Abstract
We previously identified angiotensin converting enzyme (ACE) and an endopeptidase activity that degraded angiotensin-(1-7) [Ang-(1-7)] to Ang-(1-5) and Ang-(1-4), respectively, in the cerebrospinal fluid (CSF) of 6-month old male sheep. The present study undertook a more comprehensive analysis of the CSF peptidase that converts Ang-(1-7) to Ang-(1-4) in control and in utero betamethasone exposed sheep (BMX). Characterization of the Ang-(1-7) peptidase revealed that the thiol agents 4-aminophenylmercuric acetate (APMA) and p-chloromercuribenzoic acid (PCMB), as well as the metallo-chelators o-phenanthroline and EDTA essentially abolished the enzyme activity. Additional inhibitors for serine, aspartyl, and cysteine proteases, as well as selective inhibitors against the endopeptidases neprilysin, neurolysin, prolyl and thimet oligopeptidases did not attenuate enzymatic activity. Competition studies against the peptidase revealed similar IC50's for Ang-(1-7)(5 μM) and Ang II (3 μM), but lower values for Ala1-Ang-(1-7) and Ang-(2-7) of 1.8 and 2.0 μM, respectively. In contrast, bradykinin exhibited a 6-fold higher IC50 (32 μM) than Ang-(1-7) while neurotensin was a poor competitor. Mean arterial pressure (78 ± 1 vs. 94 ± 2 mmHg, N= 4-5, P<0.01) and Ang-(1-7) peptidase activity (14.2 ± 1 vs 32 ± 1.5 fmol/min/ml CSF, N = 5, P<0.01) were higher in the BMX group, and enzyme activity inversely correlated with Ang-(1-7) content in CSF. Lower Ang-(1-7) expression in brain is linked to baroreflex impairment in hypertension and aging, thus, increased activity of an Ang-(1-7) peptidase may contribute to lower CSF Ang-(1-7) levels, elevated blood pressure and impaired reflex function in this model of fetal programming.
Keywords: Renin Angiotensin System, Ang-(1-7), Cerebrospinal Fluid, Peptidase
1. Introduction
Evidence for the influence of early prenatal events in the fetus to induce a greater susceptibility to cardiovascular and metabolic pathologies is evident in both experimental models and in humans. Although the precise nature of fetal programming events is not known, altered expression of the biochemical components and functional actions of the renin-angiotensin system (RAS) may constitute an important underlying mechanism [9, 14, 15, 18, 19, 28, 30, 31]. We utilize a sheep model of fetal programming in which pregnant ewes are administrated the glucocorticoid betamethasone at day 80 of gestation. This regimen parallels the dose and time that pregnant women are typically treated with glucocorticoids to enhance pulmonary function and reduce mortality of the fetus delivered preterm [1, 4, 12, 13, 24]. Fetal exposure to glucocorticoids in sheep results in a significant reduction in the nephron number within the kidney, an increase in mean arterial pressure (MAP), attenuation of the baroreflex sensitivity (BRS) in the control of heart rate, and increased indices of metabolic dysfunction in adult animals [14, 26-28, 30, 31]. Indeed, antenatal exposure to BM elicits decreased BRS by 6-weeks [27, 30], and elevated MAP by 6-months of age [14, 28, 31, 34].
Fetal programming events that have long-term consequences on the RAS may not solely reflect an influence on the ACE-Ang II-AT1 receptor axis. Indeed, our studies suggest a shift away from the Ang-(1-7) axis and towards the Ang II pathway in the kidney, circulation, and brain tissue of betamethasone (BM) exposed sheep [15, 18, 28]. Microinjections of the AT1 and Ang-(1-7) receptor antagonists into the solitary tract nucleus (NTS) of the dorsal brainstem of BM exposed (BMX) sheep reveal an increase in Ang II and a decrease in Ang-(1-7) pathways mediating BRS at 6-weeks of age [29]. We recently reported that Ang-(1-7) peptide levels were significantly lower in the CSF of male BMX sheep [19]. We attributed the lower expression of Ang-(1-7) to increased metabolism of the peptide by both ACE and a soluble peptidase sensitive to the thiol inhibitor PCMB. Since the CSF is in constant contact with the brain's extracellular fluid, this peptidase activity may be relevant to the regulation of Ang-(1-7) metabolism in the brain [5, 23]. Therefore, the current study performed a comprehensive characterization of the Ang-(1-7) peptidase in regards to the sensitivity of various protease/endopeptidase inhibitors and selectivity among angiotensins and other peptides. Furthermore, we examined the expression of this peptidase in the CSF of control and BMX groups that directly exhibit differences in blood pressure and Ang-(1-7) content associated with fetal programming events.
2. Materials and Methods
2.1 Animals
Sheep received saline or betamethasone acetate: phosphate 1:1 mixture (IM, 2 doses of 0.17 mg/kg, 24 hours apart) at the 80th day of gestation. Mixed breed sheep were delivered at term, farm raised, and weaned at 3-months of age. At 6-months of age, male and female offspring were brought to our Association for Assessment and Accreditation of Laboratory Animals Care (AAALAC) approved facility, where they were maintained on a normal diet, with free access to tap water and a 12-hour light/dark cycle (lights on 7 AM to 7 PM). Sheep were anesthetized with ketamine and isoflurane and euthanized by exsanguination. Cerebrospinal fluid was extracted (~3 ml per animal), taking care to avoid contamination with blood, and tubes were stored at −80°C. These procedures were approved by the Wake Forest University School of Medicine ACUC for animal care.
