Abstract
Delirium is a common and severe neuropsychiatric syndrome characterised by acute deterioration and fluctuations in mental status. It is precipitated mainly by acute illness, trauma, surgery, or drugs. Delirium affects around 1 in 8 hospital inpatients and is associated with multiple adverse consequences, including new institutionalisation, worsening of existing dementia, and death. Patients with delirium show attentional and other cognitive deficits, altered alertness (mostly reduced, but some patients develop agitation and hyperactivity), altered sleep-wake cycle and psychoses. The pathways from the various aetiologies to the heterogeneous clinical presentations are hardly studied and are poorly understood. One of the key questions, which research is only now beginning to address, is how the factors determining susceptibility interact with the stimuli that trigger delirium. Inflammatory signals arising during systemic infection evoke sickness behaviour, a coordinated set of adaptive changes initiated by the host to respond to, and to counteract, infection. It is now clear that the same systemic inflammatory signals can have severe deleterious effects on brain function when occuring in old age or in the presence of neurodegenerative disease. Multiple animal studies now show that even mild acute systemic inflammation can induce exaggerated sickness behaviour responses and cognitive dysfunction in aged animals or those with prior degenerative pathology when compared to young and/or healthy controls. These findings appear highly promising in understanding aspects of delirium. In this review our aim is to describe and assess the parallels between exaggerated sickness behaviour in vulnerable animals and delirium in older humans. We discuss inflammatory and stress-related triggers of delirium in the context of new animal models that allow us to dissect some aspects of the mechanisms underpinning these episodes. We discuss some differences between the sickness behaviour syndrome model and delirium in the context of the complexity in the latter due to other factors such as prior pathology, psychological stress and drug effects. We conclude that, with appropriate caveats, the study of sickness behaviour in the vulnerable brain offers a promising route to uncover the mechanisms of this common and serious unmet medical need.
Keywords: Delirium, Sickness behaviour, Dementia, Acetylcholine, Cognitive, Systemic inflammation, IL-1β, prostaglandin
Introduction
Delirium is a common and severe neuropsychiatric syndrome characterised by acute deterioration and fluctuations in mental status mainly precipitated by acute illness, trauma, surgery, or the side effects of drugs. The core DSM-IV diagnostic criteria are: (a) a disturbance of consciousness (that is, reduced clarity of awareness of the environment, with reduced ability to focus, sustain, or shift attention), (b) a change in cognition (eg. memory impairment) or a perceptual disturbance, and (c) onset of hours to days, and tendency to fluctuate. Delirium is one of the most common acute medical conditions. The overall prevalence in medical inpatients is greater than 10%; in older patients this rises to greater than 20% and in intensive care units and post-operative hip fracture patients the prevalence is greater than 50%. In a typical 1000-bedded hospital, around 120 people will have delirium at any given time. There are currently no licensed treatments.
Although by definition delirium has an acute onset and always involves attentional deficits, it is otherwise heterogeneous, with the variable presence of multiple other neuropsychiatric features. Prominent among these is disturbance in level of consciousness, which ranges from barely responsive to highly agitated. A reduced level of consciousness is termed hypoactive delirium, whereas agitation and increased motor activity is termed hyperactive delirium. Many patients fluctuate between these motoric subtypes. Other neuropsychiatric manifestations include impairments in memory, perception and other cognitive domains, psychosis, and disturbance of the sleep-wake cycle (Fong et al., 2009; MacLullich & Hall, 2011).
The time-course of delirium is highly variable. Delirium has conventionally been described as a transient disorder, and indeed it does resolve in the majority of cases. Some patients may experience only a few hours of the syndrome, while others may have more prolonged episodes lasting days or weeks. Recent research has found that around 20% still exhibit symptoms three or even six months after onset; this is termed ‘persistent delirium’ (Meagher et al., 2012). Notwithstanding the resolution of most delirium, these episodes have serious long term consequences: it is now known that delirium predicts multiple adverse outcomes, including increased length of stay, morbidity, institutionalisation, and mortality during admission and one year after discharge (Witlox et al., 2010). Moreover, in cognitively normal patients, an episode of delirium is associated with a higher risk of dementia in the years following the episode (MacLullich et al., 2009; Davis et al., 2012). Delirium is also often highly distressing for patients and carers (Fong et al., 2009; MacLullich & Hall, 2011) and may result in post-traumatic stress disorder (Davydow et al., 2008).
Pathogenesis of delirium: direct brain insults versus aberrant stress responses
The precipitants of delirium are legion. So numerous and diverse are they that to conceptualise delirium as a homogeneous pathological entity with a single molecular pathway is untenable. Frequent causes include peripheral and systemic infections, changes in medication, surgery, trauma, pain, psychological stress, acute CNS pathology caused by stroke or head trauma, and metabolic derangements such as hypoglycemia, hypoxia, hyponatremia and liver failure (usually termed acute hepatic encephalopathy). To provide a conceptual framework with which to begin to address why and how such multitudinous aetiological factors can induce delirium, we have previously proposed that these triggers be divided into two broad categories: direct brain insults and aberrant stress responses (MacLullich et al., 2008). The category of direct brain insults includes triggers in which the brain is adversely affected by either a direct injury, change in metabolic environment or a drug. For example, it is conceptually very simple to accept that hypoglycemia, a recognised cause of delirium, has wide-ranging effects on the CNS that are severe and of acute onset. Likewise, an overdose of morphine often leads to impairments in brain function that meet criteria for delirium. Conversely, the category of aberrant stress responses constitute a group of responses that are somehow deficient or exaggerated in certain individuals such that responses that are typically adaptive under normal circumstances become maladaptive. This mostly occurs where there is susceptibility due to old age or pre-existing brain pathology. It is clear that aging and dementia are the major risk factors for development of delirium (Fong et al., 2009) and as such, while almost any individual can experience an episode of delirium if the stimulus is severe enough, these vulnerable individuals can become delirious even upon relatively mild stimuli. Infection is an excellent example of this: intensive care unit (ICU) patients with severe sepsis commonly develop delirium (often termed septic encephalopathy), but much milder infections, that have trivial effects in a young healthy person, can produce severe delirium in older people with dementia (Fong et al., 2009). Therefore one of the key questions, which our research has begun to address (Field et al., 2012; Murray et al., 2012), is how the factors determining susceptibility interact with the stimuli that trigger delirium?
Sickness behaviour syndrome in health
Peripheral infection can lead to changes in mood, energy, motivation and, consequently, behaviour. These changes were generally seen as direct side-effects of infection until Hart proposed the idea of a coordinated sickness behaviour response (Hart, 1988). This constitutes a reorganisation of priorities by the infected individual, suppressing energy expenditure in activities such as locomotor activity, feeding and social interaction to minimise the risk of predation, to isolate the sick individual from the rest of the herd and to allow the retention of energy to raise a fever, which is metabolically costly. Though it is difficult to prove that the changes in behaviour are beneficial to the host during infection, it is clear that failure to mount a fever response is deleterious to survival (Hart, 1988). These changes in behaviour, which are all coordinated by the CNS, can be seen as adaptive and therefore beneficial to the host. We now know that inflammatory mediators such as cytokines and prostaglandins can signal to the brain, via several routes (see (Dantzer et al., 2008) for review), to effect changes in neuronal function. Some of these mechanisms are briefly summarised below and in figure 1.
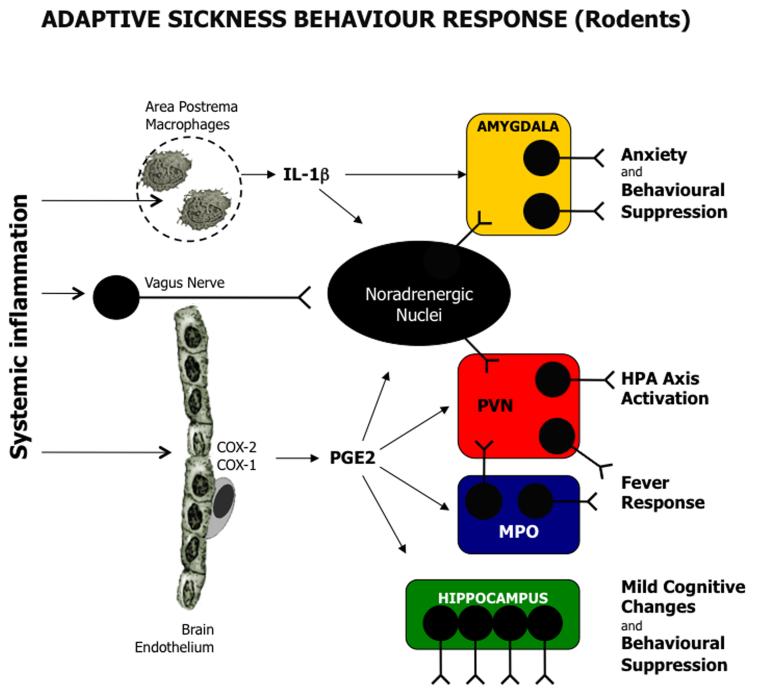
Figure 1.
Adaptive Sickness Behaviour Response: This scheme simplifies and summarises current knowledge of the sickness behaviour response as elucidated in rodents. Systemic inflammation is signalled to the brain via activation of the brain endothelium, the vagus nerve or circumventricular organs, such as the area postrema. The endothelial cells and perivascular macrophages express COX2 and COX1 to synthesize PGE2 and other prostanoids. PGE2 is known to acitvate hypothalamic centres (MPO: blue, PVN: red) via EP3 and EP1 receptors to drive fever and stress responses. Anxiety responses are mediated by the amygdala (yellow), which is activated directly by both IL-1β and brainstem noradrenaline release (indirect neuronal activation by the parabrachial nucleus detailed in main text). The hippocampus (green) can be activated by both IL-1β and PGE2 contributing to disengagement from the environment and cognitive changes. Abbreviations: PGE2: prostaglandin E2, IL-1β: interleukin-1β; COX: cyclooxygenase; HPA: hypothalamic pituitary adrenal; PVN: paraventricular nucleus; MPO: medial preoptic nucleus.
Thermoregulatory responses
The fever, or hyperthermic, response is thought to be dependent on IL-1β-induced, vascular COX-2-mediated, PGE2 production in the hypothalamus. This PGE2 binds to EP3 receptors in the median preoptic nucleus of the hypothalamus to activate GABAergic signalling in autonomic centres involved in thermogenesis (Nakamura et al., 2005; Lazarus et al., 2007).
Anxiety and the stress response
Systemic LPS or IL-1β induce neuronal activation in the amygdala, as demonstrated by expression of the immediate early gene, cFOS. Based on microdialysis (Engler et al., 2011) and neuroanatomical tracing (Gaykema et al., 2007) studies, IL-1/LPS is believed to trigger release, in the amygdala, of noradrenaline (NA) originating in brain stem areas such as the nucleus tractus solitarus, ventrolateral medulla and the locus coeruleus. These regions can be readily activated by vagal afferents and also via the circumventricular organs (CVOs: regions such as the area postrema, which lack a patent blood brain barrier). The parabrachial nucleus also ennervates the amygdala influencing anxiety and feeding responses to systemic inflammation (Gaykema et al., 2007).
The glucocorticoid response is initiated when systemic IL-1β or LPS triggers PGE2 release at the brain endothelium in the paraventricular nucleus of the hypothalamus. stimulating secretion of adrenocorticotropic hormone (ACTH) by corticotropin releasing hormone-containing neurons (see (Furuyashiki & Narumiya, 2009) for review). This ACTH stimulates the adrenal glands to secrete glucocorticoids.
Behavioural depression
Systemic inflammation suppresses locomotor activity and also decreases motivation to engage in rewarding activities (anhedonia) such as investigation of a juvenile conspecific, consumption of sweetened solutions and burrowing. The neuroanatomy of these activities is not fully understood. Pure suppression of activity versus altered motivation have not been teased apart in many studies, but activation of the amygdala and multiple hypothalamic nuclei (Park et al., 2008) and deactivation of the hippocampus are involved. Central inhibition of IL-1β using IL-1RA can reduce cFOS expression in the central nucleus of the amygdala and in the bed nucleus of the stria terminalis, reducing LPS-induced suppression of social interaction (Konsman et al., 2008). However, there is some evidence that perivascular and systemic IL-1β have a role in the reduced locomotor activity and vagotomy can also limit the induction of behavioural suppression (Konsman et al., 2000). Systemic inhibition of COX-1 also blocked inflammation-induced suppression of burrowing and glucose consumption (Swiergiel & Dunn, 2002; Teeling et al., 2010).
Cognitive changes
There are many reports of inflammation-induced cognitive deficits (Yirmiya & Goshen, 2011). However, a critical caveat is that the suppressing effects of LPS on locomotor speed, general activity, motivation by rewards and other features necessary for performance in standard rodent tests of cognitive function may confound many of the studies in this area (Cunningham & Sanderson, 2008). Consolidation of memory for context is impaired by inflammation in contextual fear conditioning experiments, and this involves both IL-1 and PGE2 (Hein et al., 2007; Frank et al., 2010). However, in other paradigms, visuospatial learning and memory and learning appears to cope rather well with even quite robust systemic infection (Jurgens et al., 2012) and it is mental flexibility, rather than visuospatial memory per se, that appears to be most impaired under the influence of systemic inflammation (Sparkman et al., 2006; Jurgens et al., 2012). It seems reasonable to state that facets of cognitive function can be impaired, but that severe, global cognitive impairment is perhaps not a core feature of sickness behaviour syndrome (SBS) in normal animals, and longer-term memories of aversive events may even be enhanced.
Human sickness behaviour
Though the concept has arisen largely in the rodent literature, it is supported by studies in humans. Injections of LPS, typhoid vaccine and interferon-α can cause similar emotional changes and subjective feelings of sickness in humans ((Grigoleit et al., 2011) and references therein). Interestingly, imaging studies in humans have demonstrated similar neural changes underpinning these subjective feelings of sickness (Harrison et al., 2009a; Harrison et al., 2009b; Inagaki et al., 2012). Evidence for systemic inflammation-induced cognitive impairments in healthy individuals is inconsistent (Reichenberg et al., 2001; Grigoleit et al., 2010). Thus, systemic inflammation in rodents can be anxiogenic and can cause affective and cognitive changes, but these are typically short lived and adaptive, and in the case of cognition, these changes are certainly mild in the otherwise healthy individual. The same appears true in healthy, young humans, where moderate doses of LPS induce mild and reversible affective and cognitive changes.
CNS responses in severe systemic inflammation
Severe infections leading to systemic inflammation and shock generally induce more pronounced behavioural changes, including delirium and even unconsciousness. The CNS changes associated with severe sepsis is often known as septic encephalopathy. This can occur in all age groups and even in the absence of prior illness. The evidence would suggest that these extreme changes in behaviour do not fall within the spectrum of adaptive SBS: humans studies show that severe systemic infections are commonly associated with significant brain injury and long-term cognitive impairment in survivors (MacLullich et al., 2009; Iwashyna et al., 2010). Caecal ligation and puncture (CLP), in which rodents are exposed to polymicrobial sepsis, is one of the most clinically relevant models of sepsis and induces significant CNS inflammation, BBB breakdown and pathology (Bozza et al., 2010; Imamura et al., 2011). Likewise, rodents challenged with large doses of bacterial endotoxin (LPS ≥ 5 mg/kg) show apoptosis, neuronal loss, glial activation and iNOS activation in several brain regions (Semmler et al., 2005). These pathological changes are associated with spatial working memory deficits in a radial arm maze at 12 weeks post-sepsis (Semmler et al., 2007). There is limited information from animal studies on the confusional state during severe sepsis, because the sickness severity in the hours and days post-infection is too great to allow any significant behavioural analysis beyond assessing suppression of locomotor activity. One study showed that animals trained on passive avoidance immediately before CLP sepsis (Shimizu et al., 1999) showed intact memory when tested 24 hours after sepsis induction but those that were trained during sepsis and tested 24 hours post-sepsis induction did show impairments 24 hours later (48 hours post-sepsis induction). Rats undergoing CLP have shown cognitive deficits, using an inhibitory avoidance paradigm, between 10 and 30 days sepsis (Barichello et al., 2005). Therefore in animals we are largely reliant on MRI, EEG and PET imaging studies to inform on early dysfunction during sepsis. These methods have demonstrated vasogenic edema, suggesting blood brain barrier breakdown in the region of the circle of Willis as soon as 6 hours post-CLP. Acute cytotoxicity (Bozza et al., 2010), and decreased glucose utilization and impaired tissue perfusion/blood flow were found in animals 24 hours post-LPS (Semmler et al., 2008). Collectively these studies suggest that decreased blood flow and energy supply might contribute to CNS dysfunction during severe sepsis, and thus direct brain injury through these mechanisms may be a significant cause of the cognitive impairment ultimately observed. As such, severe sepsis could be considered to fit the direct brain insult category discussed above (MacLullich et al., 2008). Thus, we observe a major CNS inflammatory response and cognitive dysfunction as a direct consequence of a profound systemic inflammatory response, often involving high levels of viable bacteria in the blood. It is very important to make a distinction between systemic inflammation of this intensity and the milder infectious episodes that can trigger delirium only in the aged and otherwise vulnerable populations.
