Abstract
Betamethasone is administered to accelerate lung development and improve survival of premature infants, but may be associated with hypertension later in life. In a sheep model of fetal programming resulting from exposure at 80th day of gestation to Betamethasone (Betaexposed), adult sheep at 6–9 months or 1.8 yrs of age have elevated mean arterial pressure (MAP), and attenuated spontaneous baroreflex sensitivity (sBRS) for control of heart rate compared to age-matched controls, associated with imbalances in angiotensin (Ang) II versus Ang-(1–7) tone. At 6 weeks of age, evoked BRS is already low in the Beta-exposed animals. In this study we assessed the potential contribution of the renin-angiotensin system to the impaired sBRS. Female lambs (6 weeks old) with Beta-exposure in utero had similar MAP to control lambs (78±2 vs 77±2 mm Hg, n = 4–5 per group), but lower sBRS (8±1 vs 16±3 ms/mm Hg; p < 0.05) and impaired heart rate variability (HRV). Peripheral AT1 receptor blockade using candesartan lowered MAP in both groups (~10 mm Hg) and improved sBRS and HRV in Beta-exposed lambs to a level similar to control. AT7 receptor blockade by infusion of D-ala Ang-(1–7) (700 ng/kg/min for 45 min) reduced sBRS 46±10 % in Beta-exposed vs in control lambs (p<0.15) and increased MAP in both groups (~6±2 mm Hg). Our data reveal that Beta-exposure impairs sBRS and HRV at a time point preceding the elevation in MAP via mechanisms involving an imbalance in the Ang II/Ang-(1–7) ratio consistent with a progressive loss in Ang-(1–7) function.
Keywords: Hypertension, Baroreflex Sensitivity, Spectral Analysis, lambs, Heart Rate Variability, Fetal Programming, Betamethasone
Introduction
Premature delivery occurs in about 10% of pregnancies and it is the most important cause of prenatal morbidity and mortality1. Survival rates have been greatly improved since the implementation of glucocorticoids use prenatally to accelerate lung maturation2. Betamethasone has been used to improve lung function in premature infants but recent longitudinal studies have shown that prenatal exposure to betamethasone caused an elevation in blood pressure in preterm children at 14 years of age3. Enhance femoral vascular resistance with alteration in responses to multiple dilators and constrictors has been shown in sheep exposed prenatally to betamethasone, which can induce a sustained systemic blood pressure rise4, 5. Investigating the mechanism of the vascular changes and the blood pressure elevation associated with prenatal exposure to betamethasone is of clinical importance in order to better diagnose and possibly protect these children from the consequences of prenatal betamethasone exposure.
The renin-angiotensin system (RAS) plays a vital role in the development of the fetus and alterations in the RAS have been linked to fetal programming-induced elevations in blood pressure in placental insufficiency6, maternal protein deprivation7 and other models of fetal programming8. Shifts in the balance of angiotensin (Ang) II over Ang-(1–7) in the circulating or locally within tissues are associated with impairments in autonomic function that are known risk factors for target organ damage and increased mortality. Recently we showed that exposure of the ovine fetus at 80th day of gestation to synthetic glucocorticoid perturbs normal nephrogenesis and leads to elevated mean arterial blood pressure (MAP) and impaired baroreflex sensitivity (BRS) for the heart rate control and increased left ventricle/ body weight ratio in 2 year old adult sheep. Beta-exposed sheep at 2 years of age exhibited changes in the RAS in many tissues causing a shift favoring Ang II over Ang-(1–7), similar to what happens in other models of hypertension and in aging9, 10. We showed also that in adult sheep the AT1 receptor was involved in mediating these cardiovascular effects, since MAP and BRS were improved by acute AT1 receptor blockade using Candesartan11. Both the increase in MAP and impairment in sBRS were evident as early as 6–8 months of age and the imbalance between Ang II and Ang-(1–7) was evident at that time point12, 13. More recently we reported that lambs at 6 weeks of age have normal resting MAP, but the BRS to phenylephrine-evoked increases in MAP is already reduced by ~50%.
The arterial baroreflex for control of HR is developmentally regulated, in part as a result of differential maturation rates of the parasympathetic and sympathetic branches of the autonomic nervous system. The role of the RAS in modulating cardiovascular homeostasis is greatest soon after birth (during first week) and decreases with postnatal maturation, stabilizing by 6 weeks of age at a level similar to that seen in adult sheep14.
In this study we examined the effect of antenatal betamethsone treatment in lambs at a dose and time similar to the clinical steroid treatment given to mothers expected to have a premature delivery15 on MAP, HR, sBRS, HRV, BPV at this early time point (6 weeks) to determine the contribution of enhanced Ang II actions and/ or Ang-(1–7) deficiency to theautonomic nervous system impairment at a time point that precedes the elevation in blood pressure.