2.2 Blood Pressure Measurements
Sheep were anesthetized with ketamine and isoflurane followed by catheterization into the femoral artery and vein for blood pressure measurements. After at least 5 days recovery, MAP and was recorded in conscious animals and digitized with Acknowledge software (BIOPAC 3.8.1) [31].
2.3 Sample concentration
Cerebrospinal fluid (5 ml) was pooled from individual control and BMX animals and concentrated 1:5 using molecular weight filtration tubes to remove small proteins and endogenous angiotensin peptides (30 kDa, Millipore Bedford, MA). Concentrated CSF was resuspended in a final volume of 5 ml HEPES buffer (25 mM HEPES, 125 mM NaCl, 10 μM ZnCl2, pH = 7.4) and protein concentration was measured using a Bradford protein assay.
2.4 pH Profile
Metabolism reactions were conducted with [125I]-Ang-(1-7) [0.5 nM], 100 nM Ang-(1-7), and a cocktail of inhibitors (metabolism cocktail) containing the aminopeptidase inhibitors amastatin (AM, 2 μM) and bestatin (BS, 10 μM), the chymase inhibitor chymostatin (CHYM, 10 μM), the carboxypeptidase A inhibitor benzylsuccinate (BSC, 10 μM), and the ACE inhibitor lisinopril (LIS, 10 μM). CSF (25 μl) was added to buffers ranging from pH 3-6: using 25 mM MES, 125 mM NaCl, and pH 6.5-9 using 25 mM HEPES, 125 mM NaCl).
2.5 Inhibitor profile
Various inhibitors were tested on Ang-(1-7) metabolism including PCMB, APMA, leupeptin, E-64, aprotinin, soybean trypsin inhibitor (SBTI), pepstatin A, EDTA, EGTA,o-phenanthroline, SCH 39370, 1-carboxy-3-phenyl-propyl (Ala-Ala-Phe-4-Abz-OH, CFP), Z-prolyl prolinal (ZPP), dithiothreitol (DTT), and the dipeptide Pro-Ile. Each reaction was conducted in the presence of [125I]-Ang-(1-7) [0.5 nM], 100 nM Ang-(1-7), 25 μl CSF, and the inhibitor cocktail (AM, BS, CHYM, BSC, and LIS). All inhibitors were obtained from Sigma (St. Louis, MO) except SCH 39370 (gift from Schering Plow) and CFP (Bachem, King of Prussia, PA).
2.6 Peptidase kinetics
Kinetic or competition assays were performed with [125I]-Ang-(1-7) as the substrate and increasing concentrations of either unlabeled Ang II, Ang-(1-7), Ala1-Ang-(1-7) Ang-(2-7), D-Ala7-Ang-(1-7), and D-Pro7-Ang-(1-7) as previously described [19, 32]. Reaction velocities for generation of [125I]-Ang-(1-4) from [125I]-Ang-(1-7) were expressed as nmol/min/mg protein. The assays for all peptides were performed in the inhibitor cocktail (AM, BS, CHYM, BSC, and LIS) to prevent the contribution of other peptidases in the CSF and preserve the Ang-(1-4) product. Kinetic constants (Km, Vmax, IC50) were determined using Michaelis-Menten kinetics or non-linear regression one-site competition for IC50 with no constraints in the Prism 5 statistical program. All peptides were obtained from Bachem (Torrance CA) or custom synthesized (Genscript, Piscataway, NJ).
2.7 HPLC separation
Metabolism reactions were conducted at 37°C in reaction buffer using concentrated CSF (25 μl; 35 μg) in a final volume of 250 μl. Each reaction included 0.5 nM iodinated [125I]-Ang-(1-7) and 0.1 μM non-iodinated Ang-(1-7) [28]. The reaction was stopped after 120 minutes by addition of ice-cold 1.0% phosphoric acid and centrifuged at 16,000xg. The supernatant was immediately filtered for separation by reverse-phase high-performance liquid chromatography (HPLC) on a Shimadzu equipped with an Aeris Peptide XB-C18 3.6 μm (2.1x100 mm, Phenomenex, Torrance CA). The [125I]-products were monitored by a Bioscan flow-through γ detector as described [19]. Products were identified by comparison of retention times to [125I]-standard peptides and sensitivity to peptidase inhibitors. Peptides were iodinated by the chloramine T method and purified by HPLC to a specific activity >2,000 Ci/mmol [7].
2.8 Statistics
Data are expressed as mean ± SEM. Unpaired t tests and two-way ANOVA with Bonferroni posttests were used for the statistical analysis of the data with GraphPad Prism 5.01 (GraphPad Software, San Diego, CA). The criterion for statistical significance was set at *P < 0.05.
