Abstract
IFN-lambda (IFN-λ) induces an antiviral state in many cell types and may contribute to the overall inflammatory environment following infection. Either of these effects may influence adaptive immune responses, but the role of type-3 interferons in the development of primary and memory T cell responses to infection has not been evaluated. Herein, we examined T cell responses to acute or persistent lymphocytic choriomeningitis virus (LCMV) infection in IFN-λR1-deficient mice. Following acute infection, we find that IFN-λR1-deficient mice produced normal levels of interferon, robust NK cell responses, but greater than normal CD4+ and CD8+ T cell responses compared to WT Balb/c mice. There were more T cells that were IL-7Rhi and, correspondingly, the IFN-λR-deficient mice showed a 2–3-fold increase in memory T cell number. The inhibitory effect of IFN-λR expression was independent of direct cytokine signaling into T cells. In contrast to acute infection, the IFN-λR-deficient mice generated markedly diminished T cell responses and had greater weight loss compared to WT mice when confronted with a highly disseminating variant of LCMV. These data indicate that IFN-λR limits T cell responses and memory following transient infection but augments T cell responses during persisting infection. Thus, the immune regulatory functions for IFN-λR are complex and vary with the overall inflammatory environment.
Introduction
Interferons (IFN) play a key role in limiting virus replication and stimulating adaptive immune responses against virus infections. The IFN-λs (a.k.a.: type-III IFN; IL-28/29) are a new family of interferons (1–3) that are found in many species, including humans, mice, bats, chickens, amphibians, and fish (4–7). There are three subtypes of IFN-λ in humans (λ1, λ2, λ3) and two in mice (λ2 & λ3; λ1 is a pseudogene). IFN-λ is highly conserved in human populations, implying strong evolutionary selection for these genes for protection against infections (8). Genetic polymorphisms in IFN-λ are associated with either enhanced clearance of HCV or poor outcomes (9–13). While several models demonstrate that IFN-λ signals reduce virus replication in cell lines or in vivo, the role of type-III interferons in adaptive immune responses is less well understood.
IFN-λ are induced by many cell types, including pDCs, cDCs, peritoneal macrophages, T cells, B cells, eosinophils, hepatocytes, neuronal cells, and epithelial cells, after virus infections or following activation of TLRs-3, -4, -7, -9, stimulation of RIG-I, or Ku70 (9, 14–25). IFN-λs are induced by either IRF3, IRF7, or NFkB pathways (1). The IFN-λs bind as monomers to the λR1 (IL-28Rα), which then pairs with IL-10Rβ to form the functional heterodimer receptor (2, 3). λR signals are transmitted through the JAK1/TyK2, STAT1, STAT2, STAT3, STAT5, and IRF-9 pathways to induce transcription of IFN-stimulated genes via ISGF3 (1, 26–28). These signals result in the induction of 2′-5′ oligoadenylate synthetase (OAS), serine/threonine protein kinase (PKR), ISG56, and IFN-λ2/3 (14, 28). By comparison with IFN-αβR signals, IFN-λR induces longer-lived activated (tyrosine-phosphorylated) STAT1 and STAT2 and more strongly induces interferon responsive genes (MX-1, ISG15, TRAIL, SOCS1) (29).
IFN-λ blocks the replication of numerous viruses in vitro, including encephalomyocarditis virus (14), West Nile virus (30), vaccinia virus (22), vesicular stomatitis virus (31), foot-and-mouth disease (32), herpes simplex virus 1 (33–35), influenza A virus (36–38), HIV (39), HCV and HBV (31, 40–43). The importance of this pathway in host defense is highlighted by the finding that several viruses have independently evolved mechanisms to block IFN-λ or its function. For example, the NS1 and NS2 proteins of pneumonia virus, a member of the paramyxoviridae, blocks IFN-λ induction (44). The NS3/4A protease of HCV blocks IFN-λ production (45). A secreted glycoprotein from Yaba-like disease virus blocks IFN-λ signals (46). Vaccinia Virus inhibits IFN-λR-mediated signals and gene expression and blocks the IFN-λR antiviral response through a PKR-dependent pathway (47); moreover, a recombinant-vaccinia virus that over-expresses IFN-λ shows reduced levels of replication in vivo, indicating that poxviruses are susceptible to this interferon and have evolved mechanisms to limit its activity (22). Promising results from clinical trials indicate that pegylated-IFN-λ treatment diminishes HCV RNA in patients (9, 48).
The receptor for IFN-λ is expressed on DC, macrophages, and epithelial cells in the gastrointestinal and respiratory tracts, although evidence suggests that other cell types are responsive to IFN-λ (36, 49, 50). While some evidence indicates that IFN-λR expression on peripheral leukocytes is not functional (51), other evidence shows clear antiviral innate defense in some of these cells, and IFN-λ signals stimulate monocytes and macrophages to produce IL-6, IL-8, and IL-10 (52). These findings suggest that IFN-λ has an important role in innate immunity as a first-line defense against invading pathogens through skin and mucosal surfaces but may also function during systemic infections. Mice lacking both IFNαβR and IFN-λR show increased susceptibility against respiratory viruses compared to WT, IFNαβR1-KO, or IL28Rα-KO (36, 37). Recombinant IFN-λ given to mice protects against influenza infection because the IFN-λR is expressed on the epithelial cells targeted by the virus (37). At intestinal mucosal sites, IFN-λ shows antiviral functions that are independent of type-1 interferons (50), and IFN-λR-deficient (λR-deficient) mice are more susceptible to rotavirus infection than IFN-λR-sufficient mice (50). Thus, the antiviral activities of IFN-λ largely depend on the type of virus and the route of administration of recombinant-IFN-λ (36, 37). IFN-λ may exert protective antiviral functions in multiple tissues. For example, pegylated-IFN-λ treatment improves immunity to chronic HCV (9, 48), which indicates that it can improve immunity in the liver. Together, these data suggest that IFN-λ may function in immune defense against systemic infections, since numerous immune cell types respond to IFN-λ, IFN-λ improves T cell-based vaccination (53, 54), and IFN-λ reduces hepatropic infection (9, 48).
The effect of IFN-λ on adaptive immune responses is unclear since multiple groups working in different model systems have arrived at varying conclusions. For example, in a macaque DNA vaccination model, vaccine-induced CTL responses were improved when an additional IFN-λ-expressing plasmid was included to enhance granzyme-dependent killing (53). Similarly, an IL-28B-adjuvanted DNA vaccine strongly induced IFNγ+ CD8+ T cells and long-lasting Th1-phenotype memory CD4+ T cells in macaques (54). However, IFN-λ may have immunoregulatory functions in addition to the demonstrated antiviral functions. The IFN-λRβ–chain is shared with several additional cytokine receptors, including IL-10, IL-19, IL-20, and IL-22, which have well-defined immune-modulatory functions following infections. Consistent with this, IFN-λ-stimulated DC induced the proliferation of T-reg cells in vitro (55); although over-expression of IFN-λ in vivo resulted in fewer Treg cells in a DNA vaccination model (56). IFN-λ signals inhibit the in vitro differentiation of Th2 cells but stimulate Th1 cells (57, 58). RSV-infected monocyte-derived dendritic cells secrete IFN-λ that limits the in vitro proliferation of CD4+ T cells (59). Thus, a mixture of in vitro and in vivo data show that IFN-λ mediated signals can exert positive or negative effects on T cells.
The overall influence of IFN-λ on innate and adaptive immune responses against systemic virus infections is not understood. Herein, we explored the role of IFN-λ using IFN-λR-deficient mice (24) that were given either acute LCMV-Armstrong infection or the highly disseminating variant, LCMV-Clone13. We evaluated the effects of λR-deficiency on interferon induction, NK cell frequencies, virus-specific B cell responses, and primary & memory T cell responses. We found that λR-deficient mice efficiently induced type-1 interferons and eliminated acute infection with kinetics indistinguishable from those of WT mice. Virus-specific memory B cell responses and antibody also appeared normal without IFN-λ signals. However, λR-deficient mice showed a 3-fold increase in primary & memory T cell responses compared to WT mice. In contrast, λR-deficient mice were unable to sustain T cell responses when exposed to persistent virus infection. Thus, IFN-λR signals limit T cell responses during acute infection but support T cell responses during persisting virus infection.
Materials and Methods
Mice and infections
BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and were used as controls for the IFN-λR-deficient mice. In some experiments, BALB/cBy.PL-Thy1a/ScrJ mice from the Jackson Laboratory were used as recipients of BALB/c or IFN-λR-deficient cells. Mice deficient in IFN-λ receptor-1 (IL28Rα−/−; λR-deficient) on the BALB/c background were originally generated by ZYMOGENETICS (Seattle, WA). All animal experiments were performed in accordance with the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. Adult mice (8–10 weeks old) received an intraperitoneal injection of 2×105 PFU of the Armstrong CA-1371 strain (LCMV-Arm) of LCMV. Some mice were given an intravenous injection of 2×106 PFU of LCMV-Armstrong or LCMV-Clone13. Viral stocks of plaque-purified LCMV were prepared from infected BHK-21 monolayers. The virus titer in various organs was determined by plaque assay on Vero cell monolayers (60). Some mice were infected with 1×103 colony forming units of recombinant-Listeria monocytogenes (rLM) that expresses OVA (61, 62).
