Abstract
γ-Butyrolactone autoregulator receptors of the genus Streptomyces have a common activity as DNA-binding transcriptional repressors, controlling secondary metabolism and/or morphological differentiation. A gene encoding a γ-butyrolactone autoregulator receptor was cloned from a bafilomycin B1 producer, Kitasatospora setae, for the first time from a non-Streptomyces genus of actinomycetes, and its function was evaluated by in vitro and in vivo analyses. The gene fragment was initially cloned by PCR with primers designed from two highly conserved regions of Streptomyces autoregulator receptors (BarA, FarA, ScbR, and ArpA), followed by genomic Southern hybridization yielding a 7-kb BamHI fragment on which a 654-bp receptor gene (ksbA) was identified. The recombinant KsbA protein demonstrated clear binding activity toward 3H-labeled autoregulators, especially toward [3H]SCB1, confirming that ksbA encodes a real autoregulator receptor of K. setae. To clarify the in vivo function of ksbA, a ksbA-disrupted strain was constructed by means of homologous recombination after introducing a ksbA disruption construct via transconjugation from Escherichia coli. No difference in morphology was found between the wild-type strain and the ksbA disruptants. However, the ksbA disruptants started producing bafilomycin 18 h earlier than the wild-type strain and showed a 2.4-fold-higher accumulation of bafilomycin. The phenotype was restored to the original wild-type phenotype by complementation with intact ksbA, indicating that the autoregulator receptor protein of K. setae acts as a primary negative regulator of the biosynthesis of bafilomycin but plays no role in cytodifferentiation of K. setae. This indicates that, unlike the A-factor receptor of Streptomyces griseus, the autoregulator receptor (ksbA) of K. setae belongs to a family of autoregulator receptors which control secondary metabolism but play no role in morphological differentiation.
The γ-butyrolactone autoregulators found in several species of the genus Streptomyces are regarded as microbial hormones that control secondary metabolism and/or morphological differentiation. The effectiveness of these autoregulators, which are active at nanomolar concentrations, as well as the presence of specific receptor proteins [BarA as a virginiae butanolide (VB)-specific receptor in Streptomyces virginiae (11, 12, 22), FarA as an IM-2-specific receptor in Streptomyces lavendulae FRI-5 (17, 29, 35), ScbR as an SCB1-specific receptor in Streptomyces coelicolor A3(2) (33), and ArpA as an A-factor-specific receptor in Streptomyces griseus (25)] as mediators of autoregulator signaling implies that these γ-butyrolactone autoregulators should be regarded as Streptomyces hormones. In vitro studies of the autoregulator receptors have indicated that these autoregulator receptors are dimeric DNA-binding proteins that, in the absence of autoregulators, recognize and bind to specific DNA sequences situated in the promoter region of target genes (13, 14, 16, 33). Autoregulator binding to the corresponding receptor causes the receptor to dissociate from the DNA, which in turn allows transcription of the target genes to occur. Although a common autoregulator-dependent cascade has not yet been completely clarified, all the autoregulator receptors share a common activity as DNA-binding transcriptional repressors.
However, while the autoregulator receptors in Streptomyces species have been well clarified, little is known about closely related genera such as the so-called non-Streptomyces actinomycetes—e.g., the genera Kitasatospora, Micromonospora, Actinoplanes, Amycolatopsis, Norcardia, and Actinomadura—which are also a rich source of medically important secondary metabolites. We have previously reported that γ-butyrolactone autoregulators and/or the receptor proteins were present in several non-Streptomyces actinomycetes (Kitasatospora setae IFO14216, Actinoplanes teichomyceticus IFO13999, Amycolatopsis mediterranei IFO13415, and Micromonospora echinospora IFO13250) (3, 4). In the present study, a γ-butyrolactone autoregulator receptor gene of K. setae was cloned for the first time from the genera of non-Streptomyces actinomycetes. K. setae is a producer of bafilomycin B1, a macrolide antibiotic serving as an important inhibitor of vacuolar H+-ATPase (24, 26). The in vitro and in vivo function of the autoregulator receptor was thoroughly investigated by in vitro characterization of a recombinant receptor protein, and by phenotypic comparison between the wild-type and the disruptants of the receptor gene, revealing that the autoregulator receptor in K. setae functions as the primary negative regulator of the biosynthesis of bafilomycin B1.