3. Results
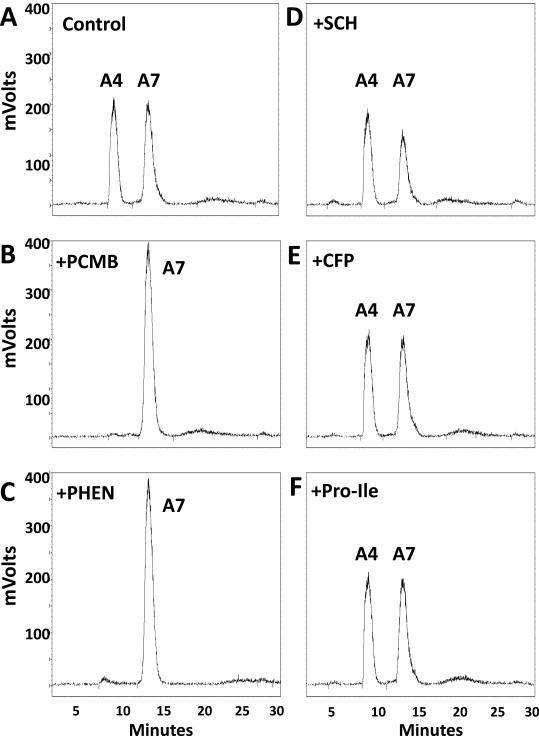
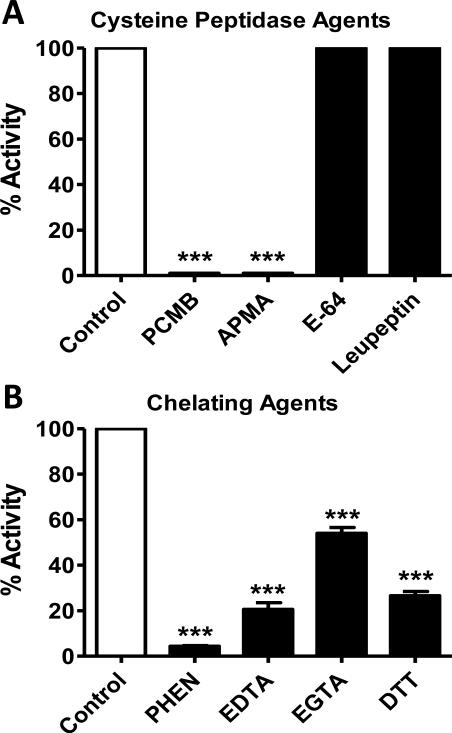
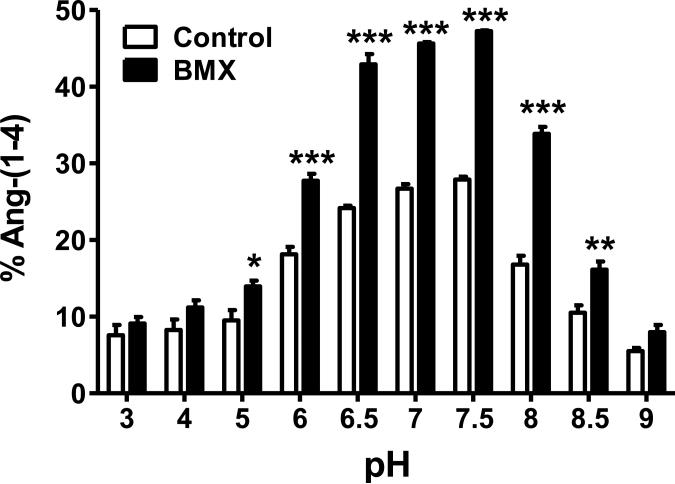
We previously reported that ACE and a PCMB-sensitive soluble peptidase contributed to the metabolism of Ang-(1-7) in sheep CSF [19]. ACE converted Ang-(1-7) to Ang-(1-5); however, the endopeptidase hydrolyzed Ang-(1-7) at the Tyr4-Ile5 bond to form the tetrapeptide Ang-(1-4). The current studies undertook a more extensive characterization of this peptidase in the CSF from both control and BMX sheep in which mean arterial pressure (MAP) and CSF levels of Ang-(1-7) were significantly altered (see Figure 5) [19]. As shown in Figure 1A, the chromatograph reveals that the CSF activity hydrolyzed [125I]-Ang-(1-7) to [125I]-Ang-(1-4). The peak of Ang-(1-4) was abolished by the thiol inhibitor PCMB and the chelating agent o-phenanthroline (PHEN, Figure 1B-C). However, selective inhibitors against neprilysin (SCH39370, SCH), thimet oligopeptidase (c-Ala-Ala-Phe-pAB, CFP) and neurolysin (Pro-Ile) failed to attenuate the metabolism of Ang-(1-7) to Ang-(1-4) (Figure 1D-F). Figure 2 presents the results from an array of inhibitors on the hydrolysis of [125I]-Ang-(1-7) to [125I]-Ang-(1-4) in the CSF. Although both the mercuri-containing agents PCMB and APMA potently inhibited Ang-(1-7) to Ang-(1-4) conversion, the prototypic cysteine protease inhibitor E-64 and the lysosomal inhibitor leupeptin did not block activity. Moreover, the reducing agent DTT, which typically activates thiol proteases by the protection of critical cysteine or methionine residues, significantly inhibited activity by 73 ± 2%. Given the mixed effects among the thiol inhibitors, we tested several chelating agents to block Ang-(1-7) metabolism. EGTA, EDTA and o-phenanthroline inhibited 46 ± 3%, 79 ± 3% and 96 ± 0.3% of Ang-(1-4) formation, respectively (Figure 2). Inhibitors against other classes of enzymes including serine (aprotinin, SBTI) and aspartyl (pepstatin) did not alter activity (Table 1). The data in Table 1 also revealed that selective inhibitors against neprilysin, thimet oligopeptidase, prolyl oligopeptidase and neurolysin did not attenuate the hydrolysis of Ang-(1-7). An optimal pH of 7.5 for [125I]-Ang-(1-7) to [125I]-Ang-(1-4) conversion was also demonstrated in both the control and BMX sheep; however the BMX pool exhibited higher activity than the control at pH 5 to 8.5 (Figure 3).
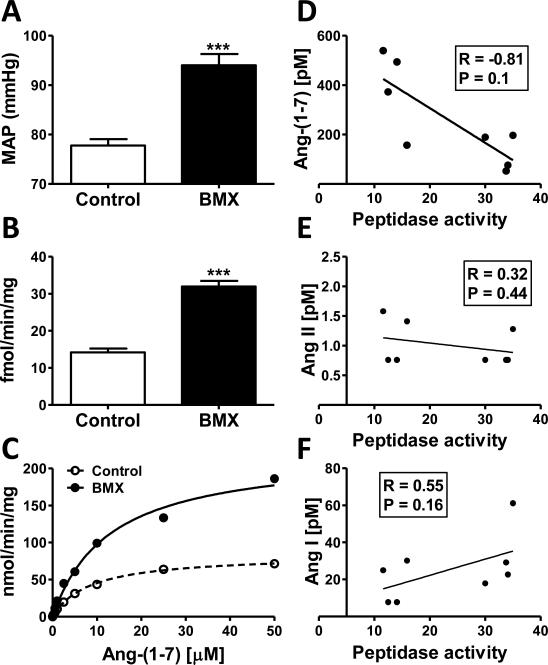
Figure 5.
Betamethasone-exposed sheep exhibit higher mean arterial pressure (MAP) and Ang-(1-7) endopeptidase activity than controls. A- Mean arterial pressure (MAP) was significantly higher in BMX animals than controls (N=4 control, N = 5 BMX). B- Peptidase activity was significantly higher in BMX animals (N = 5 per group). Activity was assayed in the CSF of control and BMX animals in the presence of 100 nM Ang-(1-7) and an inhibitor cocktail (AM, BS, CHYM, BSC, LIS) for 120 minutes at 37°C (*P < 0.05 vs. control; N=5 per group). C- The apparent Km (Km’) and maximal velocity (Vmax’) for the metabolism of 125I-Ang-(1-7) to 125I-Ang-(1-4). Control: Km’ = 8.5 μM and Vmax’ = 84 nmol/min/mg; BMX: Km’ = 13 μM and Vmax’ = 223 nmol/min/mg for BMX animals. D- Correlation of Ang-(1-7) peptide levels and peptidase activity in CSF of control and BMX animals (r = −0.81). *P < 0.05, ***P < 0.0001. Kinetic constants were derived by the GraphPad Prism 5 statistical program
Figure 1.
PCMB and o-phenanthroline abolish [125I]-Ang-(1-7) metabolism. HPLC chromatographs reveal that both PCMB (10 μM, Panel B) and o-phenanthroline (PHEN, 1 mM, Panel C) abolished conversion of 125I-Ang-(1-7) [A7] to 125I-Ang-(1-4) [A4] as compared to control (Panel A). Addition of the neprilysin inhibitor SCH39370 (SCH, 10 μM, Panel D), thimet oligopeptidase/neurolysin inhibitor CFP (100 μM, Panel E), or the neurolysin dipeptide Pro-Ile (1 mM, Panel F) did attenuate the processing of A7 to A4. Activity was assayed from pooled control and BMX CSF (N=10) in the presence of 100 nM Ang-(1-7) and an inhibitor cocktail (AM, BS, CHYM, BSC, LIS) for 120 minutes at 37°C.
Figure 2.
Cysteine peptidase inhibitors and chelating agents inhibit enzyme activity. The mercuri-containing peptidase agents PCMB (10 μM) and AMPA (10 μM) abolish activity while E-64 (10 μM) and leupeptin (100 μM) did not alter [125I]-Ang-(1-4) formation (Panel A). The chelating agents PHEN (o-phenanthroline, 1 mM), EDTA (5 mM), EGTA (5 mM), and DTT (5 mM) inhibit 125I-Ang-(1-4) formation from [125I]-Ang-(1-7) (Panel B). Extent of inhibition was determined as percent (%) [125I]-Ang-(1-4) formation compared to controls. Activity was assayed from pooled CSF of control and BMX animals (N=10) in the presence of 100 nM Ang-(1-7) and an inhibitor cocktail (AM, BS, CHYM, BSC, LIS) for 120 minutes at 37°C. N = 3, ***P<0.0001 vs. control
Table 1.
Specific inhibitors do not inhibit enzyme activity.
| Inhibitor Class | Inhibitor | % of Control (N = 3) |
|---|---|---|
| Neprilysin | SCH (10 μM) | 100 |
| Thimet oligopeptidase | CFP (10 μM) | 102 ± 3 |
| Thimet oligopeptidase | CFP (100 μM) | 97 ± 2 |
| Neurolysin | Pro-Ile (1 mM) | 101 ± 2 |
| Prolyl endopeptidase | ZPP (10 μM) | 96 ± 2 |
| Serine | Aprotinin (80 μM) | 100 |
| Serine | SBTI (100 μM) | 101 ± 2 |
| Aspartic acid | Pepstatin A (100 μM) | 97 ± 1 |
Inhibitors SCH39370 (SCH), 1-carboxy-3-phenyl-propyl (CFP), Pro-Ile, Z-prolyl prolinal (ZPP), aprotinin, soybean trypsin inhibitor (SBTI) and pepstatin A did not inhibit [125I]-Ang-(1-4) formation compared to control. Activity was assayed in a CSF pool of control and BMX animals (N=10) in the presence of 100 nM Ang-(1-7) and an inhibitor cocktail (AM, BS, CHYM, BSC, LIS) for 120 minutes at 37°C. Assays were repeated three times.
Figure 3.
