Abstract
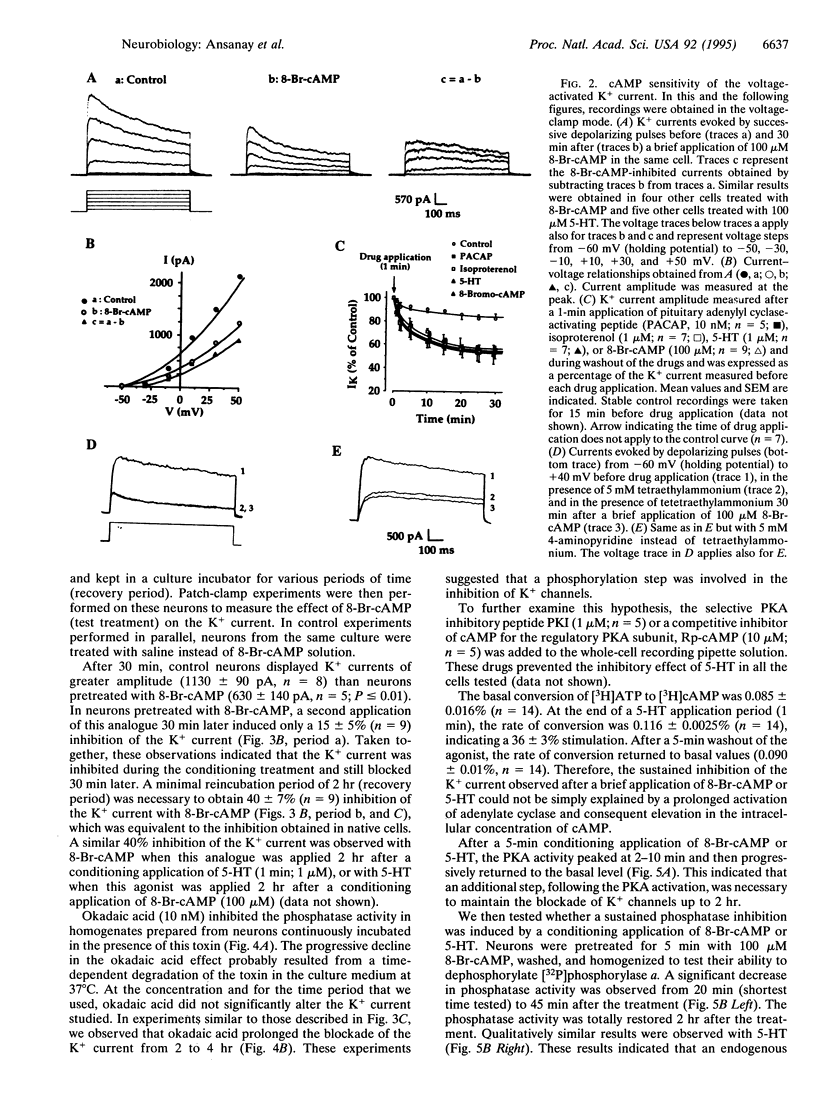
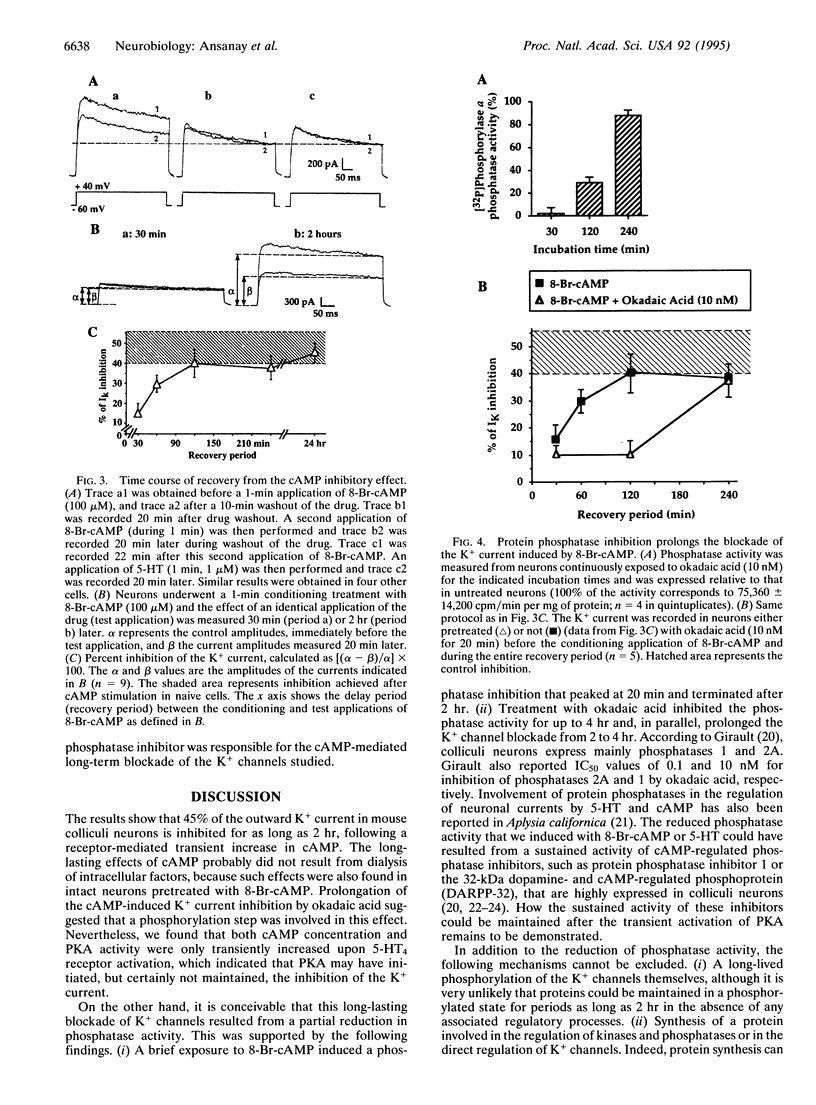
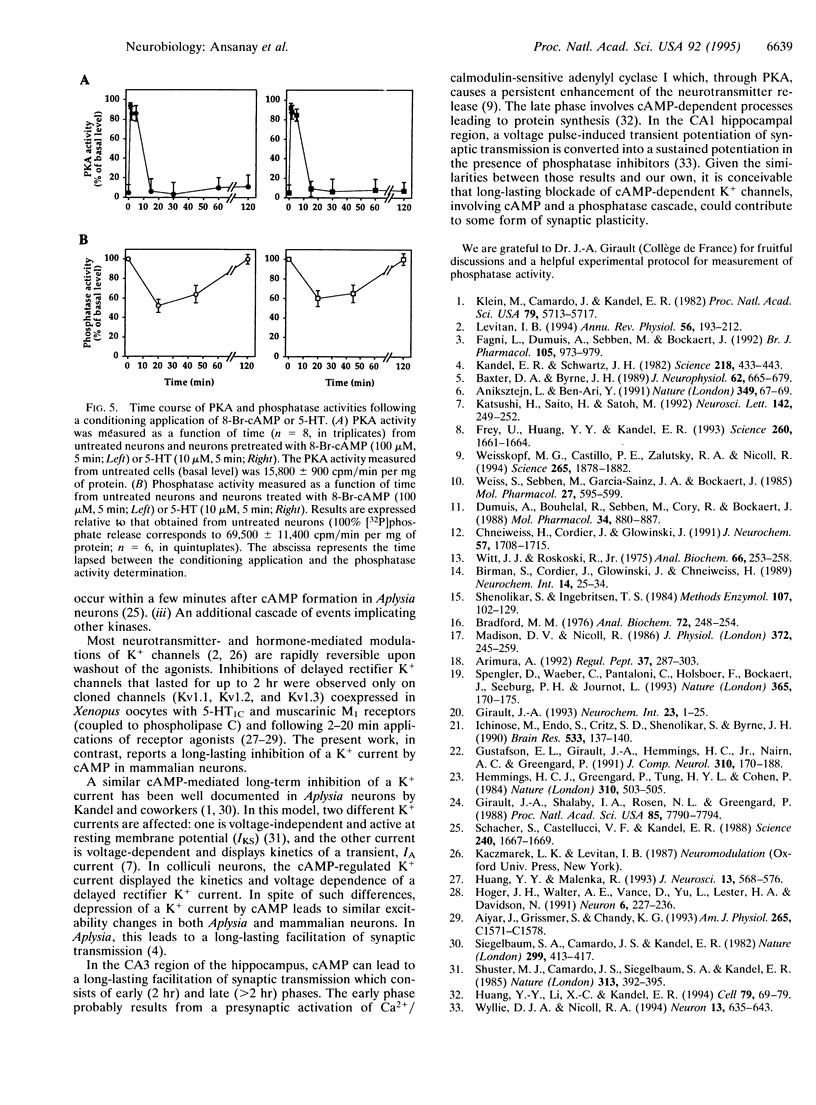
We report the long-term modulation of K+ channels by cAMP in cultured murine colliculi neurons. A short (1-2 s) application of 8-Br-cAMP induced a long-lasting broadening of the action potential, a loss of after-hyperpolarization, and a reduction in spike accommodation. In agreement with these changes, 8-Br-cAMP produced a long-lasting (2 hr) inhibition of a K+ current. These effects were also observed after a short activation of the pituitary adenylyl cyclase-activating polypeptide, beta-adrenergic, and 5-hydroxytryptamine type 4 (5-HT4) receptors, all known to increase cAMP. A transient activation of the cAMP-dependent protein kinase and a long-lasting inhibition of phosphatases (up to 2 hr) were detected. The blockade of the K+ current resulting from a brief application of 8-Br-cAMP or 5-hydroxytryptamine was prolonged from 2 to 4 hr when protein-serine/threonine phosphatases 1 and 2A were inhibited with 10 nM okadaic acid. The critical steps following the cAMP-dependent protein kinase activation and resulting in a long-term blockade of phosphatases are discussed in this report.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyar J., Grissmer S., Chandy K. G. Full-length and truncated Kv1.3 K+ channels are modulated by 5-HT1c receptor activation and independently by PKC. Am J Physiol. 1993 Dec;265(6 Pt 1):C1571–C1578. doi: 10.1152/ajpcell.1993.265.6.C1571. [DOI] [PubMed] [Google Scholar]
- Aniksztejn L., Ben-Ari Y. Novel form of long-term potentiation produced by a K+ channel blocker in the hippocampus. Nature. 1991 Jan 3;349(6304):67–69. doi: 10.1038/349067a0. [DOI] [PubMed] [Google Scholar]
- Arimura A. Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and current status of research. Regul Pept. 1992 Feb 18;37(3):287–303. [PubMed] [Google Scholar]
- Baxter D. A., Byrne J. H. Serotonergic modulation of two potassium currents in the pleural sensory neurons of Aplysia. J Neurophysiol. 1989 Sep;62(3):665–679. doi: 10.1152/jn.1989.62.3.665. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chneiweiss H., Cordier J., Glowinski J. Cyclic AMP accumulation induces a rapid desensitization of the cyclic AMP-dependent protein kinase in mouse striatal neurons. J Neurochem. 1991 Nov;57(5):1708–1715. doi: 10.1111/j.1471-4159.1991.tb06371.x. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Bouhelal R., Sebben M., Cory R., Bockaert J. A nonclassical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol Pharmacol. 1988 Dec;34(6):880–887. [PubMed] [Google Scholar]
- Fagni L., Dumuis A., Sebben M., Bockaert J. The 5-HT4 receptor subtype inhibits K+ current in colliculi neurones via activation of a cyclic AMP-dependent protein kinase. Br J Pharmacol. 1992 Apr;105(4):973–979. doi: 10.1111/j.1476-5381.1992.tb09087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U., Huang Y. Y., Kandel E. R. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993 Jun 11;260(5114):1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Girault J. A. Protein phosphorylation and dephosphorylation in mammalian central nervous system. Neurochem Int. 1993 Jul;23(1):1–25. doi: 10.1016/0197-0186(93)90139-v. [DOI] [PubMed] [Google Scholar]
- Girault J. A., Shalaby I. A., Rosen N. L., Greengard P. Regulation by cAMP and vasoactive intestinal peptide of phosphorylation of specific proteins in striatal cells in culture. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7790–7794. doi: 10.1073/pnas.85.