Methods
Mixed-breed, time-dated pregnant sheep obtained from local suppliers were maintained in open pasture with free access to food and water during pregnancy and lactation. Sheep were randomly assigned to two groups: one received two 0.17 mg/kg intramuscular injections of a 1:1 mixture of betamethasone acetate and betamethasone phosphate (Celestone Soluspan, Schering, Kenilworth, NJ), while the other group received two vehicle injections, which contained 3.4 mg of monobasic sodium phosphate, 7.1 mg of dibasic sodium phosphate, 0.1 mg of sodium ethylene diamine tetra acetic acid, and 0.2 mg of benzalkonium chlorine per milliliter. Doses were given 24 h apart at days 80 and 81 of gestation (term is ~ 145 days in our flock). The betamethasone dose given is analogous to that used in human pregnancy. Pregnancy was allowed to continue unimpeded and offspring were born naturally at term. All procedures were approved by the Institutional Animal Care and Use Committee.
Protocol
After delivery, animals were farm raised and at 5 weeks of age the pre-weanling lambs were brought to our AALAC-approved facility with their mothers. Both the lambs and ewes had free access to tap water and were housed with a 12-hour light/dark cycle (lights on 7 am to 7 pm). All lambs in this study were females (n =6 beta and 4 control) and the experiments were performed at ~ 6 weeks of age (42 ± 3 days). All experiments were initiated between 1100 and 1300 h and experiments were conducted in a quiet environment as reported previously16. Lambs were anesthetized with ketamine and isoflurane and catheters were inserted in the femoral artery and vein for blood pressure recording and drug administration. Lambs were housed in large metal cages with their mother after the surgical procedure. Five days after surgery, conscious lambs were put in a hanging sling, to acclimate while blood pressure was recorded. The arterial catheter was connected to pressure transducers and conscious pressure and HR were recorded using BIOPAC acquisition software (version 3.8.1, BIOPAC, Santa Barbara, CA). Digitized MAP and HR were used for the measurements of sBRS (as LFα, HFα, seq UP, seq DOWN and seq TOTAL), HRV (SDRR and rMSSD and LFRRI/HFRRI ratio) and BPV (LFSAP and SDMAP). sBRS, HRV and BPV were calculated by the frequency-domain analysis or sequence-time domain methods as in our previous work11, 17, 18 and reported studies4, 19–21 using analysis software designed for large animals (Nevrokard BRS, Medistar, Ljubljana, Slovenia). Measurements were made before and 45 minutes after AT1 receptor blockade using injection of 0.3 mg/kg (i.v.) Candesartan or Ang- (1–7) receptor blockade using 700 ng/kg/min D-Ala7-Ang-(1–7) continuous infusion over 1 hour. Complete blockade of AT1 receptor was tested using Ang II injection (20 ng/kg) i.v. before Candesartan injection and ~ 60 minutes after Candesartan injection at the end of the experiment. The two experiments were done on separate days.17, 18, 22, 23
Statistical procedures
All measurements were expressed as the mean ± standard error of the mean (SEM). All statistical analyses were performed with GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Two way repeated-measures ANOVA with Student-Newman-Keuls post hoc analysis was used to compare between groups at baseline and before and after Candesartan or D-ala treatment. A Student’s t-test was used to compare variables with only two conditions. The criterion for statistical significance was set at P < 0.05.
Results
Effect of Candesartan and D-ala on mean arterial blood pressure (MAP) and heart rate
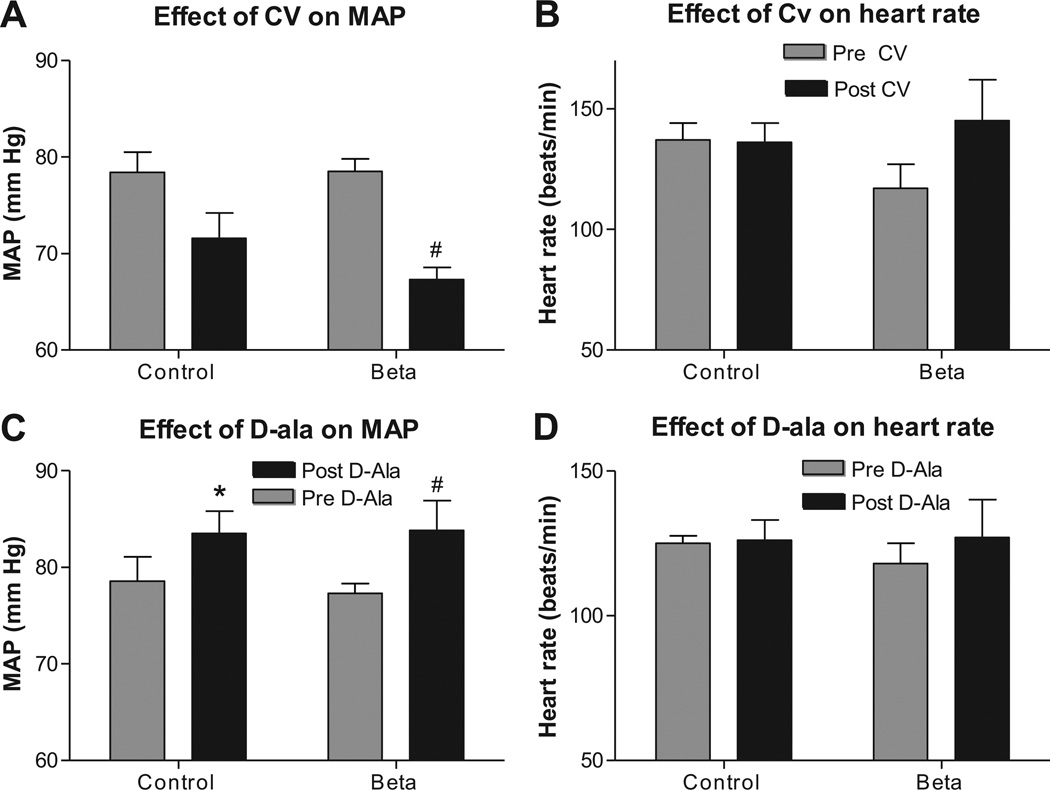
There were no difference in resting MAP or heart rate between Beta-exposed lambs and control lambs at this age. Acute AT1 blockade using Candesartan treatment (45 minutes post injection) lowered MAP in both groups but was statistically significant in the Beta-treated group only (Figure 1A, C) and had no effect on heart rate in either group (Figure 1B, D). Meanwhile, blockade of the Ang-(1–7) receptor (AT7) using D-ala increased blood pressure in both groups (Figure 1C) with no effect on HR (Figure 1D).
Figure 1.
Beta-exposed lambs had similar mean arterial pressure (MAP) compared to control lambs at 6weeks of age. AT1 receptor blockade using Candesartan (CV 11974, 0.3 mg/kg, i.v. injection) lowered MAP in beta treated group, A, while, AT7 blockade using 1 hour infusion of 700 ng/kg/min D-Ala7-Ang-(1–7) increased MAP in both groups, C. There were no effect of Beta-exposure on heart rate at this age and no effect of either of the blockers, B & D. Data are mean ± SEM, * P < 0.05 vs. control at baseline, # P< 0.05 vs Beta-exposed pre Candesartan or D-ala.
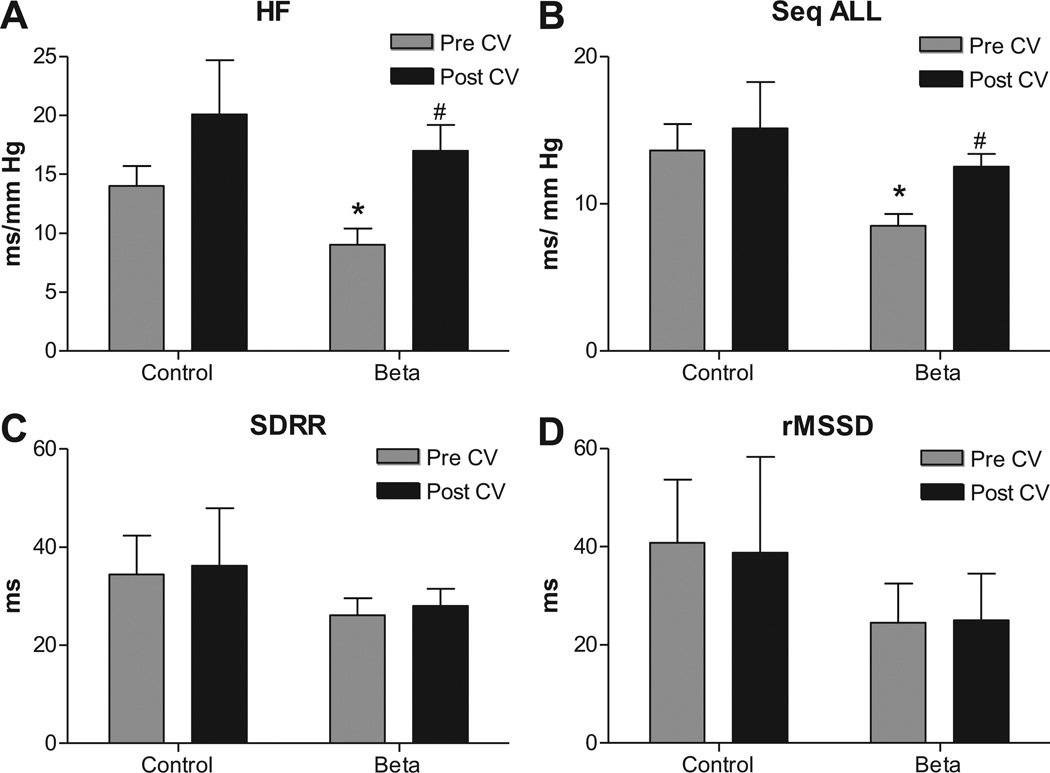
Effect of Candesartan on spontaneous baroreflex sensitivity, heart rate and blood pressure variability
sBRS for heart rate control measured by spectral analysis as HFα (Figure 2A) and via sequence method as Seq-ALL (Figure 2B) (measures for parasympathetic arm) were lower in Beta-exposed lambs on the day of the Candesartan injections. Heart rate variability measured as SDRR (Figure 2C) and rMSSD (Figure 2D) was similar in both groups. Candesartan treatment improved the sBRS in Beta-exposed lambs to values not different from the control lambs at baseline (Figure 2A, B) and had no effect on measures of HRV.