Systemic inflammation produces exaggerated CNS inflammation in the susceptible individual
It is now clear that the aged and those with chronic neurodegenerative disease can suffer a more profound CNS inflammation in response to relatively minor systemic inflammation than young healthy controls. Normally this degree of systemic inflammation would only induce adaptive CNS changes. Why is there exaggerated CNS inflammation in certain groups? The basis for this may be explained, at least partly, by the phenomenon of microglial priming. This was first described in an animal model of prion disease (Cunningham et al., 2005b) but has now been observed in multiple models of neurodegenerative injury and disease and in aging (see (Cunningham, 2012) for review). During neurodegenerative disease and ageing, microglia are “primed” by the primary pathology to make them more responsive to subsequent challenge with inflammatory stimuli. In particular, the CNS synthesis of IL-1β is exaggerated. Crucially, systemic levels of cytokines induced by the peripheral insult are equivalent in normals and aged/diseased animals (Godbout et al., 2005; Murray et al., 2012). This means that the primed microglia act as a CNS amplifier of systemic inflammatory responses. Thus, though the strength of the peripheral inflammatory signal is obviously important, the presence of primed microglia is likely to be the key determinant of the severity of the CNS inflammatory response to this peripheral signal. It should also be noted that increased BBB permeability, as has been reported to occur with aging in humans (Zlokovic, 2011), could also facilitate, increased CNS inflammatory responses in susceptible individuals even when the degree of systemic inflammation is equivalent to healthy controls. Associated with these exaggerated CNS inflammatory responses in aged and degenerating brains are exaggerated sickness behaviour responses to subsequent peripheral stimulation with LPS (Combrinck et al., 2002; Godbout et al., 2005). Thus aged and diseased animals when challenged with LPS show profoundly suppressed locomotor activity and become more hypothermic than normal animals similarly challenged. Aged animals challenged with the staphylococcal toxin SEA showed prolonged hyponeophagia compared to young animals similarly challenged (decreased consumption of a novel food, without effect on consumption of familiar foods: considered a text of anxiety) (Kohman et al., 2009). Aged animals show exaggerated and prolonged ‘depressive’ responses to systemic inflammation (Godbout et al., 2008), continuing for up to 72 hours after systemic insult. It is of interest that low plasma tryptophan, an important precursor for serotonin synthesis, has been shown to associate with delirium in a number of studies (van der Mast et al., 1991; Robinson et al., 2008). However, rodent studies have shown that systemic LPS or IL-1β actually lead to increased brain tryptophan and increased turnover of both serotonin and NA (Wieczorek et al., 2005). An alternative proposal is that altered tryptophan metabolism affects mood not via serotonin depletion but via generation of kynurenine by indoleamine 2,3 dioxygenase (Dantzer et al., 2008). Indoleamine 2,3 dioxygenase shows exaggerated induction by LPS in aged animals (Henry et al., 2009) and kynurenine to tryptophan ratio has recently been shown to be associated with delirium in ICU patients (Adams Wilson et al., 2012).
The exaggerated sickness behaviour responses described above, if encompassing anxiety, affective, sleep-wake cycle, stress axis and cognitive aspects, provides a potential conceptual framework through which we might explain the exaggerated responses of the demented and aged to relatively mild systemic infections. As we have shown here, and continue to discuss in the following section, there are data showing dysregulated or exaggerated responses in most of these domains (see figure 2).
Figure 2.
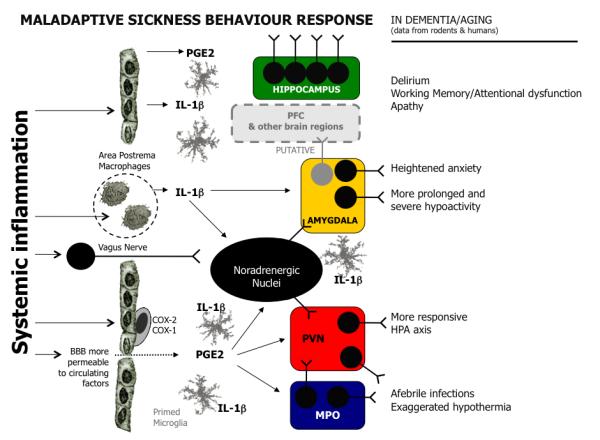
Maladaptive Sickness Behaviour response: This scheme superimposes observations from the rodent and clinical literature onto the broadly accepted model of sickness behaviour to propose ‘maladaptive sickness behaviour’. The pathways are largely as described in figure 1 with the following exceptions: increased blood brain barrier permeability may facilitate increased access of serum factors to the brain parenchyma, primed microglia (shown in grey) may cause amplified CNS responses to systemic inflammation, dysregulated HPA function may allow excessive stress responses. Putatively, altered HPA and amygdala responses, in addition to elevated inflammatory mediators may also have robust effects on the prefrontal cortex or other areas likely to be important in delirium in humans. Coupled with these changes, prior pathology in brain areas such as the cholinergic basal forebrain, the noradrenergic brainstem or the dopaminergic midbrain may have significant impacts on the presentation of inflammation-induced sickness behaviour. While the idea that delirium is compatible with an elevated and maladaptive sickness behavioural response remains speculative, evidence exists for marked hypoactivity and apathy (humans and rodents), dysregulated HPA function (humans and rodents), afebrile infections (humans) and exaggerated hypothermia (rodents), delirium (humans) and robustly impaired working memory/mental flexibility (rodents). Abbreviations: PGE2: prostaglandin E2, IL-1β: interleukin-1β; COX: cyclooxygenase; HPA: hypothalamic pituitary adrenal; PVN: paraventricular nucleus; MPO: medial preoptic nucleus.
Cognitive consequences during exaggerated sickness behaviour and clinical delirium
Although the microglial priming hypothesis has been adopted by researchers in the delirium field (van Gool et al., 2010), clear empirical evidence to support it in clinical populations has not yet been furnished. However, the psychoneuroimmunologists have addressed the hypothesis that systemic LPS stimulation would cause significant cognitive and behavioural impairments in the brains of animals made susceptible by old age or prior neurodegenerative disease. The first of these studies showed that infection with E.Coli impaired the ability of aged animals, compared to young animals, to remember and respond appropriately to the visuospatial context in which they previously received a foot-shock (Barrientos et al., 2006). This was associated with a higher CNS IL-1β response to the systemic infection. This study was an important proof of concept that exaggerated CNS inflammatory responses to equivalent systemic inflammation could produce exaggerated cognitive impairments. However, the contextual fear conditioning (CFC) paradigm appears to be especially sensitive to systemic stimulation (Pugh et al., 1998; Thomson & Sutherland, 2005). Also, it might not be the most informative for the type of cognitive impairment occuring during delirium, which is characterised by attentional deficits. This is because in the CFC task animals initially ‘attend to’ the context in the absence of an inflammatory stimulus but once inflammation is induced, immediately after the period of exploration, their memory for that context is impaired because IL-1β interferes with the consolidation of the memory. Surgical and injury induced-trauma are also frequent triggers of delirium and other post-operative cognitive dysfunction (POCD) in the population and this CFC task has also been used to interrogate POCD after tibial fracture in mice (Cibelli et al., 2010; Terrando et al., 2010). These studies have been performed only in young healthy mice and, like the earlier studies with LPS (Pugh et al., 1998; Thomson & Sutherland, 2005), have described a role for IL-1β in the deficits. These studies have the potential to be relevant to delirium, since they combine inflammatory and surgical elements of delirium risk, but the coupling of the severity of the surgical trauma to a very inflammation-sensitive cognitive task that shows impairments even under mild inflammatory stimulation in young healthy controls may limit the applicability of this paradigm to delirium.
Subsequent studies in aged rodents showed exaggerated impairments in aged animals challenged with moderate LPS in a measure of mental flexibility/working memory (Chen et al., 2008). In this study aged animals showed a reduced ability, with respect to young animals, to learn the new location of a hidden platform which is moved on each day of testing. In the aged animals LPS once again induced exaggerated inflammatory transcripts in the hippocampus, though other regions that are likely involved in the performance of this task were not examined. One interesting feature of the task employed was the daily reconfiguring of the maze: animals appear to learn that that the location of the platform will be different each day, but under the influence of LPS, this reorientation process is clearly more difficult. This is consistent with observations we have made in a shallow water Y-maze reference learning task. Animals with prior neurodegenerative disease (ME7 model of prion disease), challenged with LPS (100 μg/kg), made more errors in learning the location of the exit than did normal animals challenged with LPS. However, if diseased animals had previously learned the location of the exit, LPS had no impact on their ability to remember this location (Cunningham et al., 2009). Thus attention to/retention of new information and mental flexibility are more selectively targeted than visuospatial reference memory. This is consistent with a recent clinical study showing that patients with delirium were able to access previously learned information but showed impairments on tasks involving online processing of novel information, that is, fluid cognitive ability (Brown et al., 2011a). To directly assess whether animals with prior neurodegenerative pathology are more susceptible to working memory deficits, we developed a novel T-maze alternation task. This working memory task showed that normal animals challenged with LPS (100μg/kg) performed normally on this task, as did animals in the early stages of neurodegenerative disease. However, diseased animals challenged with LPS showed acute and transient working memory deficits (Murray et al., 2012). It is possible that these animals show an impairment of short-term memory: failing to remember the arm they had visited just 30 seconds earlier. Alternatively, the impairment might represent an attentional deficit: a failure to ‘attend to’ the location just visited. In clinical studies, the ability to maintain attention beyond approximately 30 seconds is known to be particularly badly affected in delirium, in contrast to Alzheimer’s dementia, where performance is similar to non-demented controls (Brown et al., 2011b). Thus there are parallels between the type of cognitive impairments seen in delirium and those in animals with prior age- or disease-associated vulnerability, and we have proposed the above paradigm (Murray et al., 2012) as a model with which to interrogate aspects of delirium during dementia. Collectively the data above allow us to propose that delirium, at least in its hypoactive form, represents an extreme form of sickness behaviour. However, this is not straightforward for a number of reasons, as we discuss below.
Exaggerated sickness or prior vulnerability?
In classical SBS, as induced by moderate systemic inflammation, affective and motivational changes predominate and cognition is not severely impaired. As discussed above, in the presence of a vulnerable brain, mild or moderate systemic inflammation induces significant cognitive deficits in addition to classical SBS. If the acute cognitive deficits induced by a systemic inflammatory insult superimposed on aging or neurodegenerative disease were a simple case of an exaggerated sickness behaviour response, then one might be able to replicate, in healthy young animals, these acute cognitive deficits by challenging normal/young animals with higher doses of LPS than those administered to ‘vulnerable’ animals. This would, in theory, induce an equivalent degree of sickness to that experienced in the aged/demented animals and could address the question of whether more sickness per se is sufficient to induce the observed cognitive deficit. In the case of aged animals, these experiments with higher LPS doses have not, to the best of our knowledge, been performed. With respect to animals with prior neurodegenerative disease (ME7), normal, aged-matched controls animals were challenged with 200 μg/kg and 500 μg/kg of LPS in the T-maze (Murray et al., 2012) and Y-maze (Cunningham et al., 2009) respectively, without effect on memory function in normal animals, when 100 μg/kg was sufficient to induce the cognitive deficits in diseased animals. This is an important control that verifies that deficits are indeed cognitive and not simply a sickness-related performance deficit. However, this also suggests that, with systemic inflammation, vulnerable brains are prone not only to exaggerated sickness behaviour but also appear particularly vulnerable to cognitive deficits and the results in the ME7 model make the case that the deficits observed when disease and LPS combine result from something more than an amplification of the normal adaptive pathways. The precise pathways by which this occurs remain to be defined, but we propose that prior pathology or other predisposing factors combine with exaggerated SBS to trigger the more global effects on cognition observed in delirium.
Prior pathology as a predisposing factor
In clinical populations, there are now multiple studies showing associations between plasma levels of pro-inflammatory cytokines and chemokines and delirium (Khan et al., 2011). These studies broadly confirm the hypothesis that systemic inflammation increases the likelihood of delirium and that greater severity of systemic inflammation makes delirium more likely. An important caveat is that, apart from old age, prior cognitive impairment is the major risk factor for delirium, and such impairment may also be associated with higher levels of these cytokines (Lee et al., 2009). Indeed, in some studies, associations between proinflammatory cytokines such as IL-6 and delirium are lost when adjusted for prior cognitive impairment (van Munster et al., 2010). These data argue, like those of the ME7 animal model of delirium during dementia (Murray et al., 2012) that, in humans, increased cytokines are associated with delirium but may not be sufficient to induce delirium in the absence of prior vulnerability.
The question therefore arises: does the pattern of baseline pathology predict the degree of vulnerability to developing acute cognitive dysfunction upon acute systemic inflammation and/or the phenotype of the acute dysfunction observed? The ME7 model of prion disease is a model of progressive neurodegenerative disease that is characterised by extracellular amyloidosis (PrPSc deposition), robust loss of presynaptic terminals and considerable neurodegeneration. At the time in prion disease when LPS challenges were made in the ME7 model there was already significant hippocampal synaptic loss (Murray et al., 2012) but no neuronal death (Cunningham et al., 2003). It is likely that the loss of functional synapses in the dorsal hippocampus makes these animals susceptible to acute dysfunction in this region upon systemic challenge with LPS. More recent work has shown that, as the disease progresses to further deterioration of the hippocampus and marked pathology in the dorsal thalamus (Cunningham et al., 2005a), synaptic density decreases further and these animals become progressively more susceptible to the cognition-interrupting effects of systemic LPS (Skelly & Cunningham, unpublished data). These data argue that as neuronal connectivity degenerates during disease (and perhaps ageing), the susceptibility to acute disruption grows. The clinical evidence suggests that prior cognitive impairment is the biggest risk factor predicting delirium, but studies have not yet addressed the issue of whether patients with dementia of greater severity are more likely to develop delirium than those with more mild impairments. Our data (Murray et al., 2012) rely on a model of prior hippocampal pathology and thus make predictions about subsequent acute hippocampal dysfunction. Those studies do not suggest that delirium consists of a hippocampal deficit. Rather they demonstrate that, and allow us to interrogate how, systemic inflammation and prior pathology, such as loss of synaptic integrity, interact to induce acute working memory deficits in the rodent brain. It is important to be clear that in humans delirium likely represents brain dysfunction considerably more distributed than the hippocampus (Choi et al., 2012). In many cases, delirium may not even include the hippocampus. Working memory is a hippocampal-dependent function in rodents and a frontocortical function in humans. One has to acknowledge this species difference and manipulate these models for the questions they do allow us to address. In this case, the models suggest that patterns of prior pathology influence the risk and nature of acute LPS-induced dysfunction but evidence that particular prior pathologies dictate the overall risk and symptom presentation in delirium (hypoactive/hyperactive, presence of psychotic features) is currently lacking. A systematic review of neuroimaging studies in delirium did report that increased white matter disease appeared to confer a higher degree of overall risk (Soiza et al., 2008). This finding is congruent with evidence suggesting that prior executive dysfunction, which is classically more likely to associate with vascular-type pathology than Alzheimer-type pathology, may particularly predispose to post-surgical delirium (Greene et al., 2009). These human data provide preliminary evidence that the type or region of prior pathology may influence delirium risk.
Recent animal studies also address this issue: it is believed that some delirium may be underpinned by cholinergic hypofunction, perhaps in combination with dopaminergic overactivity (Trzepacz, 2000). This hypothesis is based on the ability of anticholinergic drug treatments to induce delirium in the clinic (Tune et al., 1981). Indeed early animal models of delirium used the muscarinic receptor antagonist atropine to induce cognitive deficits and EEG changes consistent with delirium (Trzepacz et al., 1992). To address the possibility that systemic inflammation can effect dysfunction in the basal forebrain cholinergic system we have recently developed a further model to study aspects of pathophysiology of delirium during dementia (Field et al., 2012). We proposed that, by making limited lesions of the basal forebrain cholinergic system using the ribosomal toxin saporin linked to the p75 neurotrophin receptor (which is enriched on basal forebrain cholinergic neurons), we would make mice susceptible to the impact of LPS on cognitive tasks reliant on the basal forebrain cholinergic system. The novel T-maze working memory task described above was also shown to be cholinergic-dependent but neither animals with approximately 20% neuronal loss in the cholinergic medial septum and diagonal bands, nor normal animals challenged with LPS showed any deficits on this task. However, cholinergic lesioned animals, challenged 40 days later with LPS, showed acute and transient working memory deficits (Field et al., 2012). These deficits could be partially reversed by the administration of the acetylcholinesterase inhibitor donepezil, indicating that systemic inflammation induced impairments via disruption to cholinergic signalling. Thus, prior vulnerability in the cholinergic system made these animals susceptible to cognitive impairments induced by LPS (see figure 3). While there were robust systemic and CNS inflammatory responses in all LPS-treated animals there was no evidence in hypocholinergic animals of an exaggerated CNS inflammatory response to the systemic insult (Field et al., 2012). ThIs argues against the deficits being related to an exaggeration of the sickness behaviour response, at least as mediated by conventional inflammatory pathways. If prior pathology/loss of innervation contributes more significantly to delirium susceptibility than ‘inflammatory priming’, this predicts that patients and animals with prior pathology would also show increased susceptibility to insults that are not inflammatory in nature and this is certainly the case with delirium: old age, cognitive impairment and frailty predispose to all causes of delirium (MacLullich et al., 2008; Fong et al., 2009).
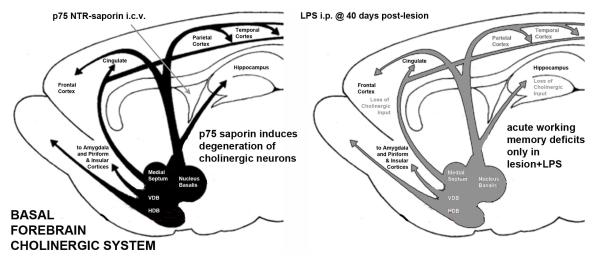
Figure 3.
Prior pathology and superimposed systemic inflammation interact to produce acute and transient working memory deficits: towards reconciling cholinergic and inflammatory hypotheses of delirium. This scheme shows the basal forebrain cholinergic nuclei (BFCN), illustrating the septohippocampal pathway, the ventral diagonal band projections to the amygdala and piriform and insular cortices and the nucleus basalis projection to the frontal, parietal and temporal cortices (after Wolff 1991). Intracerebroventricular injection (left panel) of the ribosomal toxin saporin, linked to an antibody against the p75 neurotrophin receptor, which is enriched on BFCN cells, induces selective neuronal death in these regions. This approach facilitates targeted, selective and limitable lesioning of this area and consequently decreased cholinergic tone (shown in grey, right panel) in these projection fields. In lesioned animals, LPS induces acute and transient working memory deficits, resembling delirium. Neither lesion nor LPS alone were sufficient to induce such deficits. LPS-induced CNS inflammatory responses were equivalent in lesioned and non-lesioned animals and the acetylcholinesterase inhibitor donepezil could block these deficits (Field et al., 2012). This model serves as a useful exemplar of how systemic inflammation in ‘vulnerable’ animals may be used to interrogate aspects of delirium pathophysiology.
The degree to which delirium is cholinergic-dependent has recently received increased attention in the wake of the clinical trial of of the acetylcholinesterase inhibitor, rivastigmine, to treat ICU delirium. The trial was based on the dual role of acetylcholine as a neuromodulator and as an endogenous inhibitor of systemic inflammatory responses to endotoxemia (Tracey, 2007). The trial was stopped early because of increased mortality in the treatment group and those in the treatment group actually had a greater severity and longer duration of delirium (van Eijk et al., 2010). In the light of these data it is tempting to speculate that the cholinergic hypothesis of delirium may be most relevant in those patients with prior vulnerability in the cholinergic system, as predominantly occurs in patients with dementia of the Alzheimer’s type (Ballard et al., 2011). Based on our own findings (Field et al., 2012), one might predict that acetylcholinesterase inhibitors, which boost ACh levels, might offer protection against inflammation-induced cognitive deficits, but specifically in these individuals who have a pre-existing cholinergic deficit. Since the ICU patients recently treated with rivastigmine were unlikely to have prior cholinergic deficiency, this may explain the lack of efficacy of cholinesterase inhibition in that patient group (van Eijk et al., 2010). Much has been written about acetylcholine in delirium (Trzepacz, 2000) and the parallels between Lewy body dementia, which shows marked cortical cholinergic depletion, and delirium are certainly striking (Perry & Perry, 1995). There is evidence that inflammation can directly interfere with ACh release (Taepavarapruk & Song, 2010) and the interaction between systemic inflammation and prior cholinergic depletion to induce acute and transient working memory deficits (Field et al., 2012) suggests a useful model with which to interrogate the pathophysiology of delirium, and the role of ACh after systemic inflammatory insult.
Does aetiology influence the phenotype of delirium or sickness behaviour?
Association studies lead to speculation that particular inflammatory cytokines have a key role in inducing delirium. Whether particular inflammatory mediators contribute to particular presentations of delirium has not been studied in any detail. In a cohort with Alzheimer’s disease systemic inflammatory events were found to exacerbate the rate of cognitive decline in these patients in the absence of detectable delirium (Holmes et al., 2009). These patients showed symptoms of sickness behaviour (apathy, anxiety, depression) and these were more frequent in AD patients with raised markers of systemic inflammation. They also had symptoms that often occur during delirium, such as agitation and hallucinations, which are not typically part of the sickness behaviour spectrum (Holmes et al., 2011). These data suggest that sickness behaviour is indeed exaggerated in dementia, as animal models of dementia initially suggested (Combrinck et al., 2002). These data also suggest the possibility that there may be associations between specific cytokines and different symptom profiles: IL-6 was significantly associated with apathy and hallucinations, while TNF-α was associated with agitation, depression and anxiety (Holmes et al., 2011). In the clinical setting, associations between particular inflammatory mediators and specific symptoms can not be elicited without large studies, but it is manifestly the case that some drugs are more likely to cause delirium in which sleepiness and reduced activity predominates (such as benzodiazepines), and other drugs may cause delirium with agitation (such as amphetamine). Studies examining this further would be valuable but are obviously challenging since there are usually many causes of delirium operating in concert.
Hyperactive versus hypoactive delirium
Another issue in evaluating the hypothesis of delirium as exaggerated sickness behaviour is the observation that systemic inflammation tends to induce a marked hypoactive state in animals and therefore may be incompatible with hyperactive delirium. Though a high proportion of delirious patients exhibit the hypoactive subtype, up to 20% of patients with delirium are classed as ‘hyperactive’. It is currently unknown if certain aetiologies favour certain subtypes nor if hyperactive delirium has a different neurochemical profile from hypoactive delirium, though there is limited evidence that hyperactivity occurs more readily in drug-induced cases (Meagher et al., 2011). This is a difficult question to answer because many cases of delirium show a mix of hypoactive and hyperactive phases, and some delirium shows no motor abnormalities. Interestingly, hypoactive delirium may be disproportionately more common in older people (Peterson et al., 2006). Is it possible for infection, which we so often associate with suppressed activity, to induce hyperactive or agitated states? In one study of 614 delirium cases in critically ill patients, less than 2% of these patients showed pure hyperactive delirium, although as many as 55% showed mixed subtype and 44% were hypoactive (Peterson et al., 2006), while another recent study suggested approximately 10% of ICU delirium was hyperactive (van den Boogaard et al., 2011b). In a study of admissions to a geriatric ward, hyperactive delirium was observed in one fifth of cases, while the mixed subtype was once again most common (O’Keeffe & Lavan, 1999). The same study noted, hovever, that there was no link between infection and any particular subtype. It remains difficult to explain how systemic infections/inflammation, which so often cause hypoactivity in animal models, can induce a hyperactive state in delirious patients. There is some evidence for agitation and hyperactivity as a result of various neurotropic infections in rodents, and systemic challenge with staphylococcal enterotoxins (SEA and B) can produce neuropsychological changes without suppression of activity despite robust synthesis of TNF-α (Kawashima & Kusnecov, 2002). It may be significant that SEA induces significant T-cell-mediated IL-2 synthesis, which has been shown to increase activity in mice (Zalcman et al., 1998) and to induce working memory deficits (Capuron et al., 2001) or indeed frank delirium (van Steijn et al., 2001) when given as a therapy in humans. However, it remains the case that systemic inflammation in rodents almost invariably induces hypoactivity.
Nonetheless, the available evidence does not allow us to state that infection is more likely than other aetiologies to cause hypoactive delirium. Does this invalidate the idea that exaggerated SBS is a valid model of delirium? We would argue that this simply reflects the fact that delirium in humans is more heterogeneous and complex than SBS in animal models. In the first instance, it is worth noting that the vast majority of the sickness behavior literature concerns LPS rather than active infection, thus removing several layers of complexity with respect to immunological profiles. Furthermore there is an absence, in animal models of SBS, of the heterogeneity in pre-existing brain pathology, in which patients can vary from having no pathology (i.e. young patients in intensive care units after trauma) to having various neurodegenerative disease processes present in most older people. In particular, vascular disease leading to white matter damage is almost universal in older people, and appears to be an important risk factor for delirium (Rudolph et al., 2007; Soiza et al., 2008). Yet there are no published studies examining the phenotype of SBS in animal models of vascular disease. It is conceivable that different patterns of prior pathology could alter how the brain responds to inflammatory signalling. Another factor is that in clinical practice, most delirium is multifactorial. Infection and drug side effects are commonly implicated, but there are many other likely factors. Some degree of psychological stress is present in all patients, resulting from pain, thirst, hunger, noise, temperature fluctuations and from being in an unfamiliar environment (MacLullich & Hall, 2011). Psychological stress resulting in HPA axis activation and secondary alterations in CNS neurochemistry modulates cognitive functioning towards a more vigilant, alert and cautious state (discussed below). In some patients this state could override any tendency towards somnolence stimulated by SBS processes. Sleep deprivation is also extremely common, and may persist for days, possibly leading to psychosis. Additionally, because patients with delirium are usually disorientated, they often struggle to make sense of what is happening to them. Therefore ‘agitation’, as detected on rating scales, may in some cases reflect a natural reaction to escape what is perceived to be a threatening situation and reach safety.
The hypothalamic-pituitary-axis (HPA) and delirium
An important pathway that may be activated in both hypo- and hyperactive states is the HPA axis. It is well established that systemic LPS or IL-1β challenge induces HPA axis activation and one recent study in healthy volunteers showed dose-dependent and large increases in plasma cortisol following LPS injection (Grigoleit et al., 2011). Indeed some studies of sickness behaviour and cognitive function have shown efficacy the glucocorticoid antagonists mifepristone (RU486) in reversing LPS-induced impairments (Song et al., 2004). Likewise ageing (Sapolsky et al., 1986) and dementia (de Leon et al., 1988) are associated with abnormalities in the HPA axis, with higher levels, higher trough levels in the circadian rhythm, and prolonged high levels after stress (Magri et al., 2006). Thus, there is an age-related dysregulation of the HPA axis that makes it more responsive to acute stressors. Aside from the impact of this on PFC cortex function (discussed below), this also has interesting consequences for brain inflammation: in recent years it has become clear that increased corticosterone, in rodents, actually sensitizes microglia in the hippocampus and prefrontal cortex to produce exaggerated responses to subsequent challenge with LPS (de Pablos et al., 2006; Munhoz et al., 2006). Age-related priming of microglia can be reduced significantly by adrenalectomy or RU486 treatment (Frank et al., 2012). In health, HPA activation induced by inflammation serves to mobilise energy resources, and also limit the inflammatory response.
However, where the inflammatory stimulus is prolonged or abnormal, the HPA axis may become dysfunctional. This may take the form of very high levels which can persist for weeks and which are associated with worse prognosis (Marik, 2011).
The notion that elevated endogenous cortisol might be important in precipitating delirium was suggested more than 30 years ago (Swigar et al., 1979). This idea has been refined (MacLullich et al., 2008) as we have learned more about HPA dysfunction: ageing and neurodegenerative disease are associated with dysregulation of the HPA axis such that, upon acute stress, cortisol levels take longer to return to basal levels (Otte et al., 2005; Magri et al., 2006). Sustained elevated endogenous cortisol and exogenous treatment with cortisol or analogues are associated with cognitive and/or neuropsychiatric dysfunction particularly in older and other vulnerable populations (Dubovsky et al., 2012). Several studies have directly addressed the association of the stress axis with delirium. These generally show that cortisol levels are elevated in delirium (MacLullich et al., 2008; Pearson et al., 2010; van den Boogaard et al., 2011a) and elevated cortisol levels in the cerebrospinal fluid have also been shown to associate with delirium in hip fracture patients (Pearson et al., 2010). Two further studies showed that elevated cortisol post-dexamethasone (ie failure to suppress cortisol production in the dexamethasone suppression test (DST)) were significantly more likely to show delirium (Olsson et al., 1992). More recent studies, many of which are also small, showed that elevated plasma cortisol was significantly associated with delirium in hip fracture (van Munster et al., 2010; Bisschop et al., 2011), cardiac surgery (Plaschke et al., 2010), coronary artery bypass graft (Mu et al., 2010) and sepsis (Pfister et al., 2008). Interestingly, in one study the association between plasma cortisol and delirium did not withstand adjustment for prior cognitive impairment suggesting the possibility of a strong influence of the association between elevated cortisol and prior dementia or cognitive impairment (van Munster et al., 2010). However, in an earlier study failure to suppress in the dexamethasone suppression test showed that delirium during dementia was nonetheless significantly different to dementia alone (Robertsson et al., 2001). Moreoever, higher cortisol in dementia, and an associated elevated response to stress, may be a means by which dementia confers the greater risk of delirium in these patients. Therefore, both explanations for the association are plausible and indeed may coexist. Additionally, as with systemic inflammation, it may be that old age and the presence of neurodegenerative disease render an individual more likely to react adversely to higher levels of cortisol. Notwithstanding the plausibility of the link between increased stress and delirium, causality cannot be inferred from these studies and intervention studies in animal models could usefully address this possibility.
The stress response and delirium: mechanisms
That delirium is mostly precipitated by stress, defined broadly as any form of acute threat to homeostasis, is unarguable. The evidence suggesting that there are associations between activation of stress-responsive systems such as the HPA axis and the immune system and delirium is consistent with this axiom. However, the pathways from stress stimuli to delirium are not yet defined. Given that inattention is the core cognitive feature of delirium, understanding how stress might disrupt attention would seem a reasonable place to start.
The ability of stress to induce hyperactive, inattentive and psychotic states is consistent with an existing literature on the nature of brain regulation of behaviour under conditions of significant psychological stress. Arnsten (Arnsten, 2009) has proposed that when stress pathways in the brain are strongly activated, the usual prefrontal cortex ‘top-down’ control of attention, monitoring of errors, inhibition of inappropriate responses and regulation of emotionality is disrupted. Instead, cognition and attention change to a ‘bottom up’ control exerted by the amygdala in which more rapid emotional and instinctive control along with greater vigilance and caution predominates. Activation of the amygdala, as a result of perceived threat, stimulates the basal ganglia and the hypothalamus and evokes higher levels of noradrenaline (NA) and dopamine (DA) release in the PFC. Concentrations of NA and DA modulate PFC functions such as working memory and executive attention through inverted U-shaped dose response curves. With very low concentrations of these catecholamines the PFC is insufficiently activated and there is reduced arousal. Optimal PFC function occurs when NA and DA are in the concentration range where high affinity α2-adrenoreceptors are occupied and facilitate arousal and D1 receptors and are sufficiently occupied to exert “signal to noise” suppression of inappropriate activity. Under much higher concentrations D1 receptors and α1 receptors are then fully engaged and there is generalised suppression of PFC activity: instead of DA suppression of ‘noise’ to improve signal to noise ratio, signal has been suppressed in a generalised way and PFC control of function is diminished. Spatial working memory is impaired in both rodents and primates by increased DA turnover in the PFC (Murphy et al., 1996). A detailed discussion of this important area is beyond the scope of this review but is discussed in an excellent recent review (Arnsten, 2009). Returning to systemic inflammation, there are elegant animal and human studies showing that systemic LPS can activate the amygdala, increase EEG power and increase release of NA in this area (Engler et al., 2011; Inagaki et al., 2012). Moreover, lesioning of the amygdala is sufficient to prevent the increase of NA and DA in the PFC in response to psychological stress (Goldstein et al., 1996). Consistent with this model, drugs such as amphetamine, cocaine and corticosteroids, which increase PFC monoamines, are all recognised causes of delirium.
Therefore, in amygdala-driven stimulation of catecholaminergic activation of the PFC, we have a common link between systemic inflammation and stress axis activation that is highly relevant to delirium etiology. Furthermore, HPA axis activation can perhaps link multiple stressors: psychological stress, pain, inflammation, to direct pharmacological disruptions in a final common pathway that ultimately results in increased catecholaminergic signalling and diminished PFC influence over brain function. Thus, whether the overall level of arousal is decreased (as is usually the case with systemic inflammation) or increased one may still observe significant attentional and working memory deficits underpinned by HPA axis activation and consequent increases in DA and NA. Since increased dopaminergic tone allied to decreased cholinergic tone consititutes perhaps the most adopted hypothesis of delirium etiology (Trzepacz, 2000) this makes the amygdala driven suppression of PFC activity an attractive explanation of how such a state might come about, particularly when one considers the decreased cholinergic tone apparent in most patients with dementia (Ballard et al., 2011).
However, the data supporting the above formulation mainly pertains to adaptive changes in cognition. That is, the brain has evolved to switch to a more rapid and reflexive, less thoughtful, mode of action in situations of acute stress. In health, at least, the changes above would tend to make the organism more awake and active. Most patients with delirium are hypoactive some, or all, of the time and direct evidence for amygdala activation and increased DA and NA in the PFC in delirium or models of delirium is lacking.
Conclusion and future perspective
In this review we have proposed that mapping SBS in vulnerable rodents (aged or with prior neurodegeneration) onto delirium in humans is both plausible and supported by clinical and basic research studies. However, the relationship is not simple: there are key differences between SBS in vulnerable rodents and delirium in humans with respect to aetiologies, varying levels of co-morbidity, varying phenomenology (including hyperactivity), and obviously differences between the rodent and human brains. Put simply, delirium likely results from an acute breakdown of coherent functioning of the CNS. While exaggerated SBS appears to be a prominent underlying mechanism of this phenomenon, there are clearly multiple interacting and alternative pathways that could contribute to this breakdown. These include the effects of drugs, psychological stress leading to elevated cortisol (perhaps leading to PFC/amygdala switching), and direct insults such as hypoxia, all superimposed onto heterogenous prior pathologies. However, we believe that despite the complexities of the problem, developing a better understanding of these mechanisms and pathways is feasible. The focus of this article, studying SBS in vulnerable models, provides an exemplar of a tractable approach to one probable major class of delirium pathophysiology. Future research combining systemic inflammation, psychological stress and indeed systemically administered stimulant drugs with well described prior pathologies could also begin to address the hyperactive behavioural phenotype seen in some patients with delirium.
It will also be important in the coming years to formalise measures of SBS in patients and to develop methods and clinical study designs that allow us to assess the CNS inflammation occuring during delirium in patients and to directly test the microglial priming hpothesis. A small number of studies have already provided evidence that elevated CNS IL-8 (MacLullich et al., 2011) and microglial activation (van Munster et al., 2011) are associated with delirium, but these are small studies and need to be expanded in size and scope. The paucity of research in delirium belies its enormous economic and medical importance, and the field of basic research, in particular, is in its infancy. At the extreme end of the psychoneuroimmunological spectrum seems like a sensible place to start.
DELIRIUM CASE HISTORY.
An 82-year-old man with a history of mild dementia, ischaemic heart disease and osteoarthritis who lived with his wife was taken to the emergency department with confusion and agitation, and productive cough and fever for 2 days. He had resisted being brought to the hospital initially but had then become drowsy and had to be lifted onto the ambulance trolley. Upon arrival he became agitated, saying that he didn’t want to be ‘locked up’, and he tried to get off the trolley to leave the hospital. With reassurance from his wife he became calmer. A few minutes later he was drowsy again. On examination he was able to say his name on request but did not respond meaningfully to other questions and only intermittently obeyed simple commands such as ‘lift up your arms’. The pulse was raised at 98 and the blood pressure was 108/64. Oxygen saturations were normal and the temperature was elevated at 37.8 degrees C. Chest examination revealed signs of a pneumonia but chest X-ray was not possible because the patient could not tolerate the procedure. Blood tests showed a raised white cell count and evidence of mild acute kidney injury. The patient was treated with antibiotics and intravenous fluids and within one day was less agitated. His vital signs all become normal. He became more compliant with treatment and had no further episodes of agitation. However, he remained drowsy and at times was too sleepy even to exchange a few words. When awake his speech was intermittently coherent but he was unable to sustain meaningful communication for more than a few seconds at a time. He was disorientated and unable to count backwards from 20 down to 1. His wife stated that his mental status was much worse than normal. After three more days he was more consistently awake but remained unable to sustain normal conversation. He was also unsteady when walking and required assistance to do this safely. Seven days after admission he was transferred to a rehabilitation facility, and after a further two weeks his mental and physical status had improved sufficiently such that he was discharged home. However, when visited by the general practitioner two weeks later his wife reported that though he was improving he was still sometimes disorientated, and also considerably more passive and apathetic than he had been before his illness.
Urinary Tract Infection and Delirium.
Urinary tract infections, predominantly gram-negative E. Coli infections in humans, are a very frequent trigger of delirium in older people. The reasons for this are not clear. These infections can often cause bacteremia, but this is estimated to occur in only about 15% of cases (Bahagon et al., 2007) and in many cases of UTI, there may not be a classical systemic inflammatory response (Ginde et al., 2004). In animal models of uropathogenic infection, intravenously inoculated E. Coli became systemically disseminated (Smith et al., 2010). However, when LPS was adminstered to the bladder via a catheter, inflammatory products such as iNOS were detectable in the bladder but not the kidney (Olsson et al., 1998). Thus UTI does not necessarily produce systemic inflammation and indeed UTI-induced delirium does not necessarily require clinically apparent systemic inflammation. It is unclear whether there is a specific neural mechanism of inflammatory sensing in the urinary tract, though afferents in the urothelium may potentially serve this purpose (Birder et al., 2010). It is well known that such a pathway exists to signal visceral inflammation to the brain, via activation of vagal afferents: behavioural suppression induced by systemic LPS or IL-1β can be prevented by prior subdiaphragm transection of the vagus (Konsman et al., 2000). Another possibility is that even minor infections or trauma causing macrophage activation may lead to release of circulating mediators at levels not easy to detect but which nevertheless elicit CNS change. Increased permeability of the blood brain barrier seen with ageing and in neurodegenerative disease (Zlokovic, 2011) and the priming of microglia by prior pathology (Cunningham et al., 2005b) may both contribute to a pathological CNS amplification of the signal from circulating messengers. Given the importance of UTIs as a cause of delirium in older people, the study of this interaction may provide valuable insights.
Acknowledgements
CC is supported by a Senior Fellowship from the Wellcome Trust. AM is a member of The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative. Funding from the BBSRC, EPSRC, ESRC and MRC is gratefully acknowledged.
References
- Adams Wilson J, Morandi A, Girard TD, Thompson J, Boomershine C, Shintani A, Ely E, Pandharipande P. The association of the kynurenine pathway of tryptophan metabolism with acute brain dysfunction during critical illness. Critical Care Medicine. 2012;40:835–841. doi: 10.1097/CCM.0b013e318236f62d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahagon Y, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Prevalence and predictive features of bacteremic urinary tract infection in emergency department patients. Eur J Clin Microbiol Infect Dis. 2007;26:349–352. doi: 10.1007/s10096-007-0287-3. [DOI] [PubMed] [Google Scholar]
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F. Long-term cognitive impairment in sepsis survivors. Crit Care Med. 2005;33:1671. doi: 10.1097/01.ccm.0000170192.54682.c1. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, Cruz F, Moore K, Fry CH. Is the urothelium intelligent? Neurourol Urodyn. 2010;29:598–602. doi: 10.1002/nau.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisschop PH, de Rooij SE, Zwinderman AH, van Oosten HE, van Munster BC. Cortisol, insulin, and glucose and the risk of delirium in older adults with hip fracture. J Am Geriatr Soc. 2011;59:1692–1696. doi: 10.1111/j.1532-5415.2011.03575.x. [DOI] [PubMed] [Google Scholar]
- Bozza FA, Garteiser P, Oliveira MF, Doblas S, Cranford R, Saunders D, Jones I, Towner RA, Castro-Faria-Neto HC. Sepsis-associated encephalopathy: a magnetic resonance imaging and spectroscopy study. J Cereb Blood Flow Metab. 2010;30:440–448. doi: 10.1038/jcbfm.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LJ, Ferner HS, Robertson J, Mills NL, Pessotto R, Deary IJ, MacLullich AM. Differential effects of delirium on fluid and crystallized cognitive abilities. Arch Gerontol Geriatr. 2011a;52:153–158. doi: 10.1016/j.archger.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Brown LJ, Fordyce C, Zaghdani H, Starr JM, MacLullich AM. Detecting deficits of sustained visual attention in delirium. J Neurol Neurosurg Psychiatry. 2011b;82:1334–1340. doi: 10.1136/jnnp.2010.208827. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med. 2001;63:376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Lee H, Chung TS, Park KM, Jung YC, Kim SI, Kim JJ. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry. 2012;169:498–507. doi: 10.1176/appi.ajp.2012.11060976. [DOI] [PubMed] [Google Scholar]
- Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Cunningham C. Microglia in neurodegeneration: role of systemic inflammation. GLIA. 2012 doi: 10.1002/glia.22350. in press. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Deacon R, Wells H, Boche D, Waters S, Diniz CP, Scott H, Rawlins JN, Perry VH. Synaptic changes characterize early behavioural changes in the ME7 model of murine prion disease. Eur. J. Neurosci. 2003;17:2147–2155. doi: 10.1046/j.1460-9568.2003.02662.x. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Deacon RM, Chan K, Boche D, Rawlins JN, Perry VH. Neuropathologically distinct prion strains give rise to similar temporal profiles of behavioral deficits. Neurobiol Dis. 2005a;18:258–269. doi: 10.1016/j.nbd.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Sanderson DJ. Malaise in the water maze: untangling the effects of LPS and IL-1beta on learning and memory. Brain Behav Immun. 2008;22:1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005b;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Terrera G, Keage H, Rahkonen T, Oinas M, Mathews F, Cunningham C, Polvikoski T, Sulkava R, MacLullich A, Brayne C. Delirium is a strong risk factor for dementia in the oldest old: a population-based cohort study. Brain. 2012 doi: 10.1093/brain/aws190. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30:421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, McRae T, Tsai JR, George AE, Marcus DL, Freedman M, Wolf AP, McEwen B. Abnormal cortisol response in Alzheimer’s disease linked to hippocampal atrophy. Lancet. 1988;2:391–392. doi: 10.1016/s0140-6736(88)92855-3. [DOI] [PubMed] [Google Scholar]
- de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovsky AN, Arvikar S, Stern TA, Axelrod L. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics. 2012;53:103–115. doi: 10.1016/j.psym.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Engler H, Doenlen R, Engler A, Riether C, Prager G, Niemi MB, Pacheco-Lopez G, Krugel U, Schedlowski M. Acute amygdaloid response to systemic inflammation. Brain Behav Immun. 2011;25:1384–1392. doi: 10.1016/j.bbi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Field RH, Gossen A, Cunningham C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. Journal of Neuroscience. 2012;32:6288–6294. doi: 10.1523/JNEUROSCI.4673-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5:210–220. doi: 10.1038/nrneurol.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyashiki T, Narumiya S. Roles of prostaglandin E receptors in stress responses. Curr Opin Pharmacol. 2009;9:31–38. doi: 10.1016/j.coph.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Chen CC, Goehler LE. Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: evidence for parallel viscerosensory pathways in the rat brain. Brain Res. 2007;1130:130–145. doi: 10.1016/j.brainres.2006.10.084. [DOI] [PubMed] [Google Scholar]
- Ginde AA, Rhee SH, Katz ED. Predictors of outcome in geriatric patients with urinary tract infections. J Emerg Med. 2004;27:101–108. doi: 10.1016/j.jemermed.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene NH, Attix DK, Weldon BC, Smith PJ, McDonagh DL, Monk TG. Measures of executive function and depression identify patients at risk for postoperative delirium. Anesthesiology. 2009;110:788–795. doi: 10.1097/aln.0b013e31819b5ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One. 2011;6:e28330. doi: 10.1371/journal.pone.0028330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoleit JS, Oberbeck JR, Lichte P, Kobbe P, Wolf OT, Montag T, del Rey A, Gizewski ER, Engler H, Schedlowski M. Lipopolysaccharide-induced experimental immune activation does not impair memory functions in humans. Neurobiol Learn Mem. 2010;94:561–567. doi: 10.1016/j.nlm.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009a;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009b;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stutzman DL, Bland ST, Barrientos RM, Watkins LR, Rudy JW, Maier SF. Prostaglandins are necessary and sufficient to induce contextual fear learning impairments after interleukin-1 beta injections into the dorsal hippocampus. Neuroscience. 2007;150:754–763. doi: 10.1016/j.neuroscience.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Cunningham C, Zotova E, Culliford D, Perry VH. Proinflammatory cytokines, sickness behavior, and Alzheimer disease. Neurology. 2011;77:212–218. doi: 10.1212/WNL.0b013e318225ae07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH. Systemic inflammation and disease progression in Alzheimer’s disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Wang H, Matsumoto N, Muroya T, Shimazaki J, Ogura H, Shimazu T. Interleukin-1beta causes long-term potentiation deficiency in a mouse model of septic encephalopathy. Neuroscience. 2011;187:63–69. doi: 10.1016/j.neuroscience.2011.04.063. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59:3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32:3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima N, Kusnecov AW. Effects of staphylococcal enterotoxin A on pituitary-adrenal activation and neophobic behavior in the C57BL/6 mouse. J Neuroimmunol. 2002;123:41–49. doi: 10.1016/s0165-5728(01)00486-6. [DOI] [PubMed] [Google Scholar]
- Khan BA, Zawahiri M, Campbell NL, Boustani MA. Biomarkers for delirium--a review. J Am Geriatr Soc. 2011;59(Suppl 2):S256–261. doi: 10.1111/j.1532-5415.2011.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Crowell B, Urbach-Ross D, Kusnecov AW. Influence of age on behavioral, immune and endocrine responses to the T-cell superantigen staphylococcal enterotoxin A. Eur J Neurosci. 2009;30:1329–1338. doi: 10.1111/j.1460-9568.2009.06921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Luheshi GN, Bluthe RM, Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci. 2000;12:4434–4446. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci. 2008;28:2499–2510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Lee KS, Chung JH, Choi TK, Suh SY, Oh BH, Hong CH. Peripheral cytokines and chemokines in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2009;28:281–287. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- MacLullich AM, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21:30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- MacLullich AM, Edelshain BT, Hall RJ, de Vries A, Howie SE, Pearson A, Middleton SD, Gillies F, Armstrong IR, White TO, Cunningham C, de Rooij SE, van Munster BC. Cerebrospinal Fluid Interleukin-8 Levels Are Higher in People with Hip Fracture with Perioperative Delirium Than in Controls. J Am Geriatr Soc. 2011;59:1151–1153. doi: 10.1111/j.1532-5415.2011.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65:229–238. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLullich AM, Hall RJ. Who understands delirium? Age Ageing. 2011;40:412–414. doi: 10.1093/ageing/afr062. [DOI] [PubMed] [Google Scholar]
- Magri F, Cravello L, Barili L, Sarra S, Cinchetti W, Salmoiraghi F, Micale G, Ferrari E. Stress and dementia: the role of the hypothalamicpituitary-adrenal axis. Aging Clin Exp Res. 2006;18:167–170. doi: 10.1007/BF03327435. [DOI] [PubMed] [Google Scholar]
- Marik PE. Glucocorticoids in sepsis: dissecting facts from fiction. Crit Care. 2011;15:158. doi: 10.1186/cc10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher D, Adamis D, Trzepacz P, Leonard M. Features of subsyndromal and persistent delirium. Br J Psychiatry. 2012;200:37–44. doi: 10.1192/bjp.bp.111.095273. [DOI] [PubMed] [Google Scholar]
- Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: relationship with other phenomenology, etiology, medication exposure and prognosis. J Psychosom Res. 2011;71:395–403. doi: 10.1016/j.jpsychores.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Mu DL, Wang DX, Li LH, Shan GJ, Li J, Yu QJ, Shi CX. High serum cortisol level is associated with increased risk of delirium after coronary artery bypass graft surgery: a prospective cohort study. Crit Care. 2010;14:R238. doi: 10.1186/cc9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci U S A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, Cunningham C. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging. 2012;33:603–616 e603. doi: 10.1016/j.neurobiolaging.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe ST, Lavan JN. Clinical significance of delirium subtypes in older people. Age Ageing. 1999;28:115–119. doi: 10.1093/ageing/28.2.115. [DOI] [PubMed] [Google Scholar]
- Olsson LE, Wheeler MA, Sessa WC, Weiss RM. Bladder instillation and intraperitoneal injection of Escherichia coli lipopolysaccharide upregulate cytokines and iNOS in rat urinary bladder. J Pharmacol Exp Ther. 1998;284:1203–1208. [PubMed] [Google Scholar]
- Olsson T, Marklund N, Gustafson Y, Nasman B. Abnormalities at different levels of the hypothalamic-pituitary-adrenocortical axis early after stroke. Stroke. 1992;23:1573–1576. doi: 10.1161/01.str.23.11.1573. [DOI] [PubMed] [Google Scholar]
- Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Park SM, Gaykema RP, Goehler LE. How does immune challenge inhibit ingestion of palatable food? Evidence that systemic lipopolysaccharide treatment modulates key nodal points of feeding neurocircuitry. Brain Behav Immun. 2008;22:1160–1172. doi: 10.1016/j.bbi.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson A, de Vries A, Middleton SD, Gillies F, White TO, Armstrong IR, Andrew R, Seckl JR, Maclullich AM. Cerebrospinal fluid cortisol levels are higher in patients with delirium versus controls. BMC Res Notes. 2010;3:33. doi: 10.1186/1756-0500-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Perry RH. Acetylcholine and hallucinations: disease-related compared to drug-induced alterations in human consciousness. Brain Cogn. 1995;28:240–258. doi: 10.1006/brcg.1995.1255. [DOI] [PubMed] [Google Scholar]
- Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, Ely EW. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- Pfister D, Siegemund M, Dell-Kuster S, Smielewski P, Ruegg S, Strebel SP, Marsch SC, Pargger H, Steiner LA. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12:R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, Karck M, Kopitz J. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. 2010;36:2081–2089. doi: 10.1007/s00134-010-2004-4. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Robertsson B, Blennow K, Brane G, Edman A, Karlsson I, Wallin A, Gottfries CG. Hyperactivity in the hypothalamic-pituitary-adrenal axis in demented patients with delirium. Int Clin Psychopharmacol. 2001;16:39–47. doi: 10.1097/00004850-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Robinson TN, Raeburn CD, Angles EM, Moss M. Low tryptophan levels are associated with postoperative delirium in the elderly. Am J Surg. 2008;196:670–674. doi: 10.1016/j.amjsurg.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120:807–813. doi: 10.1016/j.amjmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The adrenocortical axis in the aged rat: impaired sensitivity to both fast and delayed feedback inhibition. Neurobiol Aging. 1986;7:331–335. doi: 10.1016/0197-4580(86)90159-4. [DOI] [PubMed] [Google Scholar]
- Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, Heneka MT. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204:733–740. doi: 10.1016/j.expneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Semmler A, Hermann S, Mormann F, Weberpals M, Paxian SA, Okulla T, Schafers M, Kummer MP, Klockgether T, Heneka MT. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5:38. doi: 10.1186/1742-2094-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30:144–157. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Adachi N, Liu K, Lei B, Nagaro T, Arai T. Sepsis facilitates brain serotonin activity and impairs learning ability in rats. Brain Res. 1999;830:94–100. doi: 10.1016/s0006-8993(99)01396-7. [DOI] [PubMed] [Google Scholar]
- Smith SN, Hagan EC, Lane MC, Mobley HL. Dissemination and systemic colonization of uropathogenic Escherichia coli in a murine model of bacteremia. MBio. 2010;1 doi: 10.1128/mBio.00262-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiza RL, Sharma V, Ferguson K, Shenkin SD, Seymour DG, Maclullich AM. Neuroimaging studies of delirium: a systematic review. J Psychosom Res. 2008;65:239–248. doi: 10.1016/j.jpsychores.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Song C, Phillips AG, Leonard BE, Horrobin DF. Ethyl-eicosapentaenoic acid ingestion prevents corticosterone-mediated memory impairment induced by central administration of interleukin-1beta in rats. Mol Psychiatry. 2004;9:630–638. doi: 10.1038/sj.mp.4001462. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. Distinct Roles for Cyclooxygenases 1 and 2 in Interleukin-1-Induced Behavioral Changes. J Pharmacol Exp Ther. 2002;302:1031–1036. doi: 10.1124/jpet.102.036640. [DOI] [PubMed] [Google Scholar]
- Swigar ME, Kolakowska T, Quinlan DM. Plasma cortisol levels in depression and other psychiatric disorders: a study of newly admitted psychiatric patients. Psychol Med. 1979;9:449–455. doi: 10.1017/s0033291700031986. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Song C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: effects of omega-3 fatty acid EPA treatment. J Neurochem. 2010;112:1054–1064. doi: 10.1111/j.1471-4159.2009.06524.x. [DOI] [PubMed] [Google Scholar]
- Teeling JL, Cunningham C, Newman TA, Perry VH. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: Implications for a role of COX-1. Brain Behav Immun. 2010;24:409–419. doi: 10.1016/j.bbi.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res Bull. 2005;67:24–29. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5:132–148. doi: 10.153/SCNP00500132. [DOI] [PubMed] [Google Scholar]
- Trzepacz PT, Leavitt M, Ciongoli K. An animal model for delirium. Psychosomatics. 1992;33:404–415. doi: 10.1016/S0033-3182(92)71945-8. [DOI] [PubMed] [Google Scholar]
- Tune LE, Damlouji NF, Holland A, Gardner TJ, Folstein MF, Coyle JT. Association of postoperative delirium with raised serum levels of anticholinergic drugs. Lancet. 1981;2:651–653. doi: 10.1016/s0140-6736(81)90994-6. [DOI] [PubMed] [Google Scholar]
- van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, Pickkers P. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. 2011a;15:R297. doi: 10.1186/cc10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaard M, Schoonhoven L, van der Hoeven JG, van Achterberg T, Pickkers P. Incidence and short-term consequences of delirium in critically ill patients: A prospective observational cohort study. Int J Nurs Stud. 2011b doi: 10.1016/j.ijnurstu.2011.11.016. [DOI] [PubMed] [Google Scholar]
- van der Mast RC, Fekkes D, Moleman P, Pepplinkhuizen L. Is postoperative delirium related to reduced plasma tryptophan? Lancet. 1991;338:851–852. doi: 10.1016/0140-6736(91)91504-n. [DOI] [PubMed] [Google Scholar]
- van Eijk MM, Roes KC, Honing ML, Kuiper MA, Karakus A, van der Jagt M, Spronk PE, van Gool WA, van der Mast RC, Kesecioglu J, Slooter AJ. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376:1829–1837. doi: 10.1016/S0140-6736(10)61855-7. [DOI] [PubMed] [Google Scholar]
- van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- van Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, de Rooij SE. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res. 2011;14:615–622. doi: 10.1089/rej.2011.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Munster BC, Bisschop PH, Zwinderman AH, Korevaar JC, Endert E, Wiersinga WJ, Oosten HE, Goslings JC, Rooij SE. Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn. 2010;74:18–23. doi: 10.1016/j.bandc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- van Steijn JH, Nieboer P, Hospers GA, de Vries EG, Mulder NH. Delirium after interleukin-2 and alpha-interferon therapy for renal cell carcinoma. Anticancer Res. 2001;21:3699–3700. [PubMed] [Google Scholar]
- Wieczorek M, Swiergiel AH, Pournajafi-Nazarloo H, Dunn AJ. Physiological and behavioral responses to interleukin-1beta and LPS in vagotomized mice. Physiol Behav. 2005;85:500–511. doi: 10.1016/j.physbeh.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zalcman S, Murray L, Dyck DG, Greenberg AH, Nance DM. Interleukin-2 and -6 induce behavioral-activating effects in mice. Brain Res. 1998;811:111–121. doi: 10.1016/s0006-8993(98)00904-4. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]