Optimal pH of CSF enzyme is 7.5 in BMX and control animals. Metabolism reactions were run separately for BMX and control animals in buffers pH 3-9 and the % Ang-(1-4) formed was quantified. Buffers: pH 3-6 25 mM MES, 125 mM NaCl, and pH 6.5-9 25 mM HEPES, 125 mM NaCl. BMX animals had significantly higher metabolism than controls at pH 5-8.5. (N = 3, *P<0.05, **P<0.01, ***P<0.001)
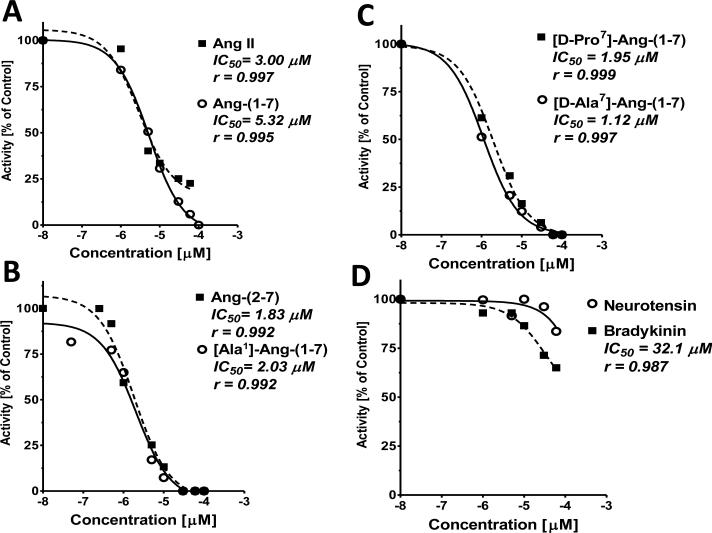
We also pooled and concentrated CSF samples from control and BMX sheep to compare the extent of inhibition (IC50) of angiotensins and other peptides for the conversion of Ang-(1-7) to Ang-(1-4). As shown in Figure 4A, Ang-(1-7) and Ang II exhibited similar IC50 values of 5.3 and 3.0 μM, respectively, although Ang II competed for approximately 75% of enzyme activity over this concentration range. The Ang-(1-7) N-terminal analogs Ang-(2-7) and Ala1-Ang-(1-7) exhibited IC50's of 1.8 and 2.0 μM, and completely inhibited activity (Figure 4B). The substituted C-terminal analogs [D-Pro7]-Ang-(1-7) and [D-Ala7]-Ang-(1-7) exhibited similar IC50 values of 2.0 and 1.1 μM and abolished activity as well (Figure 4C). Bradykinin exhibited an IC50 of 32 μM while neurotensin inhibited 15% of activity and an IC50 could not be derived over the concentration range of the peptide (Figure 4D).
Figure 4.
Analysis of the competition of angiotensin peptides for the conversion of [125I]-Ang-(1-7) to [125I]-Ang-(1-4) in pooled CSF. Activity was assayed in a CSF pool of control and BMX animals (N=10) and an inhibitor cocktail (AM, BS, CHYM, BSC, LIS) for 120 minutes at 37°C. The IC50 and goodness of fit (r) values were derived by the GraphPad Prism 5 statistical program.
We previously reported that a thiol-sensitive peptidase accounted for the majority of Ang-(1-7) metabolism in the CSF. A kinetic analysis revealed a tendency for higher activity in the BMX animals versus the controls but did not achieve a statistical difference in these groups [19]. Since the CSF Ang-(1-7) content was over 2-fold lower in the BMX animals, we examined the peptidase activity in the identical cohort of BMX and control animals in which the peptide levels and blood pressures were obtained. As shown in Figure 5A, MAP was significantly higher in BMX animals as compared to controls (78 ± 1 vs. 94 ± 2 mmHg, N = 4 control and 5 BMX, Figure 5A). The peptidase activity for [125I]-Ang-(1-7) to [125I]-Ang-(1-4) conversion was also significantly higher in the CSF of BMX animals (Figure 5B). Saturation studies on peptidase activity for Ang-(1-7) were then performed separately on pooled CSF from the same control and BMX animals. Kinetic analysis revealed an apparent Km (Km’) for Ang-(1-7) of 8.5 μM and a Vmax’ of 84 nmol/min/mg protein in controls, while the BMX pool exhibited a Km’ of 12.3 μM and Vmax’ of 223 nmol/min/mg protein (Figure 5C). Finally, Ang-(1-7), but not Ang II or Ang I, peptide levels in CSF were inversely correlated to the peptidase activity of the individual samples [p=0.01; r of 0.81] based on the peptide content previously reported in the same control and BMX sheep (Figure 5D,E,F) [19].
4. Discussion
The present study identified a soluble metallopeptidase in the CSF from control and BMX sheep. The peptidase hydrolyzed [125I]-Ang-(1-7) at the Tyr4-Ile5 bond to form the tetrapeptide [125I]-Ang-(1-4). The enzyme was highly sensitive to chelating agents (o-phenanthroline and EDTA), mercurial-based inhibitors (PCMB and APMA), and moderately sensitive to EGTA and DTT. By contrast, the peptidase was insensitive to serine, aspartyl and other cysteine protease inhibitors. The optimal pH of the Ang-(1-7) peptidase was approximately pH 7.5 and the activity was significantly higher in the CSF of BMX animals as compared to controls. Kinetic analysis confirmed that the apparent Vmax (Vmax’) of the peptidase for Ang-(1-7) was 2.5 fold higher in the BMX group consistent with higher peptidase activity in the CSF of exposed animals. Moreover, the metabolizing activity in the CSF inversely correlated to Ang-(1-7) content suggesting that alterations in peptidase activity may contribute to the lower levels of peptide in the CSF of the BMX animals [19].
Previous studies in the betamethasone model of fetal programming in sheep focused on the peptidases ACE and ACE2 that comprise the main enzymatic components of the Ang II-AT1 receptor and the Ang-(1-7)-Mas receptor pathways of the RAS [32]. Indeed, we find that the adult offspring of glucocorticoid-exposed dams exhibit alterations in both enzymes that would contribute to an imbalance in the RAS axis [28]. In the circulation, soluble ACE activity was increased while ACE2 activity decreased in the BMX sheep; the ratio of their specific activities highly correlated to blood pressure in the control and BMX groups [28]. Although renal ACE activity was unchanged, ACE2 activity was lower in the renal cortex, isolated tubules and urine of the BMX sheep which would contribute to a higher ratio of Ang II to Ang-(1-7) [28]. We have not assessed whether the expression of these peptidases are altered in the brain; however, we found that soluble ACE activity was higher in the CSF of the BMX sheep and that the peptide content of Ang-(1-7) was markedly lower [19]. ACE is well-accepted as the primary pathway to form Ang II, but the enzyme plays a key role in the metabolism of Ang-(1-7) cleaving the Ile5-His6 bond to form the pentapeptide Ang-(1-5) [2]. Indeed, ACE blockade markedly increased the half-life of Ang-(1-7) in the circulation which explains, in part, the increase in endogenous levels of the peptide following ACE inhibition [8]. In addition to CSF ACE, we detected another soluble activity that metabolized Ang-(1-7) at the Tyr4-Ile5 bond to form Ang-(1-4). The conversion of Ang-(1-7) to Ang-(1-4) in CSF was abolished by the thiol inhibitor PCMB but not the ACE inhibitor, and we had concluded that both ACE and a thiol-sensitive peptidase may metabolize Ang-(1-7) in the sheep CSF [19].
The present study undertook a more comprehensive evaluation of the CSF peptidase regarding sensitivity to both general and specific protease inhibitors. Although the peptidase was sensitive to both the mercuri-containing inhibitors PCMB and APMA, the cysteine inhibitor E-64 and lysosomal agent leupeptin did not attenuate activity while the thiol reducing agent DTT significantly reduced the metabolism of Ang-(1-7). Further analysis revealed the metallo-chelating agents EDTA and ophenanthroline effectively blocked activity, and inhibitors to other classes of peptidases were ineffective. These data indicate that the Ang-(1-7) degrading activity is likely a metalloendopeptidase with pronounced sensitivity to mercuri-containing inhibitors such as PCMB and APMA. Subsequently, a review of known endopeptidases present in the brain and sensitive to both metallo-chelating agents and thiol inhibitors suggested the potential contribution of either thimet oligopeptidase [EC3.4.24.15, TOP] or neurolysin [EC3.4.24.16] [10, 11, 19, 33]. However, the selective TOP inhibitor CFP (10 and 100 μM) [25] did not attenuate activity. We utilized a 10-fold higher concentration CFP as well as the dipeptide Pro-Ile to inhibit neurolysin, but neither agent affected Ang-(1-7) conversion in the CSF. Ferro and colleagues recently investigated whether different splice variants that dictate the cellular trafficking of neurolysin influence either substrate or inhibitor specificity of the endopeptidase; however, both cytosolic and mitochondrial forms cleaved Ang I to Ang-(1-7) and were sensitive to Pro-Ile inhibition [20]. Previous studies reported Ki's of 90 μM (Pro-Ile) and 4 μM (CFP) for neurolysin, and 180 nM (CFP) for TOP [3, 11]. Selective inhibitors against other endopeptidases including neprilysin (SCH39370, Ki of 11 nM) and prolyl oligopeptidase (ZPP, Ki of 4 nM) did not attenuate the metabolism of Ang-(1-7) to Ang-(1-4). Indeed, our previous study found no evidence for neprilysin activity (conversion of Ang I to Ang-(1-7)) in the sheep CSF [19]. The identity of the Ang-(1-7) peptidase is currently unknown; studies are in progress to obtain the purified enzyme for sequence analysis and determine whether this is a novel protein or reflects species variation of a known peptidase.
The extent of competition for the hydrolysis of [125I]-Ang-(1-7) to [125I]-Ang-(1-4) among angiotensins and other peptides was assessed in the CSF pool. Both Ang-(1-7) and Ang II exhibited similar IC50 values of 5 and 3 μM, respectively; however, the N-terminal analogs including Ala1-Ang-(1-7) and the des-Asp1 form [Ang-(2-7)] exhibited lower IC50 values of 2 μM. The D-Pro7 and D-Ala7 analogs of Ang-(1-7) also exhibited lower IC50 values than Ang-(1-7) or Ang II. In contrast, both bradykinin and neurotensin competed to a lesser degree than Ang-(1-7); the IC50 for bradykinin was 6-fold higher than Ang-(1-7) while the IC50 for neurotensin could not be obtained likely reflecting the minimal competition of the peptide. It should be noted that neurotensin, a 13-residue peptide, contains the same dipeptide sequence Tyr-Ile of Ang-(1-7) and Ang II, but at positions 11 and 12 of the peptide. Ala1-Ang-(1-7) was recently described as an endogenous peptide that may arise from decarboxylation of the aspartic residue of Ang-(1-7) or the direct conversion of Ala1-Ang II by ACE2 [17]. Ala1-Ang-(1-7) recognizes the Mas-related receptor D and the present data suggest that Ala1-Ang-(1-7) may be a potential substrate for the CSF peptidase based on its lower IC50 than Ang-(1-7) [17]. Ala1-Ang-(1-7) and Ang-(2-7) both exhibit a low IC50, suggesting that alterations to the N-terminus of the peptide do not significantly influence recognition by the peptidase. Although both the D-Ala7 and D-Pro7 forms of Ang-(1-7) are not endogenous peptides, these antagonists are frequently used in vivo and the kinetic results suggest they may interact or compete with Ang-(1-7) for the peptidase. We emphasize that the current analysis did not attempt to identify the extent and site of hydrolysis of Ala1-Ang-(1-7), Ang II or the other peptides apart from Ang-(1-7). Obtaining a purified preparation of the CSF peptidase will facilitate the kinetic studies on the optimal peptide substrates of the enzyme. However, the relatively low Km for Ang-(1-7) and that peptidase activity inversely correlated to Ang-(1-7) levels in the CSF support an endogenous role of the peptidase in the Ang-(1-7)-Mas receptor arm of the RAS. Glucocorticoid exposure in utero is associated with reduced Ang-(1-7) “tone” in the kidney and in the brain medulla which may contribute to long-term changes in blood pressure and baroreflex activity [15, 18, 19, 29]. In this regard, the immunoreactive expression of the Mas receptor was significantly lower in the brain medulla of the BMX versus the control animals while the density of the AT1 receptor was unchanged [18]. We determined that the ratio of Ang II to Ang-(1-7) in the brain medulla and CSF was significantly higher in BMX animals; thus, alterations in the metabolism of Ang-(1-7) may lead to lower tissue content of the peptide and reduced activation of the Mas receptor pathway following BM exposure [18, 19].