Figure 2.
Antenatal betamethasone exposure was associated with impaired spontaneous baroreflex sensitivity (sBRS) measured by spectral analysis methods as high frequency alpha index (HFα), A; as sequence all (Seq-ALL), B; with no significant change in heart rate variability measured by standard deviation of beat to beat intervals (SDRR), C, and by root of mean successive differences (rMSSD), D. AT1 receptor blockade with Candesartan injection (CV, 0.3 mg/kg) improved baroreflex measures in Beta-exposed lambs to a level that is not different from the control 45 minutes post injection. Data are mean ± SEM, * P < 0.05 vs. Control at baseline, # P< 0.05 vs Beta-exposed pre Candesartan.
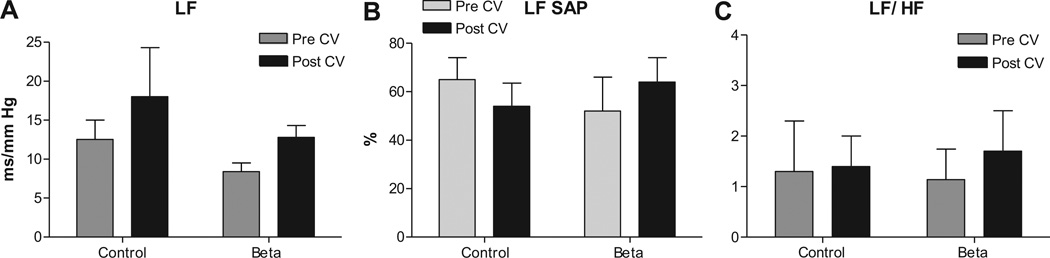
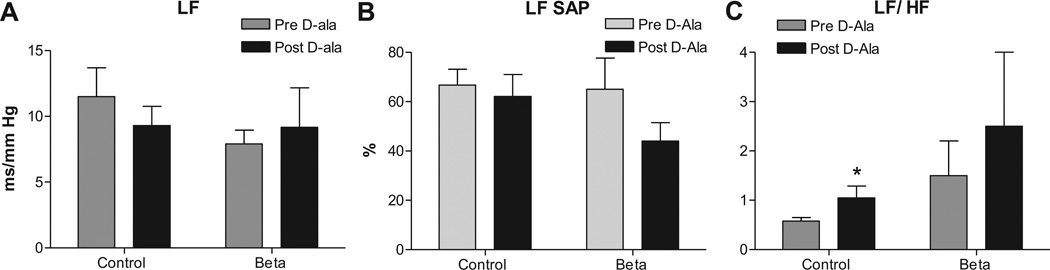
There was no effect of Beta-exposure or Candesartan treatment on sBRS measured as LFα (which is mostly a measure of sympathetic arm of the baroreflex) (Figure 3A). Antenatal betamethasone exposure had no effect on blood pressure variability measured as LFSAP (Figure 3B) and Candesartan injection had no effect on BPV in either groups. LFRRI/HFRRI, a measure of sympathovagal balance, was similar between Beta-exposed lambs and control lambs. Candesartan treatment did not alter this ratio in either group (Figure 3C)
Figure 3.
Antenatal betamethasone exposure had no significant effect on the sympathetic arc of the sBRS measured as low frequency alpha index (LFα), A, and no effect on the blood pressure variability measured as power of the spectral density of systolic arterial pressure in the low frequency range (LFSAP) in normalized units (nu), B. There was no significant effect on the sympathovagal balance measured by LFRRI/HFRRI ratio in this subgroup, C. AT1 receptor blockade with Candesartan (CV, 0.3 mg/kg) had no effect on these parameters in control or Beta-exposed lambs. Data are mean ± SEM, * P < 0.05 vs. Control at baseline.
