Abstract
Introduction
We sought to determine if restaging resection prior to initiation of induction intravesical Bacillus Calmette-Guérin improves recurrence-free rates in patients with high-risk non-muscle invasive bladder cancer.
Material and Methods
We retrospectively analyzed data on 1,021 patients treated at our institution with intravesical BCG for non-muscle invasive high-risk bladder cancer. All patients underwent a second resection, except for those already undergoing BCG treatment at the time of initial consultation and those refusing a restaging resection. All patients were assessed every 3 to 12 months for a minimum of 5 years. Univariate and multivariate regression was used to identify predictors of five-year recurrence.
Results
Restaging transurethral resection was performed on 894 (87.5%) patients. At restaging resection, 496 (55.5%) were found to have viable tumor. At 3 months, patients with a single resection had a recurrence rate of 44.3% compared to 9.6% for patients with a restaging resection (p<0.01). On multivariate analysis, a single TUR ([OR] 2.1, 95% CI 1.3, 3.3; p=0.01) was the only predictor of recurrence at 5 years. Time to recurrence for patients with a single resection was significantly shorter compared to those who underwent restaging resection (median 22 months versus 36 months, p <0.001).
Conclusion
Failure to perform a repeat resection prior to initiation of intravesical BCG therapy for high-risk non-muscle invasive bladder cancer significantly increases the risk of recurrence. We believe restaging resection should therefore be performed prior to initiating BCG therapy for all patients with high-risk non-muscle invasive bladder cancer.
Keywords: Urinary bladder neoplasms, transurethral resection, Bacillus Calmette-Guérin, recurrence
Introduction
Randomized trials involving patients with high-risk NMIBC have shown the benefits of BCG therapy compared to TUR alone or with intravesical chemotherapy.1–4 TUR with intravesical BCG is the standard of care and has been incorporated into treatment guidelines.5, 6 However, despite treatment with BCG, the majority of patients will experience recurrence of their tumor and a small percentage will experience disease progression. The quality of the TUR has been show to be directly associated with more favorable recurrence and progression rates7 and response to BCG therapy.8 Additionally, a restaging TUR has been shown to decrease the incidence of recurrence and progression and facilitates a more accurate pathologic diagnosis.9–12 The improved staging associated with restaging TUR allows patients to receive more appropriate therapeutic interventions. Therefore, restaging TUR for NMIBC has become the standard of care, according to the most recent EAU guidelines5 but the practice has not become universally accepted.
In a recent review, Vianello et al identified persistent tumor on restaging TUR in 39% and 47% of patients initially diagnosed with Ta and T1 tumors, respectively.12 We believe that having minimal tumor in the bladder prior to initiation of intravesical BCG is important for the efficacy of treatment; therefore, a restaging TUR may be important prior to BCG instillation. We evaluated our NMIBC dataset to determine if a restaging TUR prior to initiation of intravesical BCG improved recurrence- and progression-free rates compared to only a single TUR before BCG.
Material and Methods
After IRB approval, we retrospectively analyzed a prospectively maintained NMIBC (Ta, Tis and T1) database of patients treated at our institution. We identified 1,021 patients who had their bladder cancer treated with intravesical BCG by four high-volume urologists at Memorial Sloan-Kettering Cancer Center from January 1994 to December 2006. All patients were treated with six weekly instillations of Connaught strain (81 mg) BCG therapy. Patients with low-grade NMIBC were considered high-risk and treated with intravesical BCG if they had a positive cytology, were multifocal or had high-volume disease. All patients underwent a restaging resection prior to initiating intravesical BCG, except for those who were already undergoing BCG treatment at initial consultation or who refused a restaging resection. Restaging resection included aggressive resection and fulguration of all visible and suspected tumors including mucosa involved with carcinoma in situ and adequate sampling of muscle deep to suspected invasive tumors within 2–6 weeks after initial diagnostic TUR had been performed.8
Patients were assessed at 3, 6, and 12 months after completion of induction BCG with office cystoscopy, cytology, and resection of bladder tumors when indicated. After 12 months, patients were assessed every 6 to 12 months for a minimum of 5 years. A complete response was defined as a negative cystoscopy and urine cytology at follow-up. Patients with positive urine cytology or a visible tumor on cystoscopy were considered to have experienced recurrence. Patients with low-grade appearing recurrences during follow up cystoscopy were treated with fulguration in the office, while those with high-grade tumors were taken to the operating room for TUR. Progression was defined as an increase in pathologic stage (Ta to T1 or T1 to T2) on restaging resection or the development of metastatic disease. Patients with recurrent non-muscle invasive disease were eligible to receive another course of six weekly instillations of BCG. None of the patients in our study were treated with maintenance BCG.
