Abstract
STUDY DESIGN
Cross-sectional study.
OBJECTIVES
To investigate the impact of quadriceps femoris (QF) muscle strength asymmetry at the time of return to sport on self-reported function and functional performance of individuals following anterior cruciate ligament reconstruction (ACLR).
BACKGROUND
Evidence-based QF strength guidelines for return-to-sport decision making are lacking. Objective guidelines necessitate understanding the impact of QF strength deficits at the time of return to sport on function and performance.
METHODS
Fifty-five individuals (mean age, 17.3 years) who were cleared for return to sport following primary ACLR (ACLR group) and 35 uninjured individuals (mean age, 17.0 years) in a control group participated in the study. QF strength (maximum voluntary isometric contraction) was assessed, and the quadriceps index (QI) was calculated [(involved strength/uninvolved strength) × 100%]. The ACLR group was further subdivided into 2 groups, based on the QI: high quadriceps (QI of 90% or greater) and low quadriceps (QI of less than 85%). The International Knee Documentation Committee Subjective Knee Evaluation Form score was used to assess self-reported function, and hop tests were used to assess functional performance. Multivariate analysis of variance and hierarchical regression analyses were performed.
RESULTS
The individuals in the ACLR group were weaker, reported worse function, and performed worse on hop tests compared to those in the control group (P<.05). The low-quadriceps group demonstrated worse performance on the hop tests compared to the high-quadriceps group and the control group (P≤.016). Hop test performance did not differ between the high-quadriceps and control groups (P≥.14). QF strength predicted performance on the hop tests beyond graft type, presence of meniscus injury, knee pain, and knee symptoms.
CONCLUSION
At the time of return to sport, individuals post-ACLR who had weaker QF (QI of less than 85%) demonstrated decreased function, whereas those with minimal QF strength deficits (QI of 90% or greater) demonstrated functional performance similar to uninjured individuals. QF strength deficits predicted hop test performance beyond the influences of graft type, presence of meniscus injury, knee pain, and knee symptoms.
Keywords: ACL, ACL reconstruction, function, hop test, knee, performance, weakness
Anterior cruciate ligament (ACL) injury is a common and physically debilitating knee injury for athletes that may cause functional deficits,21,34,40,65 increased risk of second ACL injury,12,46,48,59 and long-term joint morbidity, such as early cartilage degeneration.32,33 For the majority of individuals, ACL rupture results in loss of functional joint stability, for which a reconstruction procedure is often recommended, particularly in those who wish to resume high-level activities. Recent studies have reported that young individuals who return to high-level activity following ACL reconstruction (ACLR) represent a high-risk population for poor outcome. Within 2 years of primary ACLR, second-injury rates are as high as 24% in young, active individuals who return to sport.46 A systematic review and meta-analysis demonstrated the low return-to-sport rates following ACLR, with only 63% resuming preinjury level of activity participation and 44% returning to competitive sport.1 Successful outcome and return to high-level activities following ACLR depend on appropriate surgical and rehabilitation decision making. Historically, research efforts have focused on assessment of variables that influence surgical outcome, such as graft choice, placement, tensioning, and fixation techniques.4 Evidence-based-medicine guidelines for rehabilitation progression and criterion-based clinical decision making are not well documented, particularly for young, active individuals who desire to return to sport.
Clinical milestones related to return to sport after ACLR can be found in the literature, with most authors advocating resolution of impairments and adequate functional performance.2,10,14,18,63 However, these criteria are vague and rely on the treating clinician for interpretation. More recently, “criterion” performance on objective measures of impairment and functional status has been proposed; however, the suggested criterion value indicative of readiness for sport participation varies widely and is not empirically based. For example, the proposed criterion value for side-to-side differences in quadriceps femoris (QF) muscle strength varies from deficits of 10% to 35%24,26,37,41,54,61 on the involved compared to the uninvolved side. The current literature lacks standard guidelines for return to sport,26 which is further reflected by the persistent deficits noted in young individuals with ACLR. Limb asymmetries in muscle strength53,65 and in performance38 and limb-loading strategies during squatting,39,58 jumping, and landing15,19,42,43,45,64 are consistently noted following return to high-level activity. The development of standardized, objective, and evidence-based recommendations is crucial for the promotion of standards of care, the optimization of activity performance, and the potential to minimize risk of future injury. The development of such guidelines necessitates further understanding of the impact of impairments on function and performance in this patient population.
Residual and persistent impairments are often cited as a limiting factor in return to preinjury levels of function and activity.1 In particular, QF weakness is a primary impairment following ACLR, and improvement of QF strength is an important factor in improving functional outcomes.51 Substantial work showed that QF weakness is related to poor functional outcomes following ACL injury9,28,57,67 and ACLR.11,24,47,66 Following ACLR, restoration of QF strength is necessary to maximize functional ability, as the return to functional activity is strongly correlated with the ability of the QF muscles to generate force.13,31,65 Of significance, poor QF strength is implicated in asymmetrical limb-loading strategies following ACLR.8 Studies to date have evaluated QF strength at specific time points (eg, 3, 6, and 12 months) following ACLR; however, the clinical decision of clearance for return to sport participation is a critical clinical time point. In an effort to progress toward evidence-based guidelines for return-to-activity decision making, understanding the impact of key impairments, such as QF strength deficits, at the time of return to sport is imperative.
The purpose of this study was to investigate the impact of QF strength asymmetry at the time of return to sport on self-reported function and the functional performance of athletes following ACLR (ACLR group). The hypothesis tested was that the individuals in the ACLR group would demonstrate QF strength deficits and reduced function compared to uninjured participants serving as a control group. The second hypothesis tested was that, within the ACLR group, larger QF strength deficits would be associated with reduced function, and that QF strength deficits would predict function on performance-based tests after controlling for graft type, presence of meniscus injury, knee pain, and knee symptoms.
METHODS
Participants
A total of 90 participants between 14 and 25 years of age were recruited from local orthopaedic practices, physical therapy clinics, and the community from 2007 to 2011. Participant demographic characteristics are shown in TABLE 1. Fifty-five individuals were recruited for the ACLR group. Participants were included in this group if they had a primary, unilateral ACLR, had completed their rehabilitation program, were cleared for return to all high-level athletic activities by their surgeon and treating physical therapist, and intended return to cutting and pivoting sports on a regular basis (50 hours or more per year). Testing occurred within 4 weeks of return-to-sport clearance and all measures were obtained during the same testing session by study personnel. Individuals with bone-tendon-bone, hamstring tendon, or allograft tissue grafts and those with meniscus repair or partial meniscectomy at the time of ACLR were included in the study. Exclusion criteria were a history of low back injury or either lower extremity injury or surgery (beyond ACL injury) requiring the care of a physician in the past year, a concomitant ligament injury (beyond grade 1 medial collateral ligament injury) in the involved limb, or being skeletally immature, as identified by an ACLR procedure that was modified due to open epiphyseal plates in the tibia or femur.
