Size fractionated particulate matter (PM) was collected in summer and winter from Beijing, China for the characterization of an expanded list of PAHs and evaluation of air pollution metrics. Summertime ΣPAHs on PM was 14.6 ± 29PM1.5, 0.88 ± 0.49PM1.5–7.2 and 0.29 ± 0.076PM7.2 ng m−3 air while wintertime concentrations were 493 ± 206PM1.5, 26.7 ± 14PM1.5–7.2 and 5.3 ± 2.5PM7.2 ng m−3 air. Greater than 90% of the carcinogenic PAHs were concentrated on PM1.5. Dibenzopyrene isomers made up a significant portion (30%) of the total carcinogenic PAH load during the winter. To our knowledge, this is the first report of dibenzopyrenes in the Beijing atmosphere and among the few studies that report these highly potent PAHs in ambient particulate matter. Lifetime risk calculations indicated that 1 out of 10000 to over 6 out of 100 Beijing residents may have an increased risk of lung cancer due to PAH concentration. Over half of the lifetime risk was attributed to Σdibenzopyrenes. The World Health Organization and Chinese daily PM10 standard was exceeded on each day of the study, however, PAH limits were only exceeded during the winter. The outcomes of the air pollution metrics were highly dependent on the individual PAHs measured and seasonal variation.
Environmental impact
Managing and assessing air quality is a world-wide challenge. Particulate matter is considered a primarily pollutant and a main indicator of air quality; however, the complexities of associated disease and illness have justified a further understanding of particulate matter composition. This work identifies an abundance of rarely monitored and highly potent carcinogens in an urban atmosphere demonstrating that investigation of particulate matter composition can significantly alter air quality assessment outcomes. Dibenzopyrenes, considered the most potent polycyclic aromatic hydrocarbons, constitute a large percentage of the calculated carcinogenic risk, showing strong seasonal influence that is not reflected by particulate matter concentration. Future air quality assessments may be impacted by considering dibenzopyrenes in routine air quality monitoring regimes.
Introduction
Particulate matter (PM) and polycyclic aromatic hydrocarbons (PAHs) are major air pollutants in urban environments. In 2005, the World Health Organization (WHO) released an estimate that two million premature deaths per year can be attributed to poor air quality and issued stricter worldwide guidelines. Reducing atmospheric PM concentration is believed to be the most crucial part of air pollution control strategies. There are many metrics to evaluate air quality, but PM concentration remains one of the main indicators and concentrations are monitored worldwide.
While the importance of monitoring PM concentration is known, PM composition can vary considerably according to particle size. Additionally, PM composition can also change with sampling locale, emission sources and season. Composition has been shown to be important for health effects and this complexity leaves room for debate concerning the most toxic aspects of PM.1 PM10 is traditionally and most frequently monitored and includes all sizes of PM <10 µm, but recent focus has shifted towards monitoring size fractionated PM since only PM <2.5 µm is thought to be respirable. PM2.5 is mainly produced by anthropogenic activity through the burning of fossil fuels and numerous studies have reported that the mutagenic and genotoxic effects of PM can be attributed to these small particles.2
One important class of pollutant contributing to PM composition is the PAHs. PAH concentration is also largely influenced by anthropogenic activity and the carcinogenic PAHs are mainly associated with the smallest PM.3 Few nations have implemented atmospheric limit values for PAHs, but atmospheric PAH concentration may also be used as an indicator of air quality. Hundreds of PAHs have been detected in the environment; however, less than twenty individual PAHs are routinely monitored. Typically, benzo[a]pyrene (BaP), the most toxicologically studied and frequently monitored PAH, is used as an indicator for atmospheric PAH concentrations, but assessing air quality according to PAH concentration presents another set of challenges.
Quantitative risk assessments for airborne PAHs are trying not only due to the complexity of the PM mixtures, but also due to different collection strategies of PM size, inadequate PAH potency information for inhalation1 and BaP has been shown to be an unpredictable indicator.1,5,6 Additionally, there is increasing evidence that carcinogenic risks of atmospheric PAHs may be overlooked by current air quality criteria and monitoring regimes that base assessments on BaP. The most potent PAHs, like the dibenzopyrenes (DBPs), are normally not included in monitoring studies.1,5 Many DBP isomers including dibenzo[a,i]pyrene, dibenzo[a,e]pyrene and dibenzo[a,h]pyrene are potent carcinogens5 and dibenzo[a,l]pyrene (D[a,l]P) is considered the most carcinogenic PAH, 1 to 100 times that of BaP.1
Until recently, the analysis of the DBPs for atmospheric research was hindered due to the presence of many structural isomers, including dibenzofluoranthenes, naphthopyrenes and naphthofluoranthenes.10,11 A few recent studies have reported concentrations of D[a,l]P and the other DBP isomers in the atmosphere7–9 and numerous groups are advocating the inclusion of DBPs into routine PAH monitoring lists.1,5 Yet, inclusion of these compounds for air quality monitoring requires justification that these PAHs are predominant in the atmosphere.
The atmosphere of Beijing, China provides an ideal location to evaluate DBP abundance and PAH cancer risk in comparison to other air quality indices, like PM concentration. PM is the primary pollutant in the Beijing atmosphere and PAH concentrations have been recorded that exceed limit values imposed by SEPA.12 Urbanization and diversified energy sources contribute to the pollution problems in Beijing13 and urban mega cities worldwide. Beijing may be considered as a representative of an urban city faced with managing air quality. The contribution of particle size and/or composition remains a predominate topic of air pollution research and this study aims to identify significant PAHs on size fractionated PM. The authors hope that this study will aid in future decision making processes for selecting the most relevant size fraction and PAHs for future monitoring studies.
Experimental
Sampling description
Air sampling was conducted in the northwestern section of downtown Beijing between the forth and fifth ring roads on the campus of Peking University. The site represents a myriad of emission sources given that the air sampler was located just above the ground level on the roof of the seven-story Geology Building at approximately 25 metres above sea level. PM was collected using a high volume air sampler adapted with a cascade impactor during summer of 2007 and in the winter of 2008. Seven days during the summer (July 2, August 18–23, 2007) and eight days during the winter (January 9–12, 15–17, 2008) are taken to represent pollution observed during the cold and hot seasons. While data from these seasons may represent the extremes of pollution in Beijing, the data are used to demonstrate the significance of DBPs on risk equations and air quality rather than as a representation of the Beijing atmosphere.