MATERIALS AND METHODS
Bacterial strains, plasmids, culture conditions, and conjugal transfer of DNA from Escherichia coli to K. setae.
K. setae (IFO14216; Institute for Fermentation, Osaka [IFO], Osaka, Japan) was used in the present study and was grown at 28°C in an ISP2 medium (Difco, Detroit, Mich.). For genetic manipulation in E. coli, strain DH5α (6) was used. pUC19 was used for the construction of a genomic library and for DNA sequencing. DNA manipulations in E. coli and in K. setae were performed as described by Sambrook and Russell (30) and Kieser et al. (10), respectively. For the seed culture preparation, 20 ml of the medium containing 1% glucose, 2% starch, 0.5% yeast extract, 0.5% peptone, and 0.4% CaCO3 (pH 7.0) (24) in 100-ml Erlenmeyer flasks was inoculated with spores (5 × 105 spores/ml of medium) and incubated at 28°C for 36 h on a reciprocating shaker (120 strokes per min). The main cultivation was performed by inoculating 1.4 ml of the seed culture into 70 ml of ISP2 medium in 500-ml Erlenmeyer flasks, followed by incubation at 28°C. The main cultivation was carried out for 12 h for preparation of cell extract and for 4 days for bafilomycin B1 assay. Conjugal DNA transfer into K. setae was performed as described by Choi et al. (4). The methylation-deficient E. coli strain ET12567 (dam-13::Tn9 dcm-6 hsdM hsdS) containing pUZ8002 was used as the donor in intergeneric conjugations (15, 27, 32). pUZ8002 is an RK2 derivative with a defective oriT (aph); it is not self-transmissible but supplies a mobilization function for oriT-containing plasmids in trans. A homologous recombination vector, pKC1132 (3.5 kb) (2), containing oriT of RK2 and an apramycin resistance gene for selection in actinomycetes and E. coli, and a site-specific integration vector, pSET152 (5.5 kb) (2), containing φC31 int and attP, were used for construction of the deletion mutants and the complemented strains, respectively. These plasmids do not contain replicative functions of actinomycete plasmids and can be maintained in recipient strains only in the chromosomally integrated state.
Manipulation of DNA and molecular cloning of ksbA.
Total DNA of K. setae was obtained by the method of Rao et al. (28). To clone an autoregulator receptor gene from K. setae, the degenerate primers AF-V (5′-CGCGGATCCGCSGCSGCSNNNGTSTTCGA-3′) and AR-1 (5′-CGCGGATCCGAAGTGGAAGTASAGSGCSCC-3′) were used (the underlined nucleotides were added to introduce a BamHI site for cloning) (Fig. 1). Southern blot hybridization was performed as described previously (35). A partial genomic library was constructed with size-fractionated BamHI fragments (7 kb) and pUC19, using E. coli DH5α as a host, and screened by colony hybridization with the 32P-labeled 102-bp PCR fragment (see Fig. 5). The DNA sequencing was carried out by the dideoxy-chain termination method (31) for both strands, using a sequencing kit (Amersham, Tokyo, Japan) and Cy5-labeled primers on a fluorescence DNA sequencer (ALFred; Pharmacia Biotech, Tokyo, Japan). Sequence analyses and homology comparisons were done on a personal computer with the GENETYX software package (Software Development Co., Ltd., Tokyo, Japan). To construct pUC19-ksbA, the coding region of ksbA was amplified by PCR. PCR was performed with primer 1 (5′-CGCGGATCCCCATGGCGGAATCCCCGCGCGCGGCCAAG-3′) and primer 2 (5′-CGCGGATCCAACCGCCCTCCAGGACGGC-3′) to generate a BamHI site at the 5′ and 3′ ends of the ksbA coding sequence, respectively (underlined). After digestion with BamHI, a 710-bp BamHI fragment was cloned into BamHI-digested pUC19, resulting in pUC19-ksbA. A 502-bp BssHII-AccIII part in the 710-bp BamHI PCR fragment was replaced with a 502-bp BssHII-AccIII fragment from the cloned 7-kb BamHI fragment to minimize nucleotide changes during the PCR. The nucleotide sequence of the ksbA region in pUC19-ksbA was confirmed by DNA sequencing.
FIG. 1.