TABLE 1. Participant Characteristics.
| ACLR Group (n = 55) | Control Group (n = 35) | P Value | |
|---|---|---|---|
| Age, y* | 17.3 ± 2.6 | 17.0 ± 2.3 | .59 |
| Height, m* | 1.68 ± 0.09 | 1.68 ± 0.09 | .69 |
| Weight, kg* | 69.7 ± 17.3 | 63.6 ± 13.7 | .06 |
| Sex, n | … | ||
| Female | 40 | 22 | |
| Male | 15 | 13 | |
| Graft type, n | … | ||
| PT BTB | 29 | … | |
| Hamstring tendon | 20 | … | |
| Allograft | 6 | … | |
| Level of sport participation, n (%) | … | ||
| Competitive collegiate | 9 (16%) | 13 (37%) | |
| Competitive middle/high school or club | 43 (78%) | 22 (63%) | |
| Recreational | 3 (6%) | 0 (0%) |
Abbreviations: ACLR, anterior cruciate ligament reconstruction; PT BTB, patellar tendon bone-tendon-bone graft.
Data are mean ± SD.
Thirty-five participants between 14 and 25 years of age were recruited from the community to serve as a control group (TABLE 1). Individuals were included in the control group if they reported no history of surgery in the low back or either lower extremity, no history of injury requiring the care of a physician in the past year in the low back or either lower extremity, and regular participation (50 hours or more per year) in cutting and pivoting sports.
The involved (test) limb was identified as the injured limb for the ACLR group and was randomly assigned for the control group. The study protocol was approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center, and all participants (or guardians, when required) provided written, informed consent and assent. Participants included in this analysis were part of an ongoing, prospective study of outcomes following ACLR.
QF Isometric Strength Assessment
QF isometric strength was quantified with an isokinetic dynamometer (Biodex Medical Systems, Inc, Shirley, NY) during a maximum voluntary isometric contraction (MVIC). This procedure has been used to quantify QF torque in individuals with ACL injury and reconstruction and has yielded reliable measurements.17,25,29,57,62 Subjects sat in the dynamometer with the trunk fully supported, the hips flexed to approximately 90°, and the knee flexed to 60°. The knee joint was aligned with the dynamometer axis, and the dynamometer resistance pad was secured to the anterior aspect of the distal shank. The pelvis and thigh were stabilized with straps. Real-time visual and verbal feedback was provided during the test to ensure maximum effort by the participant. Following 1 practice trial, 3 recorded maximum-effort trials (5 seconds in duration, separated by 15 seconds of rest) were completed for each knee. In the ACLR group, the uninvolved side was always tested first, and in the control group the order of testing was randomized. The peak torques in footpounds (ft·lb) of the 3 trials were averaged, then normalized to body weight (kg). Isometric QF peak torque values are routinely used to calculate asymmetry between the involved and uninvolved limbs.17,25,29,57,62 The average normalized peak torque value for each limb was used for further analysis and calculation of the quadriceps index (QI), as calculated with
| Equation 1 |
Subdivision of ACLR Group
To evaluate the influence of isometric QF strength on function, the ACLR group was further subdivided into 2 strength groups: a high-quadriceps group (HQ) with a QI of 90% or greater, and a low-quadriceps group (LQ) with a QI of less than 85%. Cutoff scores were based on studies that have utilized similar QF strength group values in individuals with ACLR,29 research having indicated that a side-to-side difference in peak QF force output of greater than 10% may reflect differences in the capacity of muscle performance beyond measurement error,60 and commonly reported QF strength values used as criteria to return athletes to sport.26,54 The sample-size estimate for detection of differences in performance-based measures of function between participants with high and low QIs was based on analysis of data from participants from a prior pilot study. Based on these data, a sample size of 22 participants per group was required to achieve a power of 0.80, with an alpha level of .05 (mean ± SD difference in limb symmetry index [LSI] between groups, 4% ± 4%). Of the 55 participants in the ACLR group, 23 were in the HQ group, 24 were in the LQ group, and 8 had a QI between 85% and 89%. Strength group comparisons were only made between the HQ (n = 23) and LQ (n = 24) groups, due to the small group size of those with QIs of 85% to 89% (n = 8). All participants in the ACLR group were included in all other analyses.
Measures of Function and Performance
Self-Report Measures
Self-reported function was assessed using the International Knee Documentation Committee Subjective Knee Evaluation Form (IKDC), which is scored on a 0-to-100 scale, with 100 representing higher knee function.22 The IKDC score is a reliable and valid measure of function in healthy individuals and in those following ACL injury.22,23 Knee pain and symptoms were measured with the Knee injury and Osteoarthritis Outcome Score (KOOS),55,56 which assesses 5 dimensions (pain, symptoms, function in daily living, function in sport and recreation, and knee-related quality of life) scored as separate and independent subsets.55,56 The KOOS is a valid, reliable, and responsive measure.30,55,56 All 5 subsets were completed, but only the pain (KOOS pain) and symptoms (KOOS symptoms) subsets were used for hypothesis testing. The pain subset assesses the frequency and amount of knee pain experienced during activities of daily living (including walking, twisting/pivoting, and negotiating stairs). The symptoms subset assesses the frequency of knee symptoms such as swelling, grinding, catching, and stiffness. Each subset is scored independently, and questions are scored on a 0-to-4 scale. Subset scores are transformed into a 0%-to-100% score, with 100% representing no knee problems.
Performance-Based Measures
Four single-leg hop tests40 (FIGURE 1) were used as performance-based measures of function. These hop tests are commonly used clinically and have good measurement reliability in nonimpaired individuals and in those following ACLR.5,6,16,50,52 The single-leg hop tests were performed in the following order: single hop for distance (cm), triple hop for distance (cm), triple-crossover hop for distance (cm), and 6-meter timed hop (seconds). Following a practice trial, participants completed 2 measurement trials for each limb, tested in random order. The averages of the 2 trials for the involved and uninvolved limbs were used for further analysis and to calculate an LSI for distance measures
| Equation 2 |
and for the timed hop
| Equation 3 |
The LSI is the most frequently reported criterion to quantify function with hop tests, with scores of less than 100% indicating deficits of the involved limb.3,16
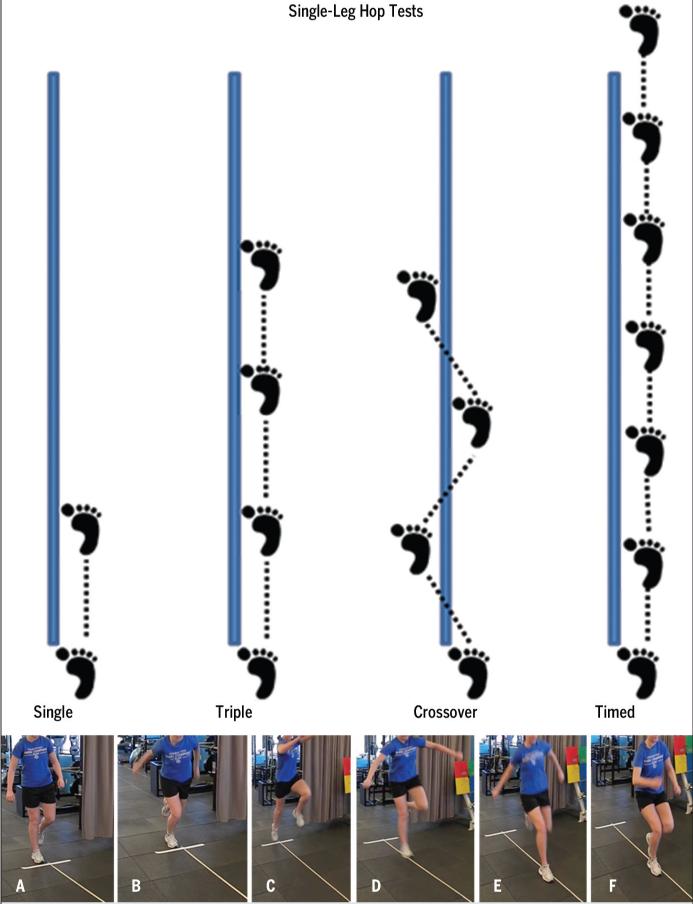
FIGURE 1.
