Abstract
Protective antigen (PA)-based vaccination is an effective countermeasure to anthrax infection. While neutralizing anti-PA antibody titers elicited by this vaccine serve as good correlates for protection against anthrax (S. Reuveny, M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan, Infect. Immun. 69:2888-2893, 2001), no data are available on the contribution of the immunological memory for PA itself to protection. We therefore developed a guinea pig model in which a primary immunization with threshold levels of PA can induce a long-term T-cell immunological memory response without inducing detectable anti-PA antibodies. A revaccination of primed animals with the same threshold PA levels was effective for memory activation, yielding a robust and rapid secondary response. A challenge with a lethal dose (40 50% lethal doses; 2,000 spores) of spores after the booster vaccinations indicated that animals were not protected at days 2, 4, and 6 postboosting. Protection was achieved only from the 8th day postboosting, concomitant with the detection of protective levels of neutralizing antibody titers in the circulation. The practical implications from the studies reported herein are that, as expected, the protective capacity of memory depends on the PA dose used for the primary immunization and that the effectiveness of booster immunizations for the postexposure treatment of anthrax may be very limited when no detectable antibodies are present in primed animals prior to Bacillus anthracis spore exposure. Therefore, to allow for the establishment of memory-dependent protection prior to the expected onset of disease, booster immunizations should not be used without concomitant antimicrobial treatment in postexposure scenarios.
Anthrax is a zoonotic disease caused by the spore-forming bacterium Bacillus anthracis. This disease most commonly occurs in wild and domestic mammals but also occurs in humans exposed to infected animals. The recent recognition that anthrax spores constitute an effective bioterrorism tool (2, 12, 21) has launched a surge of research related to protection against the disease. A major factor in the virulence of B. anthracis is the secreted toxin complex, which is comprised of two toxins, the lethal toxin and the edema toxin (9, 27). These toxins have distinct biochemically active components (LF in the lethal toxin and EF in the edema toxin), yet they share a common component, protective antigen (PA) (for a recent review, see reference 23). PA binds to a cell surface receptor where it is proteolytically activated, creating a site for LF and EF binding. Once assembled, the toxin complex can be internalized and transported into the cell cytoplasm, where the toxigenic activity is expressed (13, 22, 25).
Consistent with the central role of PA in anthrax pathophysiology, cumulative information gained over decades of research indicates that PA constitutes a major component of protective immunity against anthrax. This led to research into the development of a series of PA-based acellular vaccines and live attenuated vaccines (4, 5, 7, 8, 10, 14, 17, 19, 20, 24, 28, 32, 34). Several animal models, including guinea pigs (24, 31, 34), rhesus monkeys (11, 19), and rabbits (37), were developed to study vaccine efficacies and to evaluate PA-based vaccine formulations or vaccination regimens. Guinea pigs, the most commonly used animal model, were recently used to demonstrate that anti-PA neutralizing antibody (Ab) titers can be used as a surrogate marker for protection (31). These findings indicated that a basic level of neutralizing Ab (titer of ≥300), either passively transferred or generated by active primary immunization, is required to confer protection against a lethal challenge in guinea pigs.
One of the PA-based acellular vaccine formulations (BioThrax) has been licensed in the United States and used to vaccinate humans. The approved vaccination regimen calls for a series of three injections at short intervals followed by three injections at 6-month intervals. A recent clinical study (29) demonstrated that 18 to 24 months after receiving one, two, or three doses of a PA-based vaccine (BioThrax), the overall prevalence of anti-PA Abs detected by enzyme-linked immunosorbent assay (ELISA) was about 30%. A booster immunization with a single injection after 18 to 24 months induced high titers in 99% of the immunized humans. This indicates the establishment of solid immunological memory and suggests that a less demanding vaccination regimen may be effective. Additional preclinical and clinical studies are obviously required to assess the protective value of the booster responses. It should be noted, in this context, that the assessment of anthrax vaccines in animal models usually relies on primary immunization studies, and information on the development and protective role of long-term immunological memory is unavailable.
Immunological memory is one of the fundamental responses to vaccination and plays a major role in protection against a variety of infections. Memory is characterized by three main features: the presence of memory cells for a long period of time after the primary immunization, a rapid Ab response upon re-exposure to the antigen (within ∼7 days compared to ∼14 days for the primary response), and the generation of Abs with higher avidities upon consecutive vaccinations (reviewed in reference 38). Memory B cells are critical for protection since they proliferate rapidly and differentiate into Ab-producing plasma cells upon exposure to a pathogen (1). Thus, protective immunity can persist after immunization even when Abs are not detectable in the circulation (3, 36).
Protection against the onset of a fatal disease depends on the competition between the kinetics of disease development and the kinetics of the development of the specific protective memory response. Immunization against certain viral diseases can be protective even if the primary vaccination or booster immunization is performed after viral exposure (3). In such cases, the development of the protective Ab response is faster than the onset of the disease. In the case of anthrax, a disease that develops quite rapidly, no data are available on the ability of the immunological memory for PA itself to protect against lethal disease.
