Abstract
Objective
Describe the clinical effect and safety of low-dose buprenorphine, a kappa-opioid receptor antagonist, for treatment-resistant depression (TRD) in mid-life and older adults.
Method
Using an open-label protocol, buprenorphine was prescribed for 15 adults age 50 and older with TRD, diagnosed with the SCID for DSM-IV, between 6/2010-6/2011. The titrated dose of buprenorphine ranged from 0.2 mg-1.6 mg/day. We assessed clinical change in depression, anxiety, sleep, positive and negative affect, and quality of life. Tolerability was assessed by documenting change in vital signs, weight, cognitive function, and side effects. Clinical response durability was assessed 8 weeks after discontinuation of the buprenorphine.
Results
The average dose of buprenorphine was 0.4 mg/day (average maximum dose = 0.7 mg/day). The average depression score (MADRS) at baseline was 27.0 (SD=7.3); and at week 8, 9.5 (SD=9.5). There was a sharp decline in depression severity during the first three weeks of exposure (mean delta=−15.0 (SD=7.9)). Depression-specific items tapping pessimism and sadness improved during exposure, supporting a true antidepressant effect. Treatment emergent side effects (in particular nausea and constipation) were not sustained, vital signs and weight remained stable, and executive function and learning improved from pre- to post-treatment.
Conclusion
Low-dose buprenorphine may be a novel-mechanism medication that provides a rapid and sustained improvement for older adults with TRD. Placebo-controlled trials of longer duration are required to assess efficacy, safety, and physiological and psychological effects of extended exposure to this medication.
Introduction
Conventional treatment of major depressive disorder (MDD) to complete remission often takes many months and may be associated with persistent depressive symptoms, elevated risk of suicide, dropping out of care, and worsening medical co-morbidities. Over 50% of mid-life and older adults with depression fail to respond to traditional antidepressants (1, 2). Treatment resistant depression (TRD) – syndromal depression that does not respond to standard monoaminergic medications such as SSRIs or SNRIs – often present a therapeutic dilemma, given the lack of evidence-based alternative pharmacotherapies to traditional monoaminergics (3). When monoaminergic antidepressants are ineffective at eliciting a full response for older patients with TRD, augmentation pharmacotherapy using medications with a unique mechanism of action and rapid onset may offer relief.
Modulation of the opiate system may be a novel treatment approach for TRD.. It is established that opiate receptor subtypes modulate regulation of serotonin in the mammalian midbrain, raphe, and forebrain (4). Indeed, the periaqueductal grey matter, an area rich in opiate receptors, receives projections from the amygdala, frontal cortex, and locus coeruleus, suggesting reciprocal modulation of the opiate and monoaminergic systems (5). Owing to the observed euphoric, tranquillizing, and anti-anxiety actions of opioids, a functional deficiency of endogenous opioids has been postulated to underlie the pathogenesis of endogenous depression. This is supported by observations of mood improvement in mid-life patients treated with cyclazocine (a mixed agonist/antagonist opioid) (6), beta-endorphin infusions (7), and a synthetic enkephalin analogue (8).
Buprenorphine is a partial agonist at μ-receptors, an antagonist of kappa (κ) receptors, and also displays affinity for delta (δ) opiate receptors. Buprenorphine has a favorable safety profile with low risk of respiratory depression, and the pharmacokinetics are not affected by advanced age or renal dysfunction, supporting its use in both mid-life and older adults with TRD. The combination of μ-agonism and κ-antagonism produces less dysphoria than methadone (9), and animal studies suggest that κ-antagonism may exert antidepressant effects (10). Buprenorphine may also interact with serotonergic systems and the hypothalamic-pituitary-adrenal axis (11). Rapid improvement in mood has been observed in both younger non-opioid abusing patients with TRD (12) and opioid-dependent patients treated with buprenorphine (13). Of particular relevance for TRD, especially in older adults in which cognitive impairment is often comorbid with depression (14), is that the effects of buprenorphine on cognition may be minimal (15, 16). The unique mechanism of action, potential for early effect, and acceptable safety profile make buprenorphine an intriguing molecule to test in older adults with TRD. In this proof-of-concept, unblinded clinical trial, we describe the clinical effect, safety, and tolerability of low-dose buprenorphine for TRD in older adults.
Methods
Participants
Five subjects were recruited from an on-going study of depression in adults 60 and older (MH083660). In that study participants received unblinded treatment with venlafaxine xr, with daily doses up to 300 mg, for 12 weeks (17). Non-responders (defined as Montgomery Asberg Depression Rating Scale [MADRS] score ≥ 10) to this regimen were declared treatment resistant and offered participation in this eight-week buprenorphine pilot study. We define these subjects as treatment resistant because of the serotonergic and noradrenergic pharmacodynamic activity of venlafaxine at this higher dosing range and the rigor with which participants were monitored and adherence maintained (17). The average Antidepressant Treatment History Form (ATHF) score for these 5 participants prior to exposure to the venlafaxine was 3.6 (SD: 0.55; median = 4), indicating that they had received at least one rigorous trial of an antidepressant prior to exposure to the high-dose venlafaxine.
Entrance criteria was subsequently expanded to include retrospectively-defined TRD: community-dwelling subjects age 50 and older who had not responded to at least two different antidepressants, prescribed at an FDA-approved therapeutic dose, each for at least 6 weeks. Similar to the initial five subjects, all of these 10 additional subjects met SCID (18) criteria for major depressive disorder, recurrent or single episode, and had a MADRS score ≥ 15 at baseline. Recruitment occurred between June 2010 and June 2011.