RT-PCR
A reverse transcriptase reaction was performed to identify viral RNA (63). RNA was extracted from 5mg of spleen using RNeasy mini kit (Qiagen; www.qiagen.com) and cDNA was synthesized using SuperScript-II with random primers (Promega Corp.; www.promega.com). The cDNA was at 37°C for 20minutes followed by a denaturation step at 98°C for 5 minutes. PCR was performed using NP5-001 (TCCATGAGTGCACAGTGCGGGGTGAT) and NP3-001 (GCATGGGAAAACACAACAATTGATC) primers to amplify the NP region of LCMV S RNA. The PCR conditions were: 95°C, 15 minutes; [94°C for 30 seconds; 60°C for 90 seconds; 72°C for 90 seconds] × 35 cycles; 72°C for 10 minutes. Ten μl of the PCR product were run on gel electrophoresis on a 1.5% agarose gel using a 100bp ladder as a size reference.
Flow cytometry & ICCS
Single-cell leukocyte suspensions were prepared from spleens and erythrocytes were removed using ACK lysing buffer (Gibco-BRL, Grand Island, NY). Single-cell suspensions of splenocytes were surface stained with combinations of fluorescently labeled monoclonal antibodies that were specific for CD4 (clone RM4-5), CD8 (53–6.7), CD44 (IM7), CD62L (MEL-14), CD19 (6D5), B220 (RA3-6B2), CD11a (M17/4), KLRG1 (2F1/KLRG1), PD1 (10F.9G2), CD127 (A7R34), CD49b (DX5), CD335 (NKp46; 29A1.4), Thy1.2 (30-H12). The intracellular staining (ICCS) assay was performed as described previously (64, 65). For intracellular staining for T cells, splenocytes were cultured with or without LCMV or rLM peptide in the presence of brefeldin A. After 5.5 h of incubation, cells were stained for surface markers, washed, fixed with formaldehyde, then permeabilized and exposed to mAbs specific for IFN-γ (XMG1.2), IL-2 (JES6-5H4), TNF (MP6-XT22), or T-bet (4B10). Spleen cells were stimulated with NP118-126 peptide to quantify virus-specific CD8+ T cells, or spleen cells were stimulated with one of several peptide epitopes that bind to I-Ad (GP176-190; NP116-130), I-Ed (NP6-20), or both (Z31-45) to identify cytokine producing CD4+ T cells (66). Listeria p60217-225 or LLO91-99 were used to stimulate CD8+ T cells after rLM infection (61, 62). Antibody stained cells were detected by a FACSCalibur cytometer (BD Biosciences) and the data were analyzed with FlowJo software (Tree Star). All listed mAbs above were purchased from Biolegend, except for CD11a (eBioscience).
Memory B cell assay
An ELISPOT-based assay to identify LCMV specific memory B cells capable of differentiating into antibody secreting cells was performed as previously described (67, 68) with some modifications. Briefly, single-cell suspensions of splenocytes at a final concentration of 2×106 cells/ml were cultured in 96-well round bottom culture plates for 5.5 days in the presence of protein A from Staphylococcus aureus (Cowan strain bacteria; Sigma-Aldrich), pokeweed mitogen (Sigma-Aldrich), and CpG oligonucleotide (ODN-2006; Invitrogen); at the end of the culture, the cells were replica plated onto ELISPOT plates (MAHA N4510; Millipore) that were coated with virus-infected BHK lysates, uninfected BHK lysates, or 1μg/well donkey polyclonal anti-mouse IgG (H+L) (Jackson ImmunoResearch). The ELISPOT plates were counted by a reader (Cellular Technology Ltd.). We calculated the frequency of antigen specific B cells among total IgG positive memory B cells.
ELISA
Serum IL-10 was measured using the Quantikine Mouse IL-10 Immunoassay Kit (R&D Systems Inc., Minneapolis, MN) according to manufactur’s instructions. IFN-α was quantified by VeriKine Mouse Interferon-Alpha ELISA Kit (PBL, Piscataway, NJ) according to manufacturer’s instructions. LCMV-specific serum antibody was quantified by using ELISA plates (Greiner bio-one, Monroe, NC) that were coated with lysates from LCMV-infected BHK cells as described previously (69).
Statistics
The graphs show mean data ± sem. The statistical significance was determined by Student’s two-tailed t-test with Prism 5 software (www.graphpad.com). Comparisons were considered significantly different when P < 0.05.
Results
IFN-λR-deficient mice generate robust interferon, NK cell, and primary T cell responses to quickly resolve acute LCMV infection
Type-1 interferons are induced early after LCMV infection. Plasmacytoid-DCs are responsible for production of IFN-α by day 1, and other cell types make lower amounts of IFN-α later (70). Type-1 interferons directly inhibit virus replication, activate NK cell responses against infected cells, and stimulate adaptive T cell responses. Given the parallel relationship between IFN-λ signals and type-1 interferon expression, we considered that type-1 IFN expression might be perturbed in the λR-deficient mice. However, λR-deficiency did not impact type-1 IFN levels following virus infection, as the λR-deficient mice generated normal levels of IFN-α in the serum (Supplemental Fig. 1A).
Type-1 interferons and IFN-λ act on NK cells to increase their antiviral functions (71). To determine whether IFN-λ affects NK cell responses following infection, cohorts of WT or λR-deficient mice were given LCMV-Armstrong infection. Before infection, approximately 5% of cells in the blood were NK cells (DX5+NKp46+) in both groups of mice (Supplemental Fig. 1B). Three days after infection, 10–20% of blood cells were DX5+NKp46+ NK cells in both groups of Armstrong-infected mice, indicating that NK cell frequencies in the blood increased independently of IFN-λ signals. The number of NK cells in the spleen was similar in both groups of mice at day 3 after infection (Supplemental Fig. 1C).
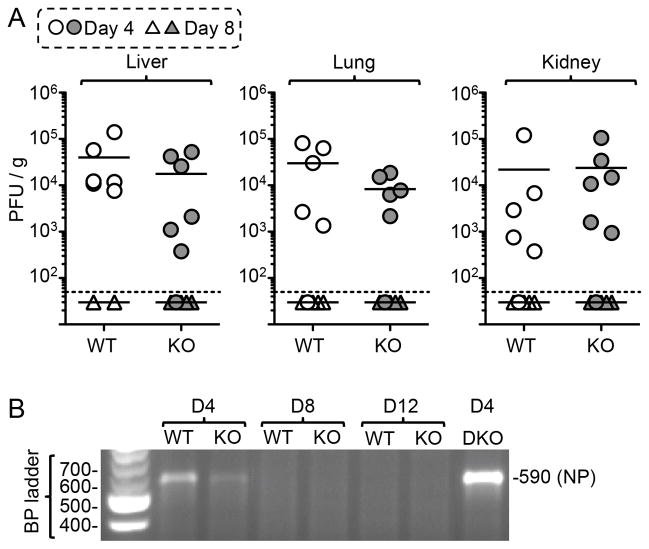
Interferon-λs induce antiviral activity against a number of viruses, including influenza, poxvirus, herpesvirus, and HCV (14, 22, 35–37, 47, 50, 72). Early analyses showed that λR-deficient mice and WT mice replicate similar levels of virus in the spleen 3 days after LCMV-Arm infection (24), but the analyses did not extend to other tissues or later times to evaluate whether the mice eventually resolved the infection. Since early virus loads can influence T cell differentiation processes, we quantified the amount of infection at several times after infection with LCMV-Armstrong. At day 4, λR-deficient mice replicated virus in the liver, lung, and kidney to levels comparable to those found in WT mice (Fig. 1A) and viral RNA could be detected in the spleens of both groups by RT-PCR (Fig. 1B). By day 8, the λR-deficient mice and the WT mice reduced the infection to levels below detection by plaque assay, and the RT-PCR analyses showed no evidence of viral RNA at day 8 or later in the spleens (Fig. 1B). IFN-λs play a key role in limiting mucosal infections (36, 37, 50, 72, 73). We considered that the λR-deficient mice might show impaired immunity to LCMV when the virus was given through a mucosal route. However, the intranasal delivery of LCMV resulted in similar levels of infection in the spleen and lung in both groups of mice at day 4 (Supplemental Fig. 2A); by day 8, both groups of mice showed major reductions in the viral load, resulting in similar levels of infection. Tissue samples from long-term immune mice were also analyzed and found to have no infectious virus (data not shown), indicating that there is no virus recrudescence in these mice. Thus, λR-signaling does not limit the early burden of virus and is not critical for the rapid resolution of LCMV-Armstrong when it is given parentally or mucosally.
Figure 1. IFN-λR-deficient mice resolve LCMV-Armstrong infection.
WT and IFN-λR-deficient (KO) mice were infected intraperitoneally with 2 × 105 PFU of LCMV-Arm. (A) The level of virus in the indicated tissues was determined by plaque assay at days 4 & 8 after infection; each symbol represents tissue from an individual mouse (circles depict measurements at day 4; triangles depict measurements at day 8), the small horizontal lines indicate the mean titers, the dotted line indicates the limit of detection. The data represent 3 experiments with 5–6 mice/group; a Student’s t-test indicated no significant difference in titer between WT and λR-KO mice. (B) The agarose gel shows the product of a representative RT-PCR reaction utilizing RNA extracted from the spleens of infected mice and DNA primers complimentary to LCMV-NP. The product of 590 base pairs was found in day 4 samples but not at day 8 or day 12. The product from day 4-infected IFNαβR−/−IFNγR−/− (DKO) mice that have very high levels of infectious virus is shown for comparison.
