Abstract
The stromal-vascular fraction (SVF) of adipose tissue is a rich source of multipotent stem cells. We and others have described 3 major populations of stem/progenitor cells in this fraction, all closely associated with small blood vessels: endothelial progenitor cells (EPC, CD45−/CD31+/CD34+), pericytes (CD45−/CD31−/CD146+) and supra-adventitial adipose stromal cells (SA-ASC, CD45−/CD31−/CD146−/CD34+). EPC are luminal, pericytes are adventitial and SA-ASC surround the vessel like a sheath.
The multipotency of the pericytes and SA-ASC compartments is strikingly similar to that of CD45−/CD34−/CD73+/CD105+/CD90+ bone marrow-derived mesenchymal stem cells (BM-MSC). Here we determine the extent to which this mesenchymal expression pattern is expressed on the 3 adipose stem/progenitor populations. Eight independent adipose tissue samples were analyzed in a single tube (CD105-FITC/CD73-PE/CD146-PETXR/CD14-PECY5/CD33-PECY5/CD235A-PECY5/CD31-PECY7/CD90-APC/CD34-A700/CD45-APCCY7/DAPI).
Adipose EPC were highly proliferative with 14.3±2.8% (mean ± SEM) having >2N DNA. About half (53.1±7.6%) coexpressed CD73 and CD105, and 71.9±7.4% expressed CD90. Pericytes were less proliferative (8.2±3.4% >2N DNA) with a smaller proportion (29.6±6.9% CD73+/CD105+, 60.5±10.2% CD90+) expressing mesenchymal associated markers. However, the CD34+ subset of CD146+ pericytes, were both highly proliferative (15.1±3.6% with >2N DNA) and of uniform mesenchymal phenotype (93.3±3.7% CD73+/CD105+, 97.8±0.7% CD90+), suggesting transit amplifying progenitor cells. SA-ASC were the least proliferative (3.7 ± 0.8%>2N DNA) but were also highly mesenchymal in phenotype (94.4±3.2% CD73+/CD105+, 95.5±1.2% CD90+).
These data imply a progenitor/progeny relationship between pericytes and SA-ASC, the most mesenchymal of SVF cells. Despite phenotypic and functional similarities to BM-MSC, SA-ASC are distinguished by CD34 expression.
Keywords: multiparameter flow cytometry, adipose stromal vascular fraction, mesenchymal stem cells, endothelial progenitor cells, perivascular cells, pericytes, immunofluorescent microscopy, supra-adventitial adipose stromal cells
Introduction
Adipose tissue is a rich source of multipotential stem cells, which are associated with small vessels and are easily recovered by mechanical and enzymatic digestion in a stromal-vascular fraction (1,2). Studies on whole adipose have revealed that the stem/progenitor components, organized around small vessels in an annular fashion, are dominated by a prevalent supra-adventitial layer of CD34+ cells of mesenchymal stromal cell (MSC)-like multipotentiality (1,3,4). These supra-adventitial adipose stromal cells (SA-ASC) surround arterioles and venules, which are colonized on their surfaces by CD146+ perivascular cells or pericytes (1,5). A CD34+/CD31+ endothelial progenitor component is associated with the luminal layer, which in adipose small vessels is proliferative. As a heterogeneous mixture, the stromal-vascular fraction is capable of differentiating in vitro along the mesodermal lineages (6–11) and forming vessel like tubules of CD31+/von Willebrand factor (vWF)+ cells in close association with α–smooth muscle actin (α–SMA) positive cells (11). In clinical application, the adipose stromal vascular fraction has been used to augment autologous lipoaspirate transfers for soft tissue reconstruction (12–16), under the premise that enrichment of stromal vascular cells will promote graft retention by promoting vascularization and adipogenesis (15,17,18). Since the plastic-adherent fraction of adipose stromal vascular cells, often referred to as adipose-derived stromal cells (ASC), resembles bone marrow derived culture-expanded MSC (7,11,19), the present study was designed to investigate the expression of MSC-associated markers on the discrete stem/progenitor subpopulations which comprise freshly isolated adipose stromal vascular cells.
Methods
Adipose samples
Subcutaneous adipose tissue was harvested during elective abdominoplasty from human adult female patients at Magee Womens Hospital (n = 8). All tissues were waste materials collected as a byproduct of surgery. De-identified samples were collected under an IRB approved exemption (number 0511186, University of Pittsburgh IRB). Samples were immediately transported to the laboratory and processed on receipt.
Immunofluorescence
Adipose samples were prepared as previously described (1). Small (~1 cm3) pieces of freshly harvested adipose tissue were washed in a solution of 15% sucrose (Sigma, St. Louis, MO) in Phosphate Buffer Saline (PBS), immersed in PBS, 7.5% gelatin (Sigma), 15% sucrose (Sigma) and held for at least 2 hours at 4°C, before being frozen by repetitive immersion in liquid nitrogen-cooled 2-methylbutane (Fisher Scientific, Fair Lawn, NJ). Sections (6 to 8 µm) were cut on a Microm cryostat, fixed for 5 min in ice cold acetone (Fisher Scientific) and stored at −80°C. Before staining, sections were dried at ambient temperature and post-fixed for 5 min in ice cold acetone. Tissue rehydration and all subsequent washes were performed by two 5-min incubations in Dako Wash Buffer (Dako, Carpinteria, CA). All incubations were completed at ambient temperature.
Rehydrated tissue sections were pretreated for 1hr at room temperature with goat serum blocking solution (5% goat serum (Sigma), 0.05% Tween 20 (Dako) in PBS) to prevent nonspecific secondary antibody binding. Specimens were then incubated with prediluted primary unconjugated antibodies for 1 hr at ambient temperature in a humid atmosphere. Washed sections were sequentially incubated for 1 hr with biotinylated secondary goat anti-mouse antibody (Dako, Cat No. E0433, 1:500, 1.58 µg/ml final concentration) and for 30 min with streptavidin-Cy3 (Sigma, Cat No. S6402, 1:500, 2 µg/mL final concentration). Tissue sections were washed twice and incubated for 1 hour at ambient temperature with either sheep anti-human FITC-conjugated vWF (1:100, US Biological, Swanpscott, MA, Cat No. V2700-01C), mouse anti-human FITC-conjugated α-SMA (1:100, Sigma, Cat No. F3777,, clone 1A4) or mouse anti-human FITC-conjugated CD146 (1:20, AbD Serotec, Raleigh, NC, Cat No. MCA2141F) antibodies. Nuclear staining was attained through 5-min incubation with 300 nM 4',6-diamidino-2-phenylindole (DAPI, Invitrogen, Carlsbad, CA, Cat. No. D1306). Slides were mounted in 1:1 PBS / glycerol (Sigma) or Prolong Gold anti-fade reagent (Invitrogen) and observed under an epi-fluorescence microscope (Nikon Eclipse TE 2000-U).
Primary antibodies used in these studies were mouse anti-human CD90 (1:10, BD Biosciences, San Jose, CA, Cat No. 550402, clone 5E10) and mouse anti-human CD73 (1:100, Invitrogen, Cat No. 41-0200). All antibodies were diluted in goat serum blocking solution. Primary antibodies were replaced by Universal Negative Control for Mouse Primary Antibodies (Ready to use, Dako cat. No. N1698) for negative controls.
Flow Cytometry
Single cell suspensions were prepared from whole fat tissue and lipoaspirates as previously described (1,20). Briefly, fat tissue was thoroughly minced with scissors. The aqueous portion of lipoaspirate was removed by aspiration after centrifugation. The resulting fatty tissue was digested for 30 min in Hanks' Balanced Salt Solution (HBSS, Invitrogen) containing 3.5% Bovine Serum Albumin (BSA, Millipore, Charlottesville, VA) and 1 mg/mL collagenase type II (Worthington, Lakewood, NJ, USA) on a shaking water bath at 37°C, and finally disaggregated through successive 425 µm and 180 µm sieves (W.S. Tyler, Mentor, OH). Mature adipocytes were eliminated by centrifugation (400 g, ambient temperature, 10 min) and cell pellets were resuspended in NH4Cl-based erythrocyte lysis buffer (Beckman Coulter, Miami, FL, Cat No. IM3630d), incubated for 10 min at ambient temperature and washed in PBS. Some samples were depleted of debris on a Ficoll-Hypaque density gradient (Histopaque®-1077, Sigma).
Freshly isolated cells from the SVF were maintained from then on ice and stained for analytical flow cytometry as previously described (21). Cell suspensions were centrifuged (200 g, 7 min) and the cell pellet was preincubated with 5 µL neat mouse serum (Sigma) to minimize non-specific antibody binding. Cells were simultaneously stained with monoclonal mouse anti-human antibodies (CD105-FITC (Fitzgerald, Acton, MA, Cat No. 61R-CD105-DHUFT), CD73-PE (BD Biosciences, Cat No. 550257), CD146-biotin (Miltenyi Biotec, Auburn, CA, Cat No. 130-092-852), CD14-PE-Cy5 (Beckman Coulter, Cat No. IM2640U), CD33-PE-Cy5 (Beckman Coulter, Cat No. IM2647U), CD235a-PE-Cy5 (Glycophorin A, BD Biosciences, Cat No. 559944), CD31-PE-Cy7 (Biolegend, San Diego, CA, Cat No. 303117), CD90-APC (BD Biosciences, Cat No. 559869), CD34 APC-Alexa 700 (Beckman Coulter, Cat No. A86354) and CD45-APC-Cy7 (BD Biosciences, Cat No. 348805), 2 µL each, on ice), washed and incubated with streptavidin- PE-Texas Red (ECD, Beckman Coulter, Cat No. IM3326).
Analytical samples were fixed with 2% methanol-free formaldehyde (Polysciences, Inc., Warrington, PA), permeabilized in PBS with 0.1% saponin (Coulter), 0.5% BSA for 10 min at ambient temperature and incubated with 7.7 µg/mL DAPI. Nine color, 14-parameter data files were acquired on a three-laser Gallios cytometer (Beckman Coulter) at a maximum of 10,000 events per second. Threshold was set on DAPI fluorescence to exclude subcellular debris and up to 1.8 million events were acquired per sample. The DAPI signal was acquired independently on two violet channels optimizing voltage settings of two individual photomultiplier tubes for removal of debris and cell cycle analysis. For compensation purposes, BD Calibrite™ beads (BD Biosciences) and single antibody-stained mouse IgG capture beads (BD Biosciences) were acquired for single fluorochromes (FITC, PE, APC) and tandem-dyes (ECD, PE-Cy5, PE-Cy7 and APC-Cy7), respectively. Initially, regions and gates were set with the aid of “fluorescence minus one” isotype controls. Offline compensation and analysis were performed using the high throughput parallel processing VenturiOne software (Applied Cytometry, Sheffield, UK). Fourteen independent samples were analyzed. Summarized results are given as arithmetic means ± SEM to indicate the precision with which mean values are known (22). A representative data file (shown in figure 2) and the associated single stained control files are included in the online the FloData Repository.
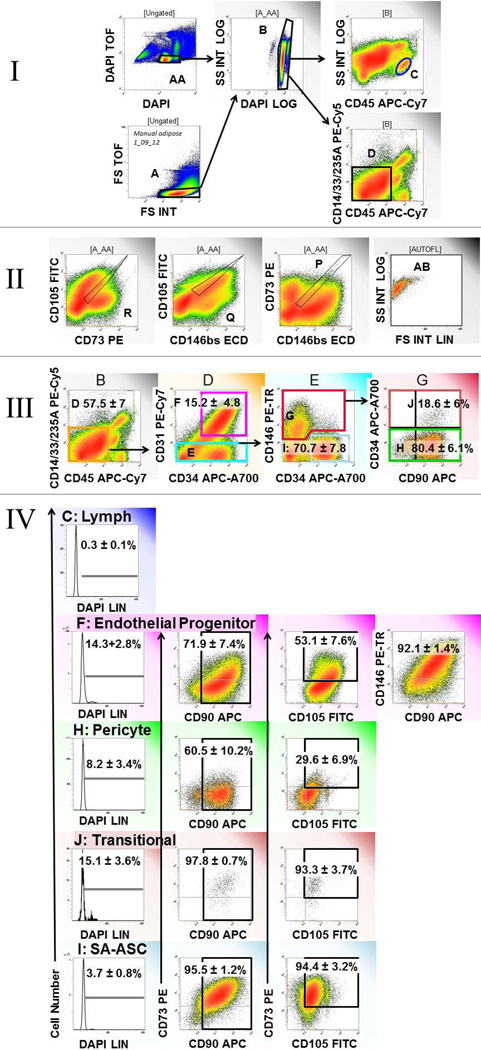
Figure 2.
Expression of mesenchymal stem cell markers on four stem/progenitor populations of the adipose stromal vascular fraction. I and II show the pregating cleanup strategy used to remove sources of artifact. I: Integral versus time of flight (DAPI, AA and forward light scatter, A) is used to identify singlet cells (A and AA), after which hypodiploid events were eliminated (B). Lymphocytes (C) and nonhematopoietic cells (D) are identified for further analysis. II: Autofluorescent cells (AB), identified by the logical gate P and Q and R, are removed from analysis. III: Four stem/progenitor populations are identified within clean non-hematopoietic cells. IV: The proportion of cycling cells (DNA >2N) is determined for lymphocytes and the four stem/progenitor populations: Endothelial progenitors (F), Pericytes (H), Transitional cells (J) and SA-ASC (I). IV: DNA profile and coexpression of mesenchymal associated markers CD90, CD73 and CD105 are shown for the four stem/progenitor populations. The lymphocyte DNA profile is shown for comparison (IV C). All fluorescence variables are scaled identically (4 decade), except DAPI vs DAPI TOF (2 log scale). A representative sample is shown; region statistics tabulate the mean and standard error of the mean for all eight samples analyzed in this series.