All participants also met the following entry criteria: 1) not using strong or moderate CYP3A4 inhibitors; 2) agreed to discontinue all opioids and alcohol; and 3) agreed to discontinue benzodiazepines other than the equivalent of lorazepam 2 mg/day that had been stably prescribed for at least 2 weeks. Subjects could not have: 1) lung disease requiring supplemental oxygen other than CPAP for obstructive sleep apnea; 2) estimated creatinine clearance < 30 mL/min; 3) hepatic impairment (AST or ALT > 1.5 times upper normal); 4) dementia, as defined by MMSE < 24 (19) and clinical evidence of dementia; 5) lifetime diagnosis of bipolar or psychotic-spectrum disorder; 6) abuse of or dependence on alcohol or other substance within the past 3 months as determined by SCID; 7) lifetime history of opioid abuse or dependence (to avoid precipitating relapse); or 8) high risk for suicide. All subjects were deemed medically stable by history and physical exam prior to entering the study.
Subjects were assessed in-person weekly. All participants provided written informed consent. This project was approved and monitored by both the University of Pittsburgh IRB and the FDA (IND 107,835).
Assessments
Diagnostic and Medical Assessment
We used the Structured Clinical Interview for DSM-IV (SCID) (18) to assess current and lifetime depression and other psychiatric disorders. Our group has maintained high inter-rater reliability with formalized training and a weekly multidisciplinary consensus conference at which final diagnoses are adjudicated (20). We used the Mini Mental State Exam (19) (MMSE) to screen for cognitive impairment. The quality of antidepressant pharmacotherapy for this episode of depression was quantified with the Antidepressant Treatment History Form (ATHF) (21).
Weekly assessments were conducted at week 0 (pretreatment) up to week 8 (termination).
Clinical
The Montgomery Asberg Depression Rating Scale (MADRS) was designed to assess treatment-sensitive change in major depressive disorder (MDD) and served as the main outcome measure for this study (22). We administered the MADRS using a published structured interview (23) and maintained Intra-class Correlation [ICC] of 0.99 among 12 raters with backgrounds ranging from Bachelor’s degree to geriatric psychiatrist. The Brief Symptom Inventory – Anxiety Subscale (BSI-anxiety) is a validated self-report scale derived from the Symptom Checklist 90-Revised (24) with strong construct validity, internal consistency, and test-retest reliability (24). We have reported that the BSI-anxiety scale has good internal consistency in older subjects with MDD (Cronbach's alpha = 0.84) (25). The Positive and Negative Affect Scales were used to assess emotional valence. These scales have high internal consistency, are largely uncorrelated, and are stable over a 2-month time period (26). The 21-item Scale for Suicide Ideation (SSI) (27) has been shown to predict completed suicide (28) and has moderately high internal consistency with Cronbach’s alphas ranging from 0.84 to 0.89 and good inter-rater reliability (27, 29). At every visit, 5 screening questions from the Scale for Suicidal Ideation were asked after the MADRS. If participants screened positive on any of these questions, the full Scale for Suicidal Ideation was administered and appropriate clinical action implemented to assure participant safety.
Cognitive Assessments
We assessed attention, psychomotor speed, executive function, and learning and memory, cognitive domains that may be affected by opioids (30). Before the first observed dose and at termination, we used two computerized tasks with repeatable conditions: a Choice Reaction Time Task and a Congruous vs. Incongruous Conditions Reaction Time Task, which have been described in detail elsewhere (31). The variables that we examined from each task include (1) accuracy (a basic measure of attention), (2) response time (a measure of psychomotor speed), and (3) a ratio comparing reaction time on the incongruous trials with that on the choice (which was neutral) reaction time task, which reflects inhibition (an executive function). We assessed memory with the Hopkins Verbal Learning Test-Revised (HVLT-R) at the same time-points, using alternate versions. The HVLT-R probes learning and memory, providing an evaluation of both the learning process and the amount of information that is both acquired and retained (32).
Tolerability, Safety, and Cognition
The Udvalg for Kliniske Undersogelser (UKU) Side Effects Rating Scale (33) was used to capture specific side effects putatively related to buprenorphine. At our geriatric depression research center, we have yearly re-training in the use of this scale. At our last reliability testing, the ICC was 0.91. General burden of side effects was assessed with the Frequency, Intensity, and Burden of Side Effects Rating (FIBSER) scale (34). The MADRS and FIBSER scores were used to guide the weekly dose of buprenorphine (see below). Vital Signs and Weight: Sitting and standing blood pressure, heart rate, and weight were assessed at every visit. Orthostasis was defined as systolic blood pressure drop of at least 20 mm/Hg, or increase in heart rate of at least 10 beats/minute (35).
Quality of Life and Sleep
We used the SF-36 (36, 37) to assess health-related quality of life. The SF-36 provides an assessment of overall physical and mental quality of life and 8 individual subscales: general health, physical functioning, mental health, vitality, pain, social functioning, role limitations due to physical health, and role limitations due to emotional health. Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI) (38). A global PSQI score > 5 distinguishes good from poor sleepers. The SF-36 and PSQI were administered monthly.
Follow-up
We used the Clinical Opiate Withdrawal Scale (COWS) (39) to monitor for signs and symptoms of withdrawal during the 2 weeks of discontinuation of the buprenorphine. The MADRS was repeated at week 16 (8 weeks after discontinuation of the buprenorphine).
Dosing Schedule and Response Criteria
Buprenorphine was provided by Reckitt Benckiser as Temgesic© 0.2 mg sublingual tablets. The first dose was administered under supervision, and subjects were reassessed 60 minutes later to assure they were not sedated or nauseated prior to leaving the clinic. For the first week, subjects took 0.2 mg/day, usually in the morning. Weekly dosing was guided by severity of depressive symptoms (MADRS) and buprenorphine-associated side effects. General burden of side effects were assessed with the Frequency, Intensity, and Burden of Side Effects Rating (FIBSER) scale (34).
Dosing was guided by MADRS and FIBSER scores. If the MADRS was ≤ 10, the dose was unchanged. If the MADRS was > 10 and the FIBSER was < 7, the dose was increased by 0.2 mg/day. If the MADRS was > 10 and the FIBSER was > 7, the dose was unchanged. Response was defined as MADRS ≤ 10. A score of 5 to 7 on the FIBSER (34) triggered additional assessment of side effects and required justification for increasing the dose, while a score of > 7 signaled no increase in dose. At the end of 8 weeks, the buprenorphine was discontinued by decreasing the dose about 20% every 2–3 days.
