Abstract
The major cause of death among pulmonary hypertension patients is right heart failure, but the biology of right heart is not well understood. Previous studies showed that mechanisms of the activation of GATA4, a major regulator of cardiac hypertrophy, in response to pressure overload are different between left and right ventricles. In the left ventricle, aortic constriction triggers GATA4 activation via post-translational modifications without influencing GATA4 expression, while pulmonary artery banding enhances GATA4 expression in the right ventricle. We found that GATA4 expression can also be increased in the right ventricle of rats treated with chronic hypoxia to induce pulmonary hypertension, and investigated the mechanism of increased GATA4 expression. Examination of Gata4 promoter revealed that CCAAT box plays an important role in gene activation; and hypoxic pulmonary hypertension promoted the binding of CBF/NF-Y to CCAAT box in the right ventricle. We found that CBF/NF-Y forms a complex with annexin A1, which inhibits DNA binding activity. In response to hypoxic pulmonary hypertension, annexin A1 gets degraded, resulting in CBF/NF-Y-dependent activation of Gata4 gene transcription. The right ventricle contains a higher level of CBF/NF-Y compared to the left ventricle, and this may allow for efficient activation in response to annexin A1 degradation. Signaling via iron-catalyzed protein oxidation, mediates hypoxic pulmonary hypertension-induced annexin A1 degradation, Gata4 gene transcription, and right ventricular hypertrophy. These results establish a right heart-specific signaling mechanism in response to pressure overload, which involves metal-catalyzed carbonylation and degradation of annexin A1 that liberates CBF/NF-Y to activate Gata4 gene transcription.
Keywords: heart failure, hypertension, hypertrophy, hypoxia, pressure overload, pulmonary hypertension, redox signaling
Introduction
Pulmonary hypertension (PH) is characterized by increased pulmonary arterial pressure and vascular resistance, which interfere with the ejection of blood by the right ventricle (RV) and ultimately causes right heart failure. While the major cause of death among PH patients is RV failure, the RV biology has not been well defined.1
GATA4 regulates transcription of genes that are expressed during adult clinical cardiac hypertrophy.2 Transgenic mice with cardiac-specific overexpression of GATA4 exhibit concentric hypertrophy of atria as well as RV and left ventricle (LV).3
While similarities and differences between adult LV and RV have not been well characterized, these two ventricles originate from different precursors.4 Further, in mouse embryo, knocking out Gata4 induced RV hypoplasia.5
Previous studies indicated that mechanisms of GATA4 activation are different between adult LV and RV. Pressure overload via aortic constriction increased GATA4 activity without enhancing GATA4 expression in LV.6 In contrast, pressure overload via pulmonary artery banding enhanced GATA4 expression in RV.7 Similarly, we found that increased pulmonary arterial pressure by chronic hypoxia (CH) increased GATA4 expression. The present study examined the mechanism of enhanced GATA4 gene expression by CH-mediated PH in RV.
Materials and Methods
Results
Effects of hypoxic PH on GATA4 in RV
Adult rats were subjected to CH at 10% O2 for 24 h per day for 2, 7 and 14 days.8 CH promoted RV hypertrophy as indicated by RV/(LV+S) mass ratio (Fig. S1A) as well as RV mass/tibia length ratio (Fig. S1B). RV mass became significantly higher compared to control rats by 7 days of hypoxia and progressively increased. No increases in LV and septum masses were noted (Fig. S1C). Lung mass was increased, consistently with the occurrence of pulmonary vascular thickening (Fig. S1D). Histological analyses of the heart showed time-dependent thickening of the RV wall (Fig. S1E), indicating the occurrence of concentric hypertrophy. Microscopic analysis of the hematoxylin and eosin (H & E) stained heart revealed increased RV myocyte thickness in animals treated with hypoxia, but LV myocytes were unchanged (Fig. S1F). Quantitative analysis showed a significant increase in RV myocyte thickness after 7 days of hypoxia (Fig. S1G). Verhoff’s Van Geison staining confirmed the occurrence of concentric RV muscle hypertrophy (Fig. S1H), without notable fibrosis as monitored by Masson Trichrome staining (Fig. S1I). Increased diastolic RV wall thickness in response to 2 weeks of CH was observed by 2-dimensional echocardiography using a VisualSonics High-Resolution In Vivo Imaging System (0.50 mm normoxia vs. 1.03 mm hypoxia). Expression of fetal genes that are often used as markers for ventricular hypertrophy such as atrial natriuretic factor (Anf) were induced in hypertrophied RV, but not in LV (Fig. S1J). Hemodynamic measurements using a Millar catheter demonstrated that CH increased RV systolic pressure (Fig. S1K).