For the present study, we developed a guinea pig model which enables the characterization of immunological memory for PA in animals in which circulatory Abs are not detected. This allows for a dissection of the protective potential of the memory response in the context of primary immunization as well as booster immunization. The interrelationship between the kinetics of anthrax disease onset and those of memory response mobilization, induced either by exposure to spores or by revaccination, is also demonstrated.
MATERIALS AND METHODS
Production and purification of PA and vaccine formulation.
B. anthracis strain V770-NPI-R (ATCC 14185) was grown anaerobically as described previously (7). After 24 h of growth, the bacteria were removed by microfiltration (0.2-μm-pore-size filter), while the PA-containing supernatant was concentrated by ultrafiltration (30K molecular weight cutoff) and dialyzed against 20 mM phosphate buffer (pH 8.0). Purification of PA was carried out by Q-Sepharose chromatography essentially as described previously (31), also yielding purified LF, which was used in neutralization assays.
The PA vaccine was prepared by adsorption of the purified PA, at a final concentration of 50 μg/ml, to an aluminum hydroxide gel (0.32% [wt/vol]) as described previously (31).
Animal studies.
Female Hartley guinea pigs (weighing 220 to 250 g) were obtained from Charles River Laboratories (Margate, United Kingdom). All animals were cared for according to the 1997 NIH guidelines for the care and use of laboratory animals; all experimental protocols were approved by the IIBR Animal Use Committee.
Immunization and challenge of guinea pigs.
Primary immunizations were performed on groups of 10 to 40 guinea pigs by single subcutaneous (s.c.) injections of 0.5 ml of the PA vaccine. Different PA vaccine doses were generated by dilutions of the original 50-μg/ml PA vaccine. A memory response was induced by booster immunizations with single injections of the same vaccine dose used for primary immunizations. At the indicated times, guinea pigs were challenged intradermally with 40 50% lethal doses (LD50) (2,000 spores) of B. anthracis strain Vollum spores (ATCC 14578), and their survival was monitored for 10 days.
ELISA for anti-PA Ab.
Ab titers were determined by a direct ELISA in 96-well microtiter plates (Nunc, Roskilde, Denmark) using PA as the capture antigen and an alkaline phosphatase-conjugated rabbit anti-guinea pig immunoglobulin G (IgG) (Sigma, St. Louis, Mo.) as the detection reagent. Plates were coated with 5 μg of purified PA (50 μl/well)/ml in NaHCO3 buffer (50 mM, pH 9.6) and subsequently blocked with TSTA buffer (50 mM Tris [pH 7.6], 142 mM sodium chloride, 0.05% sodium azide, 0.05% Tween 20, and 2% bovine serum albumin). The tested sera were serially diluted in twofold steps, and the plates were then incubated for 2 h at 37°C. The plates were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 and developed with the detection Ab-conjugate, with p-nitrophenyl phosphate (Sigma) used as a substrate, and the absorbance at 405 nm was determined. The end point was defined as the highest dilution at which the absorbance was >2 standard deviations above that of the negative control (normal guinea pig serum). Ab titers were expressed as reciprocal end-point dilutions.
Neutralization test.
Neutralizing Ab titers were determined essentially as described before (31, 33) by virtue of their ability to prevent the mortality of murine macrophage J774A.1 cells (American Type Culture Collection) induced by the PA/LF toxin complex. Aliquots of 0.2 ml of cell suspension (6 × 105 to 8 × 105 cells/ml) were plated into 96-well cell culture plates (Nunc). The tested sera were serially diluted in twofold steps in TSTA buffer containing PA (5 μg/ml) and LF (2 μg/ml). After a 1-h incubation, 10 μl of each of the PA dilutions was added to the J774A.1 cells. The plates were incubated for 5 h at 37°C in 5% CO2, and cell viability was monitored by an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, thiazolyl blue) assay (the absorbance was measured at 540 nm) (26). The end point was defined as the highest serum dilution exhibiting 0.025 absorbance units above that of the corresponding identical dilution of a control normal serum. Neutralizing Ab titers were expressed as reciprocal end-point dilutions.
Both the ELISA and the neutralizing assay were performed in duplicate. The negative control (normal serum) and a positive standard serum were added to each plate. The assays were performed by a robotic system (TECAN RMP) and validated according to the method of Halperin et al. (15, 16). The limit of detection for both assays was a titer of 50. Tests were performed on individual or pooled sera, as indicated in the text. Antibody titers below the cutoff value were given an arbitrary titer of 25 (one-half of the cutoff value) to allow for calculations of geometric mean Ab titers.
Ab avidity test.
The Ab avidity assay was performed basically by the same procedure as the ELISA test except for one additional step. NH4SCN at a concentration of 1.5 M (found to be the optimal concentration for discriminating between high and low Ab avidities) was added to the ELISA plates after the incubation with the tested sera, and the mixture was incubated for 15 min at room temperature (30). After a wash with PBS containing 0.05% Tween 20, the ELISA procedure was continued as described above.
Cell proliferation assay.