Collection of PM for chemical analysis was achieved using a three stage slotted impactor (TE 230) retrofitted to a high volume air sampler from Tisch Environmental, Inc. (Cleves, OH). Operation at 1.1 m3 min−1 allowed accumulation of three particle size fractions: >7.2 µm (stage 1), 1.5–7.2 µm (stage 3), and <1.5 µm (backup stage) collected on quartz fiber filters. The filters were baked at 350 °C for 12 h prior to field deployment. A 24 h collection time was implemented. Gas phase data were not collected since carcinogenic PAHs are predominately located on particles. Meteorological data were collected for the duration of the sample collection period using HYSPLIT by NOAA ARL with archived data. Pre- and post-sampling weights of the filters were determined gravimetrically after equilibrating to room temperature and humidity. Average humidity was 52.1 ± 8% (summer) and 39.3 ± 14% (winter). For PM mass comparison, the backup and stage 3 filters were combined during data analysis for comparison to the traditionally monitored PM10, particles with diameters less than 10 µm. Similarly, the backup filter represents particle diameters nearly identical to those of traditionally monitored, PM2.5, diameters less than 2.5 µm, and is used for direct comparison. Filters were stored in aluminium foil and transported on ice packs at less than 4 °C until sample analysis.
Chemicals
Solvents were Optima® grade or better (Fisher Scientific, Pittsburgh, PA). The following PAHs were included: naphthalene (NAP), 2-methylnaphthalene (2-mNAP), 1-methylnaphthalene (1-mNAP), naphthalene, 1,6-dimethyl (1,6-dmNAP), acenaphthylene (ACY), naphthalene, 1,2-dimethyl (1,2-dmNAP), acenaphthene (ACE), fluorene (FLU), dibenzothiophene (DBT), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLA), pyrene (PYR), retene (RET), 1-methylpyrene (1-mPYR), benz[a]anthracene (BaA), chrysene (CHR), chrysene, 6-methyl (6-mCHR), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-c,d]pyrene (I[cd]P),dibenz[a,h]anthracene (D[ah]A), benzo[ghi]perylene (B[ghi]P) and dibenzo[a,l]pyrene (D[a,l]P), dibenzo[a,e]pyrene (D[a,e]P), dibenzo[a,h]pyrene (D[a,h]P), dibenzo[a,i]pyrene (D[a,i]P), and dibenzo[a,e]fluoranthene (D[a,e]F), Accustandard, New Haven, CT. Additional standards with the molecular weight of 302 were purchased: dibenzo[j,l]fluoranthene (D[j,l]F), dibenzo[b,k]fluoranthene (D[b,k]F) and naphtho[2,3-b]fluoranthene (N[2,3-b]F), Chiron AS, Trondheim, Norway. The following deuterated PAHs were used as surrogate recovery standards: acenaphthylene-D8, benzo[a]pyrene-D12, benzo[g,h,i]perylene-D12, fluoranthene-D10, naphthalene-D8, phenanthrene-D10, pyrene-D10; and perlyene-D12 was used as the internal standard (Cambridge Isotopes Laboratories, Inc., Andover, MA). Standard Reference Materials 1491 (alkylated PAHs in toluene) and 1649b (urban dust) were obtained from National Institute of Standards and Technology (NIST, Gaithersburg, MA). A detailed table of PAHs can be found in the ESI, Table S1†.
Sample preparation
Each intact filter was equilibrated to room temperature and packed into individual cells for the extraction of analytes. Pressurized solvent extraction (ASE 300, Dionex Corporation) was performed with two cycles of 75:25 hexane:acetone, followed by two cycles of ethyl acetate (five minute static, 240 second purge, 100% flush volume, 10.3 MPa). Ten percent of the filter extract was isolated and spiked with deuterated surrogate PAHs. Samples were concentrated using N2 at ambient temperatures. Concentrated extracts were subjected to a chromatography clean-up step using 100 mg silica Discovery® solid phase extraction tubes (Supelco, Bellefonte, PA) and eluted with 2% (by volume) dichloromethane in isooctane. The final concentrated extracts were spiked with the deuterated internal standard for gas chromatography-mass spectrometry (GC-MS) analysis.
Analysis
Dibenzopyrene quantitation and molecular weight (MW) 302 isomer discrimination were accomplished using a GC method adapted from Bergvall et al. (2006).11 Two DB-17MS columns (30 m × 0.25 mm × 0.15 µm, Agilent J&W, Wilmington, DE) were joined by an Agilent Ultimate Union kit (Agilent, Wilmington, DE). The GC parameters were as follows: injection port at 310 °C, 1 mL min−1 helium flow, 80 °C initial temperature, 1 min hold, 40 °C min−1 ramp to 200 °C, then 10 °C min−1 ramp to 315 °C followed by a 45 min hold for a total run time of 61 minutes.
All other PAH analysis was accomplished using a DB-5MS column (30 m × 0.25 mm × 0.25 µm, Agilent J&W, Wilmington, DE). The GC parameters were as follows: injection port maintained at 300 °C, 1.5 mL min−1 helium flow, 55 °C initial temperature, 1 min hold, 25 °C min−1 ramp to 320 °C, followed by a 3 min hold. All samples were analyzed using Agilent 5975B GC-MS in electron impact mode (70 eV) utilizing selective ion monitoring (SIM).Mass spectrometer temperatures were operated at 150, 230 and 280 °C for the quadrapole, source, and transfer line, respectively. Sample concentrations were determined by the relative response ratio of the deuterated surrogate to the target standard in a ten point calibration curve having a correlation coefficient of greater than 0.98.
Quality assurance/control
Mean recoveries of added surrogate standards were between 50 and 95%. Lower recoveries were observed for the two- and three-ring labeled PAH surrogates from volatilization losses during laboratory analysis due to higher volatility than the four- to five-ring PAH surrogates. Since the response of the added surrogate to the target analyte (being of similar structure) is a ratio, losses that incurred during sample analysis are accounted for during concentration determination. Analyte method recoveries ranged from 74–123% with excellent repeatability of less than 12% RSD. Two NIST Standard Reference Materials (SRMs) were utilized for method validation. Less than ±10% deviation for non-polar substituted PAHs was achieved using SRM 1491a (alkylated PAHs in toluene). Average recoveries of certified values of PAHs, including two DBPs, from SRM 1649b (urban dust) ranged from 85–124%, Table S2†.
Method detection limits ranging from 1.4–14.2 pg µL−1 were calculated using the standard deviation of seven blank-subtracted replicates with 99% confidence (α = 0.01). Greater than 30% of analyzed filters consisted of field (n= 5), trip (n = 1), and laboratory blanks (n = 2), while reagent blanks accompanied each analytical run. All samples were field-blank corrected due to the presence of lighter molecular weight PAHs at low and negligible concentrations.
Risk calculations
Carcinogenic risk was calculated for inhalation exposure based on the daily measured concentrations of PAHs on PM1.5 for both seasons in this study. PAHs designated carcinogenic by the International Agency for Cancer Research (IACR) were normalized to BaP using Toxic Equivalency Factors (TEFs).14 Cancer risk is determined by multiplying PAH concentration by a unit risk (UR) value for BaP, eqn (1).
Daily and lifetime risk can be calculated assuming standard default values for body weight (70 kg) and respiratory rate (20 m3 min−1) and applying predetermined UR values. A URBaP of 0.0011 µg−1 m−3 has been used for daily inhalation risk calculations and was obtained from a rodent study.15 The WHO determined a lifetime cancer potency factor from an epidemiology study of coke-oven workers.4 This study determined that BaP constituted 0.71% of the benzene soluble coke-oven emissions, resulting in 8.7 × 10−5 per ng m−3 BaP and a respective URBaP of 0.09 µg−1 m−3.4
| (1) |
Statistics
Analysis was performed using Systat Software, Inc., SigmaPlot 11.0. An interfering compound, possibly plant waxes, prevented the quantitation of BbF and BkF in the larger PM, only during the summer. However, this exclusion did not affect risk equations since risk equations were based solely on PM1.5.
Results and discussion
DBP and MW 302 isomer measurement
Detection and separation of D[a,l]P and other DBPs in Beijing PM were confirmed using authentic standards of structural isomers known to co-elute using traditional chromatography methodologies.7,10,11 Over 20 isomers with am/z of 302 were baseline separated from SRM 1649b (urban dust) and from Beijing PM. Peaks were identified based on retention time and ion fragmentation pattern compared to the authentic standards. Excellent recoveries of certified and reference values for MW 302 isomers in SRM 1649b were demonstrated (Table S2†). Recoveries of certified values were 109 ± 6% for D[a,e]P and 105 ± 5% for D[b,k]F. Authentic standards of D[b,k]F and D[a,e]F could not be separated, therefore values of D[b,k]F may have included D[a,e]F. To our knowledge, separation of D[a,e]F and D[b,k]F isomers from matrices has not been reported. The recovery of D[a,l]P from the present study was lower than that reported as a reference value by NIST,16 but good agreement was observed for D[a,l]P and reported DBP isomers with Bergvall et al. (2006) even though a programmed temperature injection, LC step and GCguard column were employed for their analysis.11,17 Other studies have identified MW 302 isomers from cigarette smoke, diesel exhaust and tar-cork18 as well as other SRMs19 using similar LC/GC-MS methods. Schubert et al.(2003) found GC-MS methods more efficient than LC and identified the most extensive list of MW 302 isomers from a number of SRMs.20
Using methodologies and instrumentation similar to that of Schubert et al. (2003), we found that the number of peak profiles from SRM 1649b was very similar to previous reports17,20 and also to the Chinese air samples (Fig. 1). This observation was also noted from the above mentioned study comparing MW 302 isomers from Stockholm to a previous lot of urban dust, SRM 1649a. This Swedish study was one of the first to report the predominance of DBPs in diverse atmospheres and positively identified four MW 302 isomers.11 In this study, we identified seven MW 302 isomers in ambient air using authentic standards. From SRM 1649a, Schubert et al. in 2003 reported that 22 MW 302 isomers were identified based on 17 authentic standards and literature comparisons.20 From these findings, the tentative identification of most peaks from the Beijing air was possible; however, some isomers still may have co-eluted. Data from Schubert et al. (2003) suggest that the peak identified as D[a,h]P may also contain dibenzo[a,j and a,l]fluoranthene.20
Fig. 1.
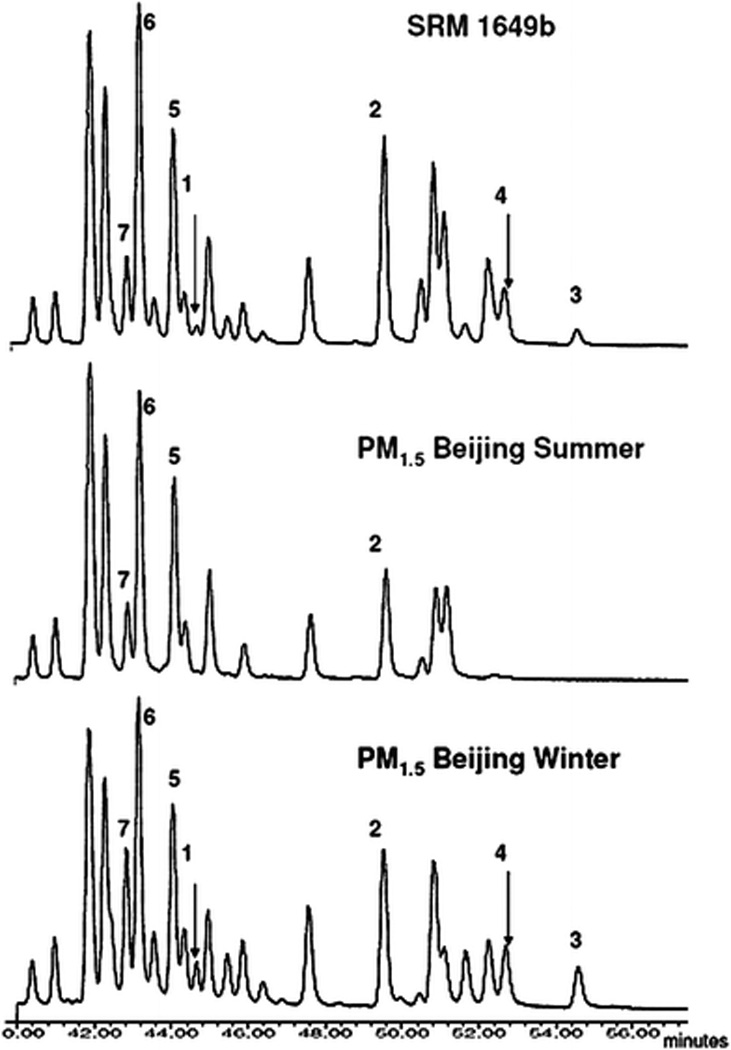
Representative chromatograms of m/z 302 from Urban Dust SRM 1649b and Beijing PM1.5. Gas chromatography-mass spectrometry scans were collected in selected ion monitoring mode. Numbered peaks correspond to PAH isomers that were positively identified using authentic standards. 1 D[a,l]P, 2 D[a,e]P, 3 D[a,h]P, 4 D[a,i]P, 5 D[j,l]F, 6 D[b,k]F and D[a,e]F, and 7 N[2,3-b]F.
Environmental occurrences of these six-ring PAHs are infrequently reported and it should be emphasized that D[a,l]P was detected in the respirable fraction (PM1.5) during both seasons in Beijing. MW 302 PAHs were present in all three particle size fractions measured during the winter and the summer. All four of the carcinogenic DBPs were detected in the Beijing atmosphere and D[a,e]P was the most frequently measured DBP. Dibenzofluoranthene and napthofluoranthene isomers were also measured at 100% detections in airborne particulate matter from Beijing. The peak profiles also clearly demonstrate the seasonal distinction in MW 302 occurrence. The peak profile from Beijing winter was more similar to that of SRM 1649b (representing a yearly composite from 1976–77 from Washington, DC) than the Beijing Summer (Fig. 1).
PAH and PM concentrations
Total PAHs
A number of intense sampling campaigns have used data from Peking University to represent the Beijing atmosphere and found that pollutant concentrations are not significantly different from other locations within the city.21 Furthermore, data are presented from both summer and winter since these seasons typically represent the lowest and highest PAH concentrations within the year and Beijing has demonstrated the same trend.21
The total PAH concentrations were 15.8 ± 3.24 ng m−3 and 524.8 ± 318 ng m−3 during the summer and winter respectively. The PAHs were 10 to 50 times more concentrated in the winter. The strong seasonal effect likely resulted from the heightened emissions for heating and the relatively increased atmospheric stability in the winter compared to the hotter summers when meteorological variables favor atmospheric mixing and removal.
During both seasons, concentrations of PAHs decreased with increasing particle size. In accordance with established principles of PAH particle partitioning, greater than 80% of the PAH load was concentrated on the smallest particles, PM1.5. Individual concentrations for the 31 monitored PAHs on PM1.5, PM1.5–7.2 and PM7.2 during the summer and winter are listed in Table S4†. Although expansion of the analyte list beyond the frequently monitored US EPA 16 priority pollutant PAHs identified other important PAHs in the Beijing atmosphere, the US EPA priority pollutant PAHs did indeed make up the bulk of the measured PAHs. The concentrations of the US EPA 16 priority pollutant PAHs remained consistent during both seasons comprising 87 ± 4% during the summer and 75 ± 11% of the total PAHs in the winter.
Observed PAH concentrations from the present study were high for a developed urban area, but not uncommon for Beijing. For example, PAH concentrations from the Beijing winter of 2005–06 were 407 ng m−3 on average.22In developed countries, PAH concentrations in urban areas typically fall in the range of 1 to 10 ng m−3 for European nations4 and 0.1 to 19 ng m−3 for the US.23 Inevitably, the number of PAHs included in the summation varied from study to study, and none of the studies included the MW 302 isomers. Our results also agreed with other recent studies from Asia concerning the particle size distribution of PAHs. Greater than 90% of nine monitored PAHs from Tokyo, Japan24 and greater than 50% of the US EPA 16 priority pollutant PAHs from a multi-city study in China25 were located on the ultrafine particulate matter.
Carcinogenic PAHs and MW 302 isomers
A significant portion, approximately half of the total PAHs during the summer and 33% of the total PAHs in the winter, was IARC classified carcinogens. As a result, the potential for exposure of carcinogenic PAHs was high, with 89% (summer) and 94% (winter) of the total carcinogenic load concentrated on PM1.5. An important finding that contributed to the high loading of carcinogenic PAHs was the measurement of DBPs in the Beijing air. In fact, the seven DBPs and MW 302 isomers made up nearly 10% of the total PAHs during the summer, but up to 30% of all PAHs during the winter on PM1.5.
Average concentrations of carcinogenic and MW 302 isomer PAHs can be found in Fig. 2. The MW 302 isomer concentrations were on the same order of magnitude as most carcinogenic PAHs suggesting that these infrequently monitored PAHs may be abundant in urban environments. D[a,l]P may be considered the most important DBP due to cancer potency, but was the least concentrated MW 302 isomer measured. However, another DBP, D[a,e]P, was measured at an order of magnitude higher concentration. Studies have shown that D[a,e]P is also carcinogenic, possibly even as potent as BaP, a widely utilized indicator for PAH concentration assessments.1 Yet, BaP was more concentrated than most DBPs; however, BbF was the most concentrated carcinogenic PAH measured (Fig. 2).
Fig. 2.
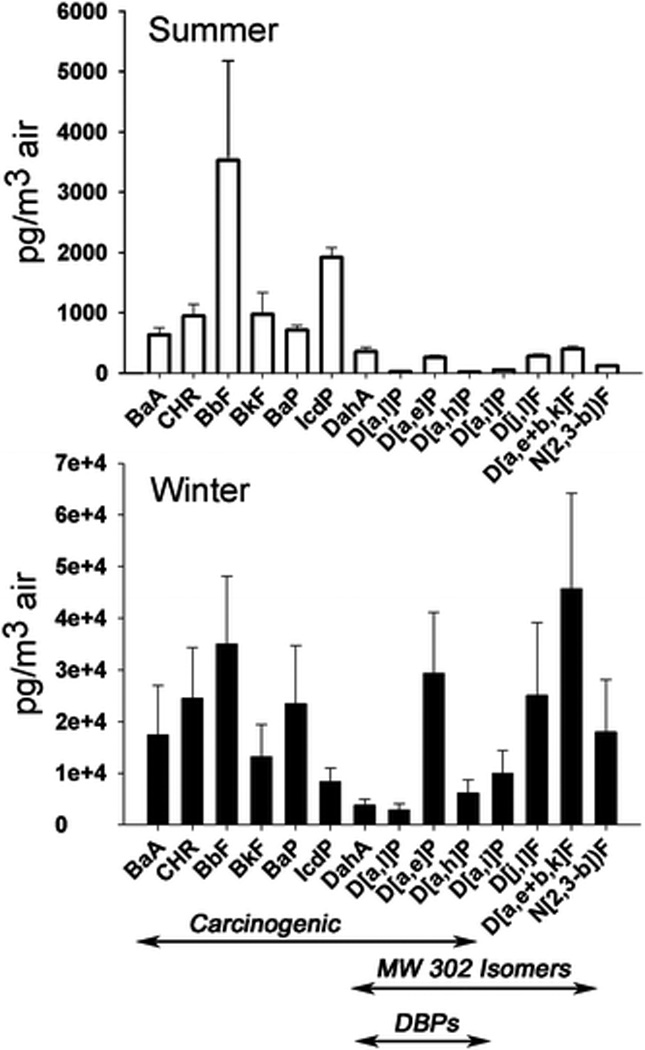
Mean PAH concentrations from Beijing PM1.5 samples, plotted with standard deviation. Summer 2007 (n = 7) and winter 2008 (n = 8) PAHs are normalized per cubic metre of air sampled. Note the scale difference portraying an order of magnitude higher concentration in the winter. Only known carcinogenic PAHs (International Agency for Research on Cancer14) are bracketed as “carcinogenic”.
Since very little data were reported for DBPs in the atmosphere, comparisons were somewhat limited. Similar to this study, Castro et al. (2009) and Bergvall et al. (2007) detected low pg concentrations of D[a,l]P, while other carcinogenic PAH concentrations were much higher in air particulates from Portugal8 and Sweden,7 respectively. Bergvall et al. (2007) also reported that D[a,l]P was the least concentrated DBP isomer from ambient air.7 However, other carcinogenic PAHs were one to two orders of magnitude higher than total DBPs in Sweden. The relationship of carcinogenic PAHs to MW 302 isomers may be influenced by source as evidenced by findings from an occupational exposure study to diesel exhaust, where D[ah]A (a carcinogenic US EPA priority pollutant PAH) and D[a,l]P were statistically equivalent during both summer and winter.9 From Beijing, no statistical difference was found between wintertime D[a,l]P and D[ah]A concentrations (p = 0.28), although D[ah]A was 10 to 60 times higher in the summer. Additionally, the MW 302 isomers were correlated to the sum of carcinogenic PAHs in the winter (R > 0.9 and p < 0.001), but not in the summer. The seasonal and source affect on the relationship between DBPs and other carcinogenic PAHs warrants further investigation. Regardless, each scenario provided evidence that DBPs and isomers were occurring at concentrations similar to other carcinogenic PAHs in ambient air.
Other PAHs
The air quality assessment was only performed on the IARC classified carcinogenic PAHs; however, a number of other PAHs detected could aide in source identification and future mitigation initiatives. Justification of human health relevance for alkylated, sulfur substituted and some MW 302 isomer PAHs was hindered by limited toxicological data, but concentrations reported here may be of use for exposure evaluations.Dibenzothiophene has been suggested as a marker for sulfur-containing fuel and the detection only in the winter could have signified the predominance of coal pollution rather than diesel vehicles.1 Furthermore, dibenzothiophenewas strongly correlated (R > 0.8 and p < 0.001) to the carcinogenic PAHs and MW 302 isomers. Alkylated PAHs were also primarily detected in the wintertime, but were not correlated with the carcinogenic PAHs. Often, the presence of certain PAHs (e.g. retene) and PAH ratios are used to diagnos combustion sources in air. To date, the main pollutant sources in Beijing are reported as coal and vehicular emissions.26,21,27 PAH diagnostic ratios from this study supported these main sources of PAHs and further explanation can be found in the ESI, Table S5†.
Particulate matter
Particles collected on the filters were predominately of the smallest size fraction: PM1.5 consisted of 58% and 76% of the total PM mass for summer and winter respectively. Approximate comparison to the traditional PM10 was achieved by summation of PM1.5 and PM1.5–7.2. Overall average concentrations were 175 µg m−3 for PM1.5 and 225 µg m−3 for PM10.
Particulate matter concentrations reported from the present study were also comparable to previous years in China. During 2002–03 similar concentrations were reported in Beijing for PM2.5 at concentrations of 77.3 and 135.7 µg m−3 for summer and winter, while PM10 was 117.2 and 184.4 µg m−3 air.26 Three days (August 18–20) included in the summertime dataset from the present study were source control trials (car bans) for preparation of the Beijing Olympics.28 Interestingly, no statistical differences in PM mass or PAH concentration between the car ban (source control) and non-source control days were found (p > 0.05). Similarly, PM mass concentrations and null source control results were also reported by Wang et al. (2009) who conducted a study on PM pollution in Beijing the following year.28
Unlike the chemical data, PM mass lacked temporal resolution for both PM1.5 and PM10 and no statistical differences between seasons were observed (Table S6†). This has also been observed by Huang et al., who in 2006 found 16 to 54 times higher concentrations of PAHs in Beijing winter compared to summer, despite similar PM mass concentrations.21 Furthermore, there was a lack of correlation between PM mass and ΣPAH concentration during the summer, but some modest positive correlations (R2 > 0.6 and p < 0.05) were observed for all three size fractions in the winter (Fig. S1†). Secondary aerosols, salts and plant waxes may have contributed to the PM mass in the summer,29 while combustion sources for heating and transportation likely contributed to the bulk of PM during the winter, thus resulting in the positive correlation with PAH concentration. Meteorological conditions are known to greatly affect atmospheric PM and PAH concentration.28 For the PM and PAH winter data, negative correlations with wind speed and positive correlations to humidity were observed. No clear trends were observed for the summer data. Meteorological data from the sampling period can be found in the ESI, Table S7†.
Air quality
Data collected in the present study were compared to daily air quality standards or maximum acceptable concentrations set by SEPA of China and international agencies for PM10 and PM2.5. This study recorded daily PM10 concentrations that exceed Chinese,30 European Union (EU)31 and WHO32 standards of 50 µg m−3 by a factor of four. Air quality standards for PM2.5 were also exceeded in every daily measurement, regardless of season, by a factor of five. The WHO set a PM2.5 maximum acceptable concentration at 35 µg m−3 over a 24 h period32 (China has not implemented a standard limit value). The WHO calculated that for every 10 µg m−3 increase in PM, there is a 0.5% increase in mortality.32 For the present study, this calculation resulted in an estimated 9% increase in mortality using daily PM averages from summer 2007 and winter 2008.
Since air quality can also be assessed by PAH concentration, the measured concentration of BaP was compared against established standards. China's SEPA set a daily limit for BaP at 10 ng m−3 air30 and, for comparison, the EU assigned an annual average standard of 1 ng m−3 air.31 Air quality assessments from the present study were based on the PAH levels measured from PM1.5 only, since this is the inhaled fraction and the majority of PAHs were concentrated on the smallest PM. It is important to note that neither BaP limit set by China or the EU was exceeded in the summer. During the winter, the daily BaP concentration was over the EU limit each day, while 75% of the days exceeded the limit set by SEPA. Daily wintertime BaP concentrations ranged from 2.5 to 38.5 ng m−3. Because the use of BaP as a reliable and predictive indicator of PAH pollution has been criticized1,5,6,9 and we aimed to consider the DBPs, a more realistic estimation of air quality was made by the summation of carcinogenic PAHs.
Carcinogenic PAHs were potency adjusted according to BaP using Toxic Equivalency Factors (TEFs). Although TEFs are based on an underlying assumption that all PAHs have the same mode of action as BaP, which is not supported by mechanistic studies, TEFs are still accepted as the most reasonable approach for assessing PAH mixtures. Many groups have reported TEFs for PAHs and values vary depending on experimental setup. For example, reported TEF values for D[a,l]P range from 1 to 100.1 The TEFs applied in the present study were chosen based on inclusion of DBP isomer data and the TEF values are commonly utilized in the literature.15 A list of PAHs and respective TEFs used in the present study can be found in Table 1. Despite the fact that relatively high concentrations of the MW 302 isomers of D[j,l]F, D[b,k]F and N[2,3-b]F were measured, they were not included since potency information was not available.
Table 1.
List of PAHs included in air quality and risk assessment calculations. Toxic Equivalency Factors or TEFs are listed for known carcinogens according to the International Agency for Research on Cancer14 and adapted from the California Environmental Protection Agency Office, Office of Environmental Health Hazard Assessment15
| Carcinogenic PAH |
|---|
| TEFs |
| Benz[a]anthracene |
| 0.1 |
| Chrysene |
| 0.01 |
| Benzo[b]fluoranthene |
| 0.1 |
| Benzo[k]fluoranthene |
| 0.1 |
| Benzo[a]pyrene |
| 1 |
| Indeno[1,2,3-c,d]pyrene |
| 0.1 |
| Dibenz[a,h]anthracene |
| 1 |
| Dibenzo[a,l]pyrene |
| 10 |
| Dibenzo[a,e]pyrene |
| 1 |
| Dibenzo[a,h]pyrene |
| 10 |
| Dibenzo[a,i]pyrene |
| 10 |
The transformation of carcinogenic PAHs into BaP equivalents resulted in ΣPAH TEF concentrations of 2.4 ± 0.25 and 231 ± 100 ng m−3 for summer and winter, respectively (Fig. 3). In contrast to considering only BaP, the TEF-adjusted PAHs exceeded the EU PAH limit value during the summer in addition to the winter.
Fig. 3.
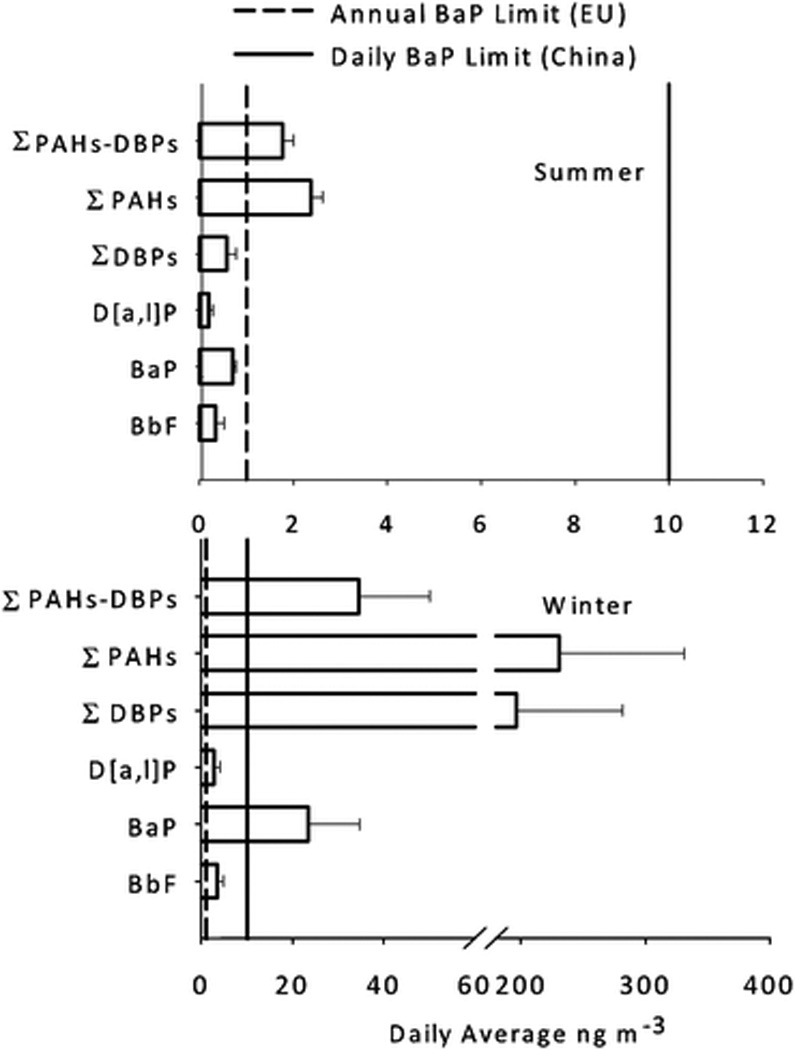
Carcinogenic PAHs from Beijing PM1.5 samples are plotted after potency adjustment using Toxic Equivalency Factors. Summer 2007 (n = 7) and winter 2008 (n = 8) average concentrations with standard deviation are shown. ΣPAHs represent only PAHs listed in Table 1. The figure highlights the contribution of notable PAHs on air quality assessments based on BaP limit values set by the European Union31 and China.30
Fig. 3 compares the contributions of important carcinogenic PAHs: BbF (the most concentrated carcinogenic PAH), BaP (air quality indicator) and D[a,l]P (most potent carcinogenic PAH), along with the ΣDBPs. These were compared to the total carcinogenic load of ΣPAHs. The ΣDBPs made the largest contribution to the overall sum in the wintertime. In the summer, BaP was the most predominate carcinogenic PAH after TEF adjustment. These data show that while BaP remained a significant atmospheric PAH, air quality assessment was affected by DBP measurement.
Using a TEF of 100 for D[a,l]P, Bergvall et al. (2007) reported that the potency adjusted concentrations of the DBPs were very similar to those of BaP from Stockholm air (autumn and spring) and also SRM 1649 (Washington, DC in 1976–77).7 Even though a lower TEF value (TEF = 10) for D[a,l]P was used in the present study, the summertime results also revealed no statistical difference in the potency of BaP and the DBPs, p > 0.3. However, during the winter, potency attributed to DBPs was eight times greater than BaP. The seasonal difference can be attributed to the individual DBPs measured. D[a,i]P, D[a,h]P (TEFs = 10) as well as D[a,e]P (TEF =1) were the main contributors to the TEF-adjusted PAH concentration in the winter, while D[a,e]P was the only DBP found at high concentration in the summer.
Risk estimations
In order to further exemplify the importance of the DBPs and other carcinogenic PAHs in air quality assessments, the data were subjected to standard risk equations. PAH concentrations from Beijing PM1.5 were used to calculate risk on a daily basis and for a lifetime using eqn (1) and respective URBaP values. The TEF-adjusted concentrations for the carcinogenic PAHs (Fig. 3) were used for the risk estimations. The calculated excess cancer risk was 2.6 ± 0.4 × 10−6 during the summer, 2.5 ± 1.6 × 10−4 during the winter and 1.2 ± 1.4 × 10−2 for a lifetime.
An important result was the influence of the DBP isomers on risk assessment. There was a statistically increased risk (p < 0.007) for inclusion of the DBPs into the ΣPAHs. The ΣDBPs account for 58 ± 32% of the lifetime risk. Specific isomers play different roles depending on season. In the summer, 12% of the total risk can be attributed to high concentrations of D[a,e]P. In the winter, 14, 21 and 48% was attributed to D[a,e]P, D[a,h]P and D[a,i]P, respectively. D[a,l]P accounted for 8% (summer) and 1% (winter) and 5% (lifetime) of the total risk. BbF was one of the most concentrated carcinogenic PAHs in PM1.5, but also had little influence on the overall risk equation since the potency is 1/100 that of D[a,l]P (Table 1).