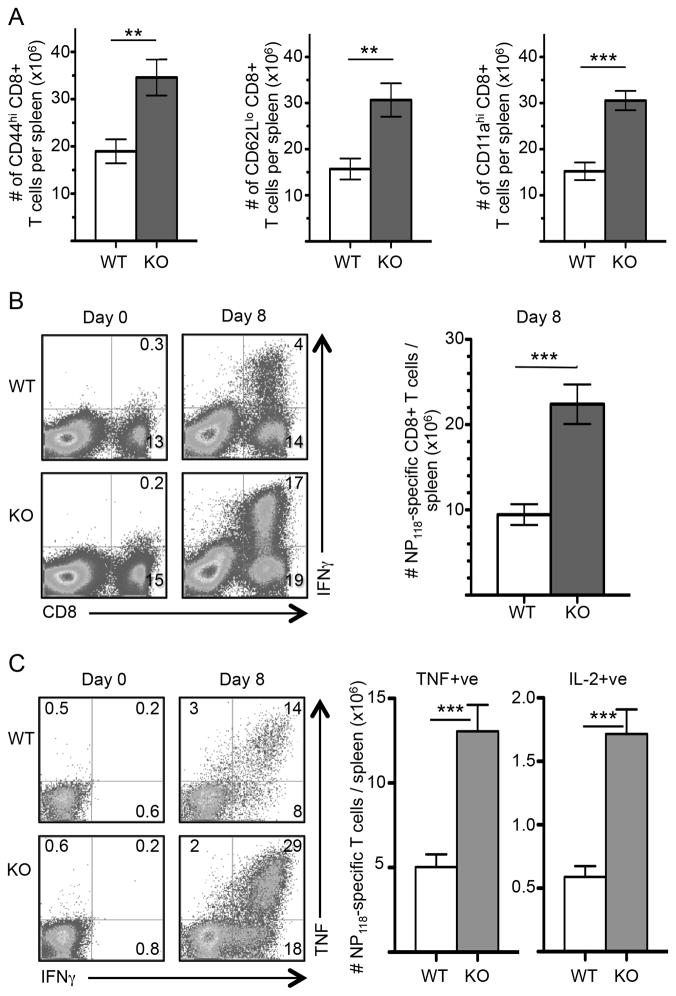
The resolution of LCMV depends upon the formation of large numbers of CD8+ CTL, so the viral clearance data above implies that λR signals are not needed to generate functional CTL. Type-1 and type-2 interferons augment antiviral T cell responses (64, 74–77). These interferons signal directly on responding T cells to increase their accumulation after acute infection. There is also evidence that these interferons can act through indirect processes to support responding T cell responses. Since the signaling pathways between IFNλR and IFNαβR overlap, we investigated whether IFN-λ signals augment virus-specific T cells by comparing peak CD8+ T cell responses in WT and λR-deficient mice. The λR-deficient mice showed normal abundances of resting CD8+ T cells before infection (Supplemental Fig. 3A & data not shown), which implies that IFN-λ signals are not involved in naïve T cell development or seeding of peripheral organs. Upon infection, there was a tremendous increase in the frequency of activated, CD44hi, CD62Llo, and CD11ahi CD8+ T cells in the spleens of WT and λR-deficient mice (Supplemental Fig. 3A); however, the frequencies of these cells were higher in the λR-deficient mice compared to the WT mice and corresponded to ~2-fold greater numbers of CD44hi, CD62Llo, and CD11ahi CD8+ T cells in the λR-deficient mice (Fig. 2A). The overall cellularity of the spleens after infection was similar in the two groups before and after infection (Supplemental Fig. 3B), indicating that there was a selective increase in activated CD8+ T cells in the λR-deficient mice.
Figure 2. Greater accumulation of virus-specific CD8+ T cells in IFN-λR-deficient mice.
Groups of WT and λR-deficient (KO) mice were infected with LCMV-Arm. (A) At day 8 after infection, spleen cells were analyzed for CD8, CD44, CD62L, and CD11a by flow cytometry. The bar graphs show cumulative data for the total number of activated CD8+ T cells that were CD44hi (left), CD62Llo (middle), CD11ahi (right) in WT and λR-deficient mice (means ± sem). The data represent 2–5 experiments with 5–11 mice/group at each time point. (B) NP118-specific CD8 T cell responses in the spleen were quantified by ICCS followed by flow cytometry analysis. The dot plots show examples of CD8+ T cell production of IFNγ in response to NP118 peptide on days 0 (uninfected) and 8 after infection; the numbers indicate the percentage of spleen cells in each quadrant. The bar graph shows cumulative data for the number of NP118-specific CD8+ T cells per spleen from 11–13 mice analyzed in 4 independent experiments (mean ± sem). (C) The representative dot plots are gated on CD8+ T cells and show the percentage of cells that co-expressed IFN-γ with TNF, as assessed by ICCS. The bar graphs show cumulative data from 11–13 mice. The left graph shows the average (± sem) number of NP118-specific CD8+ T cells per spleen that were TNF+IFNγ+; the right graph shows the number that were IL-2+IFNγ+. The data represent 2–5 experiments with 5–11 mice/group at each time point. A two-tailed Student’s t-test was used to evaluate significance with **P <0.01; ***P <0.001.
All virus-specific T cells are contained within the emergent CD44hi and CD11ahi populations of cells (78–80), so the data in Fig. 2A and Supplemental Fig. 3A suggest that there are more virus-specific CD8+ T cells in the λR-deficient mice. Therefore, intracellular cytokine staining (ICCS) was used to quantify epitope-specific CD8+ T cells in WT and λR-deficient mice before and at the peak of the T cell response. There was a vigorous NP118-specific CD8+ T cell response in the λR-deficient mice that was ~4-fold greater than that seen in the WT mice (Fig. 2B). Combined with total spleen cells counts, this frequency corresponded to >10×106 more NP118-specific CD8+ T cells in the λR-deficient mice than in the λR-sufficient mice (Fig. 2B, bar graph).
Differentiating virus-specific T cells acquire the ability to make large amounts of TNF and IL-2 as they progress into effector and memory cells. Among NP118-specific CD8+ T cells in both groups of mice, approximately 60% of IFNγ+ve cells also made TNF (Fig. 2C, left) and 10–15% of IFNγ+ve cells also made IL-2 (data not shown). There was no significant difference in the amount of IFNγ, TNF, or IL-2 produced by NP118-specific CD8+ T cells at a per cell level, as indicated by geometric mean fluorescence intensity (data not shown). However, the λR-deficient mice generated 3-fold greater numbers of IFNγ+TNF+ and IFNγ+IL-2+ CD8+ T cells at day 8 compared to λR-sufficient mice (Fig. 2C, right). Thus, λR signals do not impact the cytokine output of virus-specific CD8+ T cells but restrict T cell number. In contrast to acute LCMV infection, we observed no difference in the expansion of Listeria monocytogenes-specific CD8+ T cells in WT and λR-deficient mice (Supplemental Fig. 3C), and the mice cleared the infection in the liver as determined by colony counts on BHI agar (data not shown). Thus, the expression of IFN-λR leads to reduced T cell responses to some acute infections, perhaps correlating with the magnitude of the T cell expansion, which is far greater after LCMV than Listeria monocytogenes. Therefore, we focused our analyses on T cell responses induced by LCMV infection.
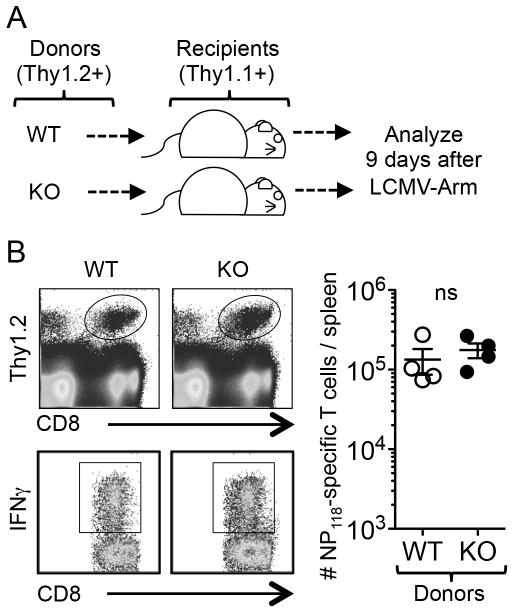
The increased T cell response in the λR-deficient mice suggests that IFN-λ is suppressive in WT mice. This effect might be mediated by direct signaling into T cells or could be an indirect consequence of IFN-λ signaling into other cell types. To determine whether T cells need to express IFN-λR, splenocytes from WT or λR-deficient mice were adoptively transferred to separate congenic recipient mice that were subsequently given acute LCMV infection (Fig. 3A). At day 9 post-infection, the NP118-specific donor cells were identified by ICCS assay in the recipient mice. The overall number of WT and λR-deficient CD8+ T cells was similar in the WT recipient mice (Fig. 3B). These data imply that IFN-λ signaling into other cell types accounts for the difference in the virus-specific T cell number when comparing WT and λR-deficient mice.
Figure 3. IFN-λ does not directly act on virus-specific T cells.
Cell transfer experiments were performed to evaluate whether IFNλ directly acts on T cells to affect their expansion after infection. (A) Single-cell suspensions of spleen cells containing 3.8×106 polyclonal CD8+ Thy1.2+ T cell populations from WT or λR-deficient mice were transferred intravenously into separate congenic Thy1.1+ mice. The recipient mice were infected with LCMV-Arm the next day and analyzed by ICCS at day 9. (B) The top dot plots show surface staining for Thy1.2 and CD8 at day 9. The ovals identify the donor cells. The bottom dot plots are gated on the donor cells and show their expression of IFNγ+ following stimulation with NP118 peptide. The graph shows the total number of IFNγ+ NP118-specific CD8+ T cells per spleen. The data represent 4 mice/group for each analysis. Based on two-tailed Student’s t-test, there was no significant difference in the expansion of WT and λR-deficient CD8+ T cells in the WT mice.