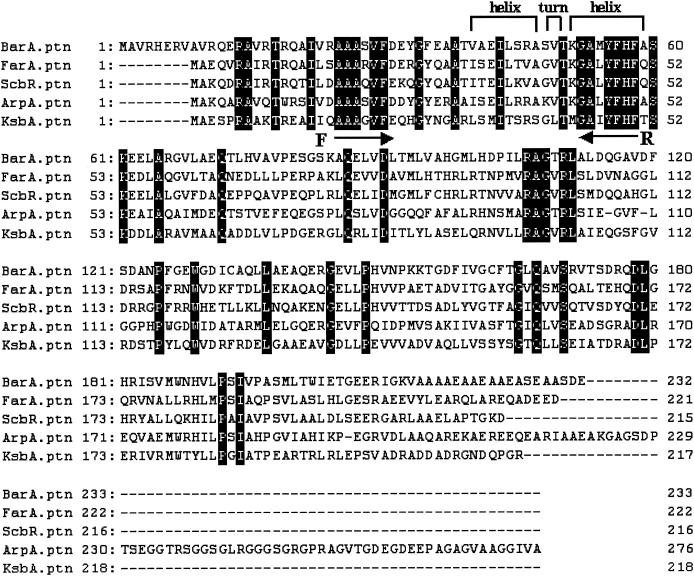
Alignment of overall amino acid sequences of BarA, FarA, ScbR, ArpA, and KsbA. Identical amino acids are indicated by black boxes. Facing arrows represent the regions of designed primers (F, AF-V; R, AR-1). BarA, VB-specific receptor (BarA) of S. virginiae (22); FarA, IM-2- specific receptor (FarA) of S. lavendulae FRI-5 (35); ScbR, SCB1 receptor (ScbR) of S. coelicolor A3(2) (33); ArpA, A-factor receptor (ArpA) of S. griseus (25); KsbA, KsbA of K. setae (this study).
FIG. 5.
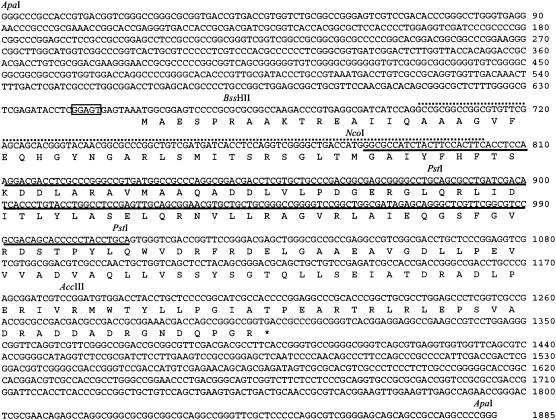
Nucleotide and deduced amino acid (one-letter notation) sequences of ksbA. The nucleotide numbering begins at the 5′-most ApaI site of the 1,885-bp ApaI fragment in the cloned 7-kb BamHI fragment. An asterisk denotes a translational stop codon. A probable ribosome-binding sequence, GGAGT (nucleotides 643 to 647), is present six nucleotides upstream of the putative ATG start codon (boxed). Underlining indicates the deleted nucleotide sequences of ksbA for construction of the ksbA deletion mutant. The regions amplified by the initial PCR with the designed primers are shown by upper dotted lines.
Preparation of cell extract.
The K. setae cells cultured for 12 h or recombinant E. coli cells cultured for 6 h were collected by centrifugation (6,000 × g) for 10 min at 4°C and washed with cold 0.9% NaCl. Wet cells (1 g) were suspended in 3 ml of buffer A (0.05 M triethanolamine-HCl [pH 7], 0.3 M KCl, 20% glycerol, 5 mM dithiothreitol, and 0.1 mM p-amidinophenylmethanesulfonyl fluoride [p-APMSF]) and disrupted by sonication (30 s × 6) at 4°C. Cell debris was removed by centrifugation (22,000 × g, 20 min) at 4°C, and the supernatant was used as crude cell extract to assay autoregulator-binding activity.
Assay of autoregulator-binding activity and protein.
