent recall. J Comp Physiol Psychol. 1972;78:248–254. doi: 10.1037/h0032178. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res Bull. 2005;67:24–29. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Sutherland RJ. Interleukin-1beta induces anorexia but not spatial learning and memory deficits in the rat. Behav Brain Res. 2006;170:302–307. doi: 10.1016/j.bbr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Voyer P, Cole MG, McCusker J, Belzile E. Prevalence and symptoms of delirium superimposed on dementia. Clin Nurs Res. 2006;15:46–66. doi: 10.1177/1054773805282299. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Mem. 2002;78:379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]