Naive and immunized guinea pigs were bled into 8-ml cell preparation tubes (CPT Vacutainer with sodium heparin; Becton Dickinson, Plymouth, United Kingdom). Peripheral blood mononuclear cells were separated by centrifugation at 380 × g for 12 min, washed three times with sterile culture medium (10% fetal calf serum-RPMI 1640 with l-glutamine), and seeded (1.5 × 105 peripheral blood mononuclear cells in 0.2 ml of culture medium and 0.12 μg of PA) in 96-well round-bottom plates (Nunc, Roskilde, Denmark). The plates were incubated in a humid environment with 5% CO2 at 37°C for 5 days. The assessment of cell proliferation was based on the measurement of BrdU incorporation during DNA synthesis into proliferating cells by use of the Biotrak cell proliferation ELISA system (Amersham Pharmacia Biotech). Stimulation indexes were calculated by dividing the optical density values measured for stimulated cultures by those measured for unstimulated cultures (mean of five replicates).
Determination of antigen-specific Ab-secreting cells.
The numbers of antigen-specific Ab-secreting cells were assessed by an ELISPOT assay as described previously (35). The spleens of immunized and control animals were removed at different time points after immunization. Mononuclear cells were isolated by mincing the spleens through wire mesh screens. Red blood cells were lysed with ammonium chloride, and the remaining cells were washed, counted, and resuspended in culture medium. Mononuclear cell suspensions containing 106 cells/ml were serially diluted and dispensed (100-μl triplicate samples) into flat-bottom wells of multiscreen 96-well nitrocellulose plates (Millipore, Molsheim, France) that were previously coated with 50 μl of 15-μg/ml PA in carbonate buffer. The plates were incubated for 12 to 15 h and washed with PBS containing 0.05% Tween 20. Rabbit anti-guinea pig IgG (Sigma) was added to each well and the plates were incubated for 1 h at 37°C, washed, and incubated again with donkey anti-rabbit IgG conjugated to alkaline phosphatase (Jackson Laboratory, Bar Harbor, Maine) for 2 h at 37°C. After the plates were washed, 5-bromo-4-chloro-3-indolyl phosphate-nitroblue tetrazolium tablets (BCIP/NBT) were added and incubated for 1 h. The reaction was stopped by extensive washing with distilled water. After drying, the numbers of spots were enumerated with a high-resolution image analyzer (Bioreader 3000 Pro system; Bio-Sys GmbH). The results are expressed as numbers of cells producing the anti-PA Ab/106 splenocytes.
RESULTS AND DISCUSSION
Defining PA doses that do not induce detectable Ab production in primary response, yet can confer immunological memory.
After the vaccination of guinea pigs with a 25-μg dose of a PA-based vaccine, high Ab titers were maintained for long periods of time (31). Such high neutralizing anti-PA Ab titers, which were shown to be protective (31), precluded the use of this well-studied immunization regimen for the evaluation of the correlation between immunological memory and protection. Therefore, a model in which primary vaccination could prime a measurable memory immune response, without an induction of detectable protective Ab titers, had to be developed.
Since PA doses equal to or higher than 3 μg were previously shown (31) to induce a protective humoral response, we vaccinated guinea pigs with single injections of 0.25 to 2.5 μg of a PA-based vaccine (Table 1). As expected, the reduction in PA led to a decrease in anti-PA Ab titers (as measured both by ELISA and by the neutralization assay). A vaccine dose of 2.5 μg of PA still led to a detectable primary response (average anti-PA titers of 260) in 10% of the immunized animals (4 of 40), while none of the guinea pigs developed detectable anti-PA Abs (titers of <50) when doses of 0.25 to 1 μg of PA were used.
TABLE 1.
Effect of PA dose on anti-PA Ab generation after primary and secondary immune responsea
| PA dose (μg) | Response on day of booster (GMT ± SD no. of [responders/total])
|
Response 14 days after booster (GMT ± SD [no. of responders/total])
|
||||||
|---|---|---|---|---|---|---|---|---|
| All sera
|
Positive sera
|
All sera
|
Positive sera
|
|||||
| Anti-PA Ab | Neutralizing Ab | Anti-PA Ab | Neutralizing Ab | Anti-PA Ab | Neutralizing Ab | Anti-PA Ab | Neutralizing Ab | |
| 25 | 10,908 ± 1.47 (13/13) | 4,525 ± 0.61 (13/13) | 10,908 ± 1.47 | 4,525 ± 0.61 | 38,000 ± 0.22 (10/10) | 15,222 ± 0.21 (10/10) | 38,000 ± 0.22 | 15,222 ± 0.21 |
| 2.5 | 60 ± 1.76 (4/40) | <50 (0/40) | 257 ± 1.13 | 936 ± 11.33 (7/11) | 621 ± 9.51 (7/11) | 4,997 ± 2.48 | 2,625 ± 3.42 | |
| 1 | <50 (0/32) | <50 (0/32) | 381 ± 6.48 (8/10) | 331 ± 4.26 (7/10) | 975 ± 4.22 | 673 ± 2.05 | ||
| 0.5 | <50 (0/32) | <50 (0/32) | 100 ± 2.53 (5/11) | 56 ± 1.32 (2/11) | 230 ± 2.14 | 100 ± 1 | ||
| 0.25 | <50 (0/32) | <50 (0/32) | <50 (0/12) | <50 (0/12) | ||||
Two weeks after the primary immunization, animals were boosted with the same dose of the PA-based vaccine. Ab titers to PA (ELISA) and neutralizing Abs were determined 14 days after the first immunization (at the day of boosting) and 14 days after the booster as described in Materials and Methods.
Once the threshold doses for anti-PA Ab induction were established, it was necessary to determine whether such low doses of PA-based vaccines could still elicit a memory response. To this end, animals were injected again, 2 weeks after the primary immunization, with the same PA dose used for the primary vaccination, and the development of anti-PA Abs was monitored for 14 days after the secondary vaccination. No response was observed for animals immunized with 0.25 μg of PA. Forty-five percent of the guinea pigs who were immunized with 0.5 μg of PA seroconverted, while with a 1-μg PA dose, about 70 to 80% of the animals exhibited a significant anti-PA Ab booster response (Table 1). Accordingly, we decided to use the 1-μg PA dose for further evaluations.
The maintenance of the memory response for an extended period of time after the primary immunization with 1 μg of PA was tested by performing revaccinations at different time intervals. While revaccination after 2 weeks yielded low Ab titers (titers of 380 and 330 for anti-PA Ab and neutralizing Ab, respectively), revaccination 4, 9, and 24 weeks after the primary immunization elicited effective humoral responses (seroconversion in 85% of guinea pigs, with geometric mean titers [GMTs] of 7,000 to 12,000 and 8,000 to 12,000 for anti-PA and neutralizing Abs, respectively), indicating the buildup of a robust, long-lasting immunological memory (Table 2).
TABLE 2.
Antibody generation after booster immunizations performed at different times after primary immunization with 1 μg of PAa
| Day of revaccination | Anti-PA Ab titer by ELISA
|
Neutralizing Ab titer
|
||
|---|---|---|---|---|
| GMT ± SD | No. of responders/total | GMT ± SD | No. of responders/total | |
| 0 | <50 ± 1 | 0/12 | <50 ± 1 | 0/12 |
| 14 | 381 ± 6.48 | 8/10 | 331 ± 4.26 | 7/10 |
| 28 | 7,000 ± 4.12 | 9/10 | 8,500 ± 3.59 | 9/10 |
| 65 | 12,000 ± 2.83 | 10/11 | 8,000 ± 4.24 | 9/11 |
| 180 | 10,000 ± 3.24 | 9/10 | 12,000 ± 2.35 | 9/10 |
The PA vaccine (1 μg) was injected (0.5 ml s.c.) into 54 guinea pigs. Groups of 10 to 12 animals were boosted with the same PA vaccine dose on day 14, 28, 65, or 180 after the primary immunization. Anti- PA (ELISA) and neutralizing Ab titers were determined 14 days later as described in Materials and Methods. The day zero group was not boosted.
Kinetics and characteristics of the immunological memory response after immunization with a threshold dose of PA.
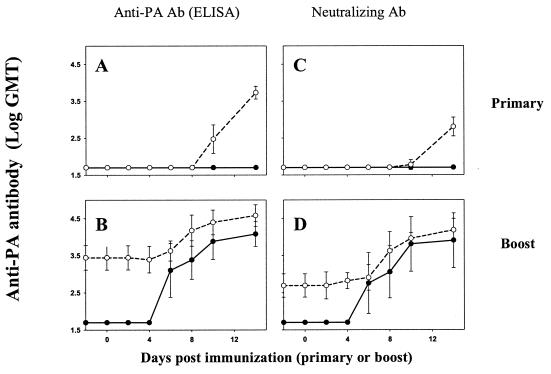
Immunological responses induced after vaccination with a dose of 1 μg of PA were compared to those elicited by 25 μg of PA (Fig. 1). The primary vaccination of guinea pigs with 25 μg of PA-based vaccine resulted in the development of high Ab titers that lasted for long periods of time, while as described above, vaccination with 1 μg of PA did not. Fourteen weeks after the first immunization, the animals were injected again with the same PA dose used for primary vaccination. The observed kinetics of PA-specific Ab production after the booster vaccination with 1 or 25 μg of PA were essentially similar (Fig. 1B and D). The onset time of both anti-PA and neutralizing Ab production was found to be earlier than day 6, which is faster than the onset time of 10 days observed after a primary immunization with 25 μg of PA (Fig. 1A and C).
FIG. 1.
Kinetics of anti-PA Ab generation after primary and secondary immunization. PA-based vaccine doses of 1 μg (solid lines) and 25 μg (dashed lines) were injected (in 0.5 ml s.c.) into 20 guinea pigs. Fourteen weeks after the immunization, the animals were boosted with the same PA-based vaccine dose. The kinetics of Ab generation in these 20 guinea pigs were monitored during the first 14 days after the primary immunization (A and C) and after the booster (B and D), as described in Materials and Methods.