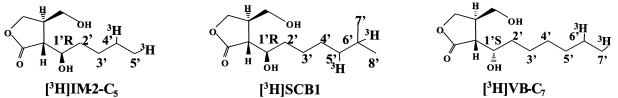
Autoregulator-binding activity was assayed in the presence of 3H-labeled autoregulator (ca. 880,000 dpm/assay) (73 nM [3H]VB-C7 [54.6 Ci/mmol], 100 nM [3H]IM-2-C5 [40 Ci/mmol], and 86 nM of [3H]SCB1 [46.3 Ci/mmol]). The autoregulator-binding assay was performed as described by Kim et al. (11). The activity was measured as the specific binding of each 3H-labeled ligand from the difference of protein-bound 3H-labeled ligand in the absence and presence of nonlabeled autoregulators (125 μM VB-C [1,710-fold excess against the 3H-labeled derivative], IM-2 [1,250-fold excess], and SCB1 [1,450-fold excess]). Protein concentrations were determined by dye-binding assay using a Bio-Rad (Hercules, Calif.) protein assay kit.
Construction of a ksbA-disrupted strain and a ksbA-complemented strain.
The ksbA disruption vector was constructed as follows. To locate ksbA in the center of the fragment, a 6.03-kb SalI (2)-BamHI fragment was recovered after partial digestion with SalI, resulting in a 971-bp deletion from the left end of the 7-kb BamHI fragment (see Fig. 4A). To construct ΔksbA, the fragment was digested with NcoI and PstI to remove a 234-bp NcoI-PstI (2) fragment containing a helix-turn-helix (HTH) motif (see Fig. 5) and was ligated after reaction with T4 DNA polymerase. The entire 5.8-kb insert containing ΔksbA was recovered as a HindIII-EcoRI fragment and inserted into the HindIII and EcoRI sites of pKC1132 to give pSUC58. E. coli ET12567(pUZ8002) transformed with pSUC58 was conjugated with K. setae. Exconjugants in which the plasmid pSUC58 had presumptively integrated at the ksbA locus by a single crossover via homologous recombination were selected with apramycin. After three rounds of incubation at 28°C on ISP2 medium in the absence of apramycin, putative ksbA-disrupted strains formed from the second crossover were detected on the basis of their apramycin sensitivities. Because all the ksbA-disrupted strains showed identical behaviors, such as morphology and growth, one of the strains was chosen for detailed analyses (strain RD1) (see Fig. 4A). To complement the ksbA-disrupted strain (strain RD1), a 1.88-kb ApaI-ApaI fragment containing the entire ksbA gene with its own promoter was isolated from pKSB7 (see Fig. 4A and 5), treated with T4 DNA polymerase, and cloned into SmaI-digested pUC19. The fragment was recovered as a BamHI-EcoRI fragment and was ligated into BamHI-EcoRI-treated pSET152, generating pKSBAC. After conjugal transfer of pKSBAC from E. coli ET12567(pUZ8002) to strain RD1, apramycin-resistant exconjugants were analyzed by PCR and Southern hybridization. The probe used was a 710-bp ksbA fragment amplified by PCR from pKSB7 using primer 1 and primer 2, as described above. All the ksbA-complemented strains showed identical phenotypes, and data for the representative strain (RDC1) are shown.
FIG. 4.
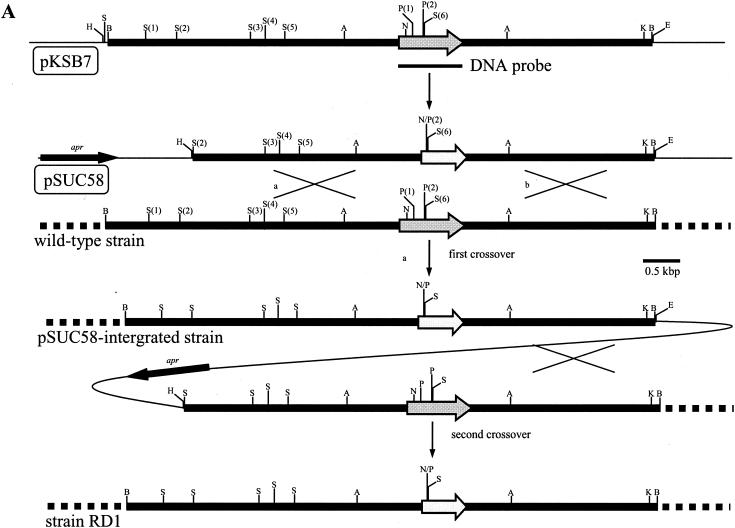
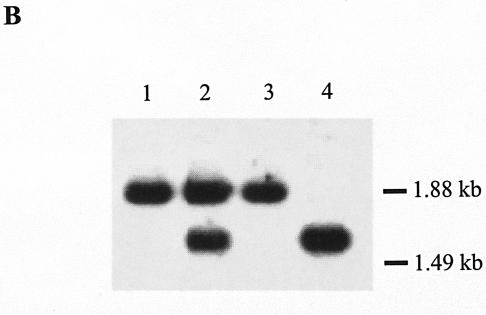