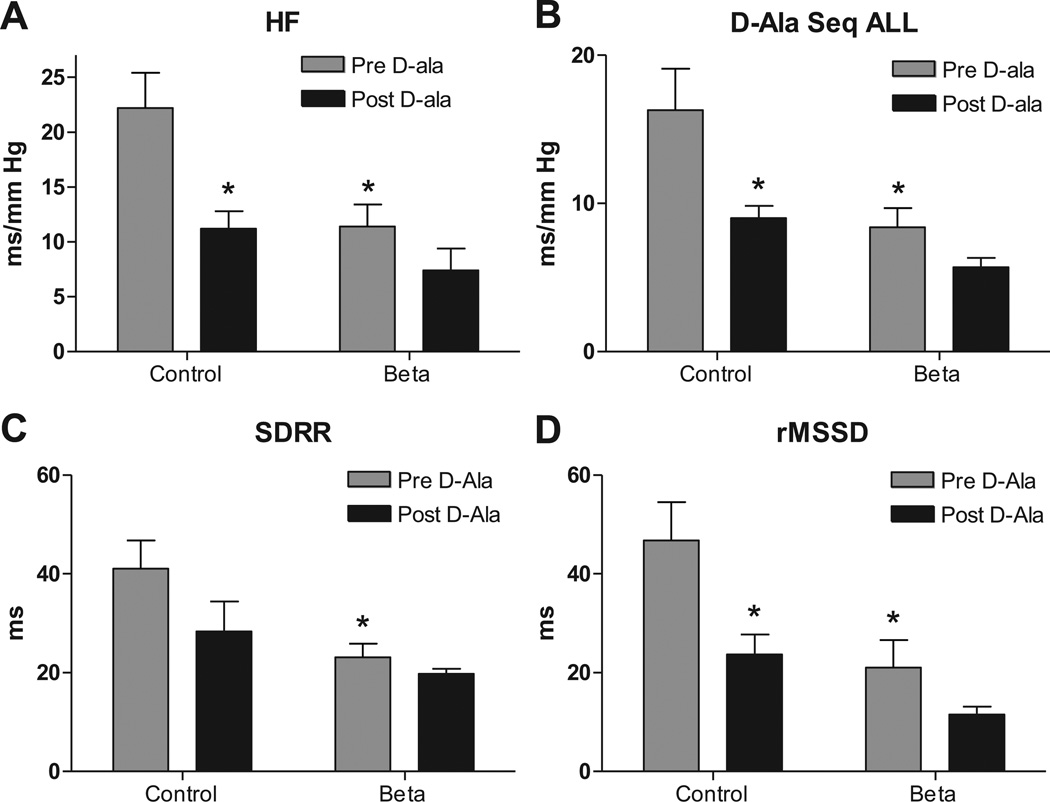
Effect of D-ala on baroreflex sensitivity, heart rate and blood pressure variability
sBRS for heart rate control measured by spectral analysis as HFα (Figure 4A) and via sequence method as Seq-ALL (Figure 4B) (which are measures for parasympathetic arm) were lower in Beta-exposed lambs on the day of the experiment for the D-ala infusions, again indicating impairment in the central control of the circulation. Heart rate variability measured as either SDRR (Figure 4C) or rMSSD (Figure 4D) was lower in Beta-exposed lambs on this treatment day. D-ala treatment impaired all 4 measures of parasympathetic activity in control lambs to values not different from the Beta-exposed lambs baseline level (Figure 4). There was no further impairment by D-ala treatment in the Beta-exposed animals.
Figure 4.
Antenatal betamethasone exposure was associated with impaired spontaneous baroreflex sensitivity measured as high frequency alpha index (HFα), A; and as sequence all (Seq-ALL), B; and lower heart rate variability measured by standard deviation of beat to beat intervals (SDRR), C, and by root of mean successive differences (rMSSD), D. AT7 receptor blockade with 700 ng/kg/min D-Ala7-Ang-(1–7) infusion impaired all 4 measures of parasympathetic function in control lambs to a level that is not different from Beta-exposed lambs at baseline. There was no significant effect for the D-ala infusion on these measures in the Beta-exposed lambs. Data are mean ± SEM, * P < 0.05 vs. Control at baseline.
There was no effect of either Beta-exposure or D-ala treatment on baroreflex measured as LFα (sympathetic arm) (Figure 5A). Antenatal betamethasone exposure had no significant effect on BPV at this time point measured as LFSAP (Figure 5B) and D-ala infusion had no effect in either group. LFRRI/HFRRI, a measure of sympathovagal balance, was similar between the two groups and D-ala treatment increased this ratio in control lambs to a value not different from the Beta-exposed lambs (Figure 5C).
Figure 5.
Antenatal betamethasone exposure had no significant effect on the sympathetic arc of the baroreflex sensitivity measured as low frequency alpha index (LFα), A, and no effect on the blood pressure variability measured as power of the spectral density of systolic arterial pressure in the low frequency range (LFSAP) in normalized units (nu), B. The sympathovagal balance measured by LFRRI/HFRRI ratio was similar in both groups at baseline, C. AT7 receptor blockade with 700 ng/kg/min D-Ala7-Ang-(1–7) infusion had no effect on LFα or LFSAP and it increased LF/HF ratio in control lambs. Data are mean ± SEM, * P < 0.05 vs. Control at baseline.