Chi-squared tests were used to analyze associations between categorical variables, and the student’s t-test was used for continuous variables. We used univariate and multivariate logistic regression to assess each of our variables as predictors of recurrence at 3, 6, and 12 months. Multivariate logistic regression was also used to identify possible predictors of overall progression and recurrence. Since all patients in our study had a minimum of 5 years of follow-up, recurrence and progression were treated as categorical variables. Kaplan–Meier curves were constructed for RFS and PFS. Differences in recurrence and progression were assessed using a log-rank test. All statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, Illinois).
Results
Of the 1,021 patients, 894 (87.5%) had a restaging TUR. The majority of our patients, 756 (75.3%), were male with a mean age of 63 (SD 11.5). No difference was found between the two treatment cohorts in gender (p=0.19) or age (p=0.75). On diagnostic resection 409 patients were diagnosed with T1 disease (40.1%) and 612 (59.9%) with Ta disease, with no difference in stage between the two treatment cohorts (p=0.42). Overall, 769 (75.3%) patients had high-grade disease and 629 (61.6%) had either concomitant or primary Tis. Patients who underwent a single TUR, compared to those having restaging TUR, were significantly more likely to have high-grade tumors (93% versus 73%, p<0.001) and carcinoma in situ (77% versus 59%, p<0.001). Residual tumor was present in 496 (55.5%) patients, with residual Tis found at the highest frequency (18.8%). (Table 1)
Table 1.
Baseline Patient Characteristics
| All Patients N=1021 |
Restaging TUR N=894 (87.5%) |
Single TUR N=127 (12.5%) |
p-value | |
|---|---|---|---|---|
| Mean age at surgery, years (SD) | 63.0 (11.5) | 62.8 (10) | 63.1 (11.7) | 0.75 |
| Gender | ||||
| Female | 265 (26.0%) | 238 (27%) | 27 (21%) | 0.19 |
| Male | 756 (74.0%) | 656 (73%) | 100 (78%) | |
| Grade | <0.001 | |||
| Low Grade | 252 (24.7%) | 243 (27%) | 9 (7%) | |
| High Grade | 769 (75.3%) | 651 (73%) | 118 (93%) | |
| Initial Stage | 0.42 | |||
| Ta | 612 (59.9%) | 525 (59%) | 87 (69%) | |
| T1 | 409 (40.1%) | 369 (41%) | 40 (31%) | |
| Presence of CIS | 629 (61.6%) | 532 (59%) | 97 (77%) | <0.001 |
| Stage at restaging TUR | ||||
| T0 | 398 (44.5%) | |||
| Papilloma | 104 (11.6%) | |||
| CIS | 168 (18.8%) | |||
| Ta | 114 (12.8%) | |||
| T1 | 110 (12.3%) |
P-value from Fisher’s exact test. (SD or %)
At 3 months, 43.3% of patients who underwent a single TUR had evidence of disease recurrence, compared to only 9.6% of patients who underwent a restaging TUR (p <0.001). For patients receiving a single TUR, the rate of recurrence increased to 44.8% and 58.3% at 6 and 12 months, respectively. Patients receiving a restaging TUR had significantly lower recurrence rates of 16.6% and 28.2% at 6 and 12 months, respectively (all p <0.001). At 5 years, patients treated with a single TUR had a 77.2% recurrence rate, compared to 61.6% for patients who underwent a restaging TUR (p<0.001). If we exclude the patients whose disease recurred at 3 months, which may be persistence of disease rather than recurrence of disease, the recurrence rates at 5 years for patients who underwent a single TUR and for those with a restaging TUR are 58.3% and 57.5%, respectively (p=0.84). (Figure 1)
Figure 1. Percentage of patients with recurrence at 3, 6 and 12 months.