Results
Expression of mesenchymal markers in whole fat
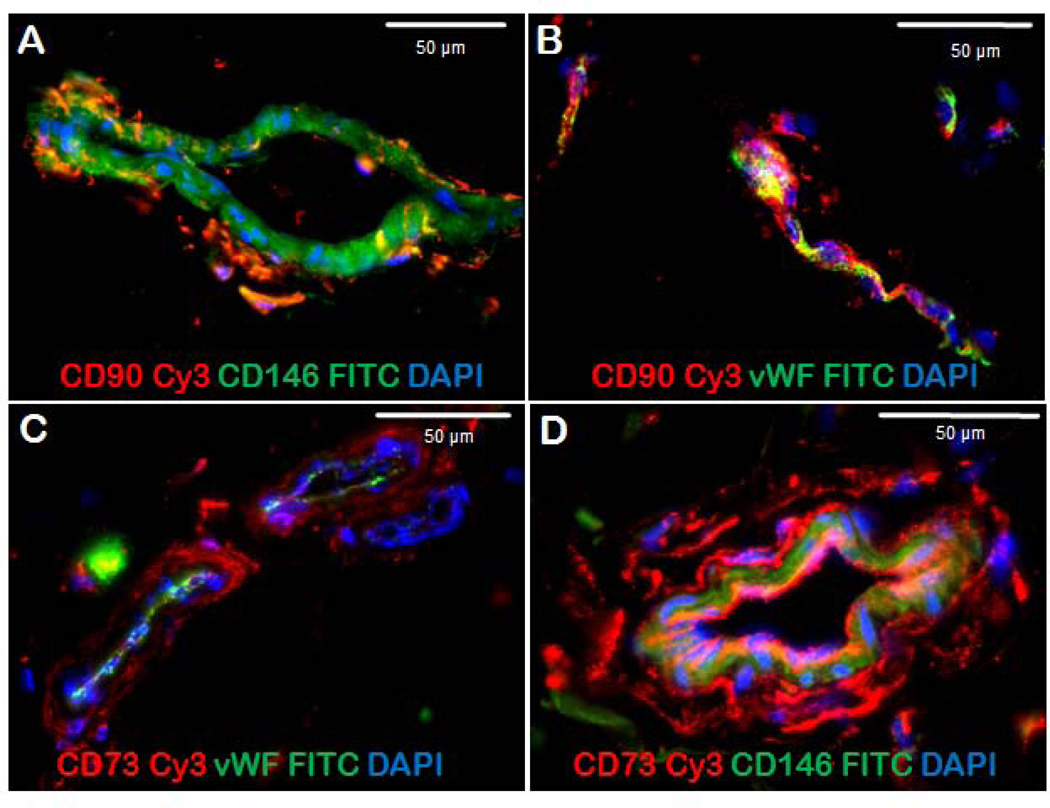
We have previously demonstrated that four distinct stem/progenitor populations are organized around the small vessels of adipose tissue in an annular fashion (1). Luminal endothelial progenitor cells are CD45−/CD31+/CD34+, pericytes (CD45−/CD31−/CD146+) are closely associated with α-SMA+ smooth muscle cells on the surface of the vessels, and supra-adventitial adipose stromal cells (CD45−/CD31−/CD146−/CD34+), sometimes called preadipocytes, surround the vessels like a sheath. The fourth population, detectable by flow cytometry but too rare to be seen by tissue staining, is intermediate between pericytes and SA-ASC, coexpressing CD146 and CD34. Based on its high proliferative activity in vivo, this appears to be a transit amplifying cell (TA) population. Figure 1 illustrates the expression of mesenchymal stem cell associated markers CD90 (Figure 2 A, B) and CD73 (Figure 1 C, D) in an adipose tissue section. Von Willebrand factor (vWF) and α-smooth muscle actin (α-SMA) served as landmarks, the former marking vascular endothelial cells and the latter marking the outer layer of the vessel. Pericytes are CD146+/vWF−. CD90 was coexpressed with vWF on capillary endothelial cells (Figure 1B), but was absent of the lumen of small vessels (Figure 1A). The mesenchymal marker CD73 was detected on the pericytic ring of small vessels, where it is coexpressed with CD146 (Figure 1D). CD90 and CD73 were both detected in the ring surrounding capillaries and small vessels.
Figure 1.
Expression of mesenchymal stromal cell associated markers CD90 and CD73 in an adipose tissue section. Panel A shows an adipose small vessel in cross-section. The lumen is marked by CD146+/CD90− endothelial cells, surrounded by CD146+/CD90+ pericytes. Panel B shows an adipose capillary in longitudinal section. The lumen is marked by Von Willebrand factor (vWF)+ cells; CD90+ cells are distinct and tightly associated with the capillary. Panels C and D show adipose vessels in cross section. vWF+ cells mark the lumen (panel C); two vessels are surrounded by CD73+/vWF− cells. A larger vessel (panel D) shows a discrete supra-adventitial layer of CD73+/CD146− cells.