Discussion
We have previously shown that antenatal exposure of sheep to betamethasone at a time point developmentally similar to that when infants of mothers threatened of premature delivery are exposed to betamethasone increased MAP and impaired BRS and HRV in adult sheep as early as 6 months of age11, 12. In addition to these cardiovascular changes, the Beta-exposed animals showed an enhanced Ang II action through the AT1 receptor and these changes were corrected by the AT1 receptor blockade11. At 6 months of age, the elevated MAP and impaired BRS for the control of heart rate in Beta-exposed sheep were accompanied by a loss of Ang-(1–7) tone.
The current study examined the effect of antenatal Betamethsone exposure in female lambs on MAP, sBRS, HRV and BPV. We also studied the effect of AT7 blockade in addition to AT1 blockade on the hemodynamic parameters in both control and Beta-exposed lambs. Our data reveal for the first time that Beta-exposure at 80th day of gestation followed by term delivery impairs sBRS and HRV at a time point preceding the elevation in MAP. This is consistent with our previous observations of an impaired BRS in response to phenylephrine-evoked increases in MAP and is accompanied by exaggerated response to several stress-related stimuli at this age, even though resting MAP is not elevated and in the absence of changes in resting ACTH and cortisol levels16. Acute AT1 blockade with Candesartan in these lambs lowered MAP in both groups revealing a contribution of Ang II to resting MAP at this time point. Candesartan also increased sBRS in Beta-exposed lambs with no effect on HRV in either group. These data support the hypothesis that antenatal Beta-exposure is associated with enhanced actions of Ang II via AT1 receptors to impair baroreflex control of heart rate at this early time point. Moreover, these data also suggests that Candesartan actions to lower MAP are independent of the effect on sBRS, since in control lambs Candesartan lowered MAP without altering sBRS but this doesn’t completely rule out the possibility that the improvement in sBRS in Beta-exposed lambs is related to the MAP reduction. Whether the increased “tone” for Ang II via AT1 receptors represents an actual increase in Ang II or AT1 receptors was not investigated in this study.
Meanwhile, interruption of Ang- (1–7) actions via AT7 receptors using D-ala increased MAP in both groups and significantly impaired sBRS and HRV measures in control lambs, illustrating that Ang-(1–7) contributes to both maintenance of resting MAP and autonomic balance at this age. While there was a trend for slight reductions in sBRS and HRV in Beta-exposed lambs suggesting that some Ang- (1–7) tone remains in the Beta-exposed lambs for improving BRS and HRV at this age that is lost as the animals age. Previous data at 6–9 months of age indicate almost total loss of Ang-(1–7) tone for MAP, sBRS and HRV in Beta-exposed sheep. Thus, the progressive loss in Ang-(1–7) function following Beta-exposure may reflect an accelerated age-related decline resulting from this prenatal event24,9.
Considerable evidence25–28 shows that Ang II and Ang-(1–7) act in an opposing manner to regulate parasympathetic control of heart rate, sympathetic control of blood pressure, and that the balance of actions is altered in aging9. We have reported previously an increase in angiotensin converting enzyme activity (ACE) and a reduction in ACE2 activity in proximal tubules isolated from Beta-exposed sheep at 1.8 years of age29, which may shift the balance between Ang II and Ang- (1–7) towards higher Ang II and lower Ang- (1–7) in the kidney and circulation29. This reduction in Ang- (1–7) appears to occur in other tissues such as brain30 and may explain the loss of Ang- (1–7) contribution to facilitate sBRS in the Beta-exposed sheep. The loss of Ang-(1–7) tone for sBRS and HRV in Beta-exposed sheep at an early time point prior to the increase in MAP, also argues that these phenomena may be at least one mechanism contributing to the increase in MAP.
Perspectives
Numerous studies indicate that impaired sBRS and HRV are associated with elevated supine MAP, cardiac hypertrophy and increased risk of stroke31, prior to and without frank hypertension. Antenatal betamethasone exposure impairs sBRS for control of heart rate and reduces HRV before the elevation of MAP in lambs associated with a progressive loss in Ang-(1–7) function as a potential mechanism for the autonomic imbalance. These findings are relevant to the follow-up of children exposed prenatally to steroids in order to detect changes in cardiovascular control systems that may precede any elevation of MAP.
Many epidemiological studies have shown that the intrauterine environment is extremely important in determining the health of the individual later in life and that perturbations at critical points during development can lead to long lasting programming effects including the development of cardiovascular diseases32. Preterm glucocorticoid administration confers a distinct prognostic advantage upon infants delivered before full gestation and is an established method to reduce neonatal mortality and morbidity2. While prematurity alone is associated with reduced nephron number and increased risk of cardiometabolic disease, it is not clear whether the exposure to antenatal steroids mitigates or aggravates the risk factors for early onset cardiovascular problems over the long term. Indeed, emerging evidence suggests that antenatal administration of glucocorticoids is associated with alteration in the development of the fetus and results in elevated mean arterial pressure (MAP) during the adolescent years33 and disturbances of metabolism in young adults19 as well as in various models of fetal programming4, 8, 34–40. Studies at earlier ages (~6 years old) indicate no difference in MAP between steroid-exposed and non-exposed groups41, consistent with our observations here in lambs.