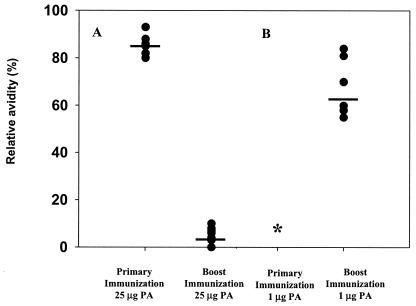
Avidity studies revealed a qualitative difference between anti-PA Abs induced by primary and booster immunizations with 25 μg of PA and those induced by primary and booster immunizations with 1 μg of PA (Fig. 2). In the case of the high-dose vaccination regimen, a significant increase in Ab avidity (as evaluated by the ability of thiocyanate to disrupt immune complexes) was observed after the booster vaccination compared to that after primary immunization, suggesting an effective maturation process. For the low-dose vaccination regimen, the absence of measurable titers after primary immunization precluded a direct evaluation of maturation. Nevertheless, while the avidity of anti-PA Abs generated after boosting with 1 μg of PA was lower than that after boosting with 25 μg of PA, this avidity was significantly higher (P = 0.015) than that found 2 weeks after the induction of the primary response by 25 μg of PA (Fig. 2). This observation indicates that partial maturation does take place after a regimen of immunization with a threshold dose of PA, suggesting that under these conditions, the development of a typical memory response can occur.
FIG. 2.
Anti-PA Ab avidity measured after primary and secondary immunization. Guinea pigs were immunized with a PA-based vaccine and boosted with the same PA dose 2 months after the first immunization. The anti-PA avidities of individual serum samples were determined 14 days after the first immunization and 14 days after the booster. Relative avidities are expressed as percentages of ELISA optical density values in the absence and presence of 1.5 M thiocyanate. (A) Primary and booster immunizations with 25 μg of PA. (B) Primary and booster immunizations with 1 μg of PA. The anti-PA Ab levels (GMT) in animals immunized with 25 μg of PA were 12,800 after primary vaccination and 25,600 after booster vaccination. In animals vaccinated with 1 μg of PA, a titer of 18,000 was reached after booster vaccination. *, no Ab titers were measured during primary immunization with 1 μg of PA.
The generation of Ab-secreting cells after the booster immunization was monitored by an ELISPOT assay. Large numbers of anti-PA Ab-secreting cells (323 ± 152 Ab-secreting cells/106 splenocytes, compared to a background of 7 ± 6 Ab-secreting cells/106 splenocytes) were found 5 days after the booster immunization with 1 μg of PA but were not detected at all after the primary immunization with 1 μg of PA, demonstrating the rapid proliferation and differentiation of B cells into Ab-secreting cells, as is expected upon the induction of a proper memory response.
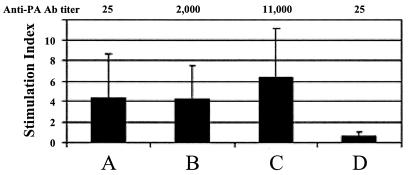
The presence of memory cells after the primary immunization was evaluated by a lymphocyte proliferation assay. An average proliferation index of 4.4 was measured 8 weeks after the primary immunization with 1 μg of PA and was maintained for a long period of time after the booster (Fig. 3). A similar proliferation index was detected after the primary immunization with 25 μg of PA (6.3), indicating that a robust T-cell response is established after priming with either 1 or 25 μg of PA. These results are notable in view of the big difference in Ab titers elicited by the two vaccination regimens (titers of ∼10,000 versus nondetectable Abs after a single immunization with 25 and 1 μg of PA, respectively). It therefore appears that in the absence of a measurable Ab titer, the proliferation test is a sensitive tool for assessing the induction of immune response in individuals vaccinated with threshold doses of PA and is indicative of immunological memory that could in turn be mobilized by a booster immunization.
FIG. 3.
Cell proliferation index measured before and after booster immunization. The PA-based vaccine was injected (in 0.5 ml s.c.) into guinea pigs, and a booster of the same PA dose was given 8 weeks after the primary immunization. Cell proliferation assays and anti-PA titer ELISAs were performed 14 days after primary and booster immunization. (A) 1-μg dose prebooster; (B) 1-μg dose postbooster; (C) 25-μg dose prebooster (positive control); (D) negative control.
Taken together, all of the immunological analyses described above demonstrate that the primary immunization of guinea pigs with doses as low as 1 μg of PA can induce a typical long-lasting immunological memory response to PA that is qualitatively similar to the response obtained by vaccination at higher doses.
Relationship between kinetics of mobilization of memory response and development of protective immunity after boosting.
The protective potential of immunological memory for PA was examined in two different manners. First, by examining whether memory can be recruited for a protective response by an exposure of primed animals to anthrax spores (relying on the infective, PA-producing bacteria as triggers of the secondary response), and second, by examining the protective effect of a booster vaccination concomitant with exposure to anthrax.