Primary CD4+ T cell responses are increased in λR-deficient mice after acute infection
Earlier studies using in vitro stimulated T cell cultures indicated that IFNλ stimulates Th1 cells and inhibits Th2 cells (57, 58, 81, 82). Therefore, we examined the effects of IFNλ on CD4+ T cell responses to infection. At day 8 after infection, modest levels of CD4+ T cell activation were revealed by slight increases in the proportion of CD4+ T cells that were either CD44hi or CD62Llo (data not shown). Several H-2d-restricted epitopes have been identified (66), which enables the quantitation of epitope-specific T cells in Balb/c mice using ICCS. In WT mice, ~0.25% of CD4+ T cells were specific for GP176 or NP6, 0.4% for NP116, and 0.13% for Z31 (Fig. 4A). These frequencies corresponded to 3–10×104 epitope-specific T cells per spleen (Fig. 4B). A similar analysis was performed for the λR-deficient mice, which showed moderately increased percentages of CD4+ T cells specific for each peptide. Overall, there was a 2-fold increase in the number of CD4+ T cells specific for GP176, NP6, NP116, and no difference in responses to Z31 (Fig. 4B). The NP116-130 peptide contains NP118-126, which efficiently stimulates H-2Ld-restricted CD8+ T cells that are the likely source of non-CD4+ T cells that made IFNγ+ in the center dot plots. In both groups of mice, the IFNγ+ CD4+ T cells specific for GP176, NP6, and Z31 also produced IL-2. There was a >2-fold increase in the number of IFNγ+IL-2+ CD4+ T cells specific for GP176 and NP6 (Fig. 4C). In total, these data indicate the CD4+ T cell response in WT H-2d mice matures into small populations capable of making IFNγ and IL-2, but those responses are only moderately increased in the absence of λR signals.
Figure 4. Improved expansion of LCMV-specific CD4+ T cells in the absence of IFN-λR interactions.
Epitope-specific CD4 T cell responses were measured by ICCS assay using spleen cells from WT (Balb/c) and IFNλR-KO mice 8 days after infection. (A) The representative dot plots show IFNγ production by CD4+ T cells in response to the indicated LCMV peptides; the numbers indicate the percentage of cells in each quadrant. (B) The bar graphs show the average (± sem) number of IFN-γ+ epitope-specific CD4+ T cells per spleen in WT and IFN-λR-KO mice. (C) The bar graphs show the average (± sem) number of IL-2+ epitope-specific CD4+ T cells per spleen in WT and IFN-λR-KO. Data are representative of 2 experiments with 7 mice/group. A two-tailed Student’s t-test was used to evaluate significance with **P <0.01 and ***P <0.001.
IFNλR is not required to generate humoral immune responses to acute LCMV
CD4+ T cell responses drive strong antiviral B cell responses after LCMV (69, 83). We examined whether the greater CD4+ T cell responses in the λR-deficient mice affected the humoral response after LCMV-Armstrong infection. WT and λR-deficient mice showed comparable numbers of CD19+ B cells before and several times after infection (Supplemental Fig. 4A & data not shown). The percentage of cells with phenotypic markers of GC B-cells (CD19+GL7+) at day 15 was higher in λR-deficient mice versus WT mice (20±1% versus 15±1%), however, when measured by an in vitro memory B cell assay (67, 68) at day 120, there was no statistically significant difference in memory B cell numbers between WT and λR-deficient mice (Supplemental Fig. 4B). Thus, the elevated GC-phenotype B cells seen at day 15 did not result in more LCMV-specific memory B cell responses. Correspondingly, LCMV-specific serum antibody levels were similar in both groups of mice at days 14 and 49 (Supplemental Fig. 4C). These data indicate that λR-signals are not involved in differentiating memory B cell or plasma cells following acute infection.
λR-deficient mice sustain elevated numbers of LCMV-specific memory CD8+ T-cells
The above data (Fig. 2 & 4) show that IFN-λR-deficiency leads to an increase in virus-specific CD8+ and CD4+ T cells. For CD8+ T cells, IFNs influence the formation of short-lived effector cells (SLEC) and long-lived, memory precursor cells (MPEC) (84). We explored whether λR signals affect the development of SLECs (KLRG1hiIL-7Rlo) or MPECs (KLRG1loIL-7Rhi). Among activated CD11a+ T cells, the λR-deficient mice showed normal frequencies of SLEC but 2-fold higher frequencies of MPEC at day 8 (Supplemental Fig. 3D), which corresponded to a 1.7-fold increase in SLEC number and a 4-fold increase in MPEC number (Supplemental Fig. 3D). These data suggest that the expression of IFN-λR restricts the accumulation of cells with memory potential, which implies there could be long-term effects on T cell memory. Therefore, cohorts of mice were infected with LCMV-Armstrong, and the virus-specific CD8+ T cell number was quantified at various times after infection. The λR-deficient mice continued to have 2–3-fold greater numbers of NP118-specific CD8+ T cells after the peak response (Fig. 5A, top). The proportions of memory T cells capable of making IFNγ with TNF (Fig. 5A, middle) or IFNγ with IL-2 (Fig. 5A, bottom) were also increased in λR-deficient mice compared to WT mice. At day 180, the λR-deficient mice continued to have nearly 3-fold more NP118-specific CD8+ T cells and more CD8+CD11a+ T cells that expressed the IL-7Rhi MPEC phenotype (Fig. 5B) that is associated with memory.
Figure 5. T cell memory is increased in IFN-λR-deficient mice.
NP118-specific CD8+ T cells were quantified by ICCS at multiple times after acute infection. (A) The line graphs show the average (± sem) number of CD8+ T cells per spleen that made IFNγ (top), IFNγ with TNF (middle), or IFNγ with IL-2 (bottom) at the indicated days after infection. 3–13 mice per group were analyzed at each time point. (B) The bar graph shown the number of CD8+CD11hiKLRG1loIL-7Rhi memory T cells in the spleens of 3–4 mice at 180 days after infection. (C) The bar graphs show cumulative data for the number of CD4+ T cells specific for the indicated MHCII-restricted LCMV epitopes as measured by IFNγ ICCS assay at day 180. (D) An example of ICCS co-staining for IFNγ and IL-2 at day180. Note the greater frequency of cytokine+ve cells in the IFN-λR-KO mice than in the immune WT mice. (E–F) WT and IFNλR-KO mice were infected intraperitoneally with 2 × 105 PFU of LCMV-Arm and, 120 days later, were rechallenged intravenously with 2 × 106 PFU of LCMV-Arm. Six days after the re-challenge, virus-specific T cell responses in the spleen and viral loads were quantified. (E) The bar graphs show the number of NP118-specific CD8+ T cells per spleen before (not striped) and after (striped) the re-challenge. The bar graphs summarize the mean (± sem) of 5 mice per group and 2 independent experiments and depict the total number of IFNγ+ve cells, IFNγ+TNF+ cells, and IFNγ+IL-2+ cells per spleen. (F) The amount of infectious virus in the liver and kidney was measured by plaque assay. The inverted triangles show the levels in re-challenged immune mice; the circles indicate levels found in naïve mice that were given the re-challenge dose and analyzed 6-days later. The data represent 2 experiments with 4–5 mice/group. A two-tailed Student’s t-test was used to evaluate significance with *P <0.05; **P <0.01; ***P <0.001.
There is no information about the number and epitope-specificity of memory LCMV-reactive CD4+ T cells in Balb/c mice. Here, we followed CD4+ T cell memory responses in WT (Balb/c) and λR-deficient mice. Virus-specific memory IFNγ+ CD4+ T cells were quantified by ICCS at late times after infection. At day 180, the NP116-specific CD4+ T cells were the largest population of epitope-specific T cells in the WT mice; that population was 2-fold greater in the λR-deficient mice (Fig. 5C). Approximately 104 CD4+ T cells per spleen were specific for GP176, NP6, and Z31 in both groups of mice. Among the NP116-reactive CD4+ T cells, a higher percentage made IL-2 in the λR-deficient mice compared to the WT mice (Fig. 5D). These data indicate that IFN-λR signals limit the abundance and cytokine content of immune-dominant memory CD4+ and CD8+ T cells.
IL-2 signals are involved in both the establishment of memory and in the recall response (85–89). CD8+ T cells in λR-deficient mice made slightly more IL-2 per cell than did CD8+ T cells from WT mice at days 40, 120, and 180 after infection (data not shown). The effect was small but significant based on an unpaired Student’s t-test P-value of 0.01–0.03 at each time, with 3–5 mice per group. A similar pattern was observed for CD4+ T cells (Fig. 5D). This suggests that λR-deficient cells are qualitatively improved compared to cells in WT mice. Therefore, cohorts of WT and λR-deficient mice were immunized and 4 months later some were re-challenged with a higher dose of LCMV-Armstrong. As expected, WT mice mounted a robust recall response by 6 days, with ~6% of CD8+ T cells specific for NP118 and able to make IFNγ (Fig. 5E); the λR-deficient mice generated 2-fold higher frequencies of these cells, and the same pattern was apparent when TNF and IL-2 production were quantified (Fig. 5E). These percentages corresponded to 5–10×106 more NP118-specific cytokine-producing CD8+ T cells per spleen in the re-challenged λR-deficient mice compared to the WT mice. The larger response in the λR-deficient mice upon re-challenge corresponded to the greater number of memory cells in these mice before challenge. Thus, there was no significant increase (Student’s t-test P-value = 0.07) for the λR-deficient mice compared to the WT mice when the ratio of effector cells after challenge was normalized to the number of memory cells before challenge. These data indicate that while IFN-λR-deficient mice establish more memory cells than WT mice, those memory cells are not inherently better at proliferative responses compared to memory cells in WT mice.
The re-call response in both groups of immune mice led to efficient control of the infection (Fig. 5F, triangles). By comparison, both groups of naïve mice given the challenge dose continued to show high levels of infection at day 6 (Fig 5F, circles). Cumulatively, these data show that λR-dependent signals are dispensable for generating protective primary and memory T cell and B cell responses after acute infection and act to restrict the size of the overall response.