Because antenatal steroids provide such distinct advantages postnatally, current guidelines support their use in threatened premature delivery. Thus, the incidence of steroid exposure in the last trimester of gestation in human subjects who are threatened with premature delivery and subsequently proceed to full term has increased42. Therefore, the question of whether in the absence of prematurity, Beta-exposure has negative consequences is also of increasing importance and will help provide information relevant to the increasing number of human subjects exposed to steroids and delivered at full term42.
Acknowledgment
Dr. Hossam A. Shaltout is currently a faculty member in the Department of Pharmacology and Toxicology, School of Pharmacy, University of Alexandria, Egypt.
Sources of Funding
National Institue of Health Grants HD-47584, HD-17644, HL-56973, HL-51952
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest / Disclosure
None
References
- 1.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 2.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 2000;98:137–142. [PubMed] [Google Scholar]
- 4.Anwar MA, Schwab M, Poston L, Nathanielsz PW. Betamethasone-mediated vascular dysfunction and changes in hematological profile in the ovine fetus. Am J Physiol. 1999;276:H1137–H1143. doi: 10.1152/ajpheart.1999.276.4.H1137. [DOI] [PubMed] [Google Scholar]
- 5.Derks JB, Giussani DA, Jenkins SL, Wentworth RA, Visser GH, Padbury JF, Nathanielsz PW. A comparative study of cardiovascular, endocrine and behavioural effects of betamethasone and dexamethasone administration to fetal sheep. J Physiol. 1997;499(Pt 1):217–226. doi: 10.1113/jphysiol.1997.sp021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang DY, Lumbers ER, Simonetta G, Wu JJ, Owens JA, Robinson JS, McMillen IC. Effects of placental insufficiency on the ovine fetal renin-angiotensin system. Exp Physiol. 2000;85:79–84. [PubMed] [Google Scholar]
- 7.Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol. 2001;16:417–422. doi: 10.1007/s004670000560. [DOI] [PubMed] [Google Scholar]
- 8.Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1–R10. doi: 10.1152/ajpregu.00417.2005. [DOI] [PubMed] [Google Scholar]
- 9.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1–7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- 10.Diz DI, Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Averill DB. Angiotensin-(1–7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol. 2008;51:542–548. doi: 10.1097/FJC.0b013e3181734a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaltout HA, Rose JC, Figueroa JP, Chappell MC, Diz DI, Averill DB. Acute AT(1)-receptor blockade reverses the hemodynamic and baroreflex impairment in adult sheep exposed to antenatal betamethasone. Am J Physiol Heart Circ Physiol. 2010;299:H541–H547. doi: 10.1152/ajpheart.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaltout HA, Figueroa JP, Rose JC, Chappell MC, Averill DB, Diz DI. Deleterious Cardiovascular Effects of Antenatal Betamethasone Exposure in Young and Adult Sheep. FASEB J. 2008;22:1129–1116. [Google Scholar]
- 13.Shaltout HA, Figueroa JP, Rose JC, Chappell MC, Averill DB, Diz DI. Evidence of Ang-(1–7) deficiency in antenatal betamethasone-treated young adult sheep. Hypertension. 2008;52:E107–E107. [Google Scholar]
- 14.Monument MJ, Smith FG. Age-dependent effects of captopril on the arterial baroreflex control of heart rate in conscious lambs. Experimental Physiology. 2003;88:761–768. doi: 10.1113/eph8802602. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatric Research. 2005;58:510–515. doi: 10.1203/01.PDR.0000179410.57947.88. [DOI] [PubMed] [Google Scholar]
- 16.Shaltout HA, Chappell MC, Rose JC, Diz DI. Exaggerated sympathetic mediated responses to behavioral or pharmacological challenges following antenatal betamethasone exposure. American Journal of Physiology-Endocrinology and Metabolism. 2011;300:E979–E985. doi: 10.1152/ajpendo.00636.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaltout HA, Abdel-Rahman AA. Mechanism of fatty acids induced suppression of cardiovascular reflexes in rats. J Pharmacol Exp Ther. 2005;314:1328–1337. doi: 10.1124/jpet.105.086314. [DOI] [PubMed] [Google Scholar]
- 18.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension. 2009;54:1001–1008. doi: 10.1161/HYPERTENSIONAHA.109.138065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, Harding JE. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. The Lancet. 2005;365:1856–1862. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 20.Laitinen T, Hartikainen J, Niskanen L, Geelen G, Lansimies E. Sympathovagal balance is major determinant of short-term blood pressure variability in healthy subjects. Am J Physiol. 1999;276:H1245–H1252. doi: 10.1152/ajpheart.1999.276.4.H1245. [DOI] [PubMed] [Google Scholar]
- 21.Parati G, Frattola A, Di Rienzo M, Castiglioni P, Pedotti A, Mancia G. Effects of aging on 24-h dynamic baroreceptor control of heart rate in ambulant subjects. Am J Physiol. 1995;268:H1606–H1612. doi: 10.1152/ajpheart.1995.268.4.H1606. [DOI] [PubMed] [Google Scholar]
- 22.Sgoifo A, de Boer SF, Westenbroek C, Maes FW, Beldhuis H, Suzuki T, Koolhaas JM. Incidence of arrhythmias and heart rate variability in wild-type rats exposed to social stress. Am J Physiol. 1997;273:H1754–H1760. doi: 10.1152/ajpheart.1997.273.4.H1754. [DOI] [PubMed] [Google Scholar]
- 23.Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994;127:1376–1381. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 24.Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1–7)-Angiotensin-Converting Enzyme 2 Attenuates Reactive Oxygen Species Formation to Angiotensin II Within the Cell Nucleus. Hypertension. 2010;55:166–171. doi: 10.1161/HYPERTENSIONAHA.109.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campagnole-Santos MJ, Diz DI, Santos RAS, Khosla MC, Brosnihan KB, Ferrario CM. Cardiovascular effects of angiotensin-(1–7) injected into the dorsal medulla of rats. American Journal of Physiology. 1989;257:H324–H329. doi: 10.1152/ajpheart.1989.257.1.H324. [DOI] [PubMed] [Google Scholar]
- 26.Campagnole-Santos MJ, Diz DI, Ferrario CM. Baroreceptor reflex modulation by angiotensin II at the nucleus tractus solitarii. Hypertension (Supplement I) 1988;11:I-167–I-171. doi: 10.1161/01.hyp.11.2_pt_2.i167. [DOI] [PubMed] [Google Scholar]
- 27.Muratani H, Averill DB, Ferrario CM. Effect of angiotensin II in the ventrolateral medulla of spontaneously hypertensive rats. American Journal of Physiology. 1991;260:R977–R984. doi: 10.1152/ajpregu.1991.260.5.R977. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1 receptors of the nucleus of the solitary tract to attenuate the baroreceptor reflex. American Journal of Physiology. 1998;275:R1611–R1619. doi: 10.1152/ajpregu.1998.275.5.R1611. [DOI] [PubMed] [Google Scholar]
- 29.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53:404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaltout HA, Rose JC, Chappell MC, Averill DB, Diz DI. Modulation of baroreflex sensitivity by endogenous angiotensins in lamb solitary tract nucleus. FASEB J. 2010;24:624.627-. [Google Scholar]
- 31.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 32.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 33.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 2000;98:137–142. [PubMed] [Google Scholar]
- 34.Roghair RD, Lamb FS, Bedell KA, Smith OM, Scholz TD, Segar JL. Late-gestation betamethasone enhances coronary artery responsiveness to angiotensin II in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;286:R80–R88. doi: 10.1152/ajpregu.00421.2003. [DOI] [PubMed] [Google Scholar]
- 35.Schwab M, Roedel M, Anwar MA, Muller T, Schubert H, Buchwalder LF, Walter B, Nathalielsz W. Effects of betamethasone administration to the fetal sheep in late gestation on fetal cerebral blood flow. J Physiol. 2000;528:619–632. doi: 10.1111/j.1469-7793.2000.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newnham JP. Is prenatal glucocorticoid administration another origin of adult disease? Clin Exp Pharmacol Physiol. 2001;28:957–961. doi: 10.1046/j.1440-1681.2001.03559.x. [DOI] [PubMed] [Google Scholar]
- 37.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 39.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- 40.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol. 2005;289:R955–R962. doi: 10.1152/ajpregu.00455.2004. [DOI] [PubMed] [Google Scholar]
- 41.Dalziel SR, Liang A, Parag V, Rodgers A, Harding JE. Blood pressure at 6 years of age after prenatal exposure to betamethasone: follow-up results of a randomized, controlled trial. Pediatrics. 2004;114:e373–e377. doi: 10.1542/peds.2004-0196. [DOI] [PubMed] [Google Scholar]
- 42.Guinn DA, Atkinson MW, Sullivan L, Lee M, MacGregor S, Parilla BV, Davies J, Hanlon-Lundberg K, Simpson L, Stone J, Wing D, Ogasawara K, Muraskas J. Single vs weekly courses of antenatal corticosteroids for women at risk of preterm delivery: A randomized controlled trial. JAMA. 2001;286:1581–1587. doi: 10.1001/jama.286.13.1581. [DOI] [PubMed] [Google Scholar]