For the first set of experiments, different groups of guinea pigs were immunized with single doses of 1 to 6 μg of the PA-based vaccine and then challenged 6 months later with 40 LD50 (2,000 spores) of the Vollum strain (Table 3). Animals vaccinated with 1 μg of PA were not protected at any time point after vaccination (>90% of the challenged guinea pigs died, with a mean time to death [MTD] similar to that of controls), in spite of the fact that such primary vaccination does induce a memory response, as shown above. When 16 guinea pigs were immunized with a higher single PA dose of 2 μg, the animals generated protective levels of neutralizing Ab (titer range, 200 to 565) during the first 4 to 6 weeks after the immunization. Thereafter, the Ab titers decreased gradually to nondetectable levels during the following 6 months. When these animals were challenged, 4 of 16 guinea pigs (25%) were protected, in spite of the fact that no circulating anti-PA Abs could be detected in their circulation at the time of challenge. In comparison, full protection was achieved in a group of guinea pigs immunized with a PA dose of 6 μg, as they exhibited anti-PA neutralizing Ab titers of 200 to 400 6 months after the primary immunization and before the challenge. The result of this set of experiments substantiates previous observations (31) that the most important factor for protection against anthrax in this animal model is the level of neutralizing Abs that are available at the time of challenge. On the other hand, it indicates that mounting a challenge-engendered anamnestic response by itself (as in the case of primary immunization with a 1-μg PA dose) cannot protect guinea pigs from anthrax infection. This appears to be the result of a quantitative or qualitative limitation in memory aptitude, since few of the animals vaccinated with a PA dose of 2 μg were able to withstand the lethal anthrax challenge in the absence of measurable anti-PA Abs (Table 3). This suggests that the administration of the somewhat larger antigenic load could have resulted in a broader precursor memory cell repertoire, which upon recruitment can generate higher levels of Abs or Abs with higher avidities (Fig. 2) that could confer protection.
TABLE 3.
Antibody production and survival from anthrax spore challenge 6 months after primary immunization with various PA dosesa
| PA dose (μg) | Average neutralizing Ab titer at 4 to 6 weeks postimmunization (range) | Average neutralizing Ab titer at 6 months postimmunization (range) | % survival (no. of live animals/total) |
|---|---|---|---|
| 0 | <50 | <50 | 0 (0/10) |
| 1 | <50 | <50 | 5 (1/20) |
| 2 | 232 (200-566) | <50 | 25 (4/16) |
| 6 | 1,599 (800-2,262) | 300 (200-400) | 100 (5/5) |
PA vaccine doses (1, 2, and 6 μg) was injected (0.5 ml s.c.) into guinea pigs. Neutralizing Ab titers were determined 4 to 6 weeks and 6 months after the immunization. Two weeks later, guinea pigs (5 to 20 animals per group) were challenged with 40 LD50 of B. anthracis Vollum spores.
To further study the protective potential of immunological memory for PA, we examined the effect of revaccination on preventing anthrax infections in animals. Revaccination with a 1-μg PA dose was performed about 3 months after the primary immunization with the same PA dose. On the day of the booster immunization and on days 2, 4, 6, 8, 12, and 14 after the booster, groups of animals were bled for titer determinations and parallel groups of animals were challenged with 40 LD50 (2,000 spores) of B. anthracis Vollum. The experiment was actually designed in such a way that all animals were bled and challenged on the same day with the same challenge culture in order to minimize variability. This synchronization was achieved by performing the booster immunization by a reverse sequential schedule (Table 4). In this experiment, protection levels again appeared to correlate well with the neutralizing Ab titers elicited by the booster immunization. On days 0, 2, and 4 after the booster, no detectable neutralizing Ab titer was generated and most (≥66%) of the challenged guinea pigs died within 2 to 4 days (MTD = 2.5, 3.75, and 3.75 days, respectively). Only 35% of the guinea pigs challenged 6 days after the booster died (MTD = 4.8 days). A protection level of 90 to 100% was achieved when guinea pigs were challenged on day 8 postboosting, at a time when average neutralizing Ab titers were about or above 250. These correlations are in good agreement with previous observations that a threshold neutralizing Ab titer of 250 to 300 is needed for protection (31).
TABLE 4.
Antibody production and survival from anthrax spore challenge after booster immunization with 1 μg of PAa
| Day postboosting | % survivalb (no. of live animals/total) | Time to death for each animal (MTD for group) | Anti-PA Ab titerc (GMT ± SD) | Neutralizing Ab titerc (GMT ± SD) |
|---|---|---|---|---|
| 0 | 0 (0/12) | 2, 2, 3, 3, 2, 3, 3, 3, 2, 2, 3, 2 (2.5) | 25 ± 1 | <50 ± 1 |
| 2 | 29 (2/7) | 4, 4, 4, 3, 4 (3.75) | 54 ± 1 | <50 ± 1 |
| 4 | 33 (3/9) | 3, 3, 4, 3, 5, 4 (3.75) | 50 ± 1 | <50 ± 1 |
| 6 | 65 (11/17) | 4, 5, 6, 7, 3, 4 (4.8) | 171 ± 5.2 | 93 ± 6.6 |
| 8 | 90 (9/10) | 5 | 617 ± 3.3 | 259 ± 5.0 |
| 12 | 100 (10/10) | 1,467 ± 3.0 | 951 ± 3.4 |
Three months after the primary immunization with 1 μg of PA, guinea pigs were boosted with 1 μg of PA vaccine on days 12, 8, 6, 4, 2, and 0 (see text).
At day zero, animals from all groups were challenged with 40 LD50 of B. anthracis Vollum spores. A control group of 10 unimmunized animals which received the same challenge died, with a MTD of 2.5 days.
Parallel groups (each with 10 immunized guinea pigs) were used for Ab titer determination.