Statistical Analysis
Baseline demographic variables and clinical differences of participants were summarized using mean (standard deviation) for continuous variables and % (n) for categorical measures. Safety, side effects, cognitive function, and clinical measures were examined over time. Clinically meaningful definition of safety and side effects were defined and % (n) of subjects meeting the criteria are reported. Graphs show group mean data standard error. Pre- to post-intervention changes in cognitive function were tested using Wilcoxon signed rank exact test.
Results
Fifteen subjects entered the study, of whom thirteen provided 8 weeks of complete data in the analyses reported here. One subject stopped taking the medication at week 3 due to reported “constipation and bloating.” Two subjects were withdrawn from participation because of worsening chronic suicidal ideation that was related to an exacerbation of psychosocial stressors. Baseline descriptors of the sample are included in Table 1. The average daily dose was 0.40 mg/day (std=0.21, median=0.40, range=0.12-0.83) with maximum average daily dose of 0.69 (std=0.37, median=0.60, range=0.20-1.40
Table 1.
Baseline descriptors of the sample (N=15)
| Total Group N=15 |
Augmentation N=13 |
Monotherapy N=2 |
|
|---|---|---|---|
| Age | 60.7 (5.6) Range=51.1 – 69.2 |
60.4 (5.3) Range=51.1 – 69.2 |
a.) 55.7 b.) 69.2 |
| % Female | 53.3 (n=8) | 53.85 (n=7) | a.) 1: F b.) 1: M |
| % Caucasian | 100 (n=15) | 100 (n=13) | 100 (n=2) |
| Medical Outcome Survey Short Form (SF-36) | |||
| Mental Component Score | 27.7 (8.2) [n=10] |
28.1 (8.6) Range=14.6 – 42.6 [n=9] |
a.) 24.6 |
| Physical Component Score | 41.5 (9.4) [n=10] |
42.5 (9.4) Range=31.9-56.8 [n=9] |
a.) 32.7 |
| Mini Mental State Examination (MMSE)/30 | 28.5 (1.8) [n=12] |
28.5 (1.9) Range=24 – 30 [n=11] |
b.) 29.0 |
| Pittsburgh Sleep Quality Index (PSQI) | 9.9 (3.5) | 9.4 (3.2) Range=5 – 15 |
a.) 17 b.) 10 |
| Duration of current episode (weeks) | 987.9 (1125.5) Median =572 Range=6 – 3502 |
668.5 (785.5) Median=260 Range=6 – 2264 |
a.) 2626 b.) 3502 |
| History of Substance Abuse or Dependence | 4: alcohol 1: cocaine 1: alcohol, cannabis, cocaine |
2: alcohol 1: cocaine 1: alcohol, cannabis, cocaine |
2 :hx alcohol |
| Anti-Depressant Treatment History Form (ATHF) |
3.7 (0.5) [n=13] |
3.8 (0.5) Range=3 – 4 [n=12] |
b.) 3 |
| Montgomery Asberg Depression Rating Scale (MADRS) |
27.0 (7.3) | 26.2 (6.4) Range=18 – 37 |
a.) 42 b.) 23 |
| Suicidal Ideation Scale (SIS) | 3.7 (6.6) | 3.2 (6.6) Range=0 – 21 |
a.) 10 |
| Positive and Negative Affect Scale (PANAS) | |||
| Negative Right Now | 21.1 (8.2) | 21.3 (8.2) Range=13 – 37 |
a.) 12 b.) 28 |
| Positive Right Now | 21.5 (8.6) | 22.7 (8.7) Range=10 – 40 |
a.) 12 b.) 16 |
| Brief Symptom Inventory – Anxiety subscale | 1.1 (0.7) | 1.1 (0.7) Range=0 – 2.7 |
a.) 1.7 b.) 1.5 |
| UKU Side Effects Rating Scale | 12.0 (3.8) | 11.7 (3.7) Range=5 – 16 |
a.) 18 b.) 10 |
Clinical Effect
Depression
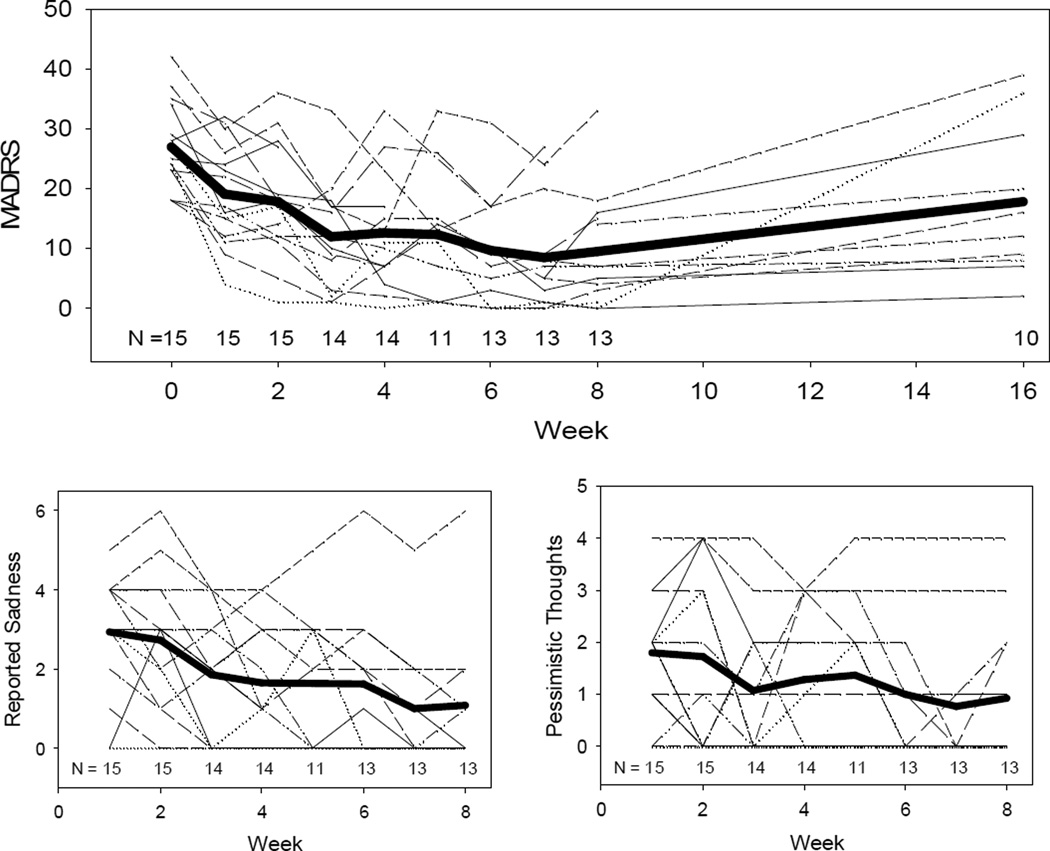
Figure 1 shows the change in depression. The average depression score (MADRS) at baseline was 27.0 (SD=7.3, median=25, range =18–42, n=15); and at week 8, 9.5 (SD=9.5, median=7, range=0–33, n=13). There was a sharp decline in depression severity during the first three weeks of exposure (mean delta=-15.0 (SD=7.9, median=-15.5, range=-25.0 to 2, n=14). To explore whether this improvement was specific to core depressive symptoms, we plotted MADRS items measuring pessimistic thoughts and reported sadness. The trajectories for these two items match the trajectory for the total MADRS score (Figure 1) and represent depression-specific clinical improvements. Response, defined as MADRS ≤ 10 at any week, was observed for 10/15 participants (66.7%, 95 CI = 38.7% to 87.0%). Response at the end of 8 weeks was observed for 8/13 participants (61.54%, 95% CI = 32.3 to 84.9).
