Abstract
Escherichia coli FepA transports certain catecholate ferric siderophores, but not others, nor any noncatecholate compounds. Direct binding and competition experiments demonstrated that this selectivity originates during the adsorption stage. The synthetic tricatecholate Fe-TRENCAM bound to FepA with 50- to 100-fold-lower affinity than Fe-enterobactin (FeEnt), despite an identical metal center, and Fe-corynebactin only bound at much higher concentrations. Neither Fe-agrobactin nor ferrichrome bound at all, even at concentrations 106-fold above the Kd. Thus, FepA only adsorbs catecholate iron complexes, and it selects FeEnt among even its close homologs. We used alanine scanning mutagenesis to study the contributions of surface aromatic residues to FeEnt recognition. Although not apparent from crystallography, aromatic residues in L3, L5, L7, L8, and L10 affected FepA's interaction with FeEnt. Among 10 substitutions that eliminated aromatic residues, Kd increased as much as 20-fold (Y481A and Y638A) and Km increased as much as 400-fold (Y478), showing the importance of aromaticity around the pore entrance. Although many mutations equally reduced binding and transport, others caused greater deficiencies in the latter. Y638A and Y478A increased Km 10- and 200-fold more, respectively, than Kd. N-domain loop deletions created the same phenotype: Δ60-67 (in NL1) and Δ98-105 (in NL2) increased Kd 10- to 20-fold but raised Km 500- to 700-fold. W101A (in NL2) had little effect on Kd but increased Km 1,000-fold. These data suggested that the primary role of the N terminus is in ligand uptake. Fluorescence and radioisotopic experiments showed biphasic release of FeEnt from FepA. In spectroscopic determinations, koff1 was 0.03/s and koff2 was 0.003/s. However, FepAY272AF329A did not manifest the rapid dissociation phase, corroborating the role of aromatic residues in the initial binding of FeEnt. Thus, the β-barrel loops contain the principal ligand recognition determinants, and the N-domain loops perform a role in ligand transport.
Even though iron is the second-most abundant metal in the earth's crust, constituting 5% by mass, its tendency to precipitate as Fe(OH)n reduces the concentration of soluble iron(III) in water to 10−38 M (47). Free iron is also scarce (∼10−18 M) in the vertebrate colon, blood, and tissues, where commensal and pathogenic bacteria must acquire it (12, 49). Such concentrations, which may vary with diet, are insufficient for bacterial growth (34, 36, 47), but microorganisms capture iron from these environments (19) by the elaboration of high-affinity iron chelators (siderophores) (48), or by direct acquisition of the iron from eukaryotic proteins (19, 37)). Certain gram-negative bacterial outer membrane (OM) proteins transport ferric siderophores (9, 20, 27, 32, 46, 64), whereas others strip iron from transferrin and lactoferrin or use the heme within hemoglobin. These processes display specificity. FepA (11), for example, transports ferric enterobactin (FeEnt), FhuA (25, 39, 67) preferentially acts on ferrichrome, and FecA (23, 65) internalizes ferric citrate. OM siderophore receptor proteins such as FepA and FhuA also serve as receptors for bacteriocins and bacteriophage (28, 66-68). They lack a nonspecific open channel, so they are not porins in the original sense of the word (44), but their structural elements include a porin-like β-barrel and a globular N-terminal domain (N-domain) that resides within the barrel and presumably regulates uptake of molecules through it (11, 23, 25, 39). Ligand binding activates these proteins to a transport-competent state (17, 33, 59). The exact nature of this competency is unknown (35), but one change that ligand binding causes is a structural rearrangement of the “TonB-box” region at the N terminus of metal transporters, which is located on the internal side of the OM bilayer (18, 22, 25).
At binding equilibrium, the external loops of the FepA, FhuA, and FecA β-barrels surround their ligands and suspend them above the N domain, prior to transport (18, 23, 25). Such binding reactions have high affinity (16, 64): both the Kd of FeEnt binding to FepA, and the Km of its transport (51, 53), are subnanomolar (∼0.2 nM). Nevertheless, the specificity of such receptors for individual siderophore is not absolute. Besides its recognition of FeEnt, bacteriophage, and colicins, FepA recognizes and transports two synthetic catecholate siderophores, ferric MECAM (20, 31) and ferric TRENCAM (FeTrn) (64). Similarly, FhuA functions to transport a variety of hydroxamate ferric siderophores (40) and a structurally unrelated antibiotic (26, 56). We found that during the binding stages FepA discriminates between FeEnt and other catecholate ferric siderophores: the receptor manifests lower affinity for even closely related tricatecholate siderophores and does not bind the dicatecholate ferric agrobactin or the hydroxamate ferrichrome. Second, we characterized the involvement of surface loop aromatic residues of FepA in ligand adsorption. Biochemical data (16, 53) suggested an initial binding site in the loop extremities (B1) and a secondary site deeper in the vestibule (B2). Crystallographic results (11) did not reveal the FeEnt contact residues but showed that both the B1 and B2 sites contain aromatic and basic amino acids with the potential to accommodate the aromatic, acidic siderophore. Analysis of 10 alanine substitutions for aromatic residues that encircle the mouth of the FepA vestibule indicated that aromaticity is a principal determinant of FeEnt adsorption affinity. Comparison of the rate of release of bound FeEnt from wild-type FepA and a double aromatic substitution mutant by radioisotopic and fluorescence techniques corroborated the participation of aromatic residues in the initial adsorption stage.
MATERIALS AND METHODS
Bacterial strains, plasmids, culture conditions, and chemicals.
We generated mutations on pITS23 (60, 61), a derivative of the low-copy vector pHSG575 (29) carrying fepA under its natural promoter. The Escherichia coli K-12 host strain was KDF541 (rpsL entA fepA fhuA cir) (59). Bacteria were grown at 37°C with vigorous shaking in Luria-Bertani (LB) broth (42) containing streptomycin (100 μg/ml) and chloramphenicol (20 μg/ml). For binding and transport measurements, we subcultured the bacteria (1%) from stationary-phase LB broth into morpholinepropanesulfonic acid (MoPS) minimal medium (45) containing the same antibiotics but without iron for 6 h, until the growth rate diminished from iron deficiency. Under these conditions the expression of the inner membrane (FepC, FepD, and FepG) and periplasmic (FepB) components of the transport system reached maximum levels, and transport of FeEnt through the OM was the rate-limiting step in its uptake (51, 63).
We purified the siderophores ferrichrome, Ent, corynebactin (Crn), and agrobactin from culture supernatants of Ustilago sphaerogena (21), E. coli (48), Bacillus subtilis (41), and Agrobacterium tumefaciens (52), respectively. TRENCAM (Trn) was a gift from D. van der Helm. We verified the authenticity of the siderophores by UV/visible spectroscopy, paper electrophoresis, and mass spectroscopy. The observed mass of Crn was 888 Da.
Site-directed mutagenesis.
Using either the M13 method (50) or Quikchange (Stratagene, San Diego, Calif.) (61), we produced site-directed substitution mutations in fepA. The former method gave rise to fepAY472A, -Y478A, -Y481A, -Y488A, -Y495A, -Y553A, and -Y638A on pITS449 (a pUC18 derivative) (2, 16). We transferred these alleles to pITS23 by restriction fragment exchange with KpnI and SstI. We directly engineered fepAW101A, -Y217A, and -Y540A on pITS23 and fepAΔ60-67 and fepAΔ98-105 on pITS449 (51) by using the QuikChange kit. We verified the mutations by DNA sequence analysis with an Alf Express sequencer (Amersham-Pharmacia) and appropriate CY-5-labeled oligonucleotide primers.
Protein expression and localization.
For measurements of protein expression, 5 × 108 bacteria were collected by centrifugation, resuspended in 100 μl of sample buffer (0.5 M Tris [pH 6.8], 30% glycerol, 3% sodium dodecyl sulfate [SDS]), and boiled for 3 min. After a brief centrifugation to pellet debris, we subjected 20-μl aliquots of the lysates to SDS-polyacrylamide gel electrophoresis (PAGE) in 10% slabs (1), transferred the resolved proteins to nitrocellulose membranes, and performed Western immunoblots with α-FepA monoclonal antibody (MAb) 45 (43) and 125I-protein A (38, 62). After overnight exposure to an imaging screen, radioactivity was quantitated on a STORMSCAN PhosphorImager (Molecular Dynamics). In other experiments we measured the concentration of FepA or mutant FepA proteins by immunoblots of purified OM fractions. We also used the OM fractions for determination of mutant protein localization. We prepared the OM fraction by Sarkosyl extraction (58), resolved the protein components by SDS-PAGE, and stained the protein components with Coomassie blue.
Colicin killing assay.