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson E. L., Girault J. A., Hemmings H. C., Jr, Nairn A. C., Greengard P. Immunocytochemical localization of phosphatase inhibitor-1 in rat brain. J Comp Neurol. 1991 Aug 8;310(2):170–188. doi: 10.1002/cne.903100204. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Greengard P., Tung H. Y., Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984 Aug 9;310(5977):503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Hoger J. H., Walter A. E., Vance D., Yu L., Lester H. A., Davidson N. Modulation of a cloned mouse brain potassium channel. Neuron. 1991 Feb;6(2):227–236. doi: 10.1016/0896-6273(91)90358-7. [DOI] [PubMed] [Google Scholar]
- Huang Y. Y., Li X. C., Kandel E. R. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994 Oct 7;79(1):69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Huang Y. Y., Malenka R. C. Examination of TEA-induced synaptic enhancement in area CA1 of the hippocampus: the role of voltage-dependent Ca2+ channels in the induction of LTP. J Neurosci. 1993 Feb;13(2):568–576. doi: 10.1523/JNEUROSCI.13-02-00568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M., Endo S., Critz S. D., Shenolikar S., Byrne J. H. Microcystin-LR, a potent protein phosphatase inhibitor, prolongs the serotonin- and cAMP-induced currents in sensory neurons of Aplysia californica. Brain Res. 1990 Nov 12;533(1):137–140. doi: 10.1016/0006-8993(90)91806-r. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Katsuki H., Saito H., Satoh M. The involvement of muscarinic, beta-adrenergic and metabotropic glutamate receptors in long-term potentiation in the fimbria-CA3 pathway of the hippocampus. Neurosci Lett. 1992 Aug 17;142(2):249–252. doi: 10.1016/0304-3940(92)90384-j. [DOI] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Cyclic adenosine 3',5'-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol. 1986 Mar;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S., Castellucci V. F., Kandel E. R. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science. 1988 Jun 17;240(4859):1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Ingebritsen T. S. Protein (serine and threonine) phosphate phosphatases. Methods Enzymol. 1984;107:102–129. doi: 10.1016/0076-6879(84)07007-5. [DOI] [PubMed] [Google Scholar]
- Shuster M. J., Camardo J. S., Siegelbaum S. A., Kandel E. R. Cyclic AMP-dependent protein kinase closes the serotonin-sensitive K+ channels of Aplysia sensory neurones in cell-free membrane patches. 1985 Jan 31-Feb 6Nature. 313(6001):392–395. doi: 10.1038/313392a0. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P. H., Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993 Sep 9;365(6442):170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Weiss S., Sebben M., Garcia-Sainz J. A., Bockaert J. D2-dopamine receptor-mediated inhibition of cyclic AMP formation in striatal neurons in primary culture. Mol Pharmacol. 1985 Jun;27(6):595–599. [PubMed] [Google Scholar]
- Weisskopf M. G., Castillo P. E., Zalutsky R. A., Nicoll R. A. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994 Sep 23;265(5180):1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Witt J. J., Roskoski R., Jr Rapid protein kinase assay using phosphocellulose-paper absorption. Anal Biochem. 1975 May 26;66(1):253–258. doi: 10.1016/0003-2697(75)90743-5. [DOI] [PubMed] [Google Scholar]
- Wyllie D. J., Nicoll R. A. A role for protein kinases and phosphatases in the Ca(2+)-induced enhancement of hippocampal AMPA receptor-mediated synaptic responses. Neuron. 1994 Sep;13(3):635–643. doi: 10.1016/0896-6273(94)90031-0. [DOI] [PubMed] [Google Scholar]