Performance of the single-leg hop tests (top), including the single hop for distance (cm), triple hop for distance (cm), crossover hop for distance (cm), and 6-meter timed hop (seconds). For the single-, triple-, and crossover-hop tests, the goal was to hop forward as far as possible, while maintaining a controlled landing on the ipsilateral limb. For the timed-hop test, the goal was to hop on the single limb, as quickly as possible, over a distance of 6 m. For this study, 2 measurement trials were obtained for each limb, tested in random order, and the average of the trials for each limb was used to calculate a limb symmetry index (LSI) for distance-hop tests (Equation 2) and the timed-hop test (Equation 3). The bottom panels show an example of a participant completing the single hop for distance. For the single hop for distance, the participant starts in a single-limb stance position on the test limb (A), moves into a semi-crouched position (B), utilizes upper extremity swing and test-limb extension to propel forward (C), and hops forward as far as possible (D), to land on the same limb (E) and maintain control of the landing without loss of balance or contralateral foot contact (F).
Statistical Analysis
Statistical analyses were performed with SPSS Version 19.0 (SPSS Inc, Chicago, IL). Variables of interest were visually inspected for normal distribution with histograms, and natural log transformations of dependent variables (IKDC score, KOOS pain score, KOOS symptoms score, hop test scores) were performed prior to group comparisons. Participant characteristics were compared between the ACLR and control groups, as well as between the 2 ACLR subgroups defined based on low and high QI, using multivariate analysis of variance (significance, α≤.05).
To test the hypothesis that the ACLR cohort would demonstrate decreased function compared to the control group, multivariate analysis of covariance was performed. The independent variable of group (ACLR versus control) and the dependent variables of IKDC score and hop test scores (LSI) were entered into the model. Multivariate analysis of covariance was also performed on the involved and uninvolved hop test scores. Participant age and sex were entered as covariates for both analyses.
To test the hypothesis that larger isometric QF strength deficits would be associated with decreased function, multivariate analysis of covariance was performed. The independent variable of group (HQ versus LQ versus control) and the dependent variables of IKDC score and hop test scores (LSI) were entered into the model. Participant age and sex were entered as covariates. To protect against type I error, Bonferroni correction was applied to post hoc comparisons, yielding a significant alpha value (α≤.02).
To test the hypothesis that isometric QF strength would predict performance-based function in the ACLR cohort, hierarchical regressions were used to evaluate the influence of graft type, presence of meniscus injury, knee pain (KOOS pain), knee symptoms (KOOS symptoms), and QI (independent variables) on single-leg hop scores (using LSI as the dependent variable) that were found to be significantly different among the strength groups. Hierarchical regression is an incremental approach to multiple regression, and the order of input of the variables was determined a priori. Variables were entered into the model separately, and the order was determined by the variables (excluding QI) thought to be most influential on performance. QI was entered into the model last to assess its influence on hop test scores after accounting for the influence of the other independent variables. As such, the hypothesis tested was that QI would predict scores on the hop tests beyond the contributions of graft type, presence of meniscus injury, knee pain, and knee symptoms. Multicollinearity among the independent variables was checked with Pearson correlation analysis and the variance inflation factor (less than 3), and it was determined that each independent variable could be entered into the regression models.
RESULTS
ACLR Group Versus Control Group Comparisons
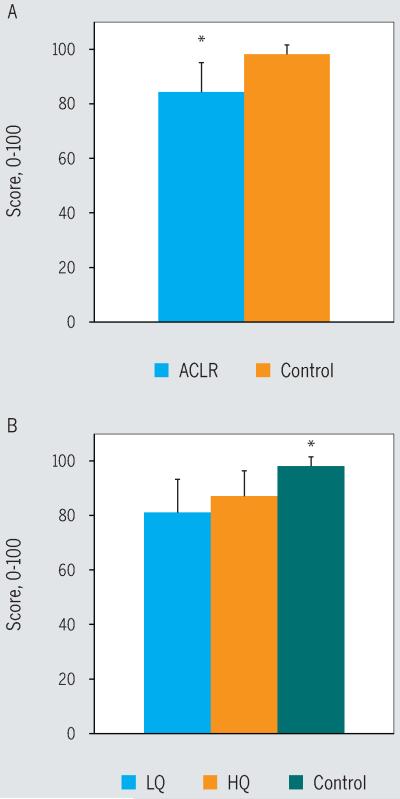
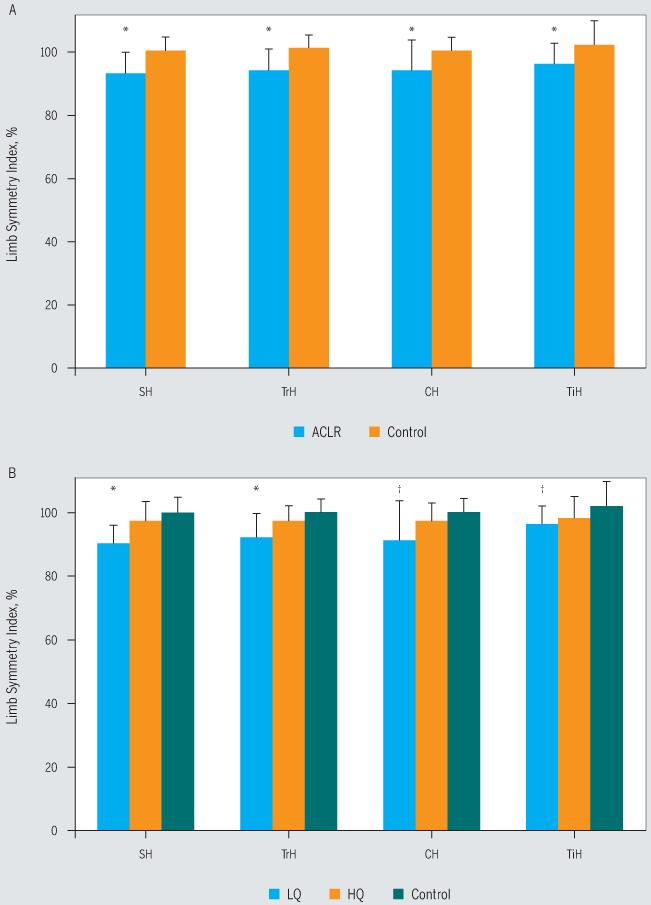
The ACLR and control groups did not differ in terms of age (P = .59), height (P = .69), or weight (P = .06) (TABLE 1). The ACLR cohort demonstrated significantly larger isometric QF strength asymmetry compared to the control group (ACLR group mean ± SD QI, 84.6% ± 15.5%; control group mean ± SD QI, 100.8% ± 7.7%; F = 31.645; P<.001). On the IKDC score, the ACLR cohort scored significantly lower than the control group (F = 37.500, P<.001) (FIGURE 2). Performance-based function for the involved limb was also significantly lower in the ACLR group, as greater asymmetry was noted on all 4 hop tests (P≤.03) (FIGURE 3). The asymmetry for the 3 hop tests for distance was due to decreased performance of the involved limb of those in the ACLR group compared to the test limb of those in the control group (P≤.016). There were no differences in performance of the uninvolved/nontest limb between the ACLR and control groups on the distance- and timed-hop scores (P>.31). On the timed-hop test, the performance of the uninvolved limb for those in the ACLR group was less than that of the nontest limb for those in the control group, but the difference was not statistically significant (P = .059).