Exaggerated loss of virus-specific T cells during disseminated virus infection
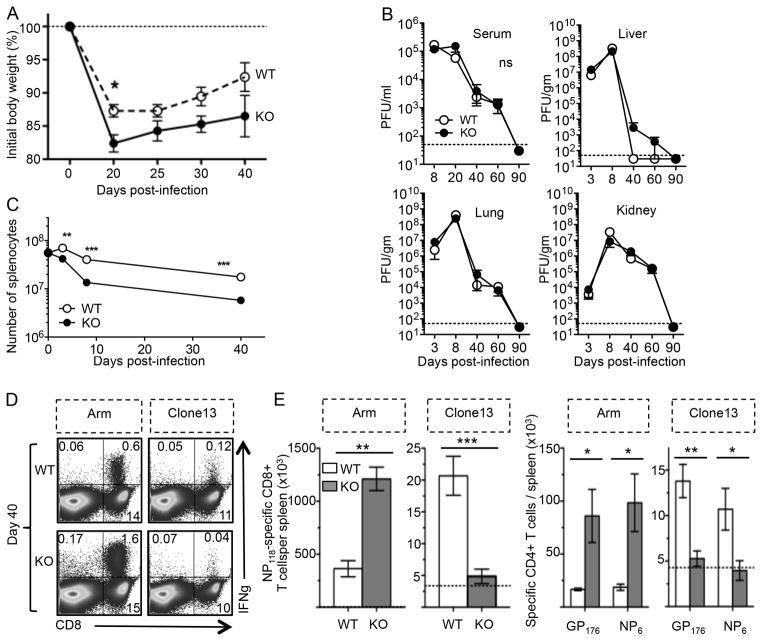
The data above indicate λR signals are dispensable for primary and memory T cell formation and protection against LCMV-Armstrong infection. The immunobiology of persisting virus infection is often very different from acute infection, and is largely impacted by the detrimental effects of sustained T cell stimulation, inhibitory molecule expression on T cells, and the presence of immune suppressive cytokines. Consequently, T cells undergo exaggerated deletion or functional inactivation during these conditions. T cell exhaustion is observed in mice with persisting LCMV infection and in people who are persistently infected with HIV or HCV. To better understand the role of IFN-λ signals on the resolution of chronic virus infection, WT and λR-deficient mice were given LCMV-Clone13, which disseminates and persists. Both groups of mice showed an initial weight loss followed by partial recovery, although the λR-deficient mice endured greater weight loss than WT mice (Fig. 6A). Both groups of mice showed similar levels of virus across time (Figure 6B). At days 9 to 20, there was 105 PFU/ml in the serum and >107 PFU/gram in the liver, lung, and kidneys in both groups. By day 40, there was a reduction in viremia and eventually both groups reduced the virus burden to the limits of detection, likely through a combination of T cell and neutralizing antibody-mediated mechanisms. Thus, there is no apparent effect of λR expression on peak viral titers or the longevity of the infection.
Figure 6. IFN-λR-deficient mice fail to sustain virus-specific T cells during persisting virus infection.
WT and IFN-λR-KO mice were infected intravenously with 2×106 PFU of LCMV-Armstrong or LCMV-Clone13. (A) Weight loss was measured across time during LCMV-Clone13 infection (mean±sem with n=6–7 mice per group per day). (B) The average (± sem) viral burden in the serum or indicated tissues was measured by plaque assay at the indicated days after Clone13 infection. The dotted line indicates the limit of detection of the plaque assay. 3–7 mice per group were analyzed at each time point. No significant difference was detected between WT and λR-KO mice using two-tailed Student’s t-test. (C) The line graph shows the total number of splenocytes (average ± sem) at the indicated days after infection. 6–11 mice per group were analyzed at each time point. A two-tailed Student’s t-test was used to evaluate significance with **P <0.01 or ***P <0.001. (D–E) Virus-specific T cells in the spleens of infected WT or KO were analyzed at day 40 by ICCS. (D) The dot plots show examples of CD8+ T cell production of IFNγ in response to NP118-126 peptide; the numbers indicate the percentage of cells in each quadrant. (E) The left bar graphs show cumulative data for the number (mean ± sem) of NP118-specific CD8+ T cells per spleen. The right bar graphs show the average (± sem) number of IFN-γ-producing GP176 or NP6-specific CD4+ T cells per spleen. The data represent 2 experiments with 3 mice/group (Arm) or 7 mice/group (CL13). Asterisks indicate statistical significance (*p < 0.05, **p < 0.01, and ***p < 0.001) by a two-tailed Student’s t-test.
Compared to the WT mice, there was a significantly greater drop in the total spleen size in the λR-deficient mice by 8 days after infection (Fig. 6C). The overall cellularity continued to decline across time to 6×106 cells by day 40 in the λR-deficient mice, whereas the WT mice had ~3-fold more splenocytes. Virus-specific T cell responses were analyzed by ICCS assay at days 8, 40, and 90 after LCMV-Clone13 infection. At day 8, the overall abundance of IFNγ+ CD8+ T cells and CD4+ T cells was similar for the two groups (data not shown). In the λR-deficient mice, a smaller percentage of activated CD8+ T cells were KLRG1hi, fewer expressed the inhibitory molecule PD-1, and more were CD127hi (data not shown), which suggested that more T cells might survive to contribute to immune control in the λR-deficient mice. However, by day 40, there were 4-fold fewer virus-specific NP118-specific CD8+ T cells (Fig. 6D) and 3-fold fewer GP176-specific or NP6-specific CD4+ T cells (Fig. 6E) in the λR-deficient mice compared to the WT mice. The reduced T cell responses were near or below the limits of detection, and this pattern was sustained to day 90 (data not shown). Much of this reduction in virus-specific T cell number was due to the lower number of spleen cells in the λR-deficient mice (Fig. 6C). In contrast to Clone13, LCMV-Armstrong led to 2–3-fold greater numbers of virus-specific CD8+ and CD4+ T cells in the λR-deficient mice compared to WT mice (Fig. 6D–E). These data show that IFN-λ functions vary with the chronicity of the infection. During persisting infection, IFN-λ signals protect against infection-induced weight loss and sustain IFNγ+ T cell responses. Following acute infection, IFN-λ limits the size of the overall T cell response and memory.
Discussion
Earlier analyses of IFN-λ focused on its role in limiting virus infection or replication, but much less is known in terms of how this pathway influences adaptive immunity. Herein, we examined primary and memory T cell responses after acute and persisting virus infection in λR-deficient mice. We found IFN-λR is not essential for mounting innate and adaptive antiviral defense and appears to regulate peak effector T cell responses and memory cell number after acute infection. In contrast, T cell responses to disseminating infection were reduced in the λR-deficient mice, implying that IFN-λ signals maintain T cell responses during persisting infection. The λR-deficient mice also showed greater weight loss that was prolonged compared to WT mice.
While the main effect of λR-deficiency was on T cells, we observed a modest trend toward increased NK cell frequencies in the blood after infection in the λR-deficient mice (Supplemental Fig. 1B &1C). The increase achieved significance after Clone13 infection but not after Armstrong infection. NK cells are induced by type-1 and type-2 interferons, so the increased NK cell response in the λR-deficient mice suggests that IFN-λ signals may counterbalance the inducing effects of these other interferons. Other investigators have shown that the antitumor activity of IFN-λ is partly mediated by positive effects on NK cell recruitment into the liver and increased tumor killing by NK cells (71). IFN-λ is being tested in clinical trials to treat HCV infection (9, 48, 90); in addition to directly limiting HCV replication in liver cells, IFN-λ may stimulate antiviral activity in hepatic NK cells to reduce HCV. NK cells restrain virus-specific T cell responses following LCMV-Clone13 infection and contribute to the formation of functionally exhausted T cells (91–94). Thus, the increased NK cell response in the Clone13-infected IFN-λR-deficient mice might contribute to greater NK cell activity and the eventual decline in virus-specific T cell responses during the chronic stage.
The levels of infectious virus early after LCMV-Armstrong infection were comparable in λR-deficient mice and WT mice (Fig. 1A, Supplemental Fig. 2), consistent with an earlier report (24), and another study showing that pre-treating mice with 10μg of recombinant-IFN-λ does not reduce LCMV levels at day 2 after infection (14). This implies that λR-deficient mice do not have new cellular targets of infection. IFN-λR signals do not limit the replication of the arenavirus, Lassa virus, in macrophages or DCs in vitro (95) and mice lacking IFN-λR did not show increased levels of Lassa virus replication compared to mice with the IFN-λR following intranasal infection (36). IFN-λR is important for the resolution of other infections at mucosal or liver sites; however, our analyses showed no difference in the lung or liver, when LCMV-Armstrong was given intranasally (Supplemental Fig. 2). LCMV causes a systemic infection that induces robust levels of IFNαβ and IFNγ. In this context, direct IFNαβR and IFNγR signals may induce sufficient antiviral activity to impair early virus replication, thus overshadowing any antiviral effects mediated by IFN-λ (24).
We found that the overall CD8+ T cell response to acute infection was increased in the absence of IFN-λR signals. There are several potential explanations for our findings. 1) pDCs express IFN-λR and are a primary source of IFNαβ immediately after LCMV infection. It is plausible that pDCs (55, 96) or a monocyte-derived lineage (52) were less active in the λR-deficient mice, leading to slightly higher antigen loads that stimulate T cells. Such slight differences in antigen-load would not be revealed by the plaque assay or RT-PCR analysis but may be sufficient to induce more T cell accumulation. 2) IFN-λ may affect T-reg activity. IFN-λ are increased during the chronic stage of HCV and act on DC to stimulate CD4+FoxP3+ T-reg cells (55, 97), perhaps suppressing immune responses to HCV. A novel population of CD4+FoxP3+CD25− T cells make IFN-λ and induce tolerance in a mouse EAE model (98), so potentially these tolerance-inducing populations of cells are induced in the infected WT mice but are diminished in the λR-deficient mice. 3) Finally, it has been shown that IFN-λ signals can induce SOCS1 and SOCS3 (99) that suppress inflammatory processes. All of these potential mechanisms are likely to be indirect as it does not appear that IFN-λ signals act directly on T cells. In some models activated T cells express λR (58, 100, 101), however, we could not detect IFN-λR expression on naïve or activated T cells using flow cytometry, and mRNA levels for IFN-λR appeared to be very low based on RT-PCR (data not shown), consistent with other findings for human T cells (102). Moreover, λR-deficient T cells did not respond better than WT T cells when the cells were placed in acutely infected WT mice (Fig. 3). Thus, the effects we observe on antiviral T cell number likely occurred as a result of IFN-λ signaling in other cell types.
Interestingly, the expression of IFN-λR was associated with sustained T cell responses during persisting infection, which contrasts with the restraining effects of IFN-λ on T cells during acute infection. We do not know why antiviral T cell responses collapsed in the chronically infected λR-deficient mice, nor do we know why the effects of IFN-λ are opposite following acute and chronic infection. The effects of cytokines on T cell responses can differ between acute and chronic infections. For example, IL-10 and type-1 IFN also can stimulate or inhibit T cell responses to LCMV, depending on the duration of the infection. IL-10 signals during acute LCMV infection increase the formation of MPECs and long-term memory cells (103) yet limit T cell responses during persisting infection (104–108). Because IL-10 has been implicated in restricting T cell responses following LCMV infection (104, 107–109), we considered that IFN-λR1-deficiency might impact the amount of IL-10 that is present in the infected mice. Serum IL-10 levels rapidly rose on day 1 and declined in both WT and λR-deficient mice after Armstrong or Clone13 infection (Supplemental Fig. 1D). An interesting rebound of serum IL-10 levels on day 15 post LCMV-Clone13 infection was observed, consistent with an earlier report (104), but there was no significant difference in serum levels of IL-10 in the WT or λR-deficient mice at any time during LCMV-Clone13 infection. Type-1 IFNs contribute to the vigorous T cell responses after acute infection but diminish T cell responses during the chronic stage of LCMV-Clone13 infection (110–112) and can induce lymphopenia after virus infections (113, 114). We observed that the Clone13-infected λR-deficient mice had somewhat smaller spleens than WT mice (Fig. 6C), and we considered that IFN-λ might affect type-1 IFN levels but found that serum levels of IFN-α were similar between WT and λR-deficient mice (Supplemental Fig. 1).
An alternative hypothesis is that IFN-λ inhibits T cell responses to both acute and chronic infections, but the differential effects observed on T cell number after LCMV-Armstrong and LCMV-Clone-13 (Fig. 5) are linked to the tropism of these strains. Thus, LCMV-Clone13 more efficiently infects fibroblastic reticular cells (FRC) (115, 116) and dendritic cells and their progenitors (117, 118). Once these cells are infected, they become targets for CTL and are destroyed, leading to generalized immune suppression and sustained T cell loss during chronic infection (115–118). FRCs provide factors that support the retention and expansion of T cell responses to acute infection. However, LCMV-clone13 infection of FRCs, leads to the disintegration of this important stromal cell network and a subsequent loss of cell populations (115, 116). Thus, a greater CTL response early on in the Clone13-infected λR-deficient mice may explain the exaggerated loss of spleen cell number in these mice (Fig. 6C). Alternatively, it may be that IFN-λ acts on FRC to protect them from destruction by CTL in the WT mice. Analogous effects could occur for dendritic cells: LCMV-Armstrong efficiently activates DCs to stimulate T cells and IFN-λ restrains T cell responses. In contrast, LCMV-Clone13 targets DCs and leads to their rapid destruction by CTL (117, 118), and IFN-λ acts to limit CTL-mediated destruction of these DCs. Thus in the presence of IFN-λ, there could be lengthier DC-driven T cell responses during chronic infection. Finally, it is possible that IFN-λ supports T cell responses during chronic infection by limiting Clone13 infection of FRC or DCs without significantly changing the overall viral burden in the spleen.
Overall, our data indicate that IFN-λ improves T cell responses during chronic LCMV infection. Recent data from clinical trials show that recombinant-IFN-λ enhances protection against HCV (48). While IFN-λ synergizes with IFN-β to reduce viral genomes in infected hepatocytes, our data in Figure 6 suggest an alternative hypothesis: recombinant-IFN-λ improves antiviral T cell number or function during persisting infection to reduce virus levels.
Supplementary Material
Acknowledgments
We would like to thank Sean Doyle of ZymoGenetics & Bristol Myers Squibb for kindly supplying the IFN-λR-deficient mice, and Jason Botten (U. Vermont) who provided helpful discussions concerning the RT-PCR analysis.
Footnotes
This research was supported in part by funds from NIH R01 grant AI074862 to J.K.W. Additional support included start-up funds from The University of North Carolina at Chapel Hill and a Small Research Grant from the UNC University Research Council.
Abbreviations: IFN-λR, interferon-lambda receptor; LCMV, lymphocytic choriomeningitis virus; MPEC, memory precursor effector cell; SLEC, short-lived effector cell.
References
- 1.Kotenko SV. IFN-lambdas. Curr Opin Immunol. 2011;23:583–590. doi: 10.1016/j.coi.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 4.Lasfar A, Lewis-Antes A, Smirnov SV, Anantha S, Abushahba W, Tian B, Reuhl K, Dickensheets H, Sheikh F, Donnelly RP, Raveche E, Kotenko SV. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66:4468–4477. doi: 10.1158/0008-5472.CAN-05-3653. [DOI] [PubMed] [Google Scholar]
- 5.Karpala AJ, Morris KR, Broadway MM, McWaters PG, O’Neil TE, Goossens KE, Lowenthal JW, Bean AG. Molecular cloning, expression, and characterization of chicken IFN -lambda. J Interferon Cytokine Res. 2008;28:341–350. doi: 10.1089/jir.2007.0117. [DOI] [PubMed] [Google Scholar]
- 6.Qi Z, Nie P, Secombes CJ, Zou J. Intron-containing type I and type III IFN coexist in amphibians: refuting the concept that a retroposition event gave rise to type I IFNs. J Immunol. 2010;184:5038–5046. doi: 10.4049/jimmunol.0903374. [DOI] [PubMed] [Google Scholar]
- 7.Zhou P, Cowled C, Todd S, Crameri G, Virtue ER, Marsh GA, Klein R, Shi Z, Wang LF, Baker ML. Type III IFNs in pteropid bats: differential expression patterns provide evidence for distinct roles in antiviral immunity. J Immunol. 2011;186:3138–3147. doi: 10.4049/jimmunol.1003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manry J, Laval G, Patin E, Fornarino S, Itan Y, Fumagalli M, Sironi M, Tichit M, Bouchier C, Casanova JL, Barreiro LB, Quintana-Murci L. Evolutionary genetic dissection of human interferons. J Exp Med. 2011;208:2747–2759. doi: 10.1084/jem.20111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagliaccetti NE, Robek MD. Interferon-lambda in HCV Infection and Therapy. Viruses. 2010;2:1589–1602. doi: 10.3390/v2081589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 11.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E, Riordan S, Sheridan D, Smedile A, Fragomeli V, Muller T, Bahlo M, Stewart GJ, Booth DR, George J. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 12.Bibert S, Roger T, Calandra T, Bochud M, Cerny A, Semmo N, Duong FH, Gerlach T, Malinverni R, Moradpour D, Negro F, Mullhaupt B, Bochud PY, Swiss Hepatitis CCS. IL28B expression depends on a novel TT/-G polymorphism which improves HCV clearance prediction. J Exp Med. 2013;210:1109–1116. doi: 10.1084/jem.20130012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antaki N, Bibert S, Kebbewar K, Asaad F, Baroudi O, Alideeb S, Hadad M, Abboud D, Sabah H, Bochud PY, Negro F. IL28B polymorphisms predict response to therapy among chronic hepatitis C patients with HCV genotype 4. J Viral Hepat. 2013;20:59–64. doi: 10.1111/j.1365-2893.2012.01621.x. [DOI] [PubMed] [Google Scholar]
- 14.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 16.Okabayashi T, Kojima T, Masaki T, Yokota S, Imaizumi T, Tsutsumi H, Himi T, Fujii N, Sawada N. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res. 2011;160:360–366. doi: 10.1016/j.virusres.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Marukian S, Andrus L, Sheahan TP, Jones CT, Charles ED, Ploss A, Rice CM, Dustin LB. Hepatitis C virus induces interferon-lambda and interferon-stimulated genes in primary liver cultures. Hepatology. 2011;54:1913–1923. doi: 10.1002/hep.24580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Megjugorac NJ, Gallagher GE, Gallagher G. IL-4 enhances IFN-lambda1 (IL-29) production by plasmacytoid DCs via monocyte secretion of IL-1Ra. Blood. 2010;115:4185–4190. doi: 10.1182/blood-2009-09-246157. [DOI] [PubMed] [Google Scholar]
- 19.Wolk K, Witte K, Witte E, Proesch S, Schulze-Tanzil G, Nasilowska K, Thilo J, Asadullah K, Sterry W, Volk HD, Sabat R. Maturing dendritic cells are an important source of IL-29 and IL-20 that may cooperatively increase the innate immunity of keratinocytes. J Leukoc Biol. 2008;83:1181–1193. doi: 10.1189/jlb.0807525. [DOI] [PubMed] [Google Scholar]
- 20.He SH, Song CH, Liu Z, Zhang H, Ma W, Zhou LF, Mahmood T, Yang PC. Eosinophil-derived interferon-lambda contributes to initiation of allergen-related inflammation in the intestine. Cytokine. 2012;58:186–192. doi: 10.1016/j.cyto.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Brann TW, Zhou M, Yang J, Oguariri RM, Lidie KB, Imamichi H, Huang DW, Lempicki RA, Baseler MW, Veenstra TD, Young HA, Lane HC, Imamichi T. Cutting edge: Ku70 is a novel cytosolic DNA sensor that induces type III rather than type I IFN. J Immunol. 2011;186:4541–4545. doi: 10.4049/jimmunol.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartlett NW, Buttigieg K, Kotenko SV, Smith GL. Murine interferon lambdas (type III interferons) exhibit potent antiviral activity in vivo in a poxvirus infection model. J Gen Virol. 2005;86:1589–1596. doi: 10.1099/vir.0.80904-0. [DOI] [PubMed] [Google Scholar]
- 23.Ank N, Paludan SR. Type III IFNs: new layers of complexity in innate antiviral immunity. Biofactors. 2009;35:82–87. doi: 10.1002/biof.19. [DOI] [PubMed] [Google Scholar]
- 24.Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 25.Yin Z, Dai J, Deng J, Sheikh F, Natalia M, Shih T, Lewis-Antes A, Amrute SB, Garrigues U, Doyle S, Donnelly RP, Kotenko SV, Fitzgerald-Bocarsly P. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J Immunol. 2012;189:2735–2745. doi: 10.4049/jimmunol.1102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J Biol Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyle SE, Schreckhise H, Khuu-Duong K, Henderson K, Rosler R, Storey H, Yao L, Liu H, Barahmand-pour F, Sivakumar P, Chan C, Birks C, Foster D, Clegg CH, Wietzke-Braun P, Mihm S, Klucher KM. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896–906. doi: 10.1002/hep.21312. [DOI] [PubMed] [Google Scholar]
- 29.Maher SG, Sheikh F, Scarzello AJ, Romero-Weaver AL, Baker DP, Donnelly RP, Gamero AM. IFNalpha and IFNlambda differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer Biol Ther. 2008;7:1109–1115. doi: 10.4161/cbt.7.7.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma D, Jiang D, Qing M, Weidner JM, Qu X, Guo H, Chang J, Gu B, Shi PY, Block TM, Guo JT. Antiviral effect of interferon lambda against West Nile virus. Antiviral Res. 2009;83:53–60. doi: 10.1016/j.antiviral.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagliaccetti NE, Eduardo R, Kleinstein SH, Mu XJ, Bandi P, Robek MD. Interleukin-29 functions cooperatively with interferon to induce antiviral gene expression and inhibit hepatitis C virus replication. J Biol Chem. 2008;283:30079–30089. doi: 10.1074/jbc.M804296200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Martin E, Weiss M, Diaz-San Segundo F, Pacheco JM, Arzt J, Grubman MJ, de Los Santos T. Bovine Type III Interferon Significantly Delays and Reduces the Severity of Foot-and-Mouth Disease in Cattle. J Virol. 2012;86:4477–4487. doi: 10.1128/JVI.06683-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Ye L, Wang X, Hu S, Ho W. Induction of interferon-lambda contributes to toll-like receptor 3-mediated herpes simplex virus type 1 inhibition in astrocytes. J Neurosci Res. 2012;90:399–406. doi: 10.1002/jnr.22758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L, Li J, Wang X, Ye L, Hou W, Ho J, Li H, Ho W. IL-29/IL-28A suppress HSV-1 infection of human NT2-N neurons. J Neurovirol. 2011;17:212–219. doi: 10.1007/s13365-011-0031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Hu S, Zhou L, Ye L, Wang X, Ho J, Ho W. Interferon lambda inhibits herpes simplex virus type I infection of human astrocytes and neurons. Glia. 2011;59:58–67. doi: 10.1002/glia.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, Sorgeloos F, Ehl S, Mayer D, Kochs G, Schwemmle M, Gunther S, Drosten C, Michiels T, Staeheli P. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84:5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mordstein M, Kochs G, Dumoutier L, Renauld JC, Paludan SR, Klucher K, Staeheli P. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4:e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svetlikova D, Kabat P, Ohradanova A, Pastorek J, Betakova T. Influenza A virus replication is inhibited in IFN-lambda2 and IFN-lambda3 transfected or stimulated cells. Antiviral Res. 2010;88:329–333. doi: 10.1016/j.antiviral.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Hou W, Wang X, Ye L, Zhou L, Yang ZQ, Riedel E, Ho WZ. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J Virol. 2009;83:3834–3842. doi: 10.1128/JVI.01773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagliaccetti NE, Chu EN, Bolen CR, Kleinstein SH, Robek MD. Lambda and alpha interferons inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities. Virology. 2010;401:197–206. doi: 10.1016/j.virol.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu H, Butera M, Nelson DR, Liu C. Novel type I interferon IL-28A suppresses hepatitis C viral RNA replication. Virol J. 2005;2:80. doi: 10.1186/1743-422X-2-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79:3851–3854. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong SH, Cho O, Kim K, Shin HJ, Kotenko SV, Park S. Effect of interferon-lambda on replication of hepatitis B virus in human hepatoma cells. Virus Res. 2007;126:245–249. doi: 10.1016/j.virusres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Heinze B, Frey S, Mordstein M, Schmitt-Graff A, Ehl S, Buchholz UJ, Collins PL, Staeheli P, Krempl CD. Both nonstructural proteins NS1 and NS2 of pneumonia virus of mice are inhibitors of the interferon type I and type III responses in vivo. J Virol. 2011;85:4071–4084. doi: 10.1128/JVI.01365-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding Q, Huang B, Lu J, Liu YJ, Zhong J. Hepatitis C virus NS3/4A protease blocks IL-28 production. Eur J Immunol. 2012;42:2374–2382. doi: 10.1002/eji.201242388. [DOI] [PubMed] [Google Scholar]
- 46.Huang J, Smirnov SV, Lewis-Antes A, Balan M, Li W, Tang S, Silke GV, Putz MM, Smith GL, Kotenko SV. Inhibition of type I and type III interferons by a secreted glycoprotein from Yaba-like disease virus. Proc Natl Acad Sci U S A. 2007;104:9822–9827. doi: 10.1073/pnas.0610352104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bandi P, Pagliaccetti NE, Robek MD. Inhibition of type III interferon activity by orthopoxvirus immunomodulatory proteins. J Interferon Cytokine Res. 2010;30:123–134. doi: 10.1089/jir.2009.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muir AJ, Shiffman ML, Zaman A, Yoffe B, de la Torre A, Flamm S, Gordon SC, Marotta P, Vierling JM, Lopez-Talavera JC, Byrnes-Blake K, Fontana D, Freeman J, Gray T, Hausman D, Hunder NN, Lawitz E. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology. 2010;52:822–832. doi: 10.1002/hep.23743. [DOI] [PubMed] [Google Scholar]
- 49.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A. 2011;108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witte K, Gruetz G, Volk HD, Looman AC, Asadullah K, Sterry W, Sabat R, Wolk K. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 2009;10:702–714. doi: 10.1038/gene.2009.72. [DOI] [PubMed] [Google Scholar]
- 52.Jordan WJ, Eskdale J, Boniotto M, Rodia M, Kellner D, Gallagher G. Modulation of the human cytokine response by interferon lambda-1 (IFN-lambda1/IL-29) Genes Immun. 2007;8:13–20. doi: 10.1038/sj.gene.6364348. [DOI] [PubMed] [Google Scholar]
- 53.Morrow MP, Yan J, Pankhong P, Shedlock DJ, Lewis MG, Talbott K, Toporovski R, Khan AS, Sardesai NY, Weiner DB. IL-28B/IFN-lambda 3 drives granzyme B loading and significantly increases CTL killing activity in macaques. Mol Ther. 2010;18:1714–1723. doi: 10.1038/mt.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrow MP, Yan J, Pankhong P, Ferraro B, Lewis MG, Khan AS, Sardesai NY, Weiner DB. Unique Th1/Th2 phenotypes induced during priming and memory phases by use of interleukin-12 (IL-12) or IL-28B vaccine adjuvants in rhesus macaques. Clin Vaccine Immunol. 2010;17:1493–1499. doi: 10.1128/CVI.00181-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mennechet FJ, Uze G. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood. 2006;107:4417–4423. doi: 10.1182/blood-2005-10-4129. [DOI] [PubMed] [Google Scholar]
- 56.Morrow MP, Pankhong P, Laddy DJ, Schoenly KA, Yan J, Cisper N, Weiner DB. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood. 2009;113:5868–5877. doi: 10.1182/blood-2008-11-190520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jordan WJ, Eskdale J, Srinivas S, Pekarek V, Kelner D, Rodia M, Gallagher G. Human interferon lambda-1 (IFN-lambda1/IL-29) modulates the Th1/Th2 response. Genes Immun. 2007;8:254–261. doi: 10.1038/sj.gene.6364382. [DOI] [PubMed] [Google Scholar]
- 58.Koltsida O, Hausding M, Stavropoulos A, Koch S, Tzelepis G, Ubel C, Kotenko SV, Sideras P, Lehr HA, Tepe M, Klucher KM, Doyle SE, Neurath MF, Finotto S, Andreakos E. IL-28A (IFN-lambda2) modulates lung DC function to promote Th1 immune skewing and suppress allergic airway disease. EMBO Mol Med. 2011;3:348–361. doi: 10.1002/emmm.201100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chi B, Dickensheets HL, Spann KM, Alston MA, Luongo C, Dumoutier L, Huang J, Renauld JC, Kotenko SV, Roederer M, Beeler JA, Donnelly RP, Collins PL, Rabin RL. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J Virol. 2006;80:5032–5040. doi: 10.1128/JVI.80.10.5032-5040.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 62.Wang N, Strugnell RA, Wijburg OL, Brodnicki TC. Systemic infection of Mice with Listeria monocytogenes to characterize host immune responses. Methods Mol Biol. 2013;1031:125–144. doi: 10.1007/978-1-62703-481-4_16. [DOI] [PubMed] [Google Scholar]
- 63.Ike F, Bourgade F, Ohsawa K, Sato H, Morikawa S, Saijo M, Kurane I, Takimoto K, Yamada YK, Jaubert J, Berard M, Nakata H, Hiraiwa N, Mekada K, Takakura A, Itoh T, Obata Y, Yoshiki A, Montagutelli X. Lymphocytic choriomeningitis infection undetected by dirty-bedding sentinel monitoring and revealed after embryo transfer of an inbred strain derived from wild mice. Comp Med. 2007;57:272–281. [PubMed] [Google Scholar]
- 64.Whitmire JK, Tan JT, Whitton JL. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foster B, Prussin C, Liu F, Whitmire JK, Whitton JL. Detection of intracellular cytokines by flow cytometry. In: Coligan John E, et al., editors. Current protocols in immunology. Unit 6. Chapter 6. 2007. p. 24. [DOI] [PubMed] [Google Scholar]
- 66.Mothe BR, Stewart BS, Oseroff C, Bui HH, Stogiera S, Garcia Z, Dow C, Rodriguez-Carreno MP, Kotturi M, Pasquetto V, Botten J, Crotty S, Janssen E, Buchmeier MJ, Sette A. Chronic lymphocytic choriomeningitis virus infection actively down-regulates CD4+ T cell responses directed against a broad range of epitopes. J Immunol. 2007;179:1058–1067. doi: 10.4049/jimmunol.179.2.1058. [DOI] [PubMed] [Google Scholar]
- 67.Blanchard-Rohner G, Galli G, Clutterbuck EA, Pollard AJ. Comparison of a limiting dilution assay and ELISpot for detection of memory B-cells before and after immunisation with a protein-polysaccharide conjugate vaccine in children. J Immunol Methods. 2010;358:46–55. doi: 10.1016/j.jim.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 68.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 69.Whitmire JK, Slifka MK, Grewal IS, Flavell RA, Ahmed R. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J Virol. 1996;70:8375–8381. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Swiecki M, Cella M, Alber G, Schreiber RD, Gilfillan S, Colonna M. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe. 2012;11:631–642. doi: 10.1016/j.chom.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 2006;176:7686–7694. doi: 10.4049/jimmunol.176.12.7686. [DOI] [PubMed] [Google Scholar]
- 72.Jewell NA, Cline T, Mertz SE, Smirnov SV, Flano E, Schindler C, Grieves JL, Durbin RK, Kotenko SV, Durbin JE. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J Virol. 2010;84:11515–11522. doi: 10.1128/JVI.01703-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khaitov MR, Laza-Stanca V, Edwards MR, Walton RP, Rohde G, Contoli M, Papi A, Stanciu LA, Kotenko SV, Johnston SL. Respiratory virus induction of alpha-, beta- and lambda-interferons in bronchial epithelial cells and peripheral blood mononuclear cells. Allergy. 2009;64:375–386. doi: 10.1111/j.1398-9995.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 74.Whitmire JK, Benning N, Whitton JL. Cutting edge: early IFN-gamma signaling directly enhances primary antiviral CD4+ T cell responses. J Immunol. 2005;175:5624–5628. doi: 10.4049/jimmunol.175.9.5624. [DOI] [PubMed] [Google Scholar]
- 75.Whitmire JK, Eam B, Benning N, Whitton JL. Direct Interferon-{gamma} Signaling Dramatically Enhances CD4+ and CD8+ T Cell Memory. J Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 76.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 78.Masopust D, Murali-Krishna K, Ahmed R. Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought. J Virol. 2007;81:2002–2011. doi: 10.1128/JVI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rai D, Pham NL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol. 2009;183:7672–7681. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 81.Srinivas S, Dai J, Eskdale J, Gallagher GE, Megjugorac NJ, Gallagher G. Interferon-lambda1 (interleukin-29) preferentially down-regulates interleukin-13 over other T helper type 2 cytokine responses in vitro. Immunology. 2008;125:492–502. doi: 10.1111/j.1365-2567.2008.02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dai J, Megjugorac NJ, Gallagher GE, Yu RY, Gallagher G. IFN-lambda1 (IL-29) inhibits GATA3 expression and suppresses Th2 responses in human naive and memory T cells. Blood. 2009;113:5829–5838. doi: 10.1182/blood-2008-09-179507. [DOI] [PubMed] [Google Scholar]
- 83.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 84.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R{alpha}-expressing cells. J Exp Med. 2007;204:547–557. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.D’Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 88.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 89.Kristensen NN, Christensen JP, Thomsen AR. High numbers of IL-2-producing CD8+ T cells during viral infection: correlation with stable memory development. J Gen Virol. 2002;83:2123–2133. doi: 10.1099/0022-1317-83-9-2123. [DOI] [PubMed] [Google Scholar]
- 90.Ramos EL. Preclinical and clinical development of pegylated interferon-lambda 1 in chronic hepatitis C. J Interferon Cytokine Res. 2010;30:591–595. doi: 10.1089/jir.2010.0066. [DOI] [PubMed] [Google Scholar]
- 91.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waggoner SN, Taniguchi RT, Mathew PA, Kumar V, Welsh RM. Absence of mouse 2B4 promotes NK cell-mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J Clin Invest. 2010;120:1925–1938. doi: 10.1172/JCI41264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brustle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Haussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A. 2012;109:1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cook KD, Whitmire JK. The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J Immunol. 2013;190:641–649. doi: 10.4049/jimmunol.1202448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baize S, Pannetier D, Faure C, Marianneau P, Marendat I, Georges-Courbot MC, Deubel V. Role of interferons in the control of Lassa virus replication in human dendritic cells and macrophages. Microbes Infect. 2006;8:1194–1202. doi: 10.1016/j.micinf.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 96.Megjugorac NJ, Gallagher GE, Gallagher G. Modulation of human plasmacytoid DC function by IFN-lambda1 (IL-29) J Leukoc Biol. 2009;86:1359–1363. doi: 10.1189/jlb.0509347. [DOI] [PubMed] [Google Scholar]
- 97.Dolganiuc A, Kodys K, Marshall C, Saha B, Zhang S, Bala S, Szabo G. Type III interferons, IL-28 and IL-29, are increased in chronic HCV infection and induce myeloid dendritic cell-mediated FoxP3+ regulatory T cells. PloS one. 2012;7:e44915. doi: 10.1371/journal.pone.0044915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rynda A, Maddaloni M, Ochoa-Reparaz J, Callis G, Pascual DW. IL-28 supplants requirement for T(reg) cells in protein sigma1-mediated protection against murine experimental autoimmune encephalomyelitis (EAE) PloS one. 2010;5:e8720. doi: 10.1371/journal.pone.0008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diebold J, Diepolder H, Adler B, Auernhammer CJ, Goke B, Dambacher J. IL-28A and IL-29 mediate antiproliferative and antiviral signals in intestinal epithelial cells and murine CMV infection increases colonic IL-28A expression. Am J Physiol Gastrointest Liver Physiol. 2005;289:G960–968. doi: 10.1152/ajpgi.00126.2005. [DOI] [PubMed] [Google Scholar]
- 100.Siebler J, Wirtz S, Weigmann B, Atreya I, Schmitt E, Kreft A, Galle PR, Neurath MF. IL-28A is a key regulator of T-cell-mediated liver injury via the T-box transcription factor T-bet. Gastroenterology. 2007;132:358–371. doi: 10.1053/j.gastro.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 101.He SH, Chen X, Song CH, Liu ZQ, Zhou LF, Ma WJ, Zhao LD, Li TL, Tang SG, Xing Z, Yang PC. Interferon-lambda mediates oral tolerance and inhibits antigen-specific, T-helper 2 cell-mediated inflammation in mouse intestine. Gastroenterology. 2011;141:249–258. 258 e241–242. doi: 10.1053/j.gastro.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 102.Dickensheets H, Sheikh F, Park O, Gao B, Donnelly RP. Interferon-lambda (IFN-lambda) induces signal transduction and gene expression in human hepatocytes, but not in lymphocytes or monocytes. J Leukoc Biol. 2013;93:377–385. doi: 10.1189/jlb.0812395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc Natl Acad Sci U S A. 2010;107:3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Biswas PS, Pedicord V, Ploss A, Menet E, Leiner I, Pamer EG. Pathogen-specific CD8 T cell responses are directly inhibited by IL-10. J Immunol. 2007;179:4520–4528. doi: 10.4049/jimmunol.179.7.4520. [DOI] [PubMed] [Google Scholar]
- 107.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hahm B, Trifilo MJ, Zuniga EI, Oldstone MB. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 2005;22:247–257. doi: 10.1016/j.immuni.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 111.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, Brooks DG. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108:3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 114.Schattner A, Meshorer A, Wallach D. Involvement of interferon in virus-induced lymphopenia. Cell Immunol. 1983;79:11–25. doi: 10.1016/0008-8749(83)90046-1. [DOI] [PubMed] [Google Scholar]
- 115.Ng CT, Nayak BP, Schmedt C, Oldstone MB. Immortalized clones of fibroblastic reticular cells activate virus-specific T cells during virus infection. Proc Natl Acad Sci U S A. 2012;109:7823–7828. doi: 10.1073/pnas.1205850109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, Larsen CP, Ahmed R. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci U S A. 2007;104:15430–15435. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.