Fig. 4 illustrates calculated risks presented as increased risk per million people for the previously discussed PAHs and the summation of PAHs. Although the seasonal differences were dramatic, the lifetime calculation (summer and winter data combined) yielded the highest risks and may be more appropriate for cancer risk estimates. Lifetime results ranged from 1 in 10000 to 6 in 100 people may have an increased risk of cancer due to exposure of the measured PAH concentrations from Beijing. By including all carcinogenic PAHs using the TEF adjustment approach, the calculated risk was up to 10 times greater than the risk based on BaP alone.
Fig. 4.
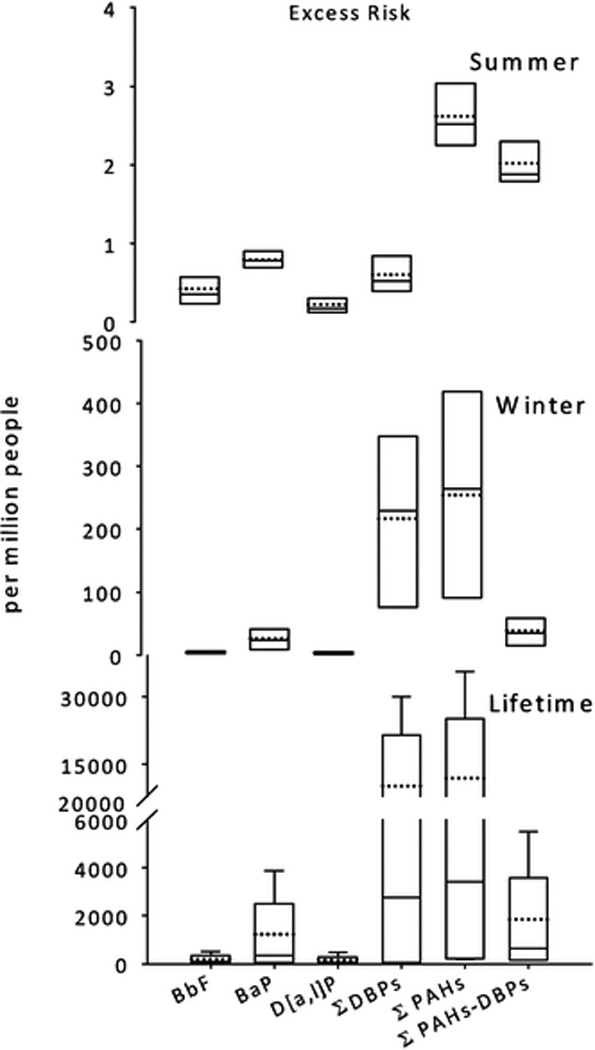
Box plots of excess risk per million people during summer 2007 (top), winter 2008 (middle), and calculated for a lifetime (bottom) from exposure to select PAHs and the sums of carcinogenic PAHs measured on PM1.5. Boxes represent the 5th and 95th percentile; dotted and solid horizontal lines represent mean and median values, respectively.
Sauvain et al. (2003) reported a five fold increase in risk when 15 measured PAHs were included in the risk assessment from an occupational study of diesel exhaust particles in Switzerland.9 The same DBP isomers were included by Sauvain et al. (2003) and risks were 2.1 × 10−3 during the summer and 1.6 × 10−2 during winter.9 While the importance of including other PAHs in risk assessment was addressed, the individual contribution of the DBPs to the risk was not discussed. Although higher risks would be expected from an occupational study, the DBP concentrations, relative to other PAHs, were much higher in the present study.
It should be reiterated that the aim of the present study was to highlight the importance of DBPs in the atmospheric PM and was not meant to quantify actual carcinogenic risk for the Beijing residents. Previous investigations by Zhang et al. (2009) have reported that annual excess lung cancer risk for Chinese people was 6.5 × 10−6 for population-weighted PAH concentrations.33 This value is only slightly higher than summertime risk calculations reported from the present study; however, Zhang et al. (2009) considered the entire country, other atmospheric variables, and population dynamics for an integrated assessment of carcinogenic risk attributed to ambient PAHs. Eastern China and urban cities, like Beijing, were noted to have an increased risk over rural and Western China, with the exception of coal coking communities.33 DBPs were not included in the emission inventories of Zhang et al. (2009).33
Additional considerations should be recognized when interpreting the presented results. Gas phase PAHs and the other particulate matter components were not taken into account and only IARC classified carcinogens on PM1.5 were included. In addition, sampling occurred at a fixed location, alternative to point of contact. Calculating lifetime risk also implies a lifetime exposure to similar concentrations measured in the study. Implementation of the TEF approach to normalize the partially characterized air samples into BaP carcinogenicity also warrants many assumptions that may over or under estimate risk. For the present study, underestimations may have occurred from use of mid-range TEFs for the two most potent PAHs, D[ah]A and D[a,l]P. Next, additivity assumptions may not accurately reflect the potency of all carcinogenic PAHs, but TEF normalization assumes the same mode of action as BaP. Another concern inherent to the TEF normalization is that TEFs were determined by other routes of exposure (not inhalation) in animal studies, resulting in an unknown degree of uncertainty.
Conclusions
The profile of MW 302 isomers found in Beijing PM was very similar to those of SRM 1649b (collected in Washington, DC) and the few other previously reported cities. The high concentration of DBPs and other MW 302 isomers, in comparison to frequently monitored PAHs, provided further evidence that DBPs are predominant carcinogens occurring in urban atmospheres around the world. Even though PM1.5 proved to be the most important particle size fraction, the need for the characterization of PM composition when assessing air quality was also demonstrated. Anthropogenic influences remained unnoticed from a PM air quality standpoint, but greatly affected air quality outcomes according to PAH standards. The disparity became larger when potent carcinogens, like DBPs, were included in the air quality assessments. This study reports concentrations of the most potent carcinogenic PAH, D[a,l]P, in urban PM. However, other DBP isomers, namely D[a,e]P, D[a,i]P and D[a,h]P, were more concentrated and therefore significant contributors of DBP carcinogenic potential. Moreover, two isomers measured here, D[j,l]F and D[a,e]F, occur at higher concentrations than DBPs, but limited carcinogenicity information was available. The DBPs and MW 302 isomers should be considered in future monitoring studies. There is a need for continued research involving further identification and measurement of MW 302 isomers in ambient air. However, these data are only useful for air quality and risk assessments if potency data become available. Spatial and temporal studies, taking into account population and exposure dynamics, are needed to further evaluate the effects of DBPs on carcinogenic risk.