Gene replacement of the K. setae ksbA gene with mutated ksbA by homologous recombination. (A) Schematic representation of the strategy used for the disruption of ksbA. The dark gray arrows indicate the location and orientation of ksbA, and the light gray arrows represent the disrupted ksbA. apr is the apramycin resistance gene. Abbreviations: A, ApaI; B, BamHI; E, EcoRI; H, HindIII; K, KpnI; N, NcoI; P, PstI; S, SalI. For the ApaI sites, two necessary sites among the seven ApaI sites were shown to simplify the map. (B) Southern hybridization analysis of chromosomal DNA from the K. setae wild-type strain (lane 1), pSUC58-integrated strain (lane 2), wild-type segregant (lane 3), and a ksbA disruptant, strain RD1 (lane 4). The probe used was the 0.7-kbp PCR-amplified fragment containing the entire ksbA gene.
FIG. 3.
Structures of tritium-labeled autoregulator analogues (12, 29, 33) used for the binding assay.
Morphological assessment and analysis of bafilomycin B1 production.
To analyze morphological differences, spores of the wild-type strain, a ksbA-disrupted strain, RD1, and a ksbA-complemented strain, RDC1, were streaked on ISP2 medium, ISP4 medium (Nihon Seiyaku, Tokyo, Japan), IFO medium 231 (9), IFO medium 266 (3), TSB agar (Oxoid, Basingstoke, United Kingdom), R5 agar (10), oatmeal agar (7), MS agar (8), modified SMMS agar (supplemental minimum medium, solid) as described by Takano et al. (34), and minimal medium agar containing 0.5% (wt/vol) mannitol as a carbon source (10), and this was followed by cultivation at 28°C for 14 days. For monitoring the bafilomycin B1 production, the seed culture and the main culture were performed as described above. The broth of the main culture was centrifuged at 13,000 × g for 10 min at 4°C to remove mycelia, and the supernatant was used for bioassay with Saccharomyces cerevisiae (24) as a test organism on yeast extract-peptone-dextrose (1% agar) medium. The clear zone was measured after incubation for 18 h at 30°C. Commercial bafilomycin B1 (Fluka) was used as a standard.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the DDBJ nucleotide sequence databases under accession numbers AB121071 and AB126048.
RESULTS AND DISCUSSION
Cloning and sequencing of ksbA.
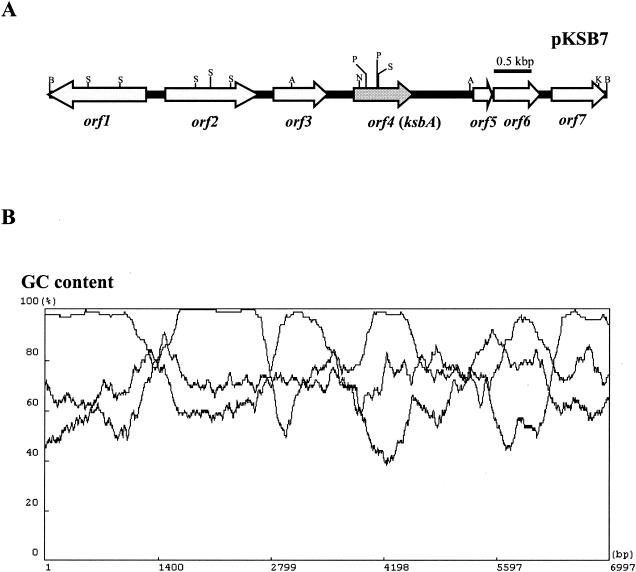
In the genus Streptomyces, there are three types of γ-butyrolactone autoregulator receptors corresponding to the three types of autoregulators: (i) BarA, as a VB-type receptor from S. virginiae (11, 12, 22, 23); (ii) FarA and ScbR, as IM-2-type receptors from S. lavendulae FRI-5 and S. coelicolor A3(2), respectively (17, 29, 33, 35); and (iii) ArpA, as an A-factor-type receptor from S. griseus (25). Alignment of the amino acid sequences of the above four receptors revealed two highly conserved regions (corresponding to amino acid residues 25 to 30 and 52 to 58 of BarA) (Fig. 1). Based on these conserved sequences, with codon usage data derived from 64 Streptomyces genes (36), degenerate oligonucleotide primers for use in PCR were designed and applied for K. setae. As described in Materials and Methods, a 102-bp PCR product clearly encoding the targeted region of a plausible autoregulator receptor was obtained, and a 7-kb BamHI fragment was cloned using the PCR fragment as a probe, yielding pKSB7 (Fig. 2A). Nucleotide sequencing of the cloned fragment revealed one truncated (orf1) and six complete open reading frames (ORFs) (orf2 to orf7). All the ORFs were in agreement with the characteristic codon usage of Streptomyces genes with extremely high G+C contents (96.5, 94.7, 95.8, 92.3, 