Figure 1.
Individual subject trajectories for MADRS total score, reported sadness, and pessimistic thoughts.
Note: Group mean trajectories included as bold line. Sample size indicated at each time point. Total MADRS graph shows telephone assessment of depression symptoms at week 16 done to assess durability of the antidepressant effect.
Discontinuation and follow-up
We used the Clinical Opiate Withdrawal Scale (COWS) during the 4 weeks of BUP discontinuation. None of the subjects experienced clinically significant withdrawal symptoms. The average score on the COWS during discontinuation was 1.9 (SD=1.3, median=1.6, range=0.3–4.3, n=12) (indicating clinically insignificant symptoms of withdrawal). Participants were contacted at week 16 (usually by telephone) to assess the durability of the antidepressant effect. Of note, three of the participants were continuing to take low-dose buprenorphine, obtained from their psychiatrists, after the first 8 weeks. The average MADRS (excluding those who continued to take buprenorphine) at week 16 was 17.8 (SD=12.9, median=14, range=2–39, n=10).
Cognitive Function
Neuropsychological Assessment
On the Choice Reaction Time Task, mean accuracy (which was at or near the test ceiling) and mean reaction time (RT) did not significantly change over the 8-week trial. On the Congruous vs. Incongruous Reaction Time Task, mean accuracy did not significantly change over the 8-week trial, but mean RT improved (i.e., RT became faster) on both the congruous and incongruous conditions, suggesting improvement in psychomotor speed. The ratio of Incongruous / Congruous Choice Reaction Time revealed a trend for improvement across the two time-points [Baseline=1.38 (std=0.23, median=1.36) vs. Week 8=1.15 (std=0.23, median=1.25); Wilcoxon signed rank exact p=0.10], suggesting improvement in the ability to inhibit automatic responses in favor of more effortful responses (Table 2).
Table 2.
Neuropsychological Changes Between Baseline and Week 8
| Baseline % or mean (SD) Median |
Week 8 % or mean (SD) Median |
Cohen D for change * p<0.05 |
|
|---|---|---|---|
| Choice Reaction Time Task – accuracy | 98.1% (5.5) 100 |
99.4% (1.1) 100 |
0.33 |
| Choice Reaction Time Task – reaction time | 545.1 ms* (174.9) 501.3 |
555.6 ms (210.9) 488.9 |
0.16 |
| Congruous Task – accuracy | 93.6% (16.10) 100 |
99.7% (0.83) 100 |
0.33 |
| Congruous Task – reaction time | 649.7 ms (182.5) 630.4 |
562.5 ms (140.2) 530.65 |
1.10* |
| Incongruous Task – accuracy | 89.6% (17.2) 97.5 |
98.9% (1.82) 100 |
0.39 |
| Incongruous Task – reaction time | 709.5 ms (233.4) 655.7 |
601.1 ms (131.2) 596.9 |
1.00* |
|
Mean (SD) Median |
Mean (SD) Median |
||
| HVLT** Trial 1-3 | 23.9 (5.5) 23.5 |
24.8 (5.1) 25.0 |
0.12 |
| HVLT Trial 4 | 8.3 (2.6) 8.0 |
9.4 (2.1) 10.0 |
0.31 |
| HVLT Percent Retained | 88.8 (13.3) 90.9 |
96.3 (14.6) 95.8 |
0.32 |
| HVLT Recognition Discriminability Index | 10.4 (1.7) 10.5 |
10.8 (1.6) 11.0 |
0.05 |
ms = milliseconds
HVLT = Hopkins Verbal Learning Test
HVLT-R
We observed improvements in all four HVLT-R measures (Trial 1–3 (learning), Trial 4 (delayed recall)), percentage of words learned that were retained over 20 minute delay (delayed recall), and Recognition Discriminability Index (percent of all words correctly recognized after 20 minute delay) (Table 2).
Other outcomes
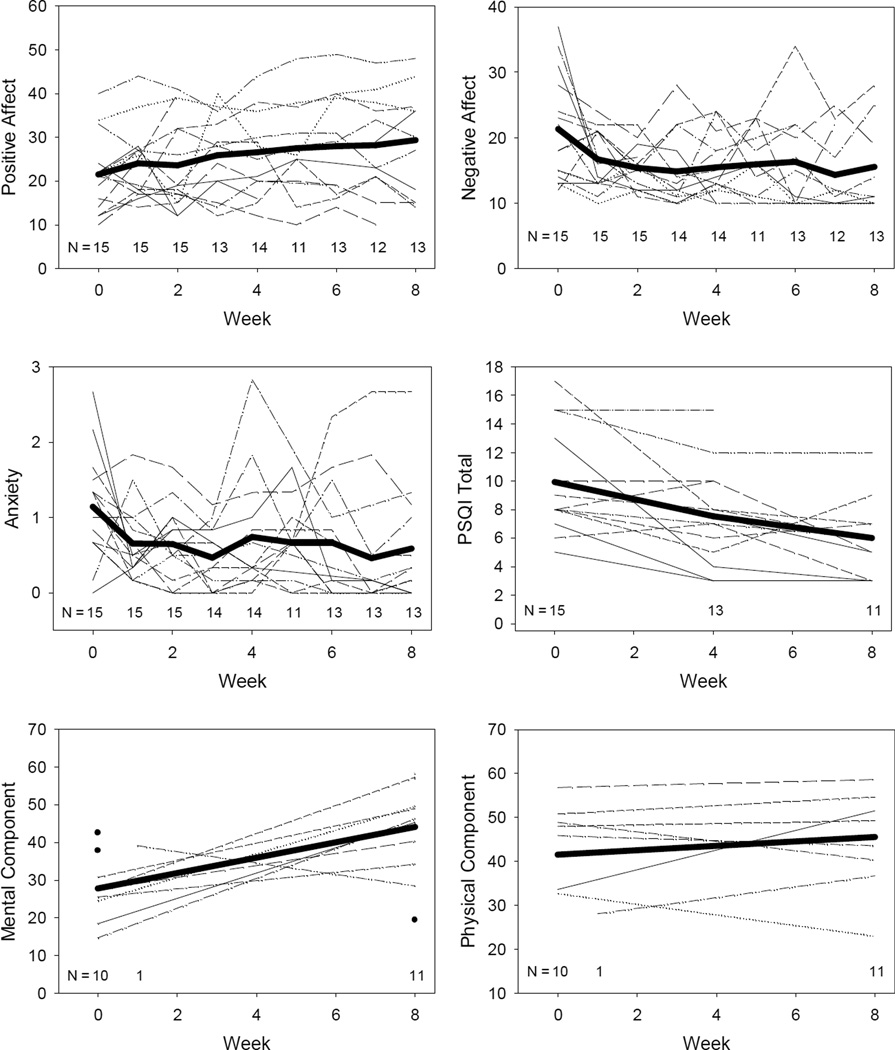
Figure 2 illustrates change in positive and negative affect, anxiety, sleep, and the mental and physical components of the SF-36. The general trend for all of these outcomes was in the direction of positive response, except for the physical component subscale of the SF-36, which was flat.
Figure 2.
Individual subject trajectories for positive and negative affect, anxiety, sleep and the mental and physical components of the SF-36.
Note: Group mean trajectories included as bold line. Sample size indicated at each time point. Circle symbol indicates subject had only one observation.
Safety and Tolerability
Vital signs and Weight
There were no sustained elevations for either systolic or diastolic blood pressure across the 8 weeks of the study. This was observed for both sitting and standing blood pressures. Three subjects experienced a greater than 20 point increase in systolic blood pressure at one assessment point during the project. Weight was stable. There were inconsistent changes in weight: 4/15 (26.7%) subjects experienced 5% decrease in weight, and 1/15 (6.7%) experienced a 5% increase in weight.
Side effects
A two point or greater change for the nausea and constipation items on the UKU side effects scale may be considered clinically significant. Five out of the 15 participants had a ≥ 2 point increase on the nausea item. Of these five subjects, the average number of weeks with a higher level of reported nausea was 1.6 (SD=0.89; median=1.0). Eight of the participants experienced a ≥ 2 point increase on the constipation item. The average number of weeks with a higher level of reported constipation was 1.29 (SD=0.49; median=1.00).
Discussion
We observed improvement in depression within the first week of starting buprenorphine. This is consistent with another small open-label trial in younger adults using buprenorphine for TRD in which much of the clinical improvement was observed by the end of week 1 (12). The improvement was sustained during exposure to buprenorphine. However, once buprenorphine was discontinued and subjects were reassessed at week 16, the average MADRS score increased to 17.8, suggesting that the antidepressant benefits of low-dose buprenorphine may require long-term dosing to be sustained.
Executive function (as represented by inhibition, or impulse control), psychomotor speed, and memory for new information, cognitive domains of particular concern during exposure to opioids, did not worsen during exposure to low-dose buprenorphine. Effective treatment of depression has been shown to improve cognitive function in studies of both mid- and late-life adults (40). Opioids may worsen cognitive function (41), but in patients who were not opioid-naïve, buprenorphine has been shown to have a neutral effect on cognition (16). It is a limitation that we do not have psychiatrically normal or opioid-naïve control participants with which to compare the repeated testing results, but the lack of slowed (and potentially improved) psychomotor speed, inhibition, and memory, suggest that low-dose buprenorphine in this sample of mid-life and older adults does not worsen, and may potentially improve, cognitive function.
The results from this pilot project also suggest that low-dose buprenorphine may be safe and well-tolerated in mid-life and older adults with TRD. The effects on vital signs and weight were not clinically actionable. This is in contrast to work by Johnson et al (35) who described clinically significant orthostasis among older adults receiving higher-dose venlafaxine. Monoamine Oxidase Reuptake Inhibitors (MAOIs), a reasonable and often effective choice for TRD, often are associated with increased heart rate and orthostasis (42, 43).
To our knowledge, there are no reports about the effect on weight of chronic administration of opioids in humans. In this study, we did not observe weight gain. This observation is relevant for patient acceptability of antidepressant pharmacotherapy. In a large population-based study with a mean follow-up of 4.8 years, depressed users of antidepressants gained 1.8 times as much as depressed individuals who did not receive antidepressant pharmacotherapy (44). While it is unclear whether the weight gain was due to depression response and improved appetite or a medication side-effect, we observed improvement in depression without increase in weight.
Constipation and nausea are among the most common opioid side effects. Buprenorphine was well-tolerated, with relatively minimal effects on these gastrointestinal symptoms. In contrast, 23.4% of patients taking duloxetine, a serotonin norepinephrine reuptake inhibitor (SNRI), experienced nausea and 10.1% experienced constipation (45). Hu and colleagues studied 401 depressed patients prescribed a SSRI (46): following 75–105 days of treatment, 86% of patients reported 1 or more side effects, while 55% reported at least 1 side effect they considered bothersome. In the Hu report, while most side effects appeared during the first two weeks of treatment, the majority of patients continued to experience side effects up to 15 weeks later. Indeed, up to 32% of patients continued to complain of nausea three months after starting treatment.
A concern with the use of buprenorphine for TRD is the risk of physiologic dependence. However, in this pilot study, we did not observe clinically significant physiologic or psychological withdrawal when the buprenorphine was tapered. This may be a result of the 1) relatively low dose used in the study; 2) the brief 8 week duration of the trial; 3) exclusion of participants with a history of opioid dependence; 4) the relatively long taper schedule; or 5) the partial mu-agonist pharmacodynamics of buprenorphine. Patients treated with FDA-approved antidepressants with relatively short half-lives such as venlafaxine and paroxetine often experience an uncomfortable withdrawal syndrome consistent with physiologic “dependence” on the medication (47). The risks of untreated TRD, which include the development of psychiatric and substance misuse comorbidity, psychosocial and economic decline, worsened medical comorbidity and early mortality, and suicide, should be taken into account when considering the risk/benefit ratio of a novel treatment. Indeed, when compared to interventions such as electroconvulsive therapy, vagal nerve stimulation, and deep brain stimulation, the risks of using buprenorphine in properly selected patients may be considered to be relatively minimal. Further research with buprenorphine or similarly acting agents, prescribed for longer duration and at higher doses, is needed to better evaluate safety and ease of discontinuation.
The off-label use of buprenorphine for indications such as pain or depression does not require the currently required waiver from the Center for Substance Abuse Treatment (a component of the Substance Abuse and Mental Health Services Administration). Off-label prescribing does require, however, a valid registration to prescribe a Schedule III controlled substance. Given the lack of placebo controlled trials indicating the efficacy of buprenorphine for treatment resistant depression, the authors do not advocate the use of this medication in routine clinical care. That being said, there are patients suffering from treatment resistant depression who may benefit from a carefully administered trial of this medication. Although we are still learning about the clinical use of buprenorphine for these challenging patients, suggested best practice procedures include, but are not limited to: 1) appropriately screening candidates to minimize the risk of diversion or relapse of an opioid misuse disorder; 2) writing the off-label indication on the prescription (such as “for depression, off-label use”); 3) maintaining meticulous records of all prescriptions; 4) requiring patients use only one pharmacy; and 5) obtaining regular urine drug screens to minimize the risk of diversion.
In summary, our study supports the further development of buprenorphine as a novel-mechanism treatment for TRD in mid-life and older adults. The findings are limited by the open label design of the study, which was necessary given the paucity of safety and efficacy data for low-dose buprenorphine, especially in a sample enriched with older individuals. The lack of ethnic diversity also needs to be remedied in future studies. The next steps in our development of buprenorphine as a novel therapeutic for TRD includes a proof-of-concept randomized clinical trial in which we further refine dosing strategies, examine response, safety and tolerability, and pilot biomarkers of response. We also plan to probe the mechanisms of action of buprenorphine using the imaging, pharmacogenetic, and physiological tools of modern clinical neuroscience.
Clinical Points.
Low-dose buprenorphine is an intriguing option for some patients with treatment resistant depression.
Clinical improvement may be observed early during treatment.
Acknowledgments
Declaration of Interest
J.F.K. receives medication supplies from Reckitt Benckiser and Pfizer for investigator initiated trials. E.J.L. has received research support from Forest, Johnson & Johnson and Roche, Rodney Lundbeck, as well as (medications only) Pfizer, Corcept, and Bristol-Myers Squibb. D.B. received research funding from a Canadian Institutes of Health Research Industry Partnered (Brainsway Ltd.) operating grant, the Campbell Family Research Institute, and the Temerty Centre for Therapeutic Brain Intervention. B.H.M. has received financial support during the past 36 months from the U.S. National Institute of Mental Health and the Canadian Institutes for Health Research and pharmaceutical supplies from Bristol-Myers Squibb, Eli Lilly, and Pfizer for his NIH-sponsored research. He has also received reimbursement for travel expenses from Roche. He owns stock in General Electric. CFR reports receiving pharmaceutical support for NIH-sponsored research studies from Bristol-Myers Squibb, Forest, Pfizer, and Lilly; receiving grants from NIH, PCORI, the Commonwealth of Pennsylvania, the John A Hartford Foundation, National Palliative Care Research Center (NPCRC), UPMC Endowment in Geriatric Psychiatry, and the American Foundation for Suicide Prevention; and serving on the American Association for Geriatric Psychiatry editorial review board. He has received an honorarium as a speaker from MedScape/WEB MD. He is the co-inventor (Licensed Intellectual Property) of Psychometric analysis of the Pittsburgh Sleep Quality Index (PSQI). His NIH funded research is supported by the following grants: P60 MD000207; P30 MH090333; UL1RR024153, UL1TR000005. MAB has served as a consultant for GlaxoSmithKline.
Supported in part by NIH: AG033575 (Karp); P30 MH090330 (Reynolds); MH083660 (Reynolds, Lenze, Mulsant); MH072947 (Butters); KL2 RR024154 (Reis), and the Brain and Behavior Research Foundation (formerly known as NARSAD) (Karp). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Reckitt Benckiser provided medication supplies for this investigator initiated trial. Reckitt Benckiser was not involved in any aspect of the design or conduct of this clinical trial. We are grateful for their support.
Footnotes
The other authors have nothing to disclose.
References
- 1.Dew MA, Whyte EM, Lenze EJ, Houck PR, Mulsant BH, Pollock BG, et al. Recovery From Major Depression in Older Adults Receiving Augmentation of Antidepressant Pharmacotherapy. Am J Psychiatry. 2007;164(6):892–899. doi: 10.1176/ajp.2007.164.6.892. [DOI] [PubMed] [Google Scholar]
- 2.Rush AJ, Trivedi M, Fava M. Depression, IV: STAR*D treatment trial for depression. American Journal of Psychiatry. 2003;160(2):237. doi: 10.1176/appi.ajp.160.2.237. [DOI] [PubMed] [Google Scholar]
- 3.Thase M, Rush A. Treatment Resistant Depression. In: Bloom F, Kupfer D, editors. Psychopharmacology - 4th Generation of Progress. 4. New York: Lippincott, Williams, and Wilkins; 1995. [Google Scholar]
- 4.Tao R, Auerbach SB. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. Journal of Pharmacology & Experimental Therapeutics. 2002 Nov;303(2):549–556. doi: 10.1124/jpet.102.037861. PubMed PMID: 12388635. [DOI] [PubMed] [Google Scholar]
- 5.Hache G, Coudore F, Gardier A, Guiard B. Monoaminergic Antidepressants in the Relief of Pain: Potential Therapeutic Utility of Triple Reuptake Inhibitors (TRIs) Pharmaceuticals. 2011;4:285–342. [Google Scholar]
- 6.Fink M, Simeon J, Itil TM, Freedman AM. Clinical antidepressant activity of cyclazocine--a narcotic antagonist. Clinical Pharmacology & Therapeutics. 1970;11(1):41–48. doi: 10.1002/cpt197011141. [DOI] [PubMed] [Google Scholar]
- 7.Gerner RH, Catlin DH, Gorelick DA, Hui KK, Li CH. beta-Endorphin. Intravenous infusion causes behavioral change in psychiatric inpatients. Archives of General Psychiatry. 1980;37(6):642–647. doi: 10.1001/archpsyc.1980.01780190040005. [DOI] [PubMed] [Google Scholar]
- 8.Ruther E, Jungkunz G, Nedopil N. Clinical effects of the synthetic analogue of methionine enkephalin FK 33-824. IIIrd World Congress of Biological Psychiatry; Stockholm. 1981 [Google Scholar]
- 9.Tenore PL. Psychotherapeutic benefits of opioid agonist therapy. Journal of Addictive Diseases. 2008;27(3):49–65. doi: 10.1080/10550880802122646. PubMed PMID: 18956529. [DOI] [PubMed] [Google Scholar]
- 10.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. Journal of Pharmacology & Experimental Therapeutics. 2003;305(1):323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 11.Sbrenna S, Marti M, Morari M, Calo G, Guerrini R, Beani L, et al. Modulation of 5-hydroxytryptamine efflux from rat cortical synaptosomes by opioids and nociceptin. British Journal of Pharmacology. 2000;130(2):425–433. doi: 10.1038/sj.bjp.0703321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. Journal of Clinical Psychopharmacology. 1995 Feb;15(1):49–57. doi: 10.1097/00004714-199502000-00008. PubMed PMID: 7714228. [DOI] [PubMed] [Google Scholar]
- 13.Kosten TR, Morgan C, Kosten TA. Depressive symptoms during buprenorphine treatment of opioid abusers. Journal of Substance Abuse Treatment. 1990;7(1):51–54. doi: 10.1016/0740-5472(90)90035-o. [DOI] [PubMed] [Google Scholar]
- 14.Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61(6):587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 15.Soyka M, Lieb M, Kagerer S, Zingg C, Koller G, Lehnert P, et al. Cognitive functioning during methadone and buprenorphine treatment: results of a randomized clinical trial. Journal of Clinical Psychopharmacology. 2008 Dec;28(6):699–703. doi: 10.1097/JCP.0b013e31818a6d38. PubMed PMID: 19011441. [DOI] [PubMed] [Google Scholar]
- 16.Dagtekin O, Gerbershagen HJ, Wagner W, Petzke F, Radbruch L, Sabatowski R. Assessing cognitive and psychomotor performance under long-term treatment with transdermal buprenorphine in chronic noncancer pain patients. Anesthesia & Analgesia. 2007;105(5):1442–1448. doi: 10.1213/01.ane.0000281078.65585.1e. PubMed PMID: 17959980. [DOI] [PubMed] [Google Scholar]
- 17.Blier P, Saint-Andre E, Hebert C, de Montigny C, Lavoie N, Debonnel G. Effects of different doses of venlafaxine on serotonin and norepinephrine reuptake in healthy volunteers. International Journal of Neuropsychopharmacology. 2007;10(1):41–50. doi: 10.1017/S1461145705006395. [DOI] [PubMed] [Google Scholar]
- 18.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Washington, D.C.: American Psychiatric Press; 1997. [Google Scholar]
- 19.Folstein M, Folstein S, PR M. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Mulsant B, Sweet R, Rifai A, Pasternak R, McEachran A, Zubenko G. The use of the Hamilton Rating Scale for Depression in elderly patients with cognitive impairment and physical illness. American Journal of Geriatric Psychiatry. 1994;2:220–229. doi: 10.1097/00019442-199400230-00006. [DOI] [PubMed] [Google Scholar]
- 21.Oquendo MA, Baca-Garcia E, Kartachov A, Khait V, Campbell CE, Richards M, et al. A computer algorithm for calculating the adequacy of antidepressant treatment in unipolar and bipolar depression. Journal of Clinical Psychiatry. 2003;64(7):825–833. doi: 10.4088/jcp.v64n0714. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 23.Williams J, Kobak K, editors. Improving Inter-Rater Reliability in Clinical Trials: Development and Validation of a Structured Interview Guide for the Montgomery-Asberg Depression Rating Scale (SIGMA); International Society for CNS Clinical Trials and Methodology 3rd Annual Scientific Congress; Washington, DC. 2007. [Google Scholar]
- 24.Derogatis L. Brief Symptom Inventory (BSI): Administration, Scoring, and Procedures Manual. 3rd ed. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- 25.Andreescu C, Lenze E, Dew M, Begley A, Mulsant B, Dombrovski A, et al. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. British Journal of Psychiatry. 2007;190(4):344–349. doi: 10.1192/bjp.bp.106.027169. [DOI] [PubMed] [Google Scholar]
- 26.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality & Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. Journal of Consulting & Clinical Psychology. 1979;47(2):343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 28.Beck A, Brown G, Steer R, Dahlsgaard K, Grisham J. Suicide ideation at its worst point: a predictor of eventual suicide in psychiatric outpatients. Suicide and Life- Threatening Behavior. 1999;29:1–9. [PubMed] [Google Scholar]
- 29.Beck AT, Brown GK, Steer RA. Psychometric characteristics of the Scale for Suicide Ideation with psychiatric outpatients. Behaviour Research & Therapy. 1997;35(11):1039–1046. doi: 10.1016/s0005-7967(97)00073-9. [DOI] [PubMed] [Google Scholar]
- 30.Rapeli P, Fabritius C, Kalska H, Alho H. Cognitive functioning in opioid-dependent patients treated with buprenorphine, methadone, and other psychoactive medications: stability and correlates. BMC Clinical Pharmacology. 2011;11:13. doi: 10.1186/1472-6904-11-13. PubMed PMID: 21854644. Pubmed Central PMCID: PMC3176473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jennings JR, Mendelson DN, Redfern MS, Nebes RD. Detecting age differences in resistance to perceptual and motor interference. Experimental Aging Research. 2011 Mar;37(2):179–197. doi: 10.1080/0361073X.2011.554512. PubMed PMID: 21424956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro A, Benedict R, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13(3):348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 33.Lingjaerde O, Ahifors U, Bech P, Dencker S, Elgen K. The UKU side effect rating scale. Acta Psychiatrica Scandinavica. 1987;34(suppl 76):1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 34.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA, for the SI. Self-rated global measure of the frequency, intensity, and burden of side effects. Journal of Psychiatric Practice. 2006;12(2):71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Johnson EM, Whyte E, Mulsant BH, Pollock BG, Weber E, Begley AE, et al. Cardiovascular Changes Associated With Venlafaxine in the Treatment of Late-Life Depression. Am J Geriatr Psychiatry. 2006;14(9):796–802. doi: 10.1097/01.JGP.0000204328.50105.b3. [DOI] [PubMed] [Google Scholar]
- 36.Ware J, Gandek B the IQOLA project group. The SF-36 health survey: Development and use in mental health research and IQOLA project. International Journal of Mental Health. 1994;23(2):49–73. [Google Scholar]
- 37.Dombrovski A, Lenze E, Dew M, Mulsant B, Pollock B, Houck P, et al. Maintenance Treatment for Old-Age Depression Preserves Health-Related Quality of Life: A Randomized, Controlled Trial of Paroxetine and Interpersonal Psychotherapy. Journal of the American Geriatrics Society. 2007;55:1325–1332. doi: 10.1111/j.1532-5415.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 38.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 39.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) Journal of Psychoactive Drugs. 2003;35(2):253–959. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 40.Raskin J, Wiltse CG, Siegal A, Sheikh J, Xu J, Dinkel JJ, et al. Efficacy of Duloxetine on Cognition, Depression, and Pain in Elderly Patients With Major Depressive Disorder: An 8-Week, Double-Blind, Placebo-Controlled Trial. Am J Psychiatry. 2007;164(6):900–909. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- 41.Chapman SL, Byas-Smith MG, Reed BA. Effects of intermediate- and long-term use of opioids on cognition in patients with chronic pain. Clinical Journal of Pain. 2002;18(4):S83–S90. doi: 10.1097/00002508-200207001-00010. [DOI] [PubMed] [Google Scholar]
- 42.Golwyn DH, Sevlie CP. Monoamine oxidase inhibitor hypertensive crisis headache and orthostatic hypotension. Journal of Clinical Psychopharmacology. 1993 Feb;13(1):77–78. doi: 10.1097/00004714-199302000-00014. PubMed PMID: 8486823. [DOI] [PubMed] [Google Scholar]
- 43.Tulen JHM, Volkers AC, van den Broek WW, Bruijn JA. Sustained effects of phenelzine and tranylcypromine on orthostatic challenge in antidepressant-refractory depression. Journal of Clinical Psychopharmacology. 2006 Oct;26(5):542–544. doi: 10.1097/01.jcp.0000236657.08663.ae. PubMed PMID: 16974207. [DOI] [PubMed] [Google Scholar]
- 44.Kivimaki M, Hamer M, Batty GD, Geddes JR, Tabak AG, Pentti J, et al. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care. 2010 Dec;33(12):2611–2616. doi: 10.2337/dc10-1187. PubMed PMID: 20823343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunton S, Wang F, Edwards SB, Crucitti AS, Ossanna MJ, Walker DJ, et al. Profile of adverse events with duloxetine treatment: a pooled analysis of placebo-controlled studies. Drug Safety. 2010 May 1;33(5):393–407. doi: 10.2165/11319200-000000000-00000. PubMed PMID: 20397739. [DOI] [PubMed] [Google Scholar]
- 46.Hu XH, Bull SA, Hunkeler EM, Ming E, Lee JY, Fireman B, et al. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: patient report versus physician estimate. Journal of Clinical Psychiatry. 2004;65(7):959–965. doi: 10.4088/jcp.v65n0712. [DOI] [PubMed] [Google Scholar]
- 47.Schatzberg AF, Haddad P, Kaplan EM, Lejoyeux M, Rosenbaum JF, Young AH, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation Consensus panel. Journal of Clinical Psychiatry. 1997;58(Suppl 7):5–10. [PubMed] [Google Scholar]