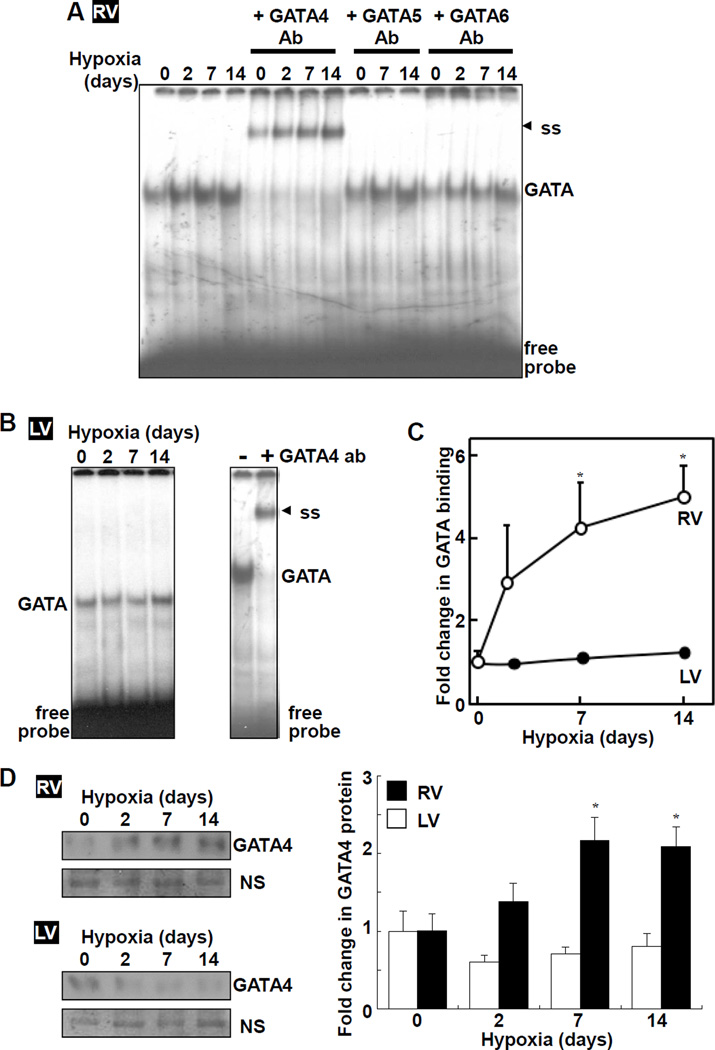
Results from electrophoretic mobility shift assays (EMSA) demonstrated that CH-induced RV hypertrophy was associated with GATA4 activation in RV (Fig. 1A), but not in LV (Fig. 1B). The increase was apparent after 2 days of hypoxia and significant increases were noted at 7 and 14 days of hypoxia (Fig. 1C). GATA binding activity in RV was reduced by the cold competitor with GATA consensus sequence TGATAA (wtGATA), but not by mutant TCTTAA (muGATA) or unrelated Oct-1 binding sequence (wtOct-1) (Fig. S2A). GATA binding activity in LV was also reduced by the cold competitor (Fig. S2B). Supershift experiments confirmed that GATA activity is due to GATA4 in RV (Fig. 1A) and LV (Fig. 1B). Hypoxia did not increase the DNA binding activity of Oct-1 (Fig. S2C). Increased GATA4 DNA binding activity in RV by CH was associated with increased GATA4 protein expression (Fig. 1D).
Fig. 1. Hypoxic PH promotes GATA4 activity and expression in the RV.
Rats were subjected to CH. Nuclear extracts from (A) RV and (B) LV were subjected to EMSA to monitor GATA DNA binding activity +/− antibodies (Ab). ss denotes supershifted bands. (C) Values represent means ± SEM. (D) GATA4 protein levels in nuclear extracts were measured by Western blot. A non-specific (NS) band suggests no differences in loading. *significantly different from normoxia control (n = 5 – 6).
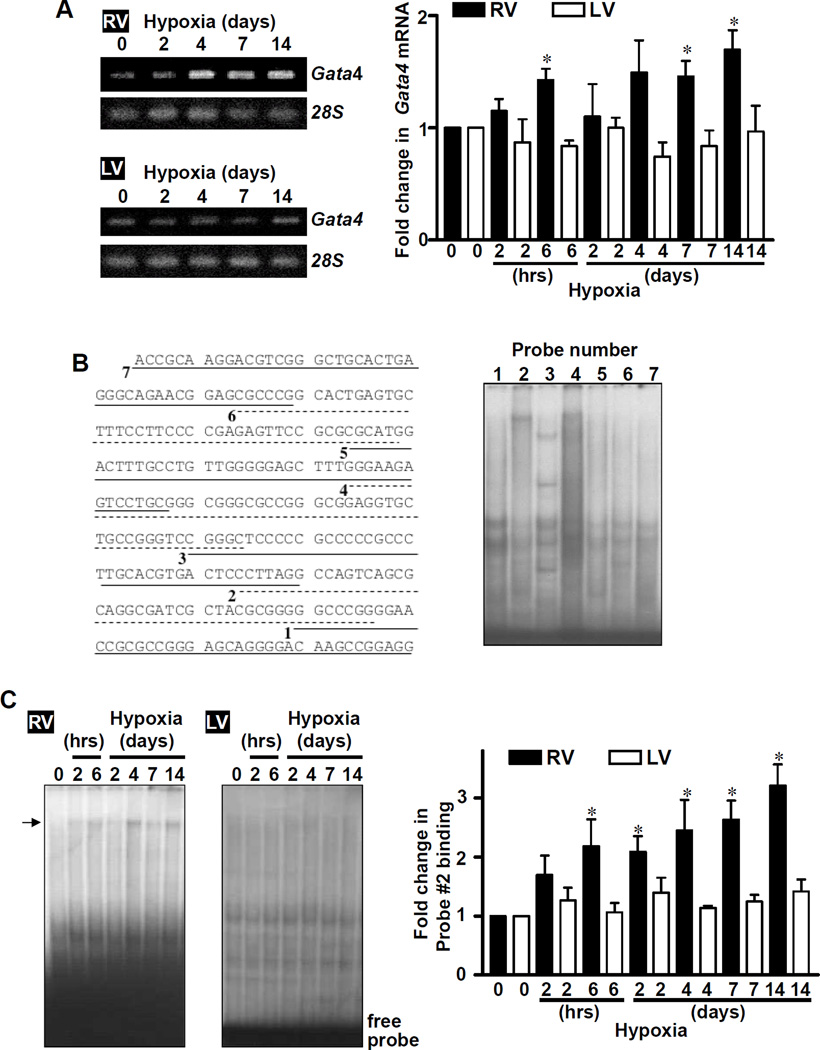
Consistent with the earlier study of pulmonary artery banding,7 induction of PH by CH also increased Gata4 mRNA expression in RV, but not in LV (Fig. 2A). In these experiments, we added earlier time points and found that significant increase in Gata4 mRNA was detected as early as 6 h of sustained hypoxia and maintained at least for 2 weeks. We did not detect the occurrence of known post-translational modification mechanisms for GATA4 such as phosphorylation9,10 (Fig. S3A), acetylation11,12 (Figs. S3B and S3C), and interactions with other transcription factors such as NFAT13 (Figs. S3D and S3E).