5. Conclusion
In consideration of the inhibitor selectivity, and the specificity exhibited against Ang-(1-7) and other angiotensin peptides, the enzyme activity characterized in the CSF of unexposed and glucocorticoid-exposed sheep may reflect a unique peptidase. Apart from this possible distinction of a novel peptidase in the sheep brain, any initial characterization regarding the specificity of a peptidase must be followed with extensive assessment of additional substrates of angiotensins and other peptide families, as well as the distribution of the activity in the brain and peripheral tissues. Initial attempts to classify peptidases based on their specificity for a particular substrate can be premature and additional pathways may become evident over time. Indeed, ACE exhibits various functions distinct from its role in the formation of Ang II that include the efficient metabolism of bradykinin, acetyl-Ser-Asp-Lys-Pro and Ang-(1-7). Neprilysin, originally termed “enkephalinase”, does not solely degrade opioid peptides and catalyzes the ACE2-independent generation of endogenous Ang-(1-7) in the circulation. Nevertheless, the demonstration of increased peptidase activity in the CSF of the exposed sheep may constitute a novel functional marker of fetal programming as a consequence of early glucocorticoid exposure. The physiological significance of this activity is presently unknown. However, in fetal programming [29], hypertension [16], and aging [21], there is increasing evidence of a role for brain medullary Ang-(1-7) in blood pressure regulation and autonomic function. Thus, attenuated Ang-(1-7) activity in pathological conditions may reveal a unique therapeutic role to target the peptidase and maintain levels of Ang-(1-7) in brain and periphery [6, 22, 30].
Highlights.
BMX sheep exhibited higher Ang-(1-7) peptidase activity and lower CSF Ang-(1-7)
Activity is metallopeptidase sensitive to chelating agents and mercuri-compounds
Peptidase activity was higher in BMX sheep and inversely correlated to Ang-(1-7)
Neuroendopeptidase may constitute novel pathway for Ang-(1-7) degradation in brain
Acknowledgements
This work was supported by the National Institutes of Health (HD-047584, HD-017644, and HL-51952), the Groskert Heart Fund, and Wake Forest Venture Fund.
The authors gratefully acknowledge Ellen Tommasi and Eric LeSaine for their technical and surgical support.
Glossary
- Ang
Angiotensin
- Ang I
[Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10]
- Ang II
[Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8]
- Ang-(1-7)
[Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7]
- Ang-(1-4)
[Asp1-Arg2-Val3-Tyr4]
- ACE
Angiotensin converting enzyme
- BM
Betamethasone
- BMX
Betamethasone Exposed
- CSF
Cerebrospinal fluid
- RAS
renin-angiotensin system
Footnotes
Conflict of Interest
The authors declare that there are no competing financial interests in the work described.
References
- 1.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–8. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 2.Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1---7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol. 2000;279:F841–50. doi: 10.1152/ajprenal.2000.279.5.F841. [DOI] [PubMed] [Google Scholar]
- 3.Barrett AJ, Brown MA, Dando PM, Knight CG, McKie N, Rawlings ND, et al. Thimet oligopeptidase and oligopeptidase M or neurolysin. Methods Enzymol. 1995;248:529–56. doi: 10.1016/0076-6879(95)48034-x. [DOI] [PubMed] [Google Scholar]
- 4.Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol. 2001;32:76–91. doi: 10.1002/ppul.1092. [DOI] [PubMed] [Google Scholar]
- 5.Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–70. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: more than regulation of blood pressure? Hypertension. 2007;50:596–9. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 7.Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1-7) in rat brain. Evidence for differential processing of angiotensin peptides. J Biol Chem. 1989;264:16518–23. [PubMed] [Google Scholar]
- 8.Chappell MC, Gomez MN, Pirro NT, Ferrario CM. Release of angiotensin-(1-7) from the rat hindlimb: influence of angiotensin-converting enzyme inhibition. Hypertension. 2000;35:348–52. doi: 10.1161/01.hyp.35.1.348. [DOI] [PubMed] [Google Scholar]
- 9.Connors N, Valego NK, Carey LC, Figueroa JP, Rose JC. Fetal and postnatal renin secretion in female sheep exposed to prenatal betamethasone. Reprod Sci. 2010;17:239–46. doi: 10.1177/1933719109351752. [DOI] [PubMed] [Google Scholar]
- 10.Dahms P, Mentlein R. Purification of the main somatostatin-degrading proteases from rat and pig brains, their action on other neuropeptides, and their identification as endopeptidases 24.15 and 24.16. Eur J Biochem. 1992;208:145–54. doi: 10.1111/j.1432-1033.1992.tb17168.x. [DOI] [PubMed] [Google Scholar]
- 11.Dauch P, Vincent JP, Checler F. Specific inhibition of endopeptidase 24.16 by dipeptides. Eur J Biochem. 1991;202:269–76. doi: 10.1111/j.1432-1033.1991.tb16372.x. [DOI] [PubMed] [Google Scholar]
- 12.Dodic M, Abouantoun T, O'Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002;40:729–34. doi: 10.1161/01.hyp.0000036455.62159.7e. [DOI] [PubMed] [Google Scholar]