Bacteria were plated on LB agar containing appropriate antibiotics. Serial dilutions of colicins B and D were freshly made in LB medium, applied to the plate by using a CloneMaster (Immusine), and the plates were incubated overnight at 37°C. Susceptibility to colicin killing was expressed in arbitrary titration units, defined as the reciprocal of the highest dilution of colicin that cleared the bacterial lawn.
FeEnt binding and uptake measurements.
For binding and transport determinations, we prepared 59Fe complexes of Ent, Trn, and Crn (specific activity, 150 to 1,000 cpm/pmol) and chromatographically purified them (66, 67). We conducted adsorption equilibrium experiments at 0°C (51), in triplicate at each concentration, with KDF541 providing negative control data that we subtracted from those of the test strains to eliminate nonspecifically adsorbed radioactivity. The Kd and capacity of 59FeEnt binding were determined by using the bound-versus-total equation of Grafit 5.09 (Erithacus, Ltd., Middlesex, United Kingdom). In each experiment, we evaluated mutant protein expression by Western immunoblot.
Siderophore nutrition tests (16, 51, 60, 66) provided qualitative determinations of FeEnt transport capability. For quantitative measurements of FeEnt transport at 37°C (51), we incubated 59FeEnt with the cells for a minimum of 10 s; bacteria expressing certain mutant FepA proteins required longer times (i.e., 1 min). After incubation we quenched the uptake with a 100-fold excess of nonradioactive FeEnt. Reactions at high concentrations of 59FeEnt (>100 nM) were not quenched because of the large amount of FeEnt required to achieve a 100-fold excess. In this case, KDF541 was a negative control, and we subtracted its values from those of the low-affinity mutants. We made measurements in triplicate for each concentration of FeEnt and determined the level of FepA expression for the strains under investigation. Transport Km and Vmax values were calculated with the enzyme kinetics equation of Grafit 5.09.
59FeEnt adsorption competitions.
We assayed KDF541/pITS23 (fepA+) (60) for its ability to bind 59FeEnt (51) in the absence or presence of the other purified siderophores FeEnt, FeTrn, FeCrn, ferric agrobactin, and ferrichrome. After growth and appropriate dilution of the bacteria, we incubated 5 × 107 bacteria, previously chilled on ice, with 59FeEnt (final concentration, 1 nM) and various concentrations of the competitor iron complexes. We premixed the competing ferric siderophores with 59FeEnt before addition to the bacteria. After incubation for 5 s, we filtered the reactions, washed them with 0.9% LiCl (aqueous), and counted the radioactivity in the filters to determine the amounts of FeEnt that adsorbed to the cells. We plotted and analyzed the data with the IC50-4 parameter logistic of Grafit 5.09.
Dissociation rate measurements.
We determined the rate of FeEnt dissociation from FepA by radioisotopic and spectroscopic methodologies. In the former case, we incubated cells prechilled at 0°C with 10 nM 59FeEnt for 1 min to allow binding to reach equilibrium (51) and then applied aliquots of the reactions (5 × 107 cells) to nitrocellulose filters and washed the cells with a series of different volumes (from 1 to 30 ml) of 0.9% LiCl either without additions or containing 50 μM FeEnt. The filtration of increasing volumes of wash solution required correspondingly longer times, and we recorded the wash duration for each sample with a stopwatch. In this way, the duration of the wash sequentially increased. Afterward, we determined and plotted the amount of radioactivity remaining on the filters versus the washing time to obtain the rate of 59FeEnt release from the bacteria.
In the spectroscopic method, KDF571 (fepA fhuA cir tonB) (59) harboring the pUC18 derivative pfepAS271C was cultured in MoPS minimal medium, labeled with fluorescein maleimide (FM) (17), and saturated with FeEnt, and 2.5 × 107 cells were incubated with an excess (7.5 × 107) of KDF541 (tonB+) harboring pITS449 (fepA+) or KDF571 (tonB)/pITS449. Changes in the fluorescence of FepAS271C-FM reflected the release of FeEnt from the tonB strain. The nonfluorescent, fepA+ bacteria acted as a repository for the released siderophore: KDF571/pITS449 bound the siderophore, and the KDF541/pITS449 cells bound and transported it.
RESULTS
Binding and transport of ferric catecholates.
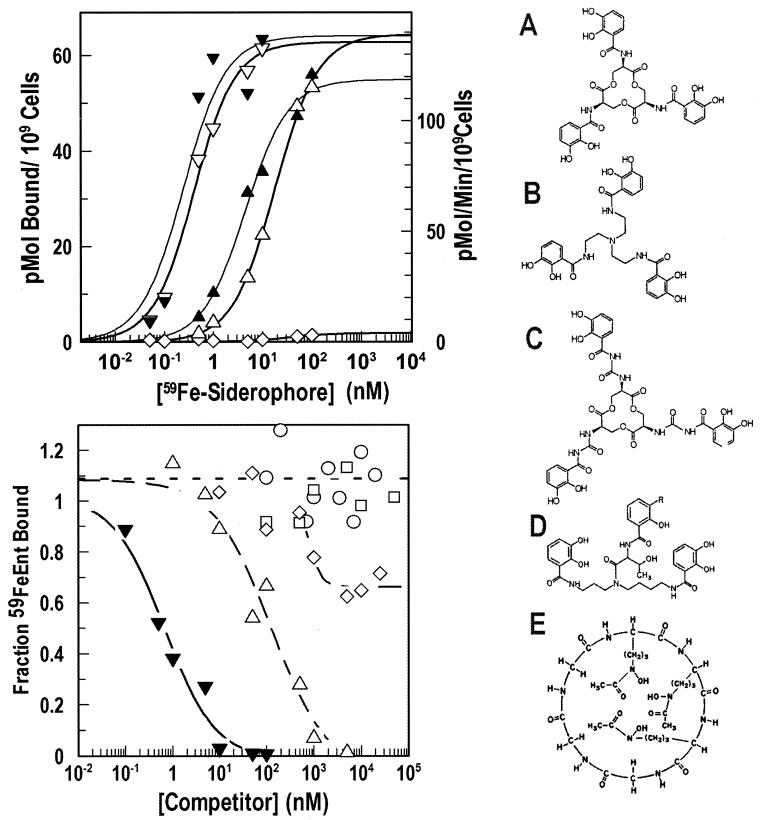
We characterized the recognition of ferric catecholate complexes by FepA by using [59Fe]siderophore binding and transport assays and by determining the ability of nonradioactive ferric siderophores to inhibit 59FeEnt adsorption. Both methods showed the receptor's preference for the native E. coli siderophore. For the direct binding determinations, we used procedures that eliminate distortions caused by ligand depletion at low concentrations (51), and these techniques revealed previously unseen differences in the affinity of FepA for different ferric catecholates (64). The Kd of 59FeTrn adsorption was 17 nM, ∼50-fold higher than that of 59FeEnt, 0.36 nM (Fig. 1) (51). 59FeCrn, another exclusively catecholate compound that is larger in size, did not adsorb to FepA at concentrations as high as 1 μM (Fig. 1). Siderophore nutrition tests (66) (data not shown) and uptake reactions with [59Fe] complexes (Fig. 1) reiterated the preference of FepA for FeEnt (Km = 0.25 nM) over FeTrn (Km = 4.5 nM) and the inability of FeCrn to supply iron to E. coli.
FIG. 1.
(Top) Binding and transport. We compared the binding (open symbols) and transport (solid symbols) of 59FeEnt (inverted triangles), 59FeTrn (triangles), and FeCrn (diamonds) by E. coli strain KDF541/pITS23. (Bottom) Competition of 59FeEnt binding to FepA by catecholate siderophores. We determined the abilities of ferrichrome (□), ferric agrobactin (○), FeTrn (▵), FeCrn (⋄), and FeEnt (▾) to inhibit the binding of 59FeEnt to E. coli strain KDF541/pITS23 (fepA+). The data were analyzed and plotted by the IC50-4 Parameter Logistic of Grafit 5.09. The right panel shows the structures of Ent (A), Trn (B), Crn (C), agrobactin A (D), and apoferrichrome (E).
In competition reactions with FeTrn, FeCrn, ferric agrobactin, and ferrichrome, aside from the identity reaction with FeEnt, only FeTrn and FeCrn inhibited the adsorption of 59FeEnt. Ferrichrome did not block the binding of 59FeEnt, even at concentrations as high as 50 μM (Fig. 1). This result was not surprising, however, in light of the fact that even the dicatecholate siderophore ferric agrobactin failed to inhibit FeEnt adsorption. The competition reactions recapitulated the results of direct binding assays: FeEnt inhibited 50% of 59FeEnt adsorption (IC50) at 0.67 nM, whereas the IC50 of FeTrn was 120 nM, i.e., 180-fold higher. In this assay we observed inhibition by FeCrn, but only at much higher concentrations: its IC50 was 1 μM (Fig. 1), indicating that its affinity for FepA was 4,000-fold lower than that of FeEnt. Furthermore, its inhibition of binding was incomplete, in that FeCrn only reduced the amount of 59FeEnt bound to 50% of its uninhibited value.
Alanine scanning mutagenesis of aromatic residues in the loop extremities.
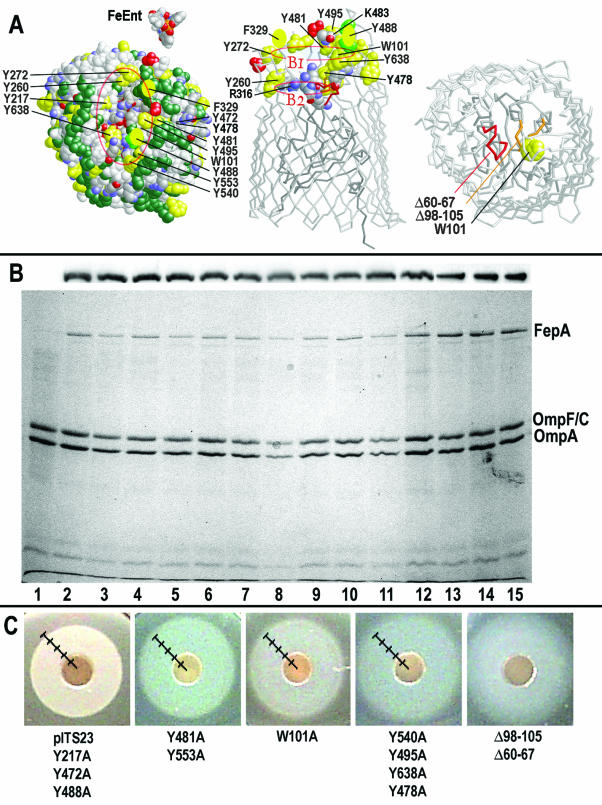
In its crystal structure, the external loops of FepA coalesce to form the mouth of the pore vestibule. Hydrophobic amino acids predominate on the exterior surfaces of the vestibule mouth, but its interior contains aromatic residues that distribute in two regions proposed to participate in ligand binding (B1 and B2; Fig. 2) (53). We were interested in the function of the exteriormost aromatic amino acids of B1 in ferric siderophore recognition, and we aligned the sequences of FeEnt transporters from several gram-negative bacteria (E. coli, Salmonella enterica serovar Typhimurium, Bordetella pertussis, Neisseria gonorrhoeae, and Xanthomonas citri) and the E. coli metal transporters (Cir, FecA, IutA, BtuB, FhuA, and FhuE) by using PILEUP (Genetics Computer Group, Madison, Wis.), and compared them to the crystal structures of FepA (Fig. 2A) (11), FhuA (25, 39), and FecA (23). The alignments identified four aromatic residues in the extremities of surface loop 7 (tyrosines 472, 478, 481 and 495 [Fig. 2A]) and one residue (W101) in the second loop of the N domain (NL2) (11) that were at least 70% conserved among the different proteins. We also found less-conserved, but well-exposed tyrosines 217 (L2), 488 (L7), 540 (L8), 553 (L8), and 638 (L10). For each of these targets we generated alanine substitutions on pITS23, a low-copy-number vector (29) that carries fepA under control of its natural promoter. We also engineered individual deletions of both N-domain loops (Fig. 2A; Δ60-67 and Δ98-105) on pITS449.
FIG. 2.
Site-directed FepA mutants. (A) Location. The model of FepA (14) depicts aromatic residues (yellow) that we changed to Ala, in space filling format viewed from the top (left). Hydrophobic residues (L, I, V, M, and A) are colored green. The locations of residues F329 and Y488 are not known (11), but the last solved residues in L4 (red) flank the approximate location of the former (yellow oval), and the last solved residues in L7 (light green) flank the approximate location of the latter residue (yellow oval). Basic residues are shown in CPK format. Residues of interest are also shown in space-filling format on a backbone representation viewed from the side (center), with the same color scheme. (Right) A backbone model, viewed from the top, shows the location of two site-directed deletions, Δ60-67 (red; in NL1) and Δ98-105 (orange; in NL2); NL2 contains residue W101 (yellow, in space-filling format). (B) Expression and localization in the OM. Cell lysates (top) or OM fractions (bottom) (58) from E. coli expressing wild-type FepA or its mutant derivatives were resolved by SDS-PAGE and either subjected to immunoblot analysis with anti-FepA MAb 45 and 125I-protein A (top) or stained with Coomassie blue (bottom). The expression levels of the mutant proteins, and their localization to the OM were related to those of OmpF and OmpA and found to be comparable to that of wild-type FepA carried on the same plasmid. Lane 1 contains a sample from KDF541, and lanes 2 to 12 contain samples from KDF51 harboring pHSG575 carrying the fepA alleles fepA+, W101A, Y271A, Y472A, Y478A, Y481A, Y488A, Y495A, Y540A, Y553A, and Y638A. Lanes 13 to 15 contains samples from KDF541 harboring pUC19 carrying the fepA alleles fepA+, ΔNL1, and ΔNL2, respectively. (C) Siderophore nutrition tests. The site-directed mutations created several different effects in qualitative uptake assays. See the text for further explanation.
The levels of mutant FepA protein expression, monitored by SDS-PAGE and by anti-FepA (MAb 45) immunoblots developed with 125I-protein A, showed few variations (Fig. 2B). KDF541 expressed all of the mutant proteins at levels similar to that of wild-type FepA, and the immunoblot determinations paralleled estimates of FepA concentration from their 59FeEnt binding capacity. The latter measurements provided the most accurate determination of the concentration of functional FepA proteins in the OM (Table 1). In addition, flow cytometric analyses (Table 1) indicated that KDF541 properly expressed all of the mutant FepA proteins and assembled them in the OM such that surface epitopes in L4 and in L5 were accessible to binding by MAbs 45 and 24 (43), respectively, at levels that were comparable to their recognition of the same residues in wild-type FepA. These localization results, together with the capacity and expression measurements, showed that the mutations did not globally disrupt the structure of FepA such that it was poorly expressed, improperly targeted or misfolded into an aberrant conformation. The data indicated that the substitution mutations created local perturbations of structure, and phenotypes that we could compare to each other and to that of wild-type FepA.
TABLE 1.
Effects of mutagenesis on FeEnt uptake
| Group and allelea | Loop | Ferric enterobactin
|
Colicine
|
Flow cytometryf
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bindingb
|
Transportc
|
||||||||||
| Kd (nM) | Cap. | Km (nM) | Vmax | Nutr. | k8d | B | D | MAb 24 | MAb 45 | ||
| fepA | |||||||||||
| fepA | NA | R | R | 0.14 (97) | 0.15 (99) | ||||||
| fepA+ | NA | 0.27 | 67 | 0.4 | 113 | 18.5 | 1.7 | 100 | 100 | 4.2 (82) | 45.2 (84) |
| fepA+g | NA | 0.41 | 150 | 0.24 | 123 | 18 | 0.8 | 100 | 100 | 4.1 (80) | 11.7 (90) |
| Class I | |||||||||||
| Y272Ag,h | 3 | 0.1 | 103 | 0.3 | 150 | 19 | 1.5 | 50 | 100 | ND | ND |
| Y472A | 7 | 1.5 | 68 | 2.6 | 105 | 19.5 | 1.5 | 10 | 100 | 8.34 (89) | 42.4 (84) |
| Y481A | 7 | 4.8 | 47 | 7.2 | 44 | 21.0 | 0.9 | 10 | 25 | 11.3 (84) | 42.6 (78) |
| Y488A | 7 | 0.5 | 38 | 0.6 | 156 | 19.5 | 4.1 | 25 | 50 | 3.5 (82) | 38.5 (86) |
| Y540A | 8 | 1.4 | 56 | 2.0 | 98 | 23.0 | 1.8 | 10 | 10 | 10.1 (90) | 45.9 (78) |
| Class II | |||||||||||
| Δ60-67g | NL1 | 7.3 | 119 | 119 | 99 | 25 | 0.8 | 10 | 10 | 6.2 (91) | 11.5 (93) |
| Δ98-105g | NL2 | 11.7 | 107 | 163 | 88 | 25 | 0.8 | 10 | 10 | 6.57 (91) | 12.6 (88) |
| W101A | NL1 | 0.85 | 12 | 393i | 269i | 20.5 | ND | 2.5 | 5 | 8.8 (92) | 54.6 (86) |
| Y217A | 2 | 0.20 | 58 | 1.3 | 122 | 18.5 | 2.1 | 20 | 50 | 3.5 (81) | 44.1 (89) |
| F329Ag,h | 4 | 0.2 | 100 | 5.5 | 135 | 19 | 1.4 | 50 | 100 | ND | ND |
| Y478A | 7 | 0.76 | 49 | 167 | 184 | 21.0 | 3.8 | 10 | 10 | 14.2 (90) | 34.8 (88) |
| Y553A | 8 | 0.40 | 46 | 2.1 | 100 | 20.5 | 2.2 | 2.5 | 5 | 7.2 (84) | 47.3 (84) |
| Y638A | 10 | 2.9 | 3 | 31 | 93 | 22.0 | 31 | 40 | 25 | 4.1 (79) | 38.5 (86) |
| Y272AF329Ag,h | 3/4 | 7.8 | 76 | 23 | 128 | 23 | 1.7 | 50 | 100 | ND | ND |
| Class III | |||||||||||
| Y495A | 7 | 0.79 | 44 | 0.9 | 17 | 21.5 | 0.4 | 20 | 10 | 8.8 (87) | 39.1 (77) |
Class I, comparable increases in binding Kd and transport Km; class II, disproportionate increase in transport Km; class III, reduction in transport rate.
Kd and capacity (Cap.; pmol bound/109 cells/min) were determined from the concentration dependence of FeEnt binding by analyzing the mean values from independent experiments with GRAFIT 5.09 by using the bound-versus total equation. The average percent errors of the Kd and capacity determinations were 32 and 7%, respectively.
Km and Vmax (pmol/109 cells/min) of uptake were determined from the concentration dependence of FeEnt transport by using GRAFIT 5.09 by using the enzyme kinetics equation. Nutrition (Nutr.) test results show the diameters of the growth halos in millimeters. The average percent errors of the Km and Vmax determinations were 34 and 10%, respectively.
The kinetic constant k8 is an expression of turnover number, relating the FeEnt transport rate to the amount bound: k8 = Vmax/capacity. It is equivalent to k3 (60) molecules/FepA protein/min.
Colicin killing was determined by measuring the susceptibility of KDF541 harboring pHSG575 or pUC18 derivatives expressing the mutant FepA proteins to limiting dilutions of colicins B and D. Results are expressed as a percentage of the killing observed for KDF541/pITS23 (wild type FepA). R, complete resistance.
We cytofluorimetrically monitored the localization of FepA in the OM and its normal overall folding with anti-FepA MAbs 45 and 24, which recognize cell surface determinants in L4 and L5, respectively. The tabulated value is the mean fluorescence intensity of 104 bacteria stained with the antibodies and fluoresceinated goat-anti-mouse immunoglobulin G. The parenthetic value is the percentage of the population within one standard deviation of the mean.
The structural gene was carried on the pUC derivative pITS449.
The mutant was previously described, and the data are reproduced here from Cao et al. (16).
We did not observe saturable transport of FeEnt through FepAW101A; Km and Vmax values were estimated. Without determination of saturation velocity, it was not possible to calculate k8 NA, not applicable; ND, no data.
Colicin susceptibility.
All of the constructs retained susceptibility to both ColB and ColD (Table 1), reiterating that neither the Ala substitution mutations nor the small deletions prevented proper folding and assembly of FepA in the OM. Most mutations decreased colicin sensitivity 2- to 10-fold, but W101A and Y553A reduced ColB susceptibility 40-fold. The effects of mutagenesis on FeEnt utilization and colicin susceptibility were usually not the same. The drop in ColB sensitivity caused byY553A, for example, contrasted with normal FeEnt binding and almost normal transport (see below). On the other hand, W101A, Y481A, and Y638A created the opposite effect: for example, Y638A yielded only a twofold decrease in colicin sensitivity but significant defects in FeEnt uptake. The N-loop deletion mutants were similar in that their loss of colicin sensitivity (10-fold) was much less than their effects on FeEnt transport (Km increased 500- to 800-fold). These data indicated that in the exteriormost loop regions of FepA different residues function in the adsorption of FeEnt and colicins B and D. Finally, killing by the two toxins involves similar recognition and uptake determinants, because the mutations in FepA usually caused comparable reductions in susceptibility to both toxins. The sole exception was Y472A, which caused a 10-fold decrease in ColB killing but did not diminish sensitivity to ColD.
Binding.
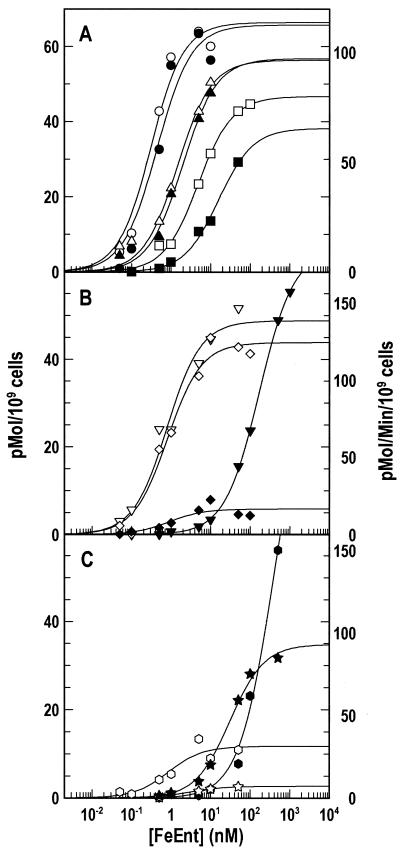
Adsorption of 59FeEnt to cells expressing the aromatic substitution mutant proteins revealed several affinity effects (Kd [Fig. 3 and Table 1]). Y217A, Y488A, and Y553A had near-wild-type binding capabilities (Kd ≤ 0.5 nM), whereas other mutations caused three- to fivefold-lower affinity (Kd ≈ 1.5 nM) in the following order of impairment: Y472 > Y540 > W101> Y495 > Y478. Substitutions Y481A and Y638A engendered greater reductions in affinity (>10- fold; Kd ≈ 5 nM), as did the deletions in NL1 and NL2 (Kd ≈ 7.3 and 11.7 nM, respectively).
FIG. 3.
59FeEnt binding and transport by Ala substitution mutants. We determined the concentration dependence of 59FeEnt binding (open symbols) and transport (solid symbols) by E. coli strain KDF541 expressing FepA substitution mutant proteins carried on the low-copy plasmid pHSG575 at six concentrations near Kd and Km, respectively, with each datum point collected in triplicate and averaged. The binding and transport data were analyzed, and curves were plotted by using the bound-versus-total and enzyme kinetics equations, respectively, of Grafit 5.09. The concentration of FeEnt is logarithmically plotted to demonstrate the decreases in affinity that some of the mutations caused. (Top) fepA+, circles; Y540A, triangles; and Y481A, squares. (Center) Y478A, inverted triangles; Y495A, diamonds. (Bottom) W101A, hexagons; Y638A, stars.
With the exception of W101A and Y638A, the mutations did not affect the maximum amount of FeEnt bound per cell (binding capacity), which concurred with the normal expression and localization of the mutant FepA proteins (Fig. 2). The capacity of bacteria expressing FepAW101A and FepAY638A was lower, even though they were expressed at normal levels and localized in the OM. Thus, their decreased capacities derived from specific detrimental effects of the Ala substitutions on FeEnt binding.
Transport.
In nutrition tests, wild-type FepA consistently gave a uniformly dense zone of growth with sharp borders, whereas the mutants produced several other types of growth halos (Fig. 2C and Table 1) that reflected their transport properties. The growth zones of KDF541 harboring Y217A, Y472A, and Y488A were comparable to that conferred by pITS23 (fepA+), which agreed with the small effects that these mutations had on 59FeEnt binding and transport (see below). The halos created by Y481A and Y553A had sharp margins like those of wild-type FepA, but they were larger in diameter (∼2 mm), and this increase reflected their detrimental effects on transport. Mutants Y478A, Y495A, Y540A, and Y638A produced fainter, larger halos with diffuse borders that indicated more pronounced transport deficiencies. The N-domain loop deletions created still larger halos with even more diffuse margins. Quantitative uptake determinations (see below) showed a general trend in the phenotypes: impaired transport resulted in larger growth halos. Mutant W101A constituted another category. Its halo was only slightly larger than that of wild type, but it had a diffuse border and a “bull's-eye” or “target” appearance.
The mutants had degrees of deficiency in 59FeEnt uptake (Table 1) that, together with their binding properties, created three categories. The decreased binding affinities created by Y472A, Y488A, Y540A, and Y481A were commensurate with their decreased uptake affinites (class I). Among these, Y481A caused the most impairment (a 17-fold increase in Kd and an 18-fold increase in Km); its Vmax was ca. 50% that of the wild type. None of the other class I mutations significantly changed Vmax. Class II mutants had more severe transport defects than binding deficiencies. Y478A and W101A created large increases in transport Km compared to their binding abilities (Km values that were 200- to 400-fold more than the Kd values) and higher Vmax values (see below). Y217A, Y553A, and Y638A also increased Km but at lower magnitudes (Km values 5- to 10-fold more than the Kd). The class II mutant FepAY638A had reduced binding capacity (5%), but its Vmax was comparable to that of wild type (93 pmol/109 cells/min). On the other hand, although W101A only slightly decreased FeEnt binding affinity (threefold), it altered the transport reaction so that it did not follow saturation kinetics. We could not accurately determine Vmax or Km despite 59FeEnt concentrations as high as 500 nM (2,000-fold that of the wild-type Km), and this kinetic distinction suggested the importance of W101 in FeEnt internalization. Y495A constituted class III: normal affinity for FeEnt (as measured by either Kd or Km) but a loss of transport velocity (fivefold drop in Vmax) that resulted from a low turnover number (k8 = 0.4/min).
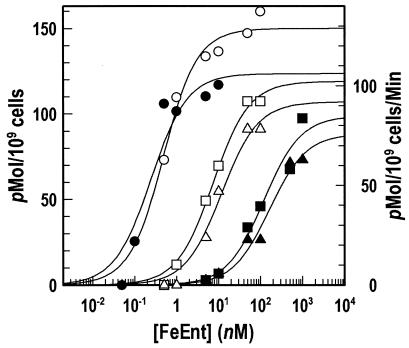
Experiments with the N-loop deletion mutations showed the importance of the N domain in FeEnt uptake. The Δ60-67 and Δ98-105 deletions impaired binding (18- and 28-fold increases in Kd, respectively), but they decreased the affinity of FeEnt uptake much more (500- and 700-fold increases in Km, respectively) (Table 1 and Fig. 4). These comparisons suggested that the dominant effect of structural alterations at the top of the N domain is an increase in transport Km. This drop in transport efficiency occurred even in the absence of severe defects in binding, as typified by W101A. It was further noteworthy that certain single residue substitutions in the surface loops (Y481A and Y638A) reduced binding affinity as much as the elimination of either N-domain loop.
FIG. 4.
59FeEnt binding and transport by N-loop deletion mutants. Methods were as described in Fig. 3. Open symbols indicate 59FeEnt binding, and solid symbols indicate 59FeEnt uptake by bacteria expressing fepA+ (circles), fepAΔ60-67 (squares), and fepAΔ98-105 (triangles) alleles. The binding and transport data were analyzed and curves were plotted by using the bound-versus-total and enzyme kinetics equations, respectively, of Grafit 5.09.
Measurements of koff in the FeEnt-FepA binding equilibrium.
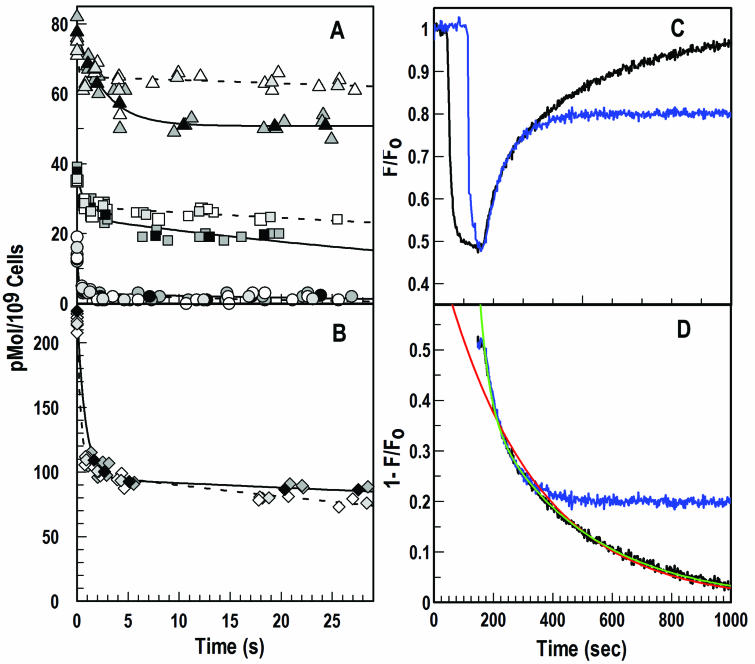
When tested by either radioisotopic or spectroscopic methods, the release of bound FeEnt from FepA displayed biphasic kinetics. The rates of these dissociations did not fit a single exponential decay process but agreed well with a double exponential decay (Fig. 5). We used 59FeEnt to characterize the release reaction in FepA-deficient bacteria (KDF541), in fepA+ strains (BN1071 and KDF541/pITS449), and in strain KDF541 expressing the aromatic substitution mutant fepAY272AF329A (16). A postadsorption wash with LiCl removed about 20 pM/109 cells, which was not bound to FepA: we observed its release from KDF541 (fepA), bringing cell-associated radioactivity to background levels. LiCl washing of cells expressing FepA from the chromosome or plasmids resulted in retention of 59FeEnt at 30 or 70 pMol/109 cells, respectively. However, the inclusion of excess nonradioactive FeEnt (50 μM) in the LiCl wash buffer released additional 59FeEnt from its specific association with FepA (10 to 15 pMol/109 cells). Most relevant to our study, the presence of FeEnt in the wash buffer did not release additional 59FeEnt from the double aromatic substitution mutant FepAY272AF329A: washing this strain in the absence or presence of FeEnt always decreased the amount of bound 59FeEnt to the same level. Comparison of the FeEnt release rates from the various strains indicated that the first, rapid phase of the discharge from FepA (Fig. 6) occurred with a kinetic constant k2 ≈ 2.5 s−1; the second stage of the dissociation was much slower (k4 ≈ 0.005 s−1).
FIG. 5.
Measurements of FeEnt dissociation from FepA and FepAY272AF329A. Light gray symbols represent data derived from bacteria washed with LiCl; the mean of these data is plotted with an open symbol, and the fitted curve (double exponential decay) from these data is shown with a dashed line. Dark gray symbols represent data derived from bacteria washed with 50 μM FeEnt; the mean of these data is plotted with a black symbol, and the fitted curve (double exponential decay) from these data is shown with a solid line. (A) Release of 59FeEnt from KDF541 (circles, fepA), BN1071 (squares, chromosomal fepA+), KDF541/pITS449 (triangles, plasmid fepA+). (B) Release of 59FeEnt from KDF541/pfepAY272AF329A (diamonds). (C and D) KDF571 (tonB)/pfepAS271C was labeled with fluorescein maleimide, and the release of FeEnt in the presence of KDF571/pITS449 (blue) or KDF541/pITS449 (black) was measured spectrophotometrically. The red and green curves derive from nonlinear fits of the release data in the presence of KDF541/pITS449 by using equations for single or double exponential decays, respectively.
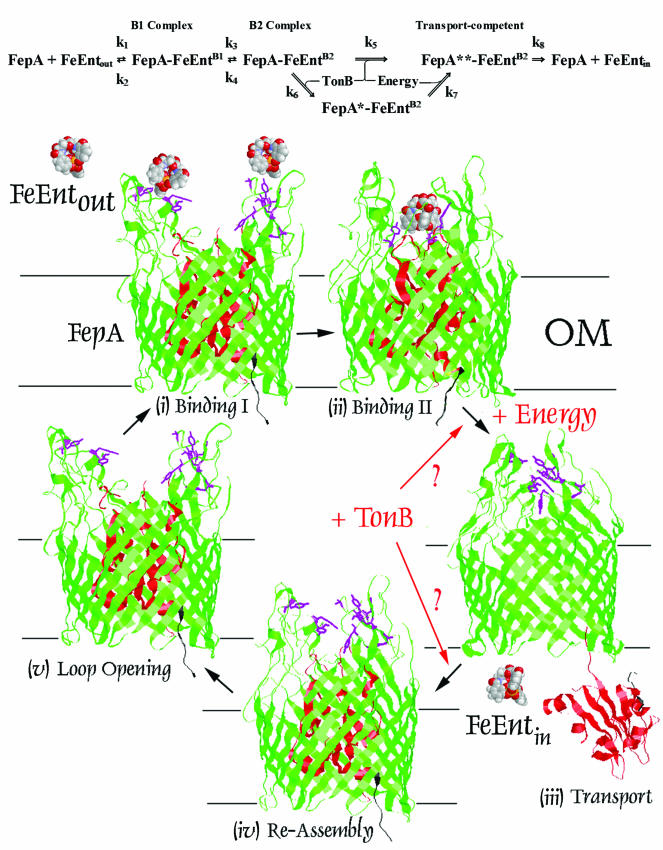
FIG. 6.
Model of FeEnt transport through FepA. (Top) Formal representation of the FepA transport process. Constants k1 to k4 are experimentally defined: fluors attached to L3 reflect both the first and second binding stages (k1 = 0.02/s and k3 = 0.005/s) (53), which are both reversible (k2 = 0.03/s and k4 = 0.003/s [the present study]). (Bottom) The FepA transport cycle is depicted as a series of conformational stages (17, 35, 60) that result in binding and internalization of FeEnt. The representations of FepA originated from its crystal structure, but they are postulated forms that were not crystallographically demonstrated. By analogy to FhuA and FecA, FeEnt binding may relocate the TonB-box region of FepA away from the β-barrel wall. Such movement may signal receptor occupancy to TonB (3-8, 10, 13-15, 18, 23-25, 39, 54, 55), but another view is that TonB-box movement away from the barrel wall frees the N-domain to dislodge from the channel. Next, the ligand passes through the C-domain channel (Transport). Theory and experiment suggest, but so far do not explicitly prove, that the input of energy is required at this stage. Similarly, TonB may or may not function during this phase of the transport reaction. A variety of findings raise the possibility that the N domain exits the pore during ligand uptake (35), but this idea is not fully substantiated: structural changes that facilitate ligand transport may take place in the N domain while it is resident in the channel. After transport the receptor reassembles, either by reinsertion of the N domain into the β-barrel, or by structural changes in situ within the pore, another potential phase for the input of energy and/or TonB. Lastly, the loops reopen to a state of maximum receptivity toward ligands.
Fluorescence measurements of the dissociation process showed comparable results.
This method did not allow examination of the effect of free FeEnt on the dissociation reaction, but the spectroscopic technique more accurately revealed the kinetics of ligand release. The first, rapid phase of FeEnt discharge occurred with a kinetic constant k2 of ≈0.03 s−1, and the second stage of dissociation was 10-fold slower (k4 ≈ 0.003 s−1). Although the rates differed, especially in the first phase, from those that we recorded by the radioisotopic method, they also showed a double exponential decay process. The standard errors for the spectroscopic calculations of k2 were less than 3%, and for k4 the standard errors were less than 0.5%. The discrepancy between the two techniques likely derives from the inherent inaccuracy of manual measurements of the first, rapid stage of FeEnt dissociation from FepA. From the overall affinity of the FeEnt-FepA equilibrium (Fig. 6; Kd = 2 × 10−10 M) (16, 17, 51) and the dissociation rate that we measured (k2 = 0.03/s), a consistent value arose for the rate at which the ferric siderophore initially encounters FepA (k1 = 1.5 × 108/s).
DISCUSSION
Radioisotopic assays of ferric siderophore binding to FepA previously found subnanomolar affinity between the ligands and the receptor in vivo (51). The Kd values from these experiments derived from quantitation of the bound ligand at equilibrium, which occurs in seconds under physiological conditions. Prior to encounters with ligands the loops of FepA, which are flexible (11), likely transpose between different conformations (53, 60, 61), and during ligand binding FepA undergoes two stages of motion that imply the existence of B1 and B2 (16). Appropriate contact with FeEnt (or colicins) presumably initiates conformational changes, driven by multiple noncovalent bonds that close the loops around the ligand, maximizing its retention on the cell surface (35). The data reported here demonstrate that, after the binding equilibrium stage, in the absence of transport, ligand dissociation occurs in a way that reproduces the two stages of association. Thus, the binding interaction between ligand and receptor, which is independent of the overall requirements for TonB and energy, is fully reversible (Fig. 6). The structural changes in the loops that occur when FeEnt binds revert when it dissociates. As observed during association (16), the elimination of Y272 and F329 changed the rapid phase but not the slow phase of dissociation. When washed with LiCl, the bacteria rapidly released nonspecifically bound 59FeEnt. The residual, specifically bound 59FeEnt was divisible into two populations, one of which was susceptible to release by washing with FeEnt (BN1071, ∼8 pmol/109 cells; KDF541/pITS449, ∼15 pmol/109 cells). The other subpopulation (BN1071, ∼22 pmol/109 cells; KDF541/pITS449, ∼55 pmol/109 cells) was resistant to release by washing. The former, loosely bound ferric siderophore was complexed by aromatic residues of the loops, because it was absent in bacteria expressing FepAY272AF329A. These data define the first binding site, which was not apparent from crystallography (11), and reprise the concept of two separate sites in the loops, through which the ligand progresses as it associates with FepA before transport.
Siderophore receptors exhibit different degrees of specificity in the reception of metal complexes. FhuA does not transport ferric catecholates but recognizes a spectrum of ferric hydroxamates with variant structures, including ferrichrome, ferricrocin, ferrichrysin, ferrirubin, ferrirhodin, and albomycin (40); it also transports the (nonmetal) rifamycin antibiotic CGP4832 (26, 56). Our results showed that conversely, FepA rejects not only hydroxamate compounds such as ferrichrome but also dicatecholates such as ferric agrobactin, and it discriminates among the three pure catecholate compounds FeEnt, FeTrn, and FeCrn. Our experiments provided some insight into the basis of FepA's recognition specificity. The conformational mobility of FepA's surface loops in vivo (61), their hydrophobic surfaces, and the aromaticity of the vestibule mouth (Fig. 2) are potentially relevant to ligand adsorption. One possible binding mechanism is that FepA initially adsorbs (hydrophobic) iron complexes by nonspecific interactions with the hydrophobic loop residues and then rejects inappropriate metal chelates as the binding equilibrium progresses further. However, the complete inability of ferrichrome and ferric agrobactin to inhibit 59FeEnt adsorption suggests that binding does not begin in this manner. The first measurable stage of ferric siderophore binding has specificity, in that it was only impaired by ferric catecholate complexes that are structurally similar to FeEnt. This specificity likely derives from the aromatic amino acids in the B1 region of the vestibule mouth and does not involve the hydrophobic residues on its surface (see also below).
The selectivity of FepA for FeEnt provides more information about the determinants of ligand recognition. Ent, Trn, and Crn all form tricatecholate, hexadentate, octahedral complexes with iron(III), but they differ in size and in the organic platforms from which their ligands arise. Unlike FeEnt, which has a trilactone backbone, the synthetic compound FeTrn contains three alkyl chains linked to a central amine that connect by amide bonds to its dihydroxybenzoic acid groups. The backbone of FeTrn (604 Da) has a backbone that is smaller than that of FeEnt, and with a pKa of 5 its central amine is essentially unprotonated at neutrality (30). The 50-fold reduction in the affinity of FeTrn binding, and 20-fold reduction in the affinity of its transport indicate that the face of its iron center, which is identical to that of FeEnt, is not the only determinant of its recognition by FepA. The structural alterations behind the iron center impair its binding, relative to that of FeEnt. The six oxygens of the natural siderophore's lactone ring provide many potential H-bond acceptors that are absent in FeTrn, suggesting a reason for its lower affinity. These data concur with the model (Fig. 6) in which the receptor's loops enwrap the ferric siderophore at binding equilibrium and thereby interact with the backbone.
Different considerations may explain the failure of FeCrn to bind to FepA. Both FeEnt and FeCrn have a cyclic ester backbone, formed by serine and threonine, respectively, from which dihydroxybenzoic acid moieties project. The latter siderophore also contains glycine spacer groups between the backbone and the chelating groups. These differences impart a 30% greater molecular mass to FeCrn, relative to FeEnt (933 versus 719 Da). Besides its greater mass, the metal center of FeCrn has opposite chirality (Λ) to that of FeEnt (Δ) and FeTrn (Δ) (57). However, although FepA recognizes the iron center of ferric siderophores (20, 31), chirality does not govern the receptor-ligand interaction: (Λ) ferric enantio-Ent with comparable affinity as FeEnt (64). Thus, the rejection of 59FeCrn was surprising and implied that its increased dimensions preclude productive adsorption to the receptor. The partial inhibition of 59FeEnt binding by FeCrn and the nonretention of 59FeCrn by cells expressing FepA suggest that the gram-positive siderophore begins to bind but does not reach a stable equilibrium, supporting the idea that its larger size prevents loop closure and hence its passage from B1 to B2.
The aromatic (tricatecholate) nature of FeEnt, combined with the aromatic residues in the mouth of the vestibule suggested a rationale for binding affinity: hydrophobic bonds and/or ring stacking interactions in the first binding stage. Our mutagenesis experiments supported this idea in that Ala substitutions for Tyr in the loop extremities affected both the kinetic and the thermodynamic parameters of the binding reaction. This initial binding event may require a specific configuration of aromatic rings in the loops that complement the symmetrical geometry of the catecholate groups of FeEnt, or it may involve less specific interactions between the ring systems: our results do not discriminate these alternatives. However, the mutagenesis data showed that interactions may occur with multiple loops and that L7, which contains five aromatic residues and K483 (61), is central to ligand adsorption. The facts that deletion of L7 inactivates FepA (51), that L7 closes when FeEnt binds (60), and that single substitutions in L7 severely affect both binding (Y481A) and transport (Y478A and Y495A) agree with this conclusion. However, elimination of residues in other loops moderately (Y272 in L3 [16]; Y540 and Y553 in L8) to severely (F329 in L4 [16]; Y638 in L10) diminished binding and or transport efficiency, indicating that interactions of FeEnt with FepA encompass multiple determinants. Finally, ionic interactions with the acidic ferric siderophore comprise another component of binding and perhaps transport: basic residues that affect affinity occur in both B1 (K483) (61) and B2 (R316) (50), and a cluster of four potentially important arginines exists at the top of the N-domain (R66, R98, R102, and R105) (Fig. 2).
After the stage of binding equilibrium, when the surface loops surround the metal complex at the top of the N-domain loops, the closed transmembrane channel opens and FeEnt passes into the periplasm. The relationship between the binding and transport phenotypes of the three mutant classes begins to illuminate this currently inscrutable uptake process. In class I mutants, the equivalent increases in binding Kd and transport Km suggest that the Ala for Tyr substitutions increase koff (originating from k2 and k4 in Fig. 6). That is, these mutations necessitate higher concentrations of FeEnt to achieve thermodynamic saturation, but they do not alter the rate-limiting transport step. In class II mutants binding affinity sometimes also decreased, but a disproportionate increase in transport Km suggested more important kinetic effects on the rate-limiting stage of uptake. That is, these mutations altered kin (originating from either k5 and k8 or from k6, k7, and k8 in Fig. 6) such that higher FeEnt concentrations were needed to saturate the overall uptake rate. Y478A and Y638A created prominent examples of this effect, which was unexpected from their positions in the loops above the channel. These data underscore our incomplete understanding of the transport process: loop residues may affect uptake, but their mechanistic contributions are unexplained and enigmatic. Likewise, Y495A created the class III phenotype: normal affinity for FeEnt, but a much reduced transport rate. In this case again, a surface loop mutation changed the efficiency of ligand uptake.
The exact function or mechanism of the first 150 residues of siderophore receptors is unknown, and we use the term “N-terminal globular domain” only because it correctly describes the protein structure. The terms “cork” and “plug” (11, 25, 39) suggest that its purpose is to obstruct the barrel. This is likely a misnomer, in that the globular domain may perform the opposite function, movement of the ligand to the periplasm. The N-domain mutations, W101A and two loop deletions, produced large class II effects, that support this conclusion. Prior experiments suggested that the N terminus is less important in ligand binding than the surface loops (60), and these current results indicate that the N-domain participates in the rate-limiting stage of transport, because all three mutations greatly increased the transport Km.
Acknowledgments
We thank Carla Taddei, Melissa Simoes, Simone Alves, and Tie Koide for constructing fepAΔ60-67 and Alexandre Moutran, Marcio Lasaro and Solange Nunes for constructing fepAΔ98-105 during the American Society for Microbiology-sponsored International Training Program for Latin America: “Molecular Biological Approaches to Bacterial Cell Wall Biochemistry,” in April 2002 at Departamento de Microbiologia, Universidade de Sao Paulo, Sao Paulo, Brasil. We thank Yi Shao and Wallace Kaserer for help in the preparation of Crn, Marj Montague for excellent technical assistance, Paul Cook and Wallace Kaserer for helpful discussions, and Alain Charbit for critiquing the manuscript.
This study was supported by OCAST grant 00072 to SMCN, NIH grant GM53836 to P.E.K., INSERM support to S.M.C.N. and P.E.K., and contributions of the U.S. Department of State and J. William Fulbright Foundation to P.E.K.
REFERENCES
- 1.Ames, G. F. 1974. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs: membrane, soluble, and periplasmic fractions. J. Biol. Chem. 249:634-644. [PubMed] [Google Scholar]
- 2.Armstrong, S. K., C. L. Francis, and M. A. McIntosh. 1990. Molecular analysis of the Escherichia coli ferric enterobactin receptor FepA. J. Biol. Chem. 265:14536-14543. [PubMed] [Google Scholar]
- 3.Braun, M., H. Killmann, and V. Braun. 1999. The β-barrel domain of FhuAΔ5-160 is sufficient for TonB-dependent FhuA activities of Escherichia coli. Mol. Microbiol. 33:1037-1049. [DOI] [PubMed] [Google Scholar]
- 4.Braun, V. 1989. The structurally related exbB and tolQ genes are interchangeable in conferring tonB-dependent colicin, bacteriophage, and albomycin sensitivity. J. Bacteriol. 171:6387-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun, V. 1997. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch. Microbiol. 167:325-331. [DOI] [PubMed] [Google Scholar]
- 6.Braun, V., J. Frenz, K. Hantke, and K. Schaller. 1980. Penetration of colicin M into cells of Escherichia coli. J. Bacteriol. 142:162-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun, V., S. Gaisser, C. Herrmann, K. Kampfenkel, H. Killmann, and I. Traub. 1996. Energy-coupled transport across the outer membrane of Escherichia coli: ExbB binds ExbD and TonB in vitro, and leucine 132 in the periplasmic region and aspartate 25 in the transmembrane region are important for ExbD activity. J. Bacteriol. 178:2836-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun, V., K. Gunter, and K. Hantke. 1991. Transport of iron across the outer membrane. Biol. Met. 4:14-22. [DOI] [PubMed] [Google Scholar]
- 9.Braun, V., R. E. Hancock, K. Hantke, and A. Hartmann. 1976. Functional organization of the outer membrane of Escherichia coli: phage and colicin receptors as components of iron uptake systems. J. Supramol. Struct. 5:37-58. [DOI] [PubMed] [Google Scholar]
- 10.Braun, V., H. Killmann, and R. Benz. 1994. Energy-coupled transport through the outer membrane of Escherichia coli small deletions in the gating loop convert the FhuA transport protein into a diffusion channel. FEBS Lett. 346:59-64. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6:56-63. [DOI] [PubMed] [Google Scholar]
- 12.Bullen, J. J., H. J. Rogers, and E. Griffiths. 1978. Role of iron in bacterial infection. Curr. Top. Microbiol. Immunol. 80:1-35. [DOI] [PubMed] [Google Scholar]
- 13.Cadieux, N., C. Bradbeer, and R. J. Kadner. 2000. Sequence changes in the Ton box region of BtuB affect its transport activities and interaction with TonB protein. J. Bacteriol. 182:5954-5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cafiso, D. S., and W. L. Hubbell. 1983. Electrogenic H+/OH− movement across phospholipid vesicles measured by spin-labeled hydrophobic ions. Biophys. J. 44:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cafiso, D. S., and W. L. Hubbell. 1982. Transmembrane electrical currents of spin-labeled hydrophobic ions. Biophys. J. 39:263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao, Z., Z. Qi, C. Sprencel, S. M. Newton, and P. E. Klebba. 2000. Aromatic components of two ferric enterobactin binding sites in Escherichia coli fepA. Mol. Microbiol. 37:1306-1317. [DOI] [PubMed] [Google Scholar]
- 17.Cao, Z., P. Warfel, S. M. Newton, and P. E. Klebba. 2003. Spectroscopic observations of ferric enterobactin transport. J. Biol. Chem. 278:1022-1028. [DOI] [PubMed] [Google Scholar]
- 18.Chimento, D. P., A. K. Mohanty, R. J. Kadner, and M. C. Wiener. 2003. Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat. Struct. Biol. 10:394-401. [DOI] [PubMed] [Google Scholar]
- 19.Cornelissen, C. N., and P. F. Sparling. 1994. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol. Microbiol. 14:843-850. [DOI] [PubMed] [Google Scholar]
- 20.Ecker, D. J., B. F. Matzanke, and K. N. Raymond. 1986. Recognition and transport of ferric enterobactin in Escherichia coli. J. Bacteriol. 167:666-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emery, T. 1971. Role of ferrichrome as a ferric ionophore in Ustilago sphaerogena. Biochemistry 10:1483-1488. [DOI] [PubMed] [Google Scholar]
- 22.Fanucci, G. E., K. A. Coggshall, N. Cadieux, M. Kim, R. J. Kadner, and D. S. Cafiso. 2003. Substrate-induced conformational changes of the periplasmic N terminus of an outer-membrane transporter by site-directed spin labeling. Biochemistry 42:1391-1400. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson, A. D., and J. Deisenhofer. 2002. TonB-dependent receptors-structural perspectives. Biochim. Biophys. Acta 1565:318-332. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson, A. D., J. Kodding, G. Walker, C. Bos, J. W. Coulton, K. Diederichs, V. Braun, and W. Welte. 2001. Active transport of an antibiotic rifamycin derivative by the outer-membrane protein FhuA. Structure 9:707-716. [DOI] [PubMed] [Google Scholar]
- 27.Fiss, E. H., P. Stanley-Samuelson, and J. B. Neilands. 1982. Properties and proteolysis of ferric enterobactin outer membrane receptor in Escherichia coli K-12. Biochemistry 21:4517-4522. [DOI] [PubMed] [Google Scholar]
- 28.Hancock, R. W., and V. Braun. 1976. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J. Bacteriol. 125:409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto-Gotoh, T., F. C. Franklin, A. Nordheim, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene 16:227-235. [DOI] [PubMed] [Google Scholar]
- 30.Hay, B. P. D., D. A. Vargas, R. Garza, J., and K. N. Raymond. 2001. Structural criteria for the rational design of selective ligands. 3. Quantitative structure-stability relationship for iron(III) complexation by Tris-catecholamide siderophores. Inorg. Chem. 40:3922-3935. [DOI] [PubMed] [Google Scholar]
- 31.Heidinger, S., V. Braun, V. L. Pecoraro, and K. N. Raymond. 1983. Iron supply to Escherichia coli by synthetic analogs of enterochelin. J. Bacteriol. 153:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollifield, W. C., Jr., and J. B. Neilands. 1978. Ferric enterobactin transport system in Escherichia coli K-12: extraction, assay, and specificity of the outer membrane receptor. Biochemistry 17:1922-1928. [DOI] [PubMed] [Google Scholar]
- 33.Jiang, X., M. A. Payne, Z. Cao, S. B. Foster, J. B. Feix, S. M. Newton, and P. E. Klebba. 1997. Ligand-specific opening of a gated-porin channel in the outer membrane of living bacteria. Science 276:1261-1264. [DOI] [PubMed] [Google Scholar]
- 34.Jurado, R. L. 1997. Iron, infections, and anemia of inflammation. Clin. Infect. Dis. 25:888-895. [DOI] [PubMed] [Google Scholar]
- 35.Klebba, P. E. 2003. Three paradoxes of ferric enterobactin uptake. Front. Biosci. 8:1422-1436. [DOI] [PubMed] [Google Scholar]
- 36.Klebba, P. E., M. A. McIntosh, and J. B. Neilands. 1982. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J. Bacteriol. 149:880-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konopka, K., and J. B. Neilands. 1984. Effect of serum albumin on siderophore-mediated utilization of transferrin iron. Biochemistry 23:2122-2127. [DOI] [PubMed] [Google Scholar]
- 38.Kronvall, G., H. M. Grey, and R. C. Williams, Jr. 1970. Protein A reactivity with mouse immunoglobulins. Structural relationship between some mouse and human immunoglobulins. J. Immunol. 105:1116-1123. [PubMed] [Google Scholar]
- 39.Locher, K. P., B. Rees, R. Koebnik, A. Mitschler, L. Moulinier, J. P. Rosenbusch, and D. Moras. 1998. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95:771-778. [DOI] [PubMed] [Google Scholar]
- 40.Luckey, M., J. R. Pollack, R. Wayne, B. N. Ames, and J. B. Neilands. 1972. Iron uptake in Salmonella typhimurium: utilization of exogenous siderochromes as iron carriers. J. Bacteriol. 111:731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.May, J. J., T. M. Wendrich, and M. A. Marahiel. 2001. The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2,3-dihydroxybenzoate-glycine-threonine trimeric ester bacillibactin. J. Biol. Chem. 276:7209-7217. [DOI] [PubMed] [Google Scholar]
- 42.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 43.Murphy, C. K., V. I. Kalve, and P. E. Klebba. 1990. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J. Bacteriol. 172:2736-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakae, T. 1976. Outer membrane of Salmonella: isolation of protein complex that produces transmembrane channels. J. Biol. Chem. 251:2176-2178. [PubMed] [Google Scholar]
- 45.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neilands, J. B. 1981. Iron absorption and transport in microorganisms. Annu. Rev. Nutr. 1:27-46. [DOI] [PubMed] [Google Scholar]
- 47.Neilands, J. B. 1974. Iron and its role in microbial physiology, p. 4-34. In J. B. Neilands (ed.), Microbial iron metabolism: a comprehensive treatise, vol. 1. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 48.Neilands, J. B. 1981. Microbial iron compounds. Annu. Rev. Biochem. 50:715-731. [DOI] [PubMed] [Google Scholar]
- 49.Neilands, J. B., T. Peterson, and S. A. Leong. 1980. High-affinity iron transport in microorganisms, p. 263-278. In A. E. Martell (ed.), Inorganic chemistry in biology and medicine, vol. 140. ACS Symposium Series. American Chemical Society, Washington, D.C.
- 50.Newton, S. M., J. S. Allen, Z. Cao, Z. Qi, X. Jiang, C. Sprencel, J. D. Igo, S. B. Foster, M. A. Payne, and P. E. Klebba. 1997. Double mutagenesis of a positive charge cluster in the ligand-binding site of the ferric enterobactin receptor, FepA. Proc. Natl. Acad. Sci. USA 94:4560-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newton, S. M., J. D. Igo, D. C. Scott, and P. E. Klebba. 1999. Effect of loop deletions on the binding and transport of ferric enterobactin by FepA. Mol. Microbiol. 32:1153-1165. [DOI] [PubMed] [Google Scholar]
- 52.Ong, S. A., T. Peterson, and J. B. Neilands. 1979. Agrobactin, a siderophore from Agrobacterium tumefaciens. J. Biol. Chem. 254:1860-1865. [PubMed] [Google Scholar]
- 53.Payne, M. A., J. D. Igo, Z. Cao, S. B. Foster, S. M. Newton, and P. E. Klebba. 1997. Biphasic binding kinetics between FepA and its ligands. J. Biol. Chem. 272:21950-21955. [DOI] [PubMed] [Google Scholar]
- 54.Postle, K. 1993. TonB and the gram-negative dilemma. Mol. Microbiol. 4:2019-2025. [DOI] [PubMed] [Google Scholar]
- 55.Postle, K. 1993. TonB protein and energy transduction between membranes. J. Bioenerg. Biomembr. 25:591-601. [DOI] [PubMed] [Google Scholar]
- 56.Pugsley, A. P., W. Zimmerman, and W. Wehrli. 1987. Highly efficient uptake of a rifamycin derivative via the FhuA-TonB-dependent uptake route in Escherichia coli. J. Gen. Microbiol. 133:3505-3511. [DOI] [PubMed] [Google Scholar]
- 57.Raymond, K. N., E. A. Dertz, and S. S. Kim.2003. Enterobactin: an archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 100:3584-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rutz, J. M., T. Abdullah, S. P. Singh, V. I. Kalve, and P. E. Klebba. 1991. Evolution of the ferric enterobactin receptor in gram-negative bacteria. J. Bacteriol. 173:5964-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rutz, J. M., J. Liu, J. A. Lyons, J. Goranson, S. K. Armstrong, M. A. McIntosh, J. B. Feix, and P. E. Klebba. 1992. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science 258:471-475. [DOI] [PubMed] [Google Scholar]
- 60.Scott, D. C., Z. Cao, Z. Qi, M. Bauler, J. D. Igo, S. M. Newton, and P. E. Klebba. 2001. Exchangeability of N termini in the ligand-gated porins of Escherichia coli. J. Biol. Chem. 276:13025-13033. [DOI] [PubMed] [Google Scholar]
- 61.Scott, D. C., S. M. Newton, and P. E. Klebba. 2002. Surface loop motion in FepA. J. Bacteriol. 184:4906-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sebestyen, G., G. Maggipinto, and A. Andre. 1986. Determination of anti-tetanus antibodies using protein A labeled with iodine 125. Rev. Fr. Transfus. Immunohematol. 29:355-376. (In French.) [DOI] [PubMed] [Google Scholar]
- 63.Sprencel, C., Z. Cao, Z. Qi, D. C. Scott, M. A. Montague, N. Ivanoff, J. Xu, K. M. Raymond, S. M. Newton, and P. E. Klebba. 2000. Binding of ferric enterobactin by the Escherichia coli periplasmic protein FepB. J. Bacteriol. 182:5359-5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thulasiraman, P., S. M. Newton, J. Xu, K. N. Raymond, C. Mai, A. Hall, M. A. Montague, and P. E. Klebba. 1998. Selectivity of ferric enterobactin binding and cooperativity of transport in gram-negative bacteria. J. Bacteriol. 180:6689-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagegg, W., and V. Braun. 1981. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein FecA. J. Bacteriol. 145:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wayne, R., K. Frick, and J. B. Neilands. 1976. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J. Bacteriol. 126:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wayne, R., and J. B. Neilands. 1975. Evidence for common binding sites for ferrichrome compounds and bacteriophage φ80 in the cell envelope of Escherichia coli. J. Bacteriol. 121:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wookey, P., and H. Rosenberg. 1978. Involvement of inner and outer membrane components in the transport of iron and in colicin B action in Escherichia coli. J. Bacteriol. 133:661-666. [DOI] [PMC free article] [PubMed] [Google Scholar]