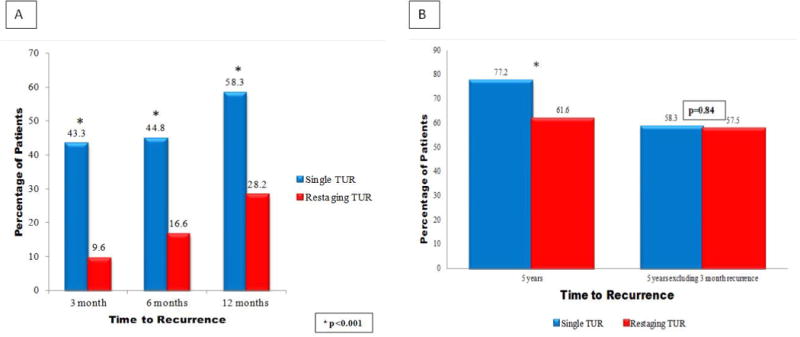
(A) Recurrence rates for patients with single or restaging TUR at 3, 6 and 12 months. (B) Recurrence rates for patients with single or restaging TUR at 5 years and at 5 years with exclusion of patients who recurred at 3 months
On univariate analysis, grade (OR 6.4, 95% CI 3.1, 13.2; p<0.001), presence of CIS (OR 2.2, 95% CI 1.4, 3.2; p<0.001), and a single TUR (OR 7.2, 95% CI 4.7, 10.9; p<0.001) were predictors of recurrence at 3 months. Similarly, grade (OR 3.9, 95% CI 2.4, 6.4; p<0.001), presence of CIS (OR 1.7, 95% CI 1.2, 2.3; p=0.003), and a single TUR (OR 4.1, 95% CI 2.8, 6.1; p<0.001) were predictors of recurrence at 6 months. On multivariate analysis grade (OR 4.9, 95% CI 2.2, 11.1; p<0.001) and a single TUR (OR 5.9, 95% CI 3.8, 9.1; p<0.001) were predictors of recurrence at 3 months. Grade (OR 3.7, 95% CI 2.1, 6.7; p<0.001) and a single TUR (OR 3.5, 95% CI 2.4, 5.3; p<0.001) were also found to be predictors of recurrence at 6 months. On multivariate analysis a single TUR (OR 2.1, 95% CI 1.3, 3.3; p=0.01) was also the only significant predictor for any recurrence during the 5-year follow up period. (Table 2) We also identified stage (OR 2.5, 95% CI 1.76, 3.45; p <0.001), grade (OR 18.7, 95% CI 5.71, 61.11; p <0.001), and a single TUR (OR 2.1, 95% CI 1.38, 3.28; p =0.01) to be predictors for progression at 5 years.
Table 2.
Multivariate logistic regression analysis for any recurrence during 5-year follow-up
| Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Single TUR | 2.1 | 1.3, 3.3 | 0.01 |
| Initial Pathology (pTa vs pT1) | 1.1 | 0.82, 1.43 | 0.6 |
| Grade (Hg vs Lg) | 1.2 | 0.81, 1.80 | 0.4 |
| Presence of pTis on initial resection | 1.18 | 0.83, 1.68 | 0.4 |
On Kaplan-Meier analysis, the median time to recurrence was significantly shorter for patients who received a single TUR compared for those who also received a restaging TUR (22 vs. 36 months, p <0.001). (Figure 2) At 5 years, a significantly shorter period of progression-free survival was seen in patients with single vs. restaging TUR, 67.2% and 81.7%, respectively (p<0.001). (Figure 3)
Figure 2.
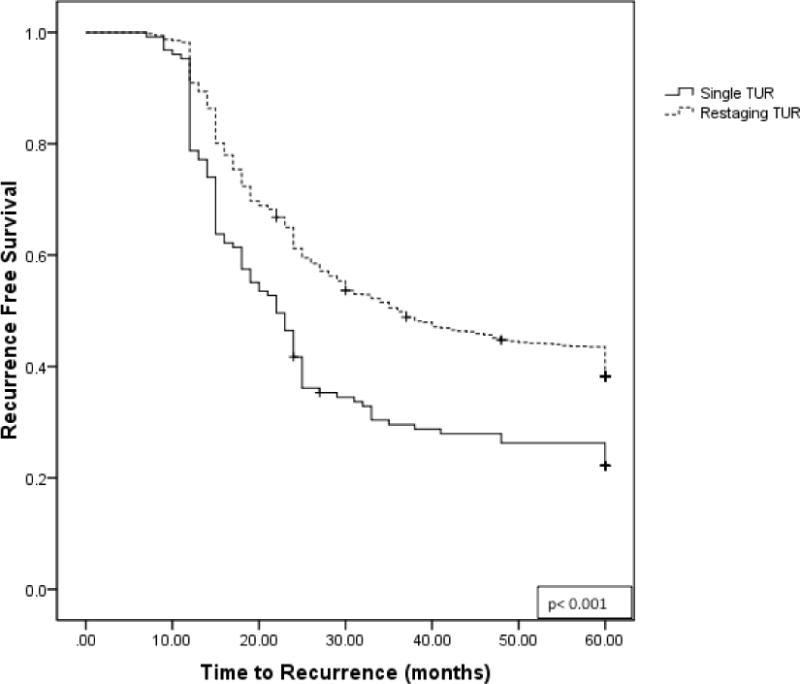
Kaplan-Meier plot for recurrence-free survival for patients receiving single TUR versus restaging TUR
Figure 3.
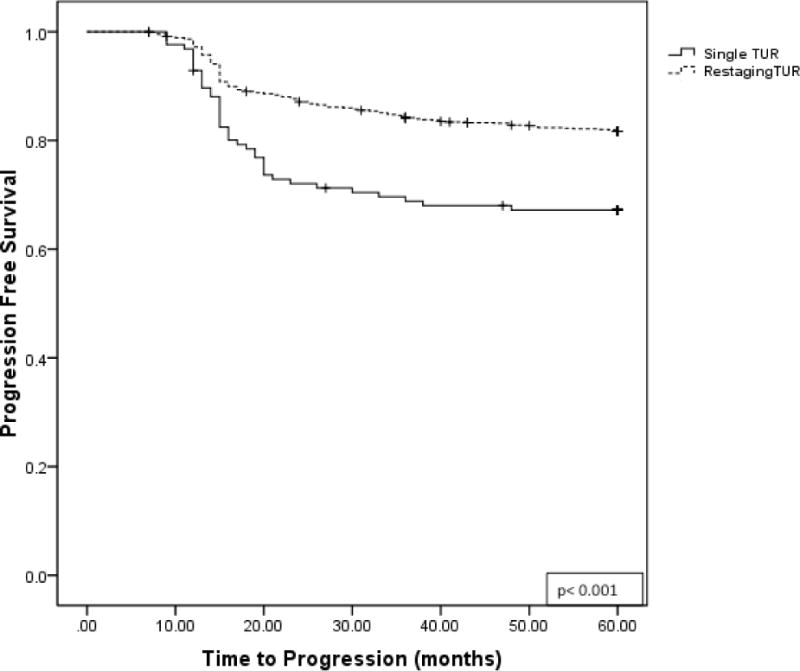
Kaplan-Meier plot for progression-free survival for patients receiving single TUR vs restaging TUR
Discussion
Accumulating evidence supports the importance of a restaging TUR for the treatment of NMIBC.9, 10, 12, 13 We report our findings in 1,021 patients treated with TUR prior to intravesical BCG. Patients who were treated with a single TUR had a 43.3% recurrence rate at 3 months compared to only 9.6% for patients who had a restaging TUR prior to intravesical BCG therapy. The relationship between restaging TUR and recurrence continued to be significant at 12 months, where 58.2% of patients with a single TUR had a recurrence compared to only 28.3% of patients with a restaging TUR. The high rates of disease found at 3 months in patients with a single TUR are most likely due to persistence of disease rather than recurrence. Excluding the patients with recurrence at 3 months, we found an almost identical recurrence rate at 5 years. These findings demonstrate the importance of having minimal viable disease prior to treatment with intravesical BCG in order to achieve optimal response.
The rates of tumor found at the time of a second TUR vary in the literature from 20% to 81.5%.15 In our series, we found residual tumor in 55.5% of the patients who underwent a restaging TUR. Thus, we strongly encourage the use of restaging TUR, not only for a more accurate pathological confirmation but also for its therapeutic effect.16, 17
In a retrospective study of patients treated with induction and maintenance BCG, at first follow up resection Guevara et al. identified a recurrence rate of 11.4% for patients without residual tumor and 27.7% for patients with residual tumor prior to treatment.14 These findings, along with our own results, lead us to believe that BCG therapy has improved efficacy when tumor burden is minimal.
In order to achieve minimal tumor burden, it is important that the highest quality TUR be performed for all patients for both initial diagnosis and restaging. Quality control guidelines for TUR put forward by our group include inspection of the surgeon’s macroscopic resection to ensure no visible tumor is remaining, to identify the presence of muscle in the final specimen, and for each urologist to monitor their recurrence rates at first follow-up.11 Using these guidelines will allow performing urologists to gauge the quality of their TUR and to optimize the effects of BCG therapy.
Furthermore, we found a single TUR to be associated with a 2-fold increased risk of recurrence at 5 years, with the greatest risk, 4.5-fold, found at 3 months. We also found a 14-month shorter median time to recurrence in patients receiving only a single TUR before BCG. These findings suggest that patients who receive a single TUR are either inappropriately staged and thus receive inadequate treatment or undergo an inadequate resection. Our findings are similar to findings by Divrik et al. who, in a prospectively randomized trial of only T1 patients either receiving a single TUR or a second TUR, found 5-year recurrence-free survival rates of 32% and 59% (p<0.001), respectively. In this study, a second TUR referred to a resection performed after an appropriate and complete initial TUR. Even when using this definition, the authors found that 33% of patients had residual tumor at time of the second TUR.9 The inclusion of Tis and Ta patients in our study is the likely reason for the difference in recurrence-free rates, but in both studies there are large differences in recurrence-free survival between patients undergoing a single TUR versus those undergoing second TUR or a restaging TUR. These findings suggest that patients who receive a single TUR are either inappropriately staged, and thus receive inadequate treatment, or undergo an inadequate resection.
While we strongly encourage a restaging TUR for its therapeutic effect, another strategy for treating these patients includes treating their tumors at the first followup cystoscopy after BCG therapy. While this is an option, at 3 months we found a 4.5-fold increase in recurrence between our two treatment groups. Therefore, a repeat TUR prior to instillation of BCG may decrease recurrence rates. Additionally, as shown in previous studies, the initial response to BCG therapy is a strong predictor of cancer outcomes.10, 18 This further adds to the importance of resecting all possible tumor prior to initiation of intravesical immunotherapy.
We had planned a randomized trial comparing the effect of BCG after one or two TURs, however the review board denied our request to conduct such a study. The reason given was our own compelling data showing that restaging TUR often discovered persistent cancer and improved staging accuracy. Since some patients already had started weekly BCG instillations when they first consulted us, we received permission to allow them to complete their induction course and then perform the second TUR (our first) about six weeks later.
This study is limited because of its retrospective methodology. Also, as our institution is a tertiary referral center, many of the diagnostic TURs were not performed here; however, dedicated genitourinary pathologists reviewed all resections for grade and stage. The generalizability of our findings is limited by the fact that we do not use maintenance BCG at our institution.
Conclusion
A single TUR was a strong predictor for recurrence as well as for progression. Patients undergoing a single TUR prior to treatment with intravesical BCG had a significantly shorter time to recurrence as well as decreased progression-free survival. The greatest difference was seen at 3 months, as we would expect if tumor were still present after an initial TUR. We believe a restaging TUR should therefore be performed prior to initiating BCG therapy for NMIBC.