- 13.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 2000;98:137–42. [PubMed] [Google Scholar]
- 14.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res. 2005;58:510–5. doi: 10.1203/01.PDR.0000179410.57947.88. [DOI] [PubMed] [Google Scholar]
- 15.Gwathmey TM, Shaltout HA, Rose JC, Diz DI, Chappell MC. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension. 2011;57:620–6. doi: 10.1161/HYPERTENSIONAHA.110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isa K, Arnold AC, Westwood BM, Chappell MC, Diz DI. Angiotensin-converting enzyme inhibition, but not AT(1) receptor blockade, in the solitary tract nucleus improves baroreflex sensitivity in anesthetized transgenic hypertensive (mRen2)27 rats. Hypertens Res. 2011;34:1257–62. doi: 10.1038/hr.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, et al. Discovery and characterization of alamandine: a novel component of the reninangiotensin system. Circ Res. 2013;112:1104–11. doi: 10.1161/CIRCRESAHA.113.301077. [DOI] [PubMed] [Google Scholar]
- 18.Marshall AC, Shaltout HA, Nautiyal M, Rose JC, Chappell MC, Diz DI. Fetal betamethasone exposure attenuates angiotensin-(1-7)-Mas receptor expression in the dorsal medulla of adult sheep. Peptides. 2013;44:25–31. doi: 10.1016/j.peptides.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall AC, Shaltout HA, Pirro NT, Rose JC, Diz DI, Chappell MC. Antenatal betamethasone exposure is associated with lower ANG-(1-7) and increased ACE in the CSF of adult sheep. Am J Physiol Regul Integr Comp Physiol. 2013;305:R679–88. doi: 10.1152/ajpregu.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rioli V, Kato A, Portaro FC, Cury GK, te Kaat K, Vincent B, et al. Neuropeptide specificity and inhibition of recombinant isoforms of the endopeptidase 3.4.24.16 family: comparison with the related recombinant endopeptidase 3.4.24.15. Biochem Biophys Res Commun. 1998;250:5–11. doi: 10.1006/bbrc.1998.8941. [DOI] [PubMed] [Google Scholar]
- 21.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, et al. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1-7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–40. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- 22.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216:R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 23.Schelling P, Ganten U, Sponer G, Unger T, Ganten D. Components of the reninangiotensin system in the cerebrospinal fluid of rats and dogs with special consideration of the origin and the fate of angiotensin II. Neuroendocrinology. 1980;31:297–308. doi: 10.1159/000123092. [DOI] [PubMed] [Google Scholar]
- 24.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–88. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 25.Serizawa A, Dando PM, Barrett AJ. Characterization of a mitochondrial metallopeptidase reveals neurolysin as a homologue of thimet oligopeptidase. J Biol Chem. 1995;270:2092–8. doi: 10.1074/jbc.270.5.2092. [DOI] [PubMed] [Google Scholar]
- 26.Shaltout HA, Figueroa JP, Rose JC, Chappell MC, Averill DB, Diz DI. Gender differences in the development of insulin resistance in adult sheep as result of antenatal betamethasone. FASEB J. 2007;21:A434. [Google Scholar]
- 27.Shaltout HA, Chappell MC, Rose JC, Diz DI. Exaggerated sympathetic mediated responses to behavioral or pharmacological challenges following antenatal betamethasone exposure. Am J Physiol Endocrinol Metab. 2011;300:E979–85. doi: 10.1152/ajpendo.00636.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53:404–8. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaltout HA, Rose JC, Chappell MC, Diz DI. Antenatal betamethasone exposure attenuates the functional role of angiotensin-(1-7) in the NTS. Hypertension. 2010;56:e103. [Google Scholar]
- 30.Shaltout HA, Rose JC, Chappell MC, Diz DI. Angiotensin-(1-7) deficiency and baroreflex impairment precede the antenatal Betamethasone exposure-induced elevation in blood pressure. Hypertension. 2012;59:453–8. doi: 10.1161/HYPERTENSIONAHA.111.185876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaltout HA, Rose JC, Figueroa JP, Chappell MC, Diz DI, Averill DB. Acute AT(1)-receptor blockade reverses the hemodynamic and baroreflex impairment in adult sheep exposed to antenatal betamethasone. Am J Physiol Heart Circ Physiol. 2010;299:H541–7. doi: 10.1152/ajpheart.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, et al. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol. 2007;292:F82–91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- 33.Shrimpton CN, Wolfson AJ, Smith AI, Lew RA. Regulators of the neuropeptide-degrading enzyme, EC 3.4.24.15 (thimet oligopeptidase), in cerebrospinal fluid. J Neurosci Res. 2003;74:474–8. doi: 10.1002/jnr.10698. [DOI] [PubMed] [Google Scholar]
- 34.Tang L, Bi J, Valego N, Carey L, Figueroa J, Chappell M, et al. Prenatal betamethasone exposure alters renal function in immature sheep: sex differences in effects. Am J Physiol Regul Integr Comp Physiol. 2010;299:R793–803. doi: 10.1152/ajpregu.00590.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]