FIGURE 2.
Scores on the International Knee Documentation Committee Subjective Knee Evaluation Form, where higher scores indicate better function. (A) *ACLR group significantly less than control group (P<.001). (B) *Control group significantly greater than both the HQ and LQ groups (P<.001). Abbreviations: ACLR, anterior cruciate ligament reconstruction; HQ, high quadriceps; LQ, low quadriceps.
FIGURE 3.
Limb symmetry index scores on the single-leg hop tests, where scores below 100% indicate deficits in the involved limb. (A) *ACLR group significantly lower than control group for all 4 hop tests (P≤.03). (B) *LQ group significantly lower than HQ and control groups (P≤.016). †LQ group significantly lower than control group (P<.003). Abbreviations: ACLR, anterior cruciate ligament reconstruction; CH, crossover hop; HQ, high quadriceps; LQ, low quadriceps; SH, single hop; TiH, timed hop; TrH, triple hop.
Strength Group Comparisons
The participants in the HQ, LQ, and control groups (TABLE 2) did not differ in terms of age (P = .57), height (P = .74), or time from surgery to return to sport (P = .57). The participants in the LQ group had the greatest weight compared to the HQ and control groups (P<.025) (TABLE 2). Multivariate analysis of variance showed that the QI, IKDC score, and all hop test symmetry scores were different among the 3 groups (P≤.007). As expected, based on group assignments, post hoc tests showed that the LQ group had significantly greater isometric QF strength asymmetry compared to the HQ and control groups (LQ mean ± SD QI, 72.8% ± 14.7%; HQ mean ± SD QI, 96.7% ± 6.6%; control mean ± SD QI, 100.8% ± 7.7%; P<.001). There was no statistically significant difference in QI between the HQ and control groups (P = .536). For the IKDC score, the control group scored significantly higher than the HQ and LQ groups (P<.001) (FIGURE 2). Between the strength groups, the LQ had a lower IKDC score than the HQ group (FIGURE 2), but this did not reach significance with our corrected alpha level (P = .052). On all of the hop tests, there were no statistically significant differences between the control and HQ groups (P≥.14). The LQ group performed worse than the HQ and control groups for the single- and triple-hop tests (P≤.016), and worse than the control group on the crossover- and timed-hop tests (P≤.003) (FIGURE 3). On the crossover hop, the HQ group performed better than the LQ group, but this did not reach statistical significance (P = .06) (FIGURE 3B).
TABLE 2. Participant Characteristics by Strength Group.
| LQ Group (n = 24)* | HQ Group (n = 23)† | Control Group (n = 35) |
P Value | |
|---|---|---|---|---|
| Age, y‡ | 17.7 ± 3.1 | 17.4 ± 2.2 | 17.0 ± 2.3 | .574 |
| Height, m‡ | 1.69 ± 0.11 | 1.68 ± 0.09 | 1.68 ± 0.09 | .735 |
| Weight, kg‡ | 75.4 ± 20.2 | 64.1 ± 11.0 | 63.6 ± 16.1 | .005§ |
| Sex, n | … | |||
| Female | 16 | 17 | 22 | |
| Male | 8 | 6 | 13 | |
| Graft type, n | … | |||
| PT BTB | 18 | 6 | … | |
| Hamstring tendon | 4 | 14 | … | |
| Allograft | 2 | 3 | … | |
| Time from ACL reconstruction, mo‡ | 6.7 ± 1.8 | 70 ± 1.5 | … | .570 |
Abbreviations: ACL, anterior cruciate ligament; HQ, high quadriceps; LQ, low quadriceps; PT BTB, patellar tendon bone-tendon-bone graft.
Individuals with a quadriceps index less than 85%.
Individuals with a quadriceps index greater than or equal to 90%.
Data are mean ± SD.
LQ greater than HQ (P = .024); LQ greater than control (P = .008).
Regression Analysis
The regression analyses were performed with the entire ACLR cohort (n = 55) for the 2 hop tests that were different between the HQ and LQ groups (the single- and triple-hop tests). Hierarchical multiple regression was used to assess the ability of the QI to predict asymmetry on the hop tests, after controlling for graft type, presence of meniscus injury, knee pain, and knee symptoms. For the single hop, knee pain (R2 change = 0.152, P = .007) was a significant predictor of performance. Graft type (R2 change = 0.041, P = .178), presence of meniscus injury (R2 change = 0.021, P = .328), and knee symptoms (R2 change = 0.052, P = .095) did not reach statistical significance as predictors. After controlling for the influence of all of these variables, the QI was a unique and significant predictor of single-hop test performance (R2 change = 0.141, P = .004). In the final model (R2 = 0.408), the QI (β = .446, P = .004) was the only statistically significant predictor. Similar results were found for the triple-hop test. Knee pain was a significant predictor (R2 change = 0.100, P = .030) and, after controlling for the influence of pain and all other variables, the QI was a significant predictor of performance (R2 change = 0.092, P = .029). In the final model (R2 = 0.280), the QI was the only statistically significant predictor of triple-hop test performance (β = .360, P = .029).