Taken together, the results underscore the delicate balance between the time required to generate functional Ab-producing cells from resting precursor memory cells and the time course of infectious disease progression. Although booster immunization with a PA-based vaccine is effective for the induction of a high-level and rapid Ab response, the activation of memory takes at least 6 days and therefore cannot compete with the rapid development of the anthrax infection, which is characterized by an MTD of 3 to 4 days in guinea pigs.
It should be noted, however, that the balance between the time to the onset of protection and the time to death could be different in other animal models. Indeed, the time to death of unimmunized animals challenged with anthrax spores varies from 1 to 2 days for rabbits to 3 to 5 days for monkeys. Interestingly, Ivins et al. (18) demonstrated that vaccinations with two doses of a PA-based vaccine protected monkeys from an aerosol spore challenge 2 years later at a time when anti-PA titers were extraordinary low. All together, it is apparent that the animal species, the initial immunization dose, and the mode of challenge may affect the protective efficiency of the memory response.
This study may have practical implications for defining postexposure anthrax treatment strategies. Primary vaccination by PA-based vaccines per se cannot ensure survival. It appears that in the guinea pig model, the presence of an appropriate neutralizing Ab titer at the time of exposure is required for effective protection. Nevertheless, certain antigen loads can elicit a memory response that is efficacious enough to allow for the production of the critical protective Ab mass in due time.
Moreover, because of the very narrow time window during which protection should be established, a booster immunization immediately after exposure would not always be effective in conferring protection, depending on the immune status of the population. It appears, therefore, that in certain postexposure scenarios (6), booster immunization would not be effective in the absence of antibiotic treatment (or passive immunotherapy) for the short period of time required to ensure the mounting of an effective anamnestic response.
Acknowledgments
We thank Yaron Kafri, Irit Shefer, Tamar Gelber, Lilach Levin, Orit Cohen, and Yossi Slomowich for their excellent technical assistance and Chanoch Kronman, Naomi Ariel, and Sara Cohen for their critical reviews of the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding the relation. Science 272:54-60. [DOI] [PubMed] [Google Scholar]
- 2.Atlas, R. M. 2001. Bioterrorism before and after September 11. Crit. Rev. Microbiol. 27:355-379. [DOI] [PubMed] [Google Scholar]
- 3.Banatvala, J. E., and P. Van Damme. 2003. Hepatitis B vaccine—do we need boosters? J. Vir. Hepatitis 10:1-6. [DOI] [PubMed] [Google Scholar]
- 4.Barnard, J. P., and A. M. Friedlander. 1999. Vaccination against anthrax with attenuated recombinant strains of Bacillus anthracis that produce protective antigen. Infect. Immun. 67:562-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belton, F. C., and R. E. Strange. 1954. Studies on a protective antigen produced in vitro from Bacillus anthracis: medium and method of production. Br. J. Exp. Pathol. 35:144-152. [PMC free article] [PubMed] [Google Scholar]
- 6.Brachman, P. S. 2002. Bioterrorism: an update with a focus on anthrax. Am. J. Epidemiol. 155:981-987. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, S., I. Mendelson, Z. Altboum, D. Kobiler, E. Elhanany, T. Bino, M. Leitner, I. Inbar, H. Rosenberg, Y. Gozes, R. Barak, M. Fisher, C. Kronman, B. Velan, and A. Shafferman. 2000. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect. Immun. 68:4549-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai, Z., J. C. Sirard, M. Mock, and T. M. Koehler. 1995. The atxA gene product activates transcription of the anthrax toxin genes and is essential for virulence. Mol. Microbiol. 16:1171-1181. [DOI] [PubMed] [Google Scholar]
- 9.Fish, D. C., J. P. Mahlandt, J. P. Dobbs, and R. E. Lincoln. 1968. Purification and properties of in vitro-produced anthrax toxin components. J. Bacteriol. 1995:907-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler, K., B. W. McBride, P. C. Turnbull, and L. W. Baillie. 1999. Immune correlates of protection against anthrax. J. Appl. Microbiol. 87:305. [DOI] [PubMed] [Google Scholar]
- 11.Friedlander, A. M., S. L. Welkos, M. L. Pitt, J. W. Ezzell, P. L. Worsham, K. J. Rose, B. E. Ivins, J. R. Lowe, G. B. Howe, and P. Mikesell. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239-1243. [DOI] [PubMed] [Google Scholar]
- 12.Gordon, S. M. 1999. The threat of bioterrorism: a reason to learn more about anthrax and smallpox. Cleveland Clinic J. Med. 66:592-595, 599-600. [DOI] [PubMed] [Google Scholar]
- 13.Gordon, V. M., S. H. Leppla, and E. L. Hewlett. 1988. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect. Immun. 56:1066-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, M. L., S. H. Leppla, and D. M. Klinman. 1999. Protection against anthrax toxin by vaccination with a DNA plasmid encoding anthrax protective antigen. Vaccine 17:340-344. [DOI] [PubMed] [Google Scholar]
- 15.Halperin, G., and H. Marcus. 2002. Application of recovery tests in the validation of immunoassays for assessing the immunogenicity of B. anthracis PA vaccine. J. Pharm. Sci. Technol. 55:150-161. [PubMed] [Google Scholar]
- 16.Halperin, G., and H. Marcus. 2002. A new assay system for accuracy validation of serum-antibody titration methods: mechanistic and operational consideration. Pharm. Forum 28:986-991. [Google Scholar]
- 17.Ivins, B. E., J. W. Ezzell, J. Jemski, K. W. Hedlund, J. D. Ristroph, and S. H. Leppla. 1986. Immunization studies with attenuated strains of Bacillus anthracis. Infect. Immun. 52:454-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivins, B. E., P. F. Fellows, M. L. Pitt, J. Estep, S. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Efficacy of a standard human anthrax vaccine against Bacillus anthracis aerosol spore challenge in rhesus monkeys. Salisbury Med. Bull. 87(Suppl.):125-126. [Google Scholar]
- 19.Ivins, B. E., M. L. Pitt, P. F. Fellows, J. W. Farchaus, G. E. Benner, D. M. Waag, S. F. Little, G. W. Anderson, P. H. Gibbs, and A. M. Friedlander. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16:1141-1148. [DOI] [PubMed] [Google Scholar]
- 20.Ivins, B. E., S. L. Welkos, S. F. Little, M. H. Crumrine, and G. O. Nelson. 1992. Immunization against anthrax with Bacillus anthracis protective antigen combined with adjuvants. Infect. Immun. 60:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jernigan, D. B., P. L. Raghunathan, B. P. Bell, R. Brechner, E. A. Bresnitz, J. C. Butler, M. Cetron, M. Cohen, T. Doyle, M. Fischer, C. Greene, K. S. Griffith, J. Guarner, J. L. Hadler, J. A. Hayslett, R. Meyer, L. R. Petersen, M. Phillips, R. Pinner, T. Popovic, C. P. Quinn, J. Reefhuis, D. Reissman, N. Rosenstein, A. Schuchat, W. J. Shieh, L. Siegal, D. L. Swerdlow, F. C. Tenover, M. Traeger, J. W. Ward, I. Weisfuse, S. Wiersma, K. Yeskey, S. Zaki, D. A. Ashford, B. A. Perkins, S. Ostroff, J. Hughes, D. Fleming, J. P. Koplan, and J. L. Gerberding. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klimpel, K. R., S. S. Molloy, G. Thomas, and S. H. Leppla. 1992. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc. Natl. Acad. Sci. USA 89:10277-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leppla, S. H. 1999. The bifactorial Bacillus anthracis lethal and oedema toxins, p. 243-263. In J. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxins. Academic Press, London, United Kingdom.
- 24.Little, S. F., B. E. Ivins, P. F. Fellows, and A. M. Friedlander. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 65:5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milne, J. C., D. Furlong, P. C. Hanna, J. S. Wall, and R. J. Collier. 1994. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J. Biol. Chem. 269:20607-20612. [PubMed] [Google Scholar]
- 26.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 27.Pezard, C., P. Berche, and M. Mock. 1991. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect. Immun. 59:3472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pezard, C., M. Weber, J. C. Sirard, P. Berche, and M. Mock. 1995. Protective immunity induced by Bacillus anthracis toxin-deficient strains. Infect. Immun. 63:1369-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittman, P. R., D. Hack, J. Mangiafico, P. Gibbs, K. T. McKee, A. M. Friedlander, and M. H. Sjogren. 2002. Antibody response to a delayed booster dose of anthrax vaccine and botulinum toxoid. Vaccine 20:2107-2115. [DOI] [PubMed] [Google Scholar]
- 30.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83-87. [DOI] [PubMed] [Google Scholar]
- 31.Reuveny, S., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh, Y., B. E. Ivins, and S. H. Leppla. 1998. Study of immunization against anthrax with the purified recombinant protective antigen of Bacillus anthracis. Infect. Immun. 66:3447-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh, Y., S. H. Leppla, R. Bhatnagar, and A. M. Friedlander. 1989. Internalization and processing of Bacillus anthracis lethal toxin by toxin-sensitive and -resistant cells. J. Biol. Chem. 264:11099-11102. [PubMed] [Google Scholar]
- 34.Turnbull, P. C., M. G. Broster, J. A. Carman, R. J. Manchee, and J. Melling. 1986. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect. Immun. 52:356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van De Verg, L. L., A. B. Hartman, A. K. Bhattacharjee, B. D. Tall, L. Yuan, K. Sasala, T. L. Hadfield, W. D. Zollinger, D. L. Hoover, and R. L. Warren. 1996. Outer membrane protein of Neisseria meningitidis as a mucosal adjuvant for lipopolysaccharide of Brucella melitensis in mouse and guinea pig intranasal immunization models. Infect. Immun. 64:5263-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West, D. J., and G. B. Calandra. 1996. Vaccine induced immunologic memory for hepatitis B surface antigen: implication for policy on booster vaccination. Vaccine 14:1019-1027. [DOI] [PubMed] [Google Scholar]
- 37.Zaucha, G. M., L. M. Pitt, J. Estep, B. E. Ivins, and A. M. Friedlander. 1998. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. Pathol. Lab. Med. 122:982-992. [PubMed] [Google Scholar]
- 38.Zinkernagel, R. M. 2002. On differences between immunity and immunological memory. Curr. Opin. Immunol. 14:523-536. [DOI] [PubMed] [Google Scholar]