Supplementary Material
Acknowledgements
Funding for this study was in part by the NSF East Asia Pacific Summer Institute Fellowship, Award ID 0714093. Julie Layshock was supported in part by NIEHS Training Grant Fellowship T32 ES007060 from the National Institutes of Health and a STAR Research Assistance Fellowship, ID Number: F07D40790, awarded by the US Environmental Protection Agency. The project described was supported in part by Award Numbers P42 ES016465 and P30 ES00210 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health nor has been formally reviewed by the EPA. The views expressed in this presentation are solely those of the authors and the EPA does not endorse any products or commercial services mentioned. Special thanks to Dr Shu Tao, Susan Genualdi and Zhang Yanxu (Michael) for help with sample collection, as well as to Jessica Murray and Rachel Huber for laboratory assistance. The authors would also like to acknowledge Glenn Wilson for technical expertise and valuable advice.
References
- 1.Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, Rannug A, Tornqvist M, Victorin K, Westerholm R. Environ. Health Perspect. 2002;110:451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claxton LD, Matthews PP, Warren SH. Mutat. Res., Rev. Mutat. Res. 2004;567:347–399. doi: 10.1016/j.mrrev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Venkataraman C, Friedlander SK. Environ. Sci. Technol. 1994;28:563–572. doi: 10.1021/es00053a006. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Air Quality Guidelines for Europe. Copenhagen: World Health Organization; 2001. [Google Scholar]
- 5.Pufulete M, Battershill J, Boobis A, Fielder R. Regul. Toxicol. Pharmacol. 2004;40:54–66. doi: 10.1016/j.yrtph.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Aubin S, Farant JP. J. Air Waste Manage. Assoc. 2000;50:2093–2101. doi: 10.1080/10473289.2000.10464236. [DOI] [PubMed] [Google Scholar]
- 7.Bergvall C, Westerholm R. Environ. Sci. Technol. 2007;41:731–737. doi: 10.1021/es062232p. [DOI] [PubMed] [Google Scholar]
- 8.Castro D, Slezakova K, Oliva-Teles MT, Delerue-Matos C, Alvim-Ferraz MC, Morais S, Pereira MC. J. Sep. Sci. 2009;32:501–510. doi: 10.1002/jssc.200800495. [DOI] [PubMed] [Google Scholar]
- 9.Sauvain JJ, Duc TV, Guillemin M. Int. Arch. Occup. Environ. Health. 2003;76:443–455. doi: 10.1007/s00420-003-0439-4. [DOI] [PubMed] [Google Scholar]
- 10.Sauvain JJ, Vu Duc T. J. Sep. Sci. 2004;27:78–88. doi: 10.1002/jssc.200301620. [DOI] [PubMed] [Google Scholar]
- 11.Bergvall C, Westerholm R. Anal. Bioanal. Chem. 2006;384:438–447. doi: 10.1007/s00216-005-0192-5. [DOI] [PubMed] [Google Scholar]
- 12.Liu YN, Tao S, Dou H, Zhang TW, Zhang XL, Dawson R. Chemosphere. 2007;66:1922–1928. doi: 10.1016/j.chemosphere.2006.07.076. [DOI] [PubMed] [Google Scholar]
- 13.Watson JG, Zhao Z. Committee on Energy Futures and Air Pollution in Urban China and the United States. National Academy of Sciences; 2007. [Google Scholar]
- 14.IARC. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Vol. 32. Lyon, France: International Agency for Research on Cancer; 2008. p. 92. [PMC free article] [PubMed] [Google Scholar]
- 15.OEHHA. Air Toxics Hot Spots Program Risk Assessment Guidelines. California Environmental Protection Agency Office of Environmental Health Hazard Assessment Air Toxicology and Epidemiology Section; 2005. [Google Scholar]
- 16.NIST. Certificate of Analysis. Gaithersburg, MD: National Institute of Standards and Technology; 2009. p. 1649b. [Google Scholar]
- 17.Bergvall C, Westerholm R. Anal. Bioanal. Chem. 2008;391:2235–2248. doi: 10.1007/s00216-008-2182-x. [DOI] [PubMed] [Google Scholar]
- 18.Seidel A, Frank H, Behnke A, Schneider D, Jacob J. Polycyclic Aromat. Compd. 2004;24:759–771. [Google Scholar]
- 19.Wise SA, Deissler A, Sander LC. Polycyclic Aromat. Compd. 1993;3:169–184. [Google Scholar]
- 20.Schubert P, Schantz MM, Sander LC, Wise SA. Anal. Chem. 2003;75:234–246. doi: 10.1021/ac0259111. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, He L, Hu M, Zhang Y. Atmos. Environ. 2006;40:2449–2458. [Google Scholar]
- 22.Wang XF, Cheng HX, Xu XB, Zhuang GM, Zhao CD. J. Hazard. Mater. 2008;157:47–56. doi: 10.1016/j.jhazmat.2007.12.092. [DOI] [PubMed] [Google Scholar]
- 23.ATSDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs) Atlanta: Agency for toxic Substances & disease Registry; 1995. [PubMed] [Google Scholar]
- 24.Kawanaka Y, Matsumoto E, Sakamoto K, Wang N, Yun SJ. Atmos. Environ. 2004;38:2125–2132. [Google Scholar]
- 25.Hattori T, Tang N, Tamura K, Hokoda A, Yang X, Igarashi K, Ohno M, Okada Y, Kameda T, Toriba A, Hayakawa K. Environ. Forensics. 2007;8:165–172. [Google Scholar]
- 26.Sun YL, Zhuang GS, Ying W, Han LH, Guo JH, Mo D, Zhang WJ, Wang ZF, Hao ZP. Atmos. Environ. 2004;38:5991–6004. [Google Scholar]
- 27.Zhou J, Wang T, Huang Y, Mao T, Zhong N. Chemosphere. 2005;61:792–799. doi: 10.1016/j.chemosphere.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Wang WT, Primbs T, Tao S, Simonich SLM. Environ. Sci. Technol. 2009;43:5314–5320. doi: 10.1021/es9007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie SD, Liu Z, Chen T, Hua L. Atmos. Chem. Phys. 2008;8:2701–2716. [Google Scholar]
- 30.SEPA. China State Environmental Protection Agency. 1996 http://www.zhb.gov.cn/english/chanel-5/GB3095-1996.doc.
- 31.EU. European Commission. 2001 doi: 10.5334/ijic.40. http://ec.europa.eu/environment/air/quality/standards.htm. [DOI] [PMC free article] [PubMed]
- 32.WHO. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide. Geneva: World Health Organization; 2005. [PubMed] [Google Scholar]
- 33.Zhang Y, Tao S, Shen H, Ma J. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21063–21067. doi: 10.1073/pnas.0905756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