Fig. 2. Identification of Gata4 promoter region that is activated by hypoxic PH in the RV.
Rats were subjected to CH. (A) RNA was isolated, and Gata4 mRNA and 28S rRNA levels were measured by RT-PCR. The bar graph represents means ± SEM (n = 4 – 5). (B) Basal DNA binding activities of RV nuclear extracts toward 32P-labeled Probes #1 – 7 constructed from the 250 bp Gata4 promoter region.14 (C) DNA binding activities in nuclear extracts toward Probe #2 were monitored by EMSA. The bar graph represents means ± SEM (n = 6). *significantly different from no hypoxia.
Studies of Gata4 promoter
We hypothesized that Gata4 gene transcription is activated by hypoxic PH in RV. We previously found that the 250 bp region immediately upstream from the transcriptional start site of mouse Gata4 gene contains key regulatory elements.14,15 To test if this region is influenced by CH in RV, EMSA probes were constructed by dissecting this 250 bp region into 7-sub-regions.14 EMSA with RV nuclear extracts showed DNA binding proteins, which interacted with these probes (Fig. 2B). While binding patterns of other probes were unaltered, using Probe #2, we identified a band that is increased in response to CH in RV (Fig. 2C). Time course studies revealed that Probe #2 binding activity was significantly increased by CH in RV as early as 6 h and remained increased up to 14 days. No activation of Probe #2 binding was noted in LV (Fig. 2C).
Role of CCAAT box
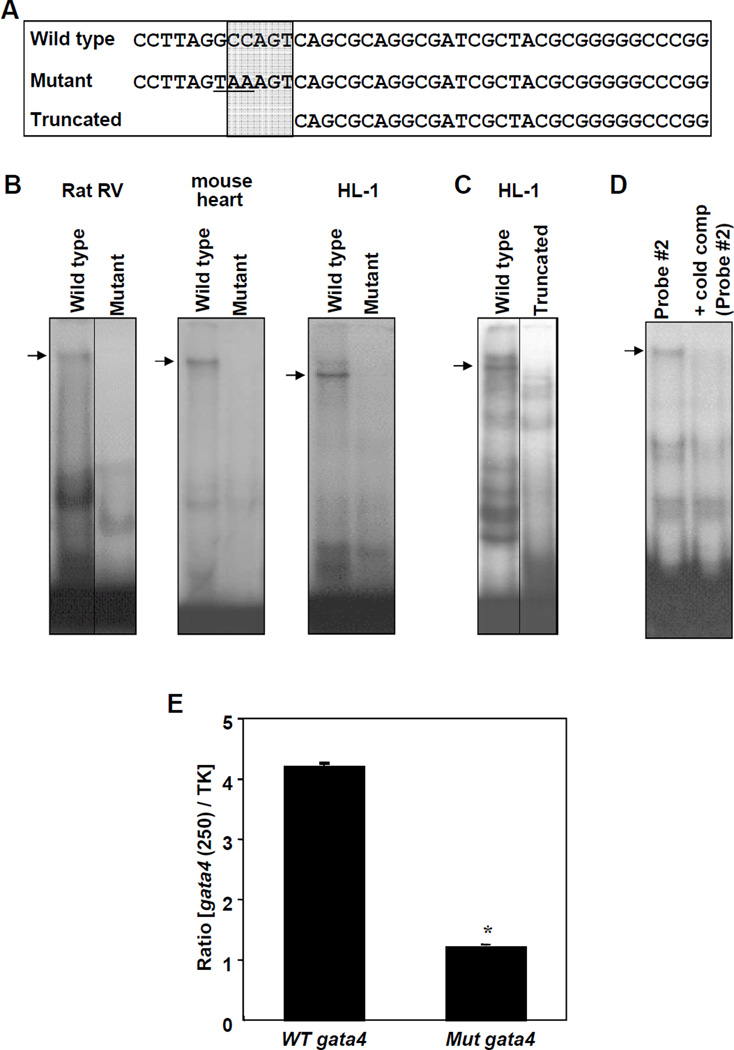
Probe #2 contains CCAAT box as depicted by the sequences indicated in Fig. 3A (shaded area). Mutation (Fig. 3B) or truncation (Fig. 3C) of CCAAT box eliminated the band that was affected by CH. A cold competitor containing CCAAT box effectively eliminated binding of the radiolabelled probe (Fig. 3D). To test if CCAAT box plays a functional role in gene transcription, we mutated the CCAAT box site (GCCAGT to TAAAGT) and constructed a luciferase vector. Transfection of HL-1 cells demonstrated that this mutation significantly attenuated the transcriptional activity of the 250 bp Gata4 promoter (Fig. 3E). These results suggest that proteins, which bind to CCAAT box within Probe #2, are affected by CH.
Fig. 3. Role of the CCAAT Box.
(A) Sequences of various double stranded EMSA probes. CCAAT box regions are indicated in the shaded area. (B) Nuclear extracts from rat RV (7-day hypoxia treated), mouse heart or HL-1 cardiac muscle cells were subjected to EMSA using wild type or mutant Probe #2. (C) Nuclear extracts were subjected to EMSA with wild type or truncated Probe #2. (D) Heart nuclear extracts were subjected to EMSA using 32P-labeled Probe #2 in the presence of excess cold competitor (unlabelled Probe #2). (E) HL-1 cells were transfected with the luciferase construct controlled by the 250 bp proximal region of the wild type (WT) or mutant (Mut) Gata4 promoter. Values represent means ± SEM of the ratio of 250 bp Gata4 promoter-controlled firefly luciferase activity to thymidine kinase (TK) promoter-controlled Renilla luciferase activity (n = 6). *significantly different from the wild type Gata4 promoter. Some images were grouped from different parts of the same gel and such arrangements are indicated by dividing lines.
Role of CBF/NF-Y
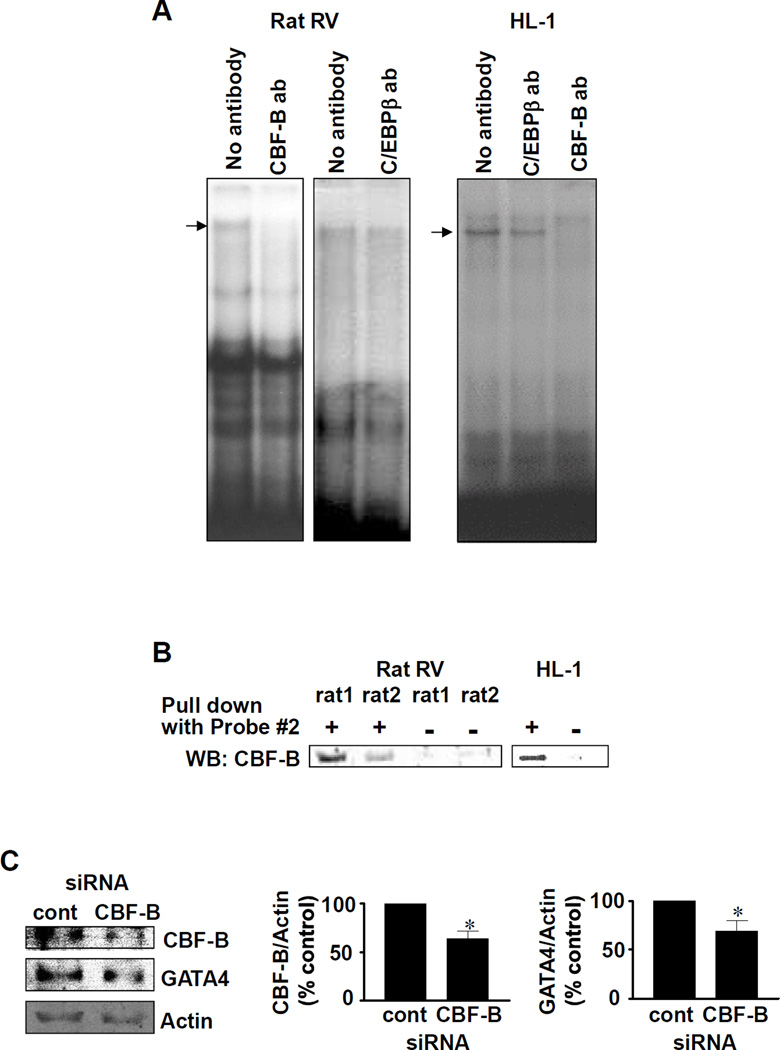
To identify the transcription factor(s) that comprise of the CCAAT box binding complex, we performed supershift experiments with antibodies against factors that are known to bind to CCAAT box including C/EBPβ, CBF/NF-Y and NF-1.16 The antibody against CBF-B (a subunit of CBF/NF-Y) eliminated the band that is affected by CH (Fig. 4A), possibly due to the interference of the DNA binding activity by the antibody. This effect was not observed with antibodies for C/EBPβ (Fig. 4A) or NF-1 (not shown). Further, experiments in which CCAAT box binding proteins were pulled down with biotinylated Probe #2 and streptavidin-agarose showed this complex contained CBF/NF-Y (Fig, 4B). Binding of CBF/NF-Y toward CCAAT box within Probe #2 of the Gata4 promoter was also observed in chromatin isolated from fixed RV tissues of CH-treated rats using chromatin immunoprecipitation assay (data not shown). To provide direct evidence that CBF/NF-Y plays a role in the regulation of Gata4 gene transcription, HL-1 cells were subjected to siRNA to knock-down CBF-B. Under such conditions, GATA4 protein expression was also downregulated (Fig. 4C). These results suggest that CBF/NF-Y binding activity toward CCAAT box within the Gata4 promoter is activated by CH in RV.
Fig. 4. Identification of CCAAT box binding protein.
(A) EMSA was performed with Probe #2 and nuclear extracts from rat RV (7-day hypoxia treated) or HL-1 cells. Supershift experiments were performed with antibodies (ab) against C/EBPβ and CBF-B. (B) Nuclear extracts from rat RV (7-day hypoxia treated) or HL-1 cells were mixed with biotinylated Probe #2 and streptavidin-agarose. Samples were centrifuged, washed and boiled. Supernatant of the final spin was subjected to SDS-PAGE and immunoblotted with CBF-B antibody. Lanes denoted minus pull down show control experiments without biotinylated Probe #2. (C) HL-1 cells were transfected with siRNA for CBF-B, nuclear extracts were prepared and subjected to Western blotting. Values in bar graphs represent means ± SEM (n = 6).
Regulation by annexin A1 carbonylation
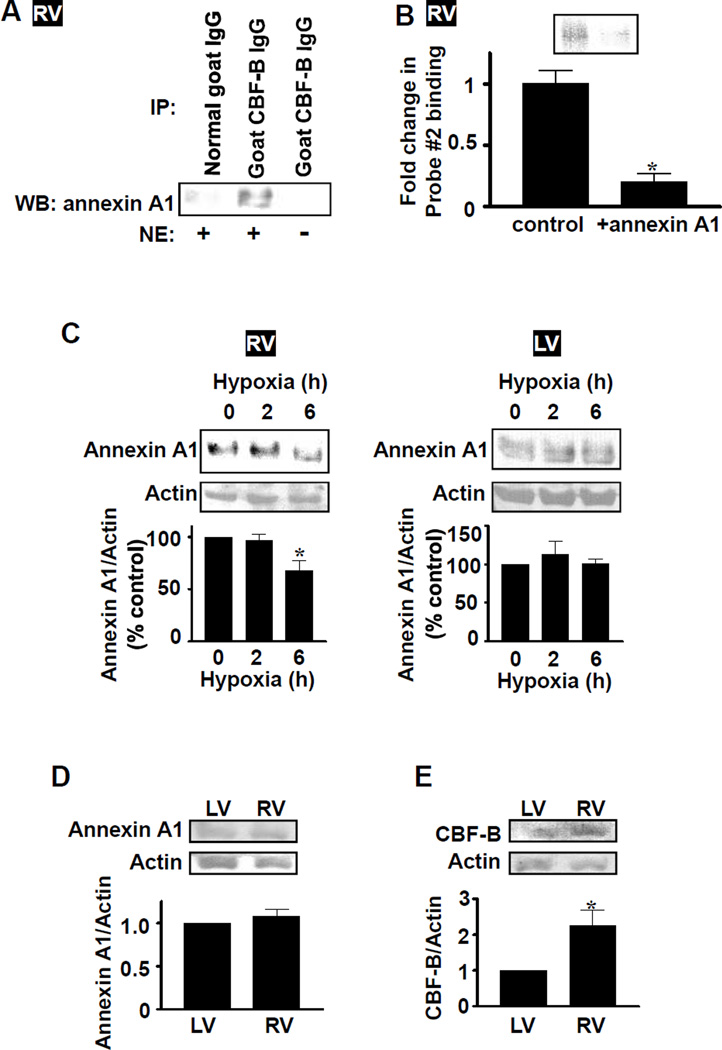
To understand the mechanism of CBF/NF-Y activation, which might promote Gata4 gene transcription, we searched for proteins that interact with CBF/NF-Y. Immunoprecipitation/immunoblotting revealed that annexin A1 can interact with CBF/NF-Y. Immunoprecipitation of rat RV nuclear extracts with CBF-B antibody followed by immunoblotting with annexin A1 antibody showed a strong band, whereas control experiments with normal IgG or in the absence of nuclear extracts did not (Fig. 5A). Similar results were obtained when immunoprecipitation with annexin A1 antibody was followed by blotting with CBF-B antibody (Fig. S4A). This interaction of annexin A1 is inhibitory to CBF/NF-Y for its DNA binding activity toward CCAAT box, as the inclusion of recombinant annexin A1 in binding reaction mixtures for EMSA significantly inhibited Probe #2 binding activity (Fig. 5B). Thus, CBF/NF-Y can bind to annexin A1, which serves as a negative regulator for the DNA binding activity. We further found that CH decreased the level of annexin A1 in RV, but not in LV (Fig. 5C), suggesting a mechanism for CBF/NF-Y activation by hypoxic PH in RV, which mediates the degradation of a negative regulator, annexin A1. Time course studies revealed that decrease in annexin A1 protein expression occurs at 6 h without changes in CBF-B protein expression (Figs. 5C, S4B and S4C). While no differences in levels of protein expression of annexin A1 in normal RV and LV were noted (Fig. 5D), we found a significantly higher level of CBF/NF-Y protein expression in normal RV compared to LV (Fig. 5E).
Fig. 5. Regulation of CBF/NF-Y by annexin A1.
(A) Rat RV nuclear extracts (NE) or buffer alone control were immunoprecipitated with CBF-B antibody or normal goat IgG control, and immuno-blotted with mouse annexin A1 antibody. (B) Recombinant human annexin A1 was added to binding reaction mixtures for EMSA with Probe #2 and RV nuclear extracts from rats subjected to 14 days of hypoxia. Values in the bar graph represent means ± SEM (n = 6). (C) Rats were subjected to hypoxia. Annexin A1 protein levels in RV and LV homogenates were monitored by Western blot. Values in bar graphs represent means ± SEM (n = 6). (D) Annexin A1 expression was monitored in LV and RV homogenates from normal rats (n = 6). (E) CBF-B protein expression was monitored in LV and RV homogenates from normal rats (n = 6). *significant difference.
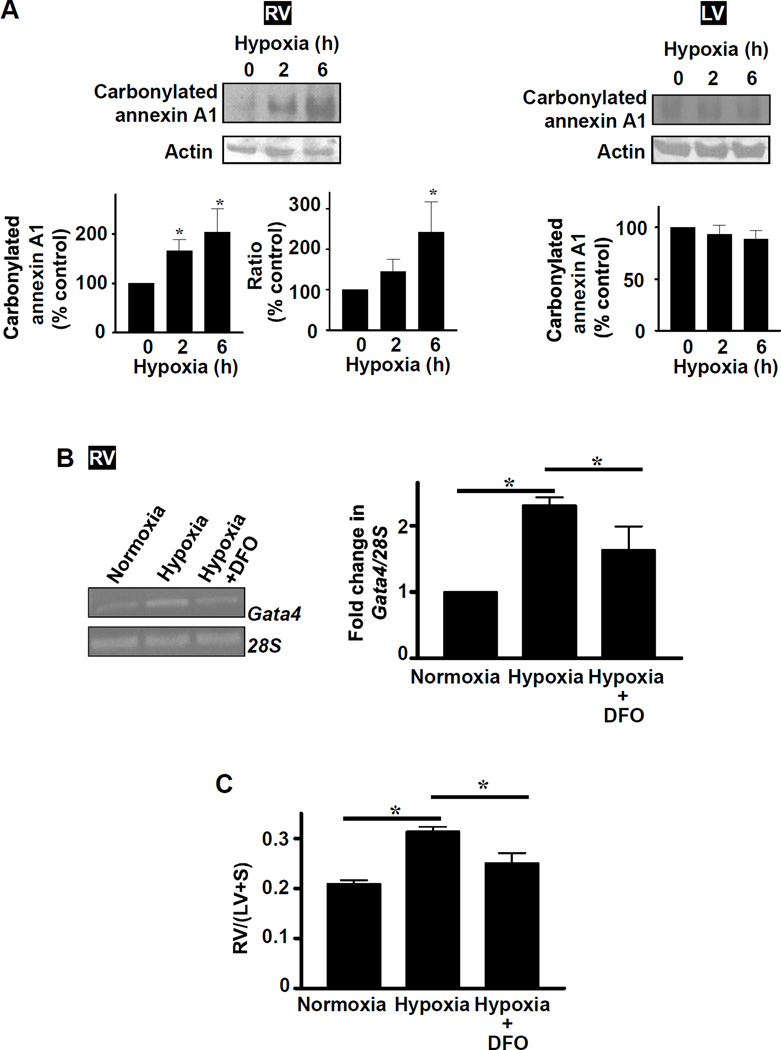
Our laboratory previously found that annexin A1 undergoes proteasome-dependent degradation via protein carbonylation signaling in smooth muscle cells.17 Similarly, in RV, hypoxia promoted carbonylation of annexin A1 (Fig. 6A). These results suggest a possible mechanism for CBF/NF-Y activation, which involves annexin A1 carbonylation and subsequent degradation, suppressing the inhibitory activity of annexin A1 toward CBF/NF-Y. To directly test this hypothesis, effects of deferoxamine (an iron chelator that inhibits iron-catalyzed protein carbonylation) and MG132 (a proteasome inhibitor) were evaluated. Administration of deferoxamine caused inhibition of hypoxic PH-mediated increase in annexin A1 carbonylation (Fig. S5) and Gata4 mRNA expression in RV (Fig. 6B) as well as RV hypertrophy (Fig. 6C). Neither hypoxia nor deferoxamine influenced the expression of other genes such as Gapdh (Fig. S6A), suggesting that these events do not occur non-specifically. Deferoxamine injection to normoxic control animals did not influence the Gata4 mRNA expression (Fig. S6B), RV mass (Fig. S6C), or RV systolic pressure (22.7 ± 1.0 mmHg untreated vs. 22.4 ± 0.5 deferoxamine-treated). Similarly, MG132 injection into rats inhibited hypoxic PH-induced increase in Gata4 mRNA expression in the RV (Fig. S7). MG132 injection to normoxic control animals did not influence the Gata4 mRNA expression (Fig. S7) or RV systolic pressure (22.7 ± 1.0 mmHg untreated vs. 23.3 ± 1.6 MG132-treated).
Fig. 6. Role of metal-catalyzed protein carbonylation in Gata4 gene expression and the development of RV hypertrophy.
(A) Rats were subjected to hypoxia, and RV and LV homogenates were prepared. Carbonylated annexin A1 was monitored by labeling carbonylated proteins with 2,4-dinitrophenylhydrazine (DNPH), immunoprecipitated with the antibody for DNPH-derivatized proteins, and Western blotting with annexin A1 antibody. Values in bar graphs represent means ± SEM of carbonylated annexin A1 and the ratio of carbonylated annexin A1 to annexin A1 protein level (n = 6). *significantly different from control. (B) Rats were injected with deferoxamine (DFO; 20 mg/kg) or saline daily during the 4 day exposure to hypoxia. Gata4 mRNA and 28s rRNA levels were monitored by RT-PCR. Values in the bar graph represent means ± SEM (n = 5). (C) Masses of RV, LV and the septum (S) were measured and RV/(LV+S) values were calculated as an estimate of RV hypertrophy (n = 3). *significantly different from each other.
Discussion
Right heart failure is the major cause of death among PH patients, however, pathophysiology of the right heart is not well understood. Since agents to treat left heart failure, do not necessarily work to treat right heart failure, understanding of the right heart biology is needed to develop therapeutic strategies to specifically prevent/treat right heart failure in PH patients. The present work investigated regulatory mechanisms of GATA4 in RV of rats subjected to CH to induce PH. We found a novel mechanism of GATA4 activation, which involves the promotion of Gata4 gene transcription that is regulated by signaling via metal-catalyzed oxidation. This may define the difference between hypertrophic signaling mechanisms between RV and LV.
GATA4 is a major transcriptional regulator of cardiac hypertrophy.2 GATA4 activation mechanisms are well understood in the context of LV hypertrophy, and involve post-translational modifications. In the LV of rats subjected to pressure overload by aortic constriction, GATA4 activity was increased without changes in GATA4 expression.6 Thus, activation mechanisms of GATA4 in LV seem to involve post-translational modifications. In contrast, in RV, increased mRNA expression of Gata4 was noted in rats subjected to PH either by pulmonary artery banding2 or by CH as described in this study. These results suggest that activation mechanisms of GATA4 in response to pressure overload are different between LV and RV.
Since understanding RV-specific mechanisms responding to pressure overload has great clinical significance for finding treatment/management strategies for PH patients, the present study examined the detailed mechanism of increased Gata4 gene transcription in RV. We cloned the Gata4 promoter and identified a major regulatory element, CCAAT box. This element is necessary for the basal expression of GATA4 and the binding toward this element is activated in response to hypoxic PH in RV. The regulatory mechanism of Gata4 gene transcription involves CBF/NF-Y, which exerts differential regulation on a wide variety of genes through its interaction with CCAAT box.18 As roles of CBF/NF-Y in the heart have not been studied, the finding in this study that pressure overload activates this transcription factor is novel and may have revealed an important biologic mechanism in the heart.
To understand the activation mechanism, we screened for proteins that can interact with CBF/NF-Y. We found that annexin A1 interacts with CBF/NF-Y. The addition of recombinant annexin A1 in the reaction mixture for EMSA inhibited the binding of CBF/NF-Y to CCAAT box, revealing that annexin A1 is a negative regulator of CBF/NF-Y. Our laboratory previously reported a novel oxidant signaling mechanism involving protein carbonylation and subsequent proteasome-dependent degradation of annexin A1.17 In RV of rats subjected to hypoxic PH, annexin A1 is carbonylated and its expression is reduced. The reduced annexin A1 expression should liberate CBF/NF-Y, promoting its DNA binding activity and thus Gata4 gene transcription.
Protein carbonylation often occurs in response to metal-catalyzed oxidation. The role of this mechanism in PH-mediated increase in Gata4 gene transcription as well as RV hypertrophy is supported by our observations that an iron chelator, deferoxamine, effectively inhibited hypoxic PH-mediated increase in Gata4 mRNA expression and RV hypertrophy. Since deferoxamine does not inhibit hypoxic pulmonary vasoconstriction,19 deferoxamine likely directly influences the RV hypertrophic mechanism. These results also suggest the possible use of deferoxamine or other inhibitors of metal-catalyzed oxidation to inhibit the development of RV hypertrophy in PH patients.
We propose two possible mechanisms by which the signaling pathway described in this study may preferentially occur in RV compared to LV. We found that the expression of CBF/NF-Y is higher in RV compared to LV. This may increase the sensitivity of RV to activate CBF/NF-Y by being liberated from annexin A1 as this protein gets degraded. In the previous study,20 we found that protein carbonylation mediated by serotonin is more pronounced in RV than in LV, indicating that the sensitivity to carbonylation signaling might be higher in RV. We attributed this to be due to differential expression of monoamine oxidase between RV and LV. Thus, this is another possible mechanism in which the activation of Gata4 gene transcription in response to pressure overload preferentially occurs in RV.