Acknowledgments
Funding: Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers. Dr. Sfakianos is a research fellow in urologic oncology supported by NIH T32-CA82088.
References
- 1.Lamm DL, Thor DE, Harris SC, et al. Bacillus Calmette-Guerin immunotherapy of superficial bladder cancer. J Urol. 1980;124:38. doi: 10.1016/s0022-5347(17)55282-9. [DOI] [PubMed] [Google Scholar]
- 2.Herr HW, Pinsky CM, Whitmore WF, Jr, et al. Effect of intravesical Bacillus Calmette-Guerin (BCG) on carcinoma in situ of the bladder. Cancer. 1983;51:1323. doi: 10.1002/1097-0142(19830401)51:7<1323::aid-cncr2820510724>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guerin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325:1205. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- 4.Malmstrom PU, Wijkstrom H, Lundholm C, et al. 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study Group. J Urol. 1999;161:1124. [PubMed] [Google Scholar]
- 5.Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Montie JE, Clark PE, Eisenberger MA, et al. Bladder cancer. J Natl Compr Canc Netw. 2009;7:8. doi: 10.6004/jnccn.2009.0002. [DOI] [PubMed] [Google Scholar]
- 7.Brausi M, Collette L, Kurth K, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol. 2002;41:523. doi: 10.1016/s0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 8.Herr HW. Restaging transurethral resection of high risk superficial bladder cancer improves the initial response to bacillus Calmette-Guerin therapy. J Urol. 2005;174:2134. doi: 10.1097/01.ju.0000181799.81119.fc. [DOI] [PubMed] [Google Scholar]
- 9.Divrik RT, Sahin AF, Yildirim U, et al. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur Urol. 2010;58:185. doi: 10.1016/j.eururo.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Herr HW, Donat SM. A re-staging transurethral resection predicts early progression of superficial bladder cancer. BJU Int. 2006;97:1194. doi: 10.1111/j.1464-410X.2006.06145.x. [DOI] [PubMed] [Google Scholar]
- 11.Herr HW, Donat SM. Quality control in transurethral resection of bladder tumours. BJU Int. 2008;102:1242. doi: 10.1111/j.1464-410X.2008.07966.x. [DOI] [PubMed] [Google Scholar]
- 12.Vianello A, Costantini E, Del Zingaro M, et al. Repeated white light transurethral resection of the bladder in nonmuscle-invasive urothelial bladder cancers: systematic review and meta-analysis. J Endourol. 2011;25:1703. doi: 10.1089/end.2011.0081. [DOI] [PubMed] [Google Scholar]
- 13.Dalbagni G, Herr HW, Reuter VE. Impact of a second transurethral resection on the staging of T1 bladder cancer. Urology. 2002;60:822. doi: 10.1016/s0090-4295(02)01920-9. [DOI] [PubMed] [Google Scholar]
- 14.Guevara A, Salomon L, Allory Y, et al. The role of tumor-free status in repeat resection before intravesical bacillus Calmette-Guerin for high grade Ta, T1 and CIS bladder cancer. J Urol. 2010;183:2161. doi: 10.1016/j.juro.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Backhaus M, Dominguez-Escrig J, Collado A, et al. Restaging transurethral resection of bladder tumor for high-risk stage Ta and T1 bladder cancer. Curr Urol Rep. 2012;13:109. doi: 10.1007/s11934-012-0234-4. [DOI] [PubMed] [Google Scholar]
- 16.Mariappan P, Zachou A, Grigor KM, et al. Detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience. Eur Urol. 2010;57:843. doi: 10.1016/j.eururo.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Ali MH, Ismail IY, Eltobgy A, et al. Evaluation of second-look transurethral resection in restaging of patients with nonmuscle-invasive bladder cancer. J Endourol. 2010;24:2047. doi: 10.1089/end.2010.0319. [DOI] [PubMed] [Google Scholar]
- 18.Grimm MO, Steinhoff C, Simon X, et al. Effect of routine repeat transurethral resection for superficial bladder cancer: a long-term observational study. J Urol. 2003;170:433. doi: 10.1097/01.ju.0000070437.14275.e0. [DOI] [PubMed] [Google Scholar]