DISCUSSION
The purpose of this study was to investigate the impact of QF strength at the time of return to high-level sport activities on the function and performance of individuals following ACLR. All participants in the ACLR group had been cleared for, and intended return to, unrestricted participation in cutting and pivoting sports. The ACLR cohort was weaker and demonstrated decreased function compared to the uninjured control group. Within the ACLR cohort, greater QF strength deficits were associated with lower self-report and performance-based measures of function. IKDC scores were lowest among individuals with ACLR with the largest QF strength deficits (LQ group). Hop test scores also discriminated functional ability, as those with the largest QF strength deficits (LQ group) performed worse on the hop tests compared to the HQ and control groups. Furthermore, confirming our hypothesis, QF strength deficits predicted performance-based function even after the contributions of graft type, presence of meniscus injury, knee pain, and knee symptoms. To our knowledge, this is the first report of QF strength impairments and function specifically at the time of return to sport in individuals following ACLR. The decision to return to sport is a critical point in the rehabilitation and medical decision-making process. Clearance for return to sport indicates the medical and rehabilitation team’s confidence in the readiness of the individual to participate in activities that place a large, and sometimes unanticipated, demand on knee joint structures and musculature, mainly the QF muscles. The results of this study indicate that deficits in isometric QF strength negatively impact functional performance. Whether the impact of QF strength deficits extends into the biomechanics of the knee joint and movement patterns is the focus of our current work.
In the literature, clinical milestones and criterion performance related to return to sport vary widely and typically involve vague criteria, such as resolution of impairments and adequate functional performance.2,7,10,14,18,34,35,47,53,63,66 Our inclusion criteria specified inclusion of individuals who were cleared for unrestricted participation in high-level activities; however, the participants in our study demonstrated a range of abilities in terms of isometric QF strength impairments and functional status. In the ACLR group, 44% demonstrated strength deficits greater than 15% (QI range, 30%-84%). The variability of isometric QF strength measures among those cleared for return to sport indicates lack of standard of care in determination of readiness for sport participation. The findings of this study provide evidence of the need to empirically develop objective, criterion-based QF strength guidelines for return to sport after ACLR.
Although this study was not designed to specifically delineate a criterion value for QF strength and return-to-sport criteria, the results do indicate that isometric QF strength deficits of greater than 15% negatively impact function and performance. The single-leg hop tests used in this study were chosen because of their clinical utility to assess functional performance capacity and dynamic neuromuscular control mechanisms in a controlled manner during activities that challenge knee stability and mimic the greater demands imposed on the knee during higher-level activity.16,27,28,57 Although many factors other than muscle strength contribute to functional performance capacity, the results of this study provide initial insight into QF muscle strength criterion values that may allow for optimal performance during high-level activities that are indicators of sport performance. In the literature, the most common recommendations for return to activity are side-to-side differences of QF strength no greater than 10% to 15%,24,26,37,41,54 corresponding to QI values between 85% and 90%. Our results indicate that individuals with a QI of 90% or greater perform similarly to uninjured individuals. Future plans include further comparisons of the function and performance of individuals in our study with a QI from 85% to 89%; however, we were unable to do so in this study due to the small sample size of this group. The discrimination of an appropriate QF strength criterion (QI of 85% versus QI of 90% or another value) remains a focus of our ongoing work.
The current findings are consistent with previous studies reporting QF strength deficits at similar and longer times following ACLR.7,34,53,65 At an average of 6.5 months (26 weeks) following ACLR, Wilk and colleagues65 reported that 59% of their sample demonstrated greater than 21% side-to-side QF deficits (a QI of 79% or less). Deficits in QF strength persist after ACLR, as significant differences in QF muscle capacity have been reported at an average of 18 and 22 months following surgery.7,34 At 2 years following surgery, Risberg and colleagues53 reported greater than 15% QF deficit in over a quarter (28%) of their sample. In the current study, the time of testing (time of return to sport) was an average of 6.9 months following ACLR. Our future analysis on this cohort from subsequent testing sessions will provide insight as to whether these QF strength deficits will persist in our sample, as was previously described in other studies.
The relationship between QF muscle strength and hop test scores is inconsistent among previous works.16 Methodological differences among studies, such as sample characteristics, time from ACLR, hop tests performed, and method of muscle performance, contributed to the various findings. For the hop tests used in our study, fair to good correlations (r = 0.36-0.69) with QF strength have previously been reported.47,53,65 In this study, the unique contribution of QF strength deficits to hop test scores was specifically assessed with hierarchical regression analysis. In the regression, potential factors that could impact performance, such as graft type, presence of meniscus injury, knee pain, and knee symptoms, were accounted for in the analysis. There are other potential factors that may influence performance on hop tests, which is indicated by the moderate R2 values resulting from our analysis. Nonetheless, our results show the QI to be a unique and significant predictor of performance on single- and triple-hop tests, even after controlling for graft type, presence of meniscus injury, knee pain, and knee symptoms. Between the LQ and HQ groups in this study, differences in distance-hop scores were apparent, whereas timed-hop scores were similar. This is consistent with previous work that has found the timed-hop test to be less discriminatory of function compared to distance-hop tests.21,40 It is likely that the demand on the QF muscle is less during the timed-hop test compared to the distance-hop test. During the distance hops, individuals are instructed to “jump as far as possible and stick the landing,” requiring them to land the hop “in control.” The combination of a maximum concentric QF effort on take-off and the eccentric demand on the QF musculature to execute a controlled landing is likely more discriminatory of QF strength ability. For the timed-hop test, the hops occur in quick succession and it is likely that there is much less focal demand on the QF muscles, and this may allow individuals with QF weakness to compensate with other musculature.
Typically, muscles around the knee function to protect joint structures, such as articular cartilage, by controlling joint motion and attenuating joint forces. When muscle strength is compromised, movement patterns may be altered and excessive forces may be transferred directly to the joint surfaces,36 which could promote cartilage damage. Limb asymmetries during squatting, landing, and jumping activities15,19,39,42,43,45,58,64 and ensuing compensation patterns observed in individuals with ACLR may be related to deficits in QF strength.8 At this time, it is not known whether QF strength deficits demonstrated at the time of return to sport are associated with altered movement patterns or long-term joint morbidity. It is the focus of ongoing work in this subject cohort to evaluate the influence of QF strength deficits on movement patterns and joint biomechanics.