There are limitations in this study. First, while the previous study has shown that pulmonary arterial banding increases Gata4 gene transcription, the present study using the CH model does not distinguish between direct effects of pressure overload and possible influence by hypoxia on specific pathways we propose. However, mechanisms described in this study are not merely hypoxia-responsive cardiac signaling, because hypoxia did not trigger these events in LV. Second, in our studies of intact animals, we observed the activation of sequential signaling events, which comprise of annexin A1 carbonylation/degradation, CBF/NF-Y activation, and Gata4 gene transcription. These observed events in vivo, which occur slower than what might be expected in cell signaling from studies of cultured cells, may offer invaluable pathophysiologically relevant information.
Perspectives
The present study, for the first time, demonstrates a RV-specific signaling mechanism in response to pressure overload. This pathway involves metal-catalyzed carbonylation and subsequent degradation of annexin A1, which liberates CBF/NF-Y for the activation of Gata4 gene transcription (Fig. S8). We propose that this mechanism may influence clinically important events of RV hypertrophy in PH patients. Further understanding of these mechanisms may lead to the development of therapeutic strategies to reduce morbidity and mortality from right heart failure.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported in part by National Institutes of Health (R01HL67340, R01HL72844 and R01HL97514) and American Heart Association (0855337E) to YJS.
Footnotes
Conflict of Interest/Disclosure
None.
References
- 1.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 2.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and-6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 3.Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem. 2001;276:30245–30253. doi: 10.1074/jbc.M102174200. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–1531. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hautala N, Tokola H, Luodonpää M, Puhakka J, Romppanen H, Vuolteenaho O, Ruskoaho H. Pressure overload increases GATA4 binding activity via endothelin-1. Circulation. 2001;103:730–735. doi: 10.1161/01.cir.103.5.730. [DOI] [PubMed] [Google Scholar]
- 7.Bär H, Kreuzer J, Cojoc A, Jahn L. Upregulation of embryonic transcription factors in right ventricular hypertrophy. Basic Res Cardiol. 2003;98:285–294. doi: 10.1007/s00395-003-0410-2. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Taegtmeyer H, Adrogue J, Razeghi P, Sen S, Ngumbela K, Essop MF. Dynamic changes of gene expression in hypoxia-induced right ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2004;286:H1185–H1192. doi: 10.1152/ajpheart.00916.2003. [DOI] [PubMed] [Google Scholar]
- 9.Kitta K, Clément SA, Remeika J, Blumberg JB, Suzuki YJ. Endothelin-1 induces phosphorylation of GATA-4 transcription factor in the HL-1 atrial-muscle cell line. Biochem J. 2001;359:375–380. doi: 10.1042/0264-6021:3590375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto T, Hasegawa K, Kaburagi S, Kakita T, Wada H, Yanazume T, Sasayama S. Phosphorylation of GATA-4 is involved in alpha 1-adrenergic agonist-responsive transcription of the endothelin-1 gene in cardiac myocytes. J Biol Chem. 2000;275:13721–13726. doi: 10.1074/jbc.275.18.13721. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki YJ, Nagase H, Day RM, Das DK. GATA-4 regulation of myocardial survival in the preconditioned heart. J Mol Cell Cardiol. 2004;37:1195–1203. doi: 10.1016/j.yjmcc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Yanazume T, Hasegawa K, Morimoto T, Kawamura T, Wada H, Matsumori A, Kawase Y, Hirai M, Kita T. Cardiac p300 is involved in myocyte growth with decompensated heart failure. Mol Cell Biol. 2003;23:3593–3606. doi: 10.1128/MCB.23.10.3593-3606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park AM, Nagase H, Vinod Kumar S, Suzuki YJ. Acute intermittent hypoxia activates myocardial cell survival signaling. Am J Physiol Heart Circ Physiol. 2007;292:H751–H757. doi: 10.1152/ajpheart.01016.2006. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki YJ, Nagase H, Wong CM, Kumar SV, Jain V, Park AM, Day RM. Regulation of Bcl-xL expression in lung vascular smooth muscle. Am J Respir Cell Mol Biol. 2007;36:678–687. doi: 10.1165/rcmb.2006-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabath DE, Koehler KM, Yang WQ, Phan V, Wilson J. DNA-protein interactions in the proximal zeta-globin promoter: identification of novel CCACCC- and CCAAT-binding proteins. Blood Cells Mol Dis. 1998;24:183–198. doi: 10.1006/bcmd.1998.0185. [DOI] [PubMed] [Google Scholar]
- 17.Wong CM, Cheema AK, Zhang L, Suzuki YJ. Protein carbonylation as a novel mechanism in redox signaling. Circ Res. 2008;102:310–318. doi: 10.1161/CIRCRESAHA.107.159814. [DOI] [PubMed] [Google Scholar]
- 18.Mantovani R. The molecular biology of the CCAAT-binding factor NF-Y. Gene. 1999;239:15–27. doi: 10.1016/s0378-1119(99)00368-6. [DOI] [PubMed] [Google Scholar]
- 19.Balanos GM, Dorrington KL, Robbins PA. Desferrioxamine elevates pulmonary vascular resistance in humans: potential for involvement of HIF-1. J Appl Physiol. 2002;92:2501–2507. doi: 10.1152/japplphysiol.00965.2001. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Marcocci L, Wong CM, Park AM, Suzuki YJ. Serotonin-mediated protein carbonylation in the right heart. Free Radic Biol Med. 2008;45:847–854. doi: 10.1016/j.freeradbiomed.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.