Multiparameter analysis on single cells
Once the tissue location of key markers is known, multiparameter flow cytometry on disaggregated tissue can be interpreted. The stromal-vascular fraction of human adipose tissue contained: Endothelial progenitors, 15.4 ± 4.8% (mean ± standard error), pericytes 2.0 ± 1.1%, CD146+/CD34+ transitional cells 0.5 ± 0.3, and SA-ASC 59.0 ± 10.0% of nonhematopoietic (CD45−/CD14−/CD33−/glycophorin A−) singlet cells. The expression of mesenchymal markers CD73, CD90 and CD105 on each of the four stem/progenitor populations is tabulated for the entire data set in Figure 2, which illustrates the analytical histograms for a representative sample. The percentages shown within histogram regions tabulate the mean ± standard error for the eight patients studied. Described from luminal surface outward, adipose EPC were highly proliferative with 14.3± 2.8% (mean ± SEM) having >2N DNA. About half (53.1 ±7.6%) coexpressed CD73 and CD105, and 71.9 ± 7.4% expressed CD90. Pericytes were less proliferative (8.2 ± 3.4%) and the least mesenchymal of the four subpopulations (29.6 ± 6.9% CD73+/CD105+, 60.5 ± 10.2% CD90+). However, the CD34+ subset of CD146+ pericytes, hypothesized to be transit amplifying progenitor cells leading to the generation of SA-ASC from pericytes, were both highly proliferative (15.1 ± 3.6%) and uniformly mesenchymal (93.3 ± 3.7 CD73+/CD105+, 97.8 ± 0.7% CD90+). The most prevalent subpopulation, SA-ASC, were the least proliferative (3.7 ± 0.8%) but were also highly mesenchymal (94.4 ± 3.2% CD73+/CD105+, 95.5 ± 1.2% CD90+).
Discussion
Adult stem/progenitor cells, especially those of mesodermal origin, have been the subject of intense work during the last decade, in part because of their proposed use for regenerative and anti-inflammatory therapy (23). In contrast to the hematopoietic system, where the differentiation hierarchy is well established, stromal stem cell biology remains unclear. Mesenchymal stem cells (MSC) (24–26), the prototype for stroma-derived adult stem cells were identified and characterized first in bone marrow and then demonstrated in a variety of other organs (27). The similarities in phenotype, transcriptome and potentiality between pericytes, which populate blood vessel adventitia in both fetal and adult human tissues, and mesenchymal stem cells have been noted (28,29).
Adipose tissue is an attractive source of adult stem cells due to its abundance and surgical accessibility. Adherent fat progenitors were isolated in 1976 by 2 independent groups (30,31). Cultured adipose-derived stem cells (ASC) were later shown to have the ability to differentiate along mesenchymal lineages (2) and express the MSC associated markers CD105 and CD44 (7). Culture expanded ASC, which like bone marrow derived MSC are expanded from plastic-adherent cells, share many properties with MSC. However, their characterization in situ in whole tissue resulted in the identification of several discrete populations organized around small vessels (1,4). We were able to clarify the relationship between these populations by the combined use of immunohistostaining and multiparameter flow cytometry (1), demonstrating the histologic relationship between multipotent stem/progenitor populations (32), and inferring the existence of a highly proliferative transitional population between pericytes and SA-ASC (5). We proposed the term SA-ASC to replace pre-adipocyte or adipose stromal/stem cell, because the latter terms confounded several distinct populations (3,33–36).
Recent studies have concluded, on the basis of retention of unique phenotype profiles in culture, that pericytes and SA-ASC are of distinct origin (37). In the present study we used multidimensional flow cytometry to determine the relationship between adipose stromal populations and classically defined adult MSC. The salient findings were that the mesenchymal phenotype, as determined by the expression of CD73, CD105 and CD90, is expressed on a lower proportion of pericytes compared to SA-ASC which are essentially homogeneously positive with respect to mesenchymal markers. The existence of a rare highly proliferative population intermediate in phenotype between pericytes and SA-ASC, and also homogeneous for mesenchymal marker expression, suggests a transitional population between pericytes and SA-ASC. Interestingly, about half of the CD34+/CD31+ EPC detected in the adipose stromal vascular fraction expressed mesenchymal markers as well as CD146, consistent with a pericytic origin for these cells as well. All of the four populations had a detectable proportion of cells in cycle compared to passenger lymphocytes (Figure 2, IV), indicating the dynamic nature of the stromal vascular fraction of adipose tissue. Since our data are phenotypic and cross-sectional, the developmental relationship between MSC, pericytes, EPCs, and adipose progenitor populations can only be inferred. Conclusive demonstration of the proposed progenitor/progeny relationships will require in vivo tracking studies.
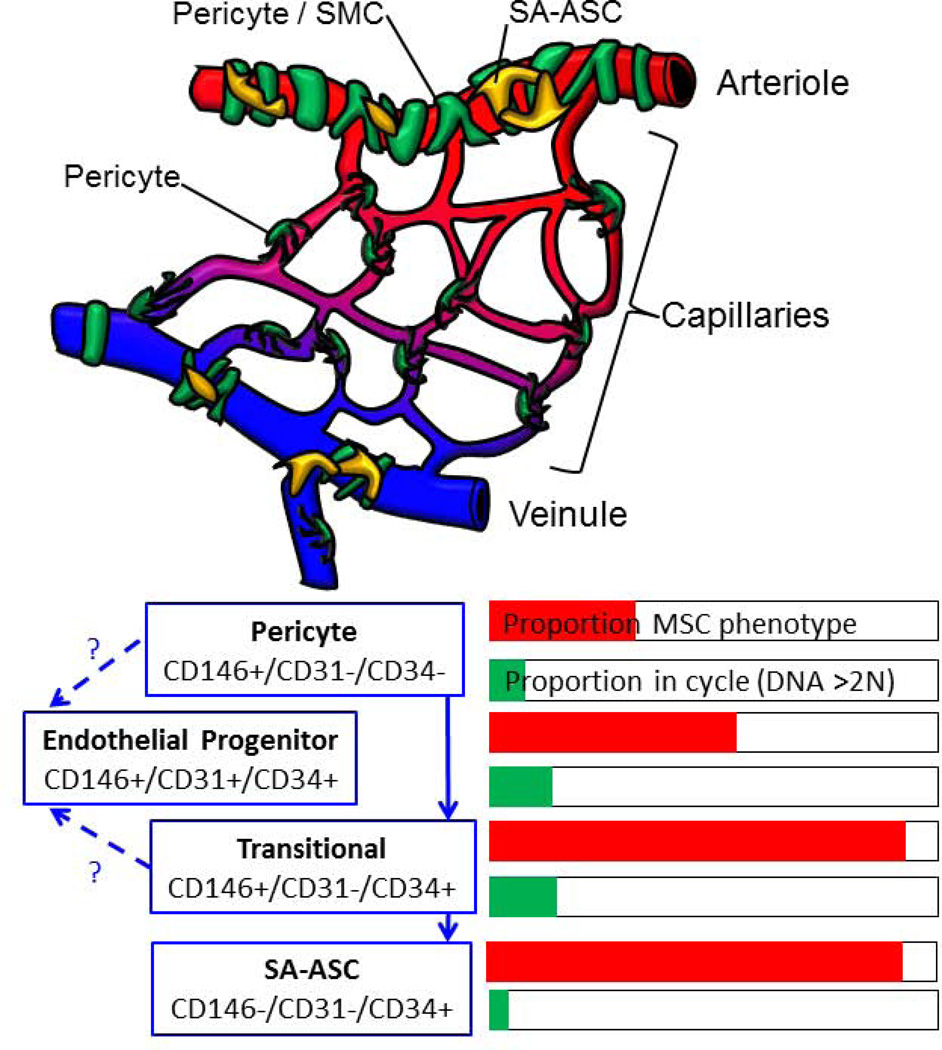
Our working model for SVF differentiation, illustrated in Figure 3, is also supported by recent developmental studies. In the mouse, PPAR+ fat progenitors develop during the first postnatal month from a pool of CD34+ precursors (38). These CD34+ cells proliferate and populate the adventitia of all adipose depot vasculature, where they display a pericytic phenotype (α-SMA, PDGFR-β, NG2), and lack expression of hematopoietic (CD45−) or endothelial (CD31−) markers. The coexpression of CD34 and pericyte markers is maintained in the adult mouse (39). This is reminiscent of the phenotype of perivascular progenitor populations in the human dermis (40), as well as the highly proliferative transitional subpopulation of human adult fat pericytes detected here (Figure 2, IV) (1,5,20).
Figure 3.
Schematic representation of the organization of adipose SVC and a working model of SVF differentiation. The upper figure is a schematic representation drawn from previously published work. Cells associated with the small vessels of adipose tissue are coded as follows: Endothelial cells (red), pericytes and smooth muscle cells (green), and supra-adventitial adipose stromal cells (yellow). The lower diagram is a working model based on the present results. For each cell type defined by phenotype, the bar graphs show the proportion of cells with a mesenchymal phenotype (CD73+/CD105+ as shown in Figure 2) and the proportion of proliferating cells.
Interestingly, while white adipocytes from the head are derived from the neural crest (41), most other fat depots seems to have a distinct, presumably mesodermal, origin (42). Similarly, during embryogenesis, primitive mesenchymogenic cells can arise from both the neural crest and the mesoderm (43,44). Pericytes have been proposed to be the precursors of MSC in both fetal and adult tissues (29). According to in vitro hESC–based models, primitive MSC may express CD34 (45,46), a phenotype shared in vivo by some rare adult bone marrow mesenchymal progenitors (47,48) but also a larger subset of adipose pericytes (5). However, most classical definitions of adult human mesenchymal stem cells include the absence of CD34 expression (26,49).
Recently, multipotent CD34+ mesodermal vasculogenic precursors have been identified using hESC/hiPSC–based methodologies (50,51). These early CD34+ precursors can give rise simultaneously to mesenchymal/pericyte (CD146+/CD31−/CD45−) and endothelial (CD31+) lineages (52,53), through the emergence of a common bipotent precursor, the “mesenchymoangioblast”, which exhibits a mesenchymal (CD105+/CD73+/CD90+) pericytic (CD140a+/CD146+) non-endothelial, non-hematopoietic (CD31−/CD43−/CD45−) phenotype. Notably, mesoangioblasts, a primitive CD34+ population of angiogenic and mesenchymal progenitors, emerge from the mouse dorsal aorta (54) during early embryogenesis and are believed to be the ancestors of postnatal multipotent pericytes. Thus the progression documented in developmental biology is consistent with the concept that adult pericytes may give rise to both EPC and SA-ASC, possibly through a common CD146+/CD34+ intermediate (Figure 3).
Acknowledgements
This work was supported by grants BC032981 and BC044784 from the Department of Defense, grant R01CA 114246 from the NIH, the Hillman Foundation, the Glimmer of Hope Foundation, the Commonwealth of Pennsylvania, through the McGowan Institute of Regenerative Medicine, the NHLBI (Production Assistance for Cellular Therapy (PACT) N01-HB-37165), and the Department of Defense Biomedical Translational Initiative (W911QY-09-C-0209). Vera Donnenberg is a congressionally directed medical research program Era of Hope Scholar. The authors would like to thank Ms. Melanie Pfeifer and James Arbore for their expert technical assistance, Dr. Charles Sfeir for the use of his microscopy facilities, and E. Michael Meyer in the UPCI Cytometry Facility. We would also like to thank Diana Napper from The Glimmer of Hope Foundation for her support. The UPCI Cytometry Facility is supported by CCSG P30CA047904.
References
- 1.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77A:22–30. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 3.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The Tunica Adventitia of Human Arteries and Veins as a Source of Mesenchymal Stem Cells. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmerlin L, Donnenberg VS, Donnenberg AD. Pericytes: a universal adult tissue stem cell? Cytometry A. 2012;81A:12–14. doi: 10.1002/cyto.a.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 8.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 9.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 10.Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, Penicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czerny V. Plastischer Ersatz der Brustdruse durch ein Lipom. Chir Kong Verhandl. 1895;2:216. [Google Scholar]
- 13.Neuber GA. Fettransplantation. Chir Kongr Verhandl Deutsche Gesellschaft für Chirurgie. 1893;22:66. [Google Scholar]
- 14.Yoshimura K, Matsumoto D, Gonda K. A clinical trial of soft tissue augmentation by lipoinjection with adipose-derived stromal cells (ASCs) 2005:9–10. [Google Scholar]
- 15.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48–55. doi: 10.1007/s00266-007-9019-4. discussion 56-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T, Sato T, Aiba-Kojima E, Iizuka F, Inoue K, Suga H, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 17.Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006;118:121S–128S. doi: 10.1097/01.prs.0000234609.74811.2e. [DOI] [PubMed] [Google Scholar]
- 18.Zhu M, Zhou Z, Chen Y, Schreiber R, Ransom JT, Fraser JK, Hedrick MH, Pinkernell K, Kuo HC. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64:222–228. doi: 10.1097/SAP.0b013e31819ae05c. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerlin L, Donnenberg VS, Donnenberg AD. Rare event detection and analysis in flow cytometry: bone marrow mesenchymal stem cells, breast cancer stem/progenitor cells in malignant effusions, and pericytes in disaggregated adipose tissue. Methods Mol Biol. 2011;699:251–273. doi: 10.1007/978-1-61737-950-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnenberg VS, Landreneau RJ, Donnenberg AD. Tumorigenic stem and progenitor cells: Implications for the therapeutic index of anti-cancer agents. J Control Release. 2007;122:385–391. doi: 10.1016/j.jconrel.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnenberg AD. Statistics of Immunological Testing. In: O'Gorman MR, Donnenberg AD, editors. Handbook of Human Immunology. 2nd ed. Boca Raton: CRC Press Taylor and Francis; 2008. pp. 29–62. [Google Scholar]
- 23.Nery AA, Nascimento I, Glaser T, Bassaneze V, Krieger J, Ulrich H. Human mesenchymal stem cells: fromg immunophenotyping by flow cytometry to clinical applications. Cytometry A. 2012 doi: 10.1002/cyto.a.22205. in press. [DOI] [PubMed] [Google Scholar]
- 24.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 25.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 27.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 28.Covas DT, Panepucci RA, Fontes AM, Silva WA, Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146(+) perivascular cells and fibroblasts. Exp Hematol. 2008 doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Van RL, Bayliss CE, Roncari DA. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976;58:699–704. doi: 10.1172/JCI108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dardick I, Poznanski WJ, Waheed I, Setterfield G. Ultrastructural observations on differentiating human preadipocytes cultured in vitro. Tissue Cell. 1976;8:561–571. doi: 10.1016/0040-8166(76)90013-6. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Zimmerlin L, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP. Adipogenic potential of adipose stem cell subpopulations. Plast Reconstr Surg. 2011;128:663–672. doi: 10.1097/PRS.0b013e318221db33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 34.Andersen DC, Schroder HD, Jensen CH. Non-cultured adipose-derived CD45- side population cells are enriched for progenitors that give rise to myofibres in vivo. Exp Cell Res. 2008;314:2951–2964. doi: 10.1016/j.yexcr.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Khan WS, Tew SR, Adesida AB, Hardingham TE. Human infrapatellar fat pad-derived stem cells express the pericyte marker 3G5 and show enhanced chondrogenesis after expansion in fibroblast growth factor-2. Arthritis Res Ther. 2008;10:R74. doi: 10.1186/ar2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 37.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem cells and development. 2012;21:1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 40.Yamanishi H, Fujiwara S, Soma T. Perivascular localization of dermal stem cells in human scalp. Exp Dermatol. 2012;21:78–80. doi: 10.1111/j.1600-0625.2011.01407.x. [DOI] [PubMed] [Google Scholar]
- 41.Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, Kessaris N, Dani C, Dupin E. The generation of adipocytes by the neural crest. Development. 2007;134:2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wrage PC, Tran T, To K, Keefer EW, Ruhn KA, Hong J, Hattangadi S, Trevino I, Tansey MG. The neuro-glial properties of adipose-derived adult stromal (ADAS) cells are not regulated by Notch 1 and are not derived from neural crest lineage. PLoS One. 2008;3:e1453. doi: 10.1371/journal.pone.0001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 44.Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. The Journal of experimental medicine. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopher RA, Penchev VR, Islam MS, Hill KL, Khosla S, Kaufman DS. Human embryonic stem cell-derived CD34+ cells function as MSC progenitor cells. Bone. 2010;47:718–728. doi: 10.1016/j.bone.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbet R, Peiffer I, Hatzfeld A, Charbord P, Hatzfeld JA. Comparison of Gene Expression in Human Embryonic Stem Cells, hESC-Derived Mesenchymal Stem Cells and Human Mesenchymal Stem Cells. Stem cells international. 2011;2011:368192. doi: 10.4061/2011/368192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaiser S, Hackanson B, Follo M, Mehlhorn A, Geiger K, Ihorst G, Kapp U. BM cells giving rise to MSC in culture have a heterogeneous CD34 and CD45 phenotype. Cytotherapy. 2007;9:439–450. doi: 10.1080/14653240701358445. [DOI] [PubMed] [Google Scholar]
- 48.Simmons DJ, Seitz P, Kidder L, Klein GL, Waeltz M, Gundberg CM, Tabuchi C, Yang C, Zhang RW. Partial characterization of rat marrow stromal cells. Calcified tissue international. 1991;48:326–334. doi: 10.1007/BF02556152. [DOI] [PubMed] [Google Scholar]
- 49.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 50.Levenberg S, Ferreira LS, Chen-Konak L, Kraehenbuehl TP, Langer R. Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells. Nat Protoc. 2010;5:1115–1126. doi: 10.1038/nprot.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park SW, Jun Koh Y, Jeon J, Cho YH, Jang MJ, Kang Y, Kim MJ, Choi C, Sook Cho Y, Chung HM, et al. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood. 2010;116:5762–5772. doi: 10.1182/blood-2010-04-280719. [DOI] [PubMed] [Google Scholar]
- 52.Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, Germanguz I, Amit M, Itskovitz-Eldor J. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125:87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- 54.Cossu G, Bianco P. Mesoangioblasts--vascular progenitors for extravascular mesodermal tissues. Current opinion in genetics & development. 2003;13:537–542. doi: 10.1016/j.gde.2003.08.001. [DOI] [PubMed] [Google Scholar]