Study Limitations
QF muscle strength performance is a commonly used clinical criterion related to return to sport in individuals following ACLR. The importance of QF muscle performance in this patient population and the absence of empirical information regarding clinical milestones for return to sport prompted this study. There are many potential contributing factors beyond QF muscle strength that impact function and performance, which the present study did not address. A number of studies have indicated the importance of hip and trunk muscle strength and activation for lower extremity control and knee biomechanics.20,46,49,68 Lack of proximal muscle control has been implicated as a risk for both initial ACL injury20,49,68 and second ACL injury following ACLR and return to sport.46 In this study, we did not analyze the contributions of proximal muscle function to overall function and performance. It is likely that consideration of both proximal muscle and QF muscle function is important in guiding rehabilitation. The study sample of young, active individuals was specifically chosen with regard to the prevalence of ACL injury within this demographic. However, the results of this study provide insight into the impact of QF strength deficits on functional performance, and consideration of the findings may be appropriate for a broader spectrum of individuals following ACLR when establishing return to activity or physical therapy discharge criteria. Consideration for the young, female athlete is appropriate, as recent studies have reported sex differences in movement patterns and second injury following ACLR in young athletes.44,45 The ratio of females to males between the ACLR and control groups in this study was not similar, but sample size limited further analysis of the influence of sex on our study. In this analysis, we accounted for sex as a covariate in our statistical models; however, the influence of sex on strength and limb asymmetries following ACLR in young, active individuals remains a focus of our ongoing work.
CONCLUSION
In young, active Individuals at the time of return to sport following ACLR, QF strength deficits were associated with decreased function and predictive of functional performance beyond the influences of knee pain, knee symptoms, or graft type. Specifically, QF strength deficits greater than 15% on the involved limb (QI of less than 85%) negatively affected function and performance. Individuals with more symmetrical QF strength (side-to-side differences of 10% or less and a QI of 90% or greater) demonstrate functional performance similar to that of uninjured individuals. Further investigation of the impact of QF strength deficits and other potential contributing factors on joint mechanics, sport performance, reinjury, and long-term joint integrity is warranted.
KEY POINTS.
FINDINGS
At the time of return to sport, individuals post-ACLR have weaker QF muscles (QI of less than 85%), report worse function, and perform worse on hop tests compared to an uninjured control group and individuals post-ACLR who have a QI equal to or greater than 90%. QF strength deficits predict hop test performance beyond the influences of graft type, presence of meniscus injury, knee pain, and knee symptoms.
IMPLICATIONS
Despite being cleared for return to sport, QF weakness and functional deficits are observed in young, active individuals following ACLR.
CAUTION
Due to the study design, specific recommendations for the QF strength criterion value for return to sport cannot be made.
ACKNOWLEDGEMENTS
We would like to thank the staff at the Sports Medicine Biodynamics Center, specifically Kevin Ford, Gregory Myer, and Staci Thomas, as well as the Sports and Orthopaedic Team in the Division of Occupational and Physical Therapy at Cincinnati Children’s Hospital Medical Center, for their contribution to this work.
This work was funded in part by support from the National Institutes of Health grants F32-AR055844, R01-AR049735, R01-AR056259, and R01-AR055563, and the National Football League Charities Medical Research Grants 2007, 2008, and 2009. The Cincinnati Children’s Hospital Medical Center Institutional Review Board approved the protocol for this study.
REFERENCES
- 1.Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45:596–606. doi: 10.1136/bjsm.2010.076364. http://dx.doi.org/10.1136/bjsm.2010.076364. [DOI] [PubMed] [Google Scholar]
- 2.Aune AK, Holm I, Risberg MA, Jensen HK, Steen H. Four-strand hamstring tendon autograft compared with patellar tendon-bone autograft for anterior cruciate ligament reconstruction. A randomized study with two-year follow-up. Am J Sports Med. 2001;29:722–728. doi: 10.1177/03635465010290060901. [DOI] [PubMed] [Google Scholar]
- 3.Barber SD, Noyes FR, Mangine RE, McCloskey JW, Hartman W. Quantitative assessment of functional limitations in normal and anterior cruciate ligament-deficient knees. Clin Orthop Relat Res. 1990:204–214. [PubMed] [Google Scholar]
- 4.Beynnon BD, Johnson RJ, Fleming BC. The science of anterior cruciate ligament rehabilitation. Clin Orthop Relat Res. 2002:9–20. doi: 10.1097/00003086-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bolgla LA, Keskula DR. Reliability of lower extremity functional performance tests. J Orthop Sports Phys Ther. 1997;26:138–142. doi: 10.2519/jospt.1997.26.3.138. [DOI] [PubMed] [Google Scholar]
- 6.Brosky JA, Jr., Nitz AJ, Malone TR, Caborn DN, Rayens MK. Intrarater reliability of selected clinical outcome measures following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1999;29:39–48. doi: 10.2519/jospt.1999.29.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Bush-Joseph CA, Hurwitz DE, Patel RR, et al. Dynamic function after anterior cruciate ligament reconstruction with autologous patellar tendon. Am J Sports Med. 2001;29:36–41. doi: 10.1177/03635465010290011101. [DOI] [PubMed] [Google Scholar]
- 8.Chmielewski TL. Asymmetrical lower extremity loading after ACL reconstruction: more than meets the eye. J Orthop Sports Phys Ther. 2011;41:374–376. doi: 10.2519/jospt.2011.0104. http://dx.doi.org/10.2519/jospt.2011.0104. [DOI] [PubMed] [Google Scholar]
- 9.Chmielewski TL, Rudolph KS, Fitzgerald GK, Axe MJ, Snyder-Mackler L. Biomechanical evidence supporting a differential response to acute ACL injury. Clin Biomech (Bristol, Avon) 2001;16:586–591. doi: 10.1016/s0268-0033(01)00050-x. [DOI] [PubMed] [Google Scholar]
- 10.De Carlo M, Shelbourne KD, Oneacre K. Rehabilitation program for both knees when the contralateral autogenous patellar tendon graft is used for primary anterior cruciate ligament reconstruction: a case study. J Orthop Sports Phys Ther. 1999;29:144–153. doi: 10.2519/jospt.1999.29.3.144. discussion 154-159. [DOI] [PubMed] [Google Scholar]
- 11.Decker MJ, Torry MR, Noonan TJ, Riviere A, Sterett WI. Landing adaptations after ACL reconstruction. Med Sci Sports Exerc. 2002;34:1408–1413. doi: 10.1097/00005768-200209000-00002. http://dx.doi.org/10.1249/01.MSS.0000027627.82650.1F. [DOI] [PubMed] [Google Scholar]
- 12.Dunn WR, Lyman S, Lincoln AE, Amoroso PJ, Wickiewicz T, Marx RG. The effect of anterior cruciate ligament reconstruction on the risk of knee reinjury. Am J Sports Med. 2004;32:1906–1914. doi: 10.1177/0363546504265006. [DOI] [PubMed] [Google Scholar]
- 13.Eitzen I, Moksnes H, Snyder-Mackler L, Risberg MA. A progressive 5-week exercise therapy program leads to significant improvement in knee function early after anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2010;40:705–721. doi: 10.2519/jospt.2010.3345. http://dx.doi.org/10.2519/jospt.2010.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ejerhed L, Kartus J, Sernert N, Kohler K, Karlsson J. Patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction? A prospective randomized study with a two-year follow-up. Am J Sports Med. 2003;31:19–25. doi: 10.1177/03635465030310011401. [DOI] [PubMed] [Google Scholar]
- 15.Ernst GP, Saliba E, Diduch DR, Hurwitz SR, Ball DW. Lower extremity compensations following anterior cruciate ligament reconstruction. Phys Ther. 2000;80:251–260. [PubMed] [Google Scholar]
- 16.Fitzgerald GK, Lephart SM, Hwang JH, Wainner RS. Hop tests as predictors of dynamic knee stability. J Orthop Sports Phys Ther. 2001;31:588–597. doi: 10.2519/jospt.2001.31.10.588. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald GK, Piva SR, Irrgang JJ. A modified neuromuscular electrical stimulation protocol for quadriceps strength training following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2003;33:492–501. doi: 10.2519/jospt.2003.33.9.492. [DOI] [PubMed] [Google Scholar]
- 18.Gobbi A, Diara A, Mahajan S, Zanazzo M, Tuy B. Patellar tendon anterior cruciate ligament reconstruction with conical press-fit femoral fixation: 5-year results in athletes population. Knee Surg Sports Traumatol Arthrosc. 2002;10:73–79. doi: 10.1007/s00167-001-0265-8. http://dx.doi.org/10.1007/s00167-001-0265-8. [DOI] [PubMed] [Google Scholar]
- 19.Gokeler A, Hof AL, Arnold MP, Dijkstra PU, Postema K, Otten E. Abnormal landing strategies after ACL reconstruction. Scand J Med Sci Sports. 2010;20:e12–e19. doi: 10.1111/j.1600-0838.2008.00873.x. http://dx.doi.org/10.1111/j.1600-0838.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- 20.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. http://dx.doi.org/10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 21.Hopper DM, Strauss GR, Boyle JJ, Bell J. Functional recovery after anterior cruciate ligament reconstruction: a longitudinal perspective. Arch Phys Med Rehabil. 2008;89:1535–1541. doi: 10.1016/j.apmr.2007.11.057. http://dx.doi.org/10.1016/j.apmr.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 22.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29:600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 23.Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34:1567–1573. doi: 10.1177/0363546506288855. http://dx.doi.org/10.1177/0363546506288855. [DOI] [PubMed] [Google Scholar]
- 24.Keays SL, Bullock-Saxton JE, Newcombe P, Keays AC. The relationship between knee strength and functional stability before and after anterior cruciate ligament reconstruction. J Orthop Res. 2003;21:231–237. doi: 10.1016/S0736-0266(02)00160-2. http://dx.doi.org/10.1016/S0736-0266(02)00160-2. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011;29:633–640. doi: 10.1002/jor.21316. http://dx.doi.org/10.1002/jor.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med. 2004;34:269–280. doi: 10.2165/00007256-200434040-00006. [DOI] [PubMed] [Google Scholar]
- 27.Lephart SM, Kocher MS, Harner CD, Fu FH. Quadriceps strength and functional capacity after anterior cruciate ligament reconstruction. Patellar tendon autograft versus allograft. Am J Sports Med. 1993;21:738–743. doi: 10.1177/036354659302100519. [DOI] [PubMed] [Google Scholar]
- 28.Lephart SM, Perrin DH, Fu FH, Gieck JH, McCue FC, Irrgang JJ. Relationship between selected physical characteristics and functional capacity in the anterior cruciate ligament-insufficient athlete. J Orthop Sports Phys Ther. 1992;16:174–181. doi: 10.2519/jospt.1992.16.4.174. [DOI] [PubMed] [Google Scholar]
- 29.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2002;17:56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 30.Liang MH, Larson MG, Cullen KE, Schwartz JA. Comparative measurement efficiency and sensitivity of five health status instruments for arthritis research. Arthritis Rheum. 1985;28:542–547. doi: 10.1002/art.1780280513. [DOI] [PubMed] [Google Scholar]
- 31.Liu-Ambrose T, Taunton JE, MacIntyre D, McConkey P, Khan KM. The effects of proprioceptive or strength training on the neuromuscular function of the ACL reconstructed knee: a randomized clinical trial. Scand J Med Sci Sports. 2003;13:115–123. doi: 10.1034/j.1600-0838.2003.02113.x. [DOI] [PubMed] [Google Scholar]
- 32.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. http://dx.doi.org/10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 33.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. http://dx.doi.org/10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 34.Mattacola CG, Perrin DH, Gansneder BM, Gieck JH, Saliba EN, McCue FC., 3rd Strength, functional outcome, and postural stability after anterior cruciate ligament reconstruction. J Athl Train. 2002;37:262–268. [PMC free article] [PubMed] [Google Scholar]
- 35.McHugh MP, Tyler TF, Browne MG, Gleim GW, Nicholas SJ. Electromyographic predictors of residual quadriceps muscle weakness after anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:334–339. doi: 10.1177/03635465020300030601. [DOI] [PubMed] [Google Scholar]
- 36.Mikesky AE, Meyer A, Thompson KL. Relationship between quadriceps strength and rate of loading during gait in women. J Orthop Res. 2000;18:171–175. doi: 10.1002/jor.1100180202. http://dx.doi.org/10.1002/jor.1100180202. [DOI] [PubMed] [Google Scholar]
- 37.Möller E, Forssblad M, Hansson L, Wange P, Weidenhielm L. Bracing versus nonbracing in rehabilitation after anterior cruciate ligament reconstruction: a randomized prospective study with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2001;9:102–108. doi: 10.1007/s001670000192. [DOI] [PubMed] [Google Scholar]
- 38.Myer GD, Schmitt LC, Brent JL, et al. Utilization of modified NFL combine testing to identify functional deficits in athletes following ACL reconstruction. J Orthop Sports Phys Ther. 2011;41:377–387. doi: 10.2519/jospt.2011.3547. http://dx.doi.org/10.2519/jospt.2011.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neitzel JA, Kernozek TW, Davies GJ. Loading response following anterior cruciate ligament reconstruction during the parallel squat exercise. Clin Biomech (Bristol, Avon) 2002;17:551–554. doi: 10.1016/s0268-0033(02)00063-3. [DOI] [PubMed] [Google Scholar]
- 40.Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19:513–518. doi: 10.1177/036354659101900518. [DOI] [PubMed] [Google Scholar]
- 41.Noyes FR, Berrios-Torres S, Barber-Westin SD, Heckmann TP. Prevention of permanent arthrofibrosis after anterior cruciate ligament reconstruction alone or combined with associated procedures: a prospective study in 443 knees. Knee Surg Sports Traumatol Arthrosc. 2000;8:196–206. doi: 10.1007/s001670000126. [DOI] [PubMed] [Google Scholar]
- 42.Orishimo KF, Kremenic IJ, Mullaney MJ, McHugh MP, Nicholas SJ. Adaptations in single-leg hop biomechanics following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18:1587–1593. doi: 10.1007/s00167-010-1185-2. http://dx.doi.org/10.1007/s00167-010-1185-2. [DOI] [PubMed] [Google Scholar]
- 43.Paterno MV, Ford KR, Myer GD, Heyl R, Hewett TE. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17:258–262. doi: 10.1097/JSM.0b013e31804c77ea. http://dx.doi.org/10.1097/JSM.0b013e31804c77ea. [DOI] [PubMed] [Google Scholar]
- 44.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22:116–121. doi: 10.1097/JSM.0b013e318246ef9e. http://dx.doi.org/10.1097/JSM.0b013e318246ef9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Hewett TE. Effects of sex on compensatory landing strategies upon return to sport after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2011;41:553–559. doi: 10.2519/jospt.2011.3591. http://dx.doi.org/10.2519/jospt.2011.3591. [DOI] [PubMed] [Google Scholar]
- 46.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38:1968–1978. doi: 10.1177/0363546510376053. http://dx.doi.org/10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petschnig R, Baron R, Albrecht M. The relationship between isokinetic quadriceps strength test and hop tests for distance and one-legged vertical jump test following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1998;28:23–31. doi: 10.2519/jospt.1998.28.1.23. [DOI] [PubMed] [Google Scholar]
- 48.Pinczewski LA, Lyman J, Salmon LJ, Russell VJ, Roe J, Linklater J. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35:564–574. doi: 10.1177/0363546506296042. http://dx.doi.org/10.1177/0363546506296042. [DOI] [PubMed] [Google Scholar]
- 49.Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40:42–51. doi: 10.2519/jospt.2010.3337. http://dx.doi.org/10.2519/jospt.2010.3337. [DOI] [PubMed] [Google Scholar]
- 50.Reid A, Birmingham TB, Stratford PW, Alcock GK, Giffin JR. Hop testing provides a reliable and valid outcome measure during rehabilitation after anterior cruciate ligament reconstruction. Phys Ther. 2007;87:337–349. doi: 10.2522/ptj.20060143. http://dx.doi.org/10.2522/ptj.20060143. [DOI] [PubMed] [Google Scholar]
- 51.Risberg MA, Holm I. The long-term effect of 2 postoperative rehabilitation programs after anterior cruciate ligament reconstruction: a randomized controlled clinical trial with 2 years of follow-up. Am J Sports Med. 2009;37:1958–1966. doi: 10.1177/0363546509335196. http://dx.doi.org/10.1177/0363546509335196. [DOI] [PubMed] [Google Scholar]
- 52.Risberg MA, Holm I, Ekeland A. Reliability of functional knee tests in normal athletes. Scand J Med Sci Sports. 1995;5:24–28. doi: 10.1111/j.1600-0838.1995.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 53.Risberg MA, Holm I, Tjomsland O, Ljunggren E, Ekeland A. Prospective study of changes in impairments and disabilities after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1999;29:400–412. doi: 10.2519/jospt.1999.29.7.400. [DOI] [PubMed] [Google Scholar]
- 54.Roi GS, Creta D, Nanni G, Marcacci M, Zaffagnini S, Snyder-Mackler L. Return to official Italian First Division soccer games within 90 days after anterior cruciate ligament reconstruction: a case report. J Orthop Sports Phys Ther. 2005;35:52–61. doi: 10.2519/jospt.2005.35.2.52. discussion 61-66. http://dx.doi.org/10.2519/jospt.2005.1583. [DOI] [PubMed] [Google Scholar]
- 55.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. http://dx.doi.org/10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 57.Rudolph KS, Axe MJ, Snyder-Mackler L. Dynamic stability after ACL injury: who can hop? Knee Surg Sports Traumatol Arthrosc. 2000;8:262–269. doi: 10.1007/s001670000130. [DOI] [PubMed] [Google Scholar]
- 58.Salem GJ, Salinas R, Harding FV. Bilateral kinematic and kinetic analysis of the squat exercise after anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2003;84:1211–1216. doi: 10.1016/s0003-9993(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 59.Salmon LJ, Refshauge KM, Russell VJ, Roe JP, Linklater J, Pinczewski LA. Gender differences in outcome after anterior cruciate ligament reconstruction with hamstring tendon autograft. Am J Sports Med. 2006;34:621–629. doi: 10.1177/0363546505281806. http://dx.doi.org/10.1177/0363546505281806. [DOI] [PubMed] [Google Scholar]
- 60.Sapega AA. Muscle performance evaluation in orthopaedic practice. J Bone Joint Surg Am. 1990;72:1562–1574. [PubMed] [Google Scholar]
- 61.Shelbourne KD, Klootwyk TE, Wilckens JH, De Carlo MS. Ligament stability two to six years after anterior cruciate ligament reconstruction with autogenous patellar tendon graft and participation in accelerated rehabilitation program. Am J Sports Med. 1995;23:575–579. doi: 10.1177/036354659502300510. [DOI] [PubMed] [Google Scholar]
- 62.Snyder-Mackler L, Delitto A, Stralka SW, Bailey SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74:901–907. doi: 10.1093/ptj/74.10.901. [DOI] [PubMed] [Google Scholar]
- 63.Webster KE, Feller JA, Hameister KA. Bone tunnel enlargement following anterior cruciate ligament reconstruction: a randomised comparison of hamstring and patellar tendon grafts with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2001;9:86–91. doi: 10.1007/s001670100191. [DOI] [PubMed] [Google Scholar]
- 64.Webster KE, Gonzalez-Adrio R, Feller JA. Dynamic joint loading following hamstring and patellar tendon anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12:15–21. doi: 10.1007/s00167-003-0400-9. http://dx.doi.org/10.1007/s00167-003-0400-9. [DOI] [PubMed] [Google Scholar]
- 65.Wilk KE, Romaniello WT, Soscia SM, Arrigo CA, Andrews JR. The relationship between subjective knee scores, isokinetic testing, and functional testing in the ACL-reconstructed knee. J Orthop Sports Phys Ther. 1994;20:60–73. doi: 10.2519/jospt.1994.20.2.60. [DOI] [PubMed] [Google Scholar]
- 66.Wojtys EM, Huston LJ. Longitudinal effects of anterior cruciate ligament injury and patellar tendon autograft reconstruction on neuromuscular performance. Am J Sports Med. 2000;28:336–344. doi: 10.1177/03635465000280030901. [DOI] [PubMed] [Google Scholar]
- 67.Wojtys EM, Huston LJ. Neuromuscular performance in normal and anterior cruciate ligament-deficient lower extremities. Am J Sports Med. 1994;22:89–104. doi: 10.1177/036354659402200116. [DOI] [PubMed] [Google Scholar]
- 68.Zazulak BT, Hewett TE, Reeves NP, Goldberg B, Cholewicki J. Deficits in neuromuscular control of the trunk predict knee injury risk: a prospective biomechanical-epidemiologic study. Am J Sports Med. 2007;35:1123–1130. doi: 10.1177/0363546507301585. http://dx.doi.org/10.1177/0363546507301585. [DOI] [PubMed] [Google Scholar]