86.4, 87.8, and 96.0% for orf1 to orf7, respectively) as determined by the FRAME analysis developed by Bibb et al. (1) (Fig. 2B). The orf1 product shows highest similarity to a putative metallopeptidase protein of Streptomyces avermitilis (63% identity and 73% similarity; accession no. AB126048), the orf2 product shows highest similarity to a hypothetical protein of Methanosarcina acetivorans C2A (39% identity, 54% similarity; accession no. AB126048), the orf3 product shows no clear similarity; the orf4 product shows highest similarity to several autoregulator receptors, such as FarA of S. lavendulae FRI-5 (42% identity, 64% similarity; accession no. AB121071); the orf5 product shows highest similarity to BarB of S. virginiae (50% identity, 65% similarity; accession no. AB126048); the orf6 product shows highest similarity to a probable extracytoplasmic function family sigma factor of S. coelicolor A3(2) (33% identity, 65% similarity; accession no. AB126048); and the orf7 product shows highest similarity to methyltransferase of Bordetella bronchiseptica (39% identity, 55% similarity; accession no. AB126048). Among the seven ORFs, orf4 (654 bp encoding a 217-amino-acid protein; DDBJ accession no. AB121071) (Fig. 2) was judged to encode the putative γ-butyrolactone autoregulator receptor, from the presence of the well-conserved HTH DNA-binding motif (Fig. 1) and from its estimated pI value of 4.8. So far, two groups of Streptomyces proteins that contain the conserved HTH motif have been recognized. One group consists of real receptors of biochemically established binding activity toward one type of autoregulator, and the other consists of pseudoreceptors for which all the efforts to observe the autoregulator-binding activity failed, suggesting that the proteins belonging to the second group do not bind any of the autoregulators. The clearest difference between the two groups is the difference of their pI values; that is, the proteins belonging to the real receptor group have a pI of around 5 (pI of 5.1 for ArpA, 5.1 for BarA, 5.3 for FarA, and 5.4 for ScbR), while those belonging to the second group have very basic pI values (pI of 10.3 for BarB, 11.5 for BarZ, 9.8 for CprA, and 10.0 for CprB) (DDBJ/EMBL/GenBank accession no. AB001609, AB035547, AB000384, and AB000385, respectively). On the basis of the pI value of 4.8 for the orf4 product, orf4 is most likely to encode a real autoregulator receptor protein, and thus it was named ksbA (for K. setae butyrolactone autoregulator).
FIG. 2.
(A) Gene organization in the cloned 7-kb BamHI DNA fragment in K. setae. orf1 is truncated. Abbreviations: A, ApaI; B, BamHI; K, KpnI; N, NcoI; P, PstI; S, SalI. For the ApaI sites, two necessary sites among the seven ApaI sites were shown to simplify the map. (B) Frame analysis of the 7-kb BamHI-BamHI region.
With respect to the gene organization around ksbA, it is noteworthy that a gene (orf5) homologous to barB of S. virginiae is present downstream of ksbA. BarB has been clarified to be a regulator controlling the initial stage of virginiamycin biosynthesis (18). In S. virginiae, the barB gene is situated immediately downstream of barA encoding the VB-specific autoregulator receptor, and the transcription of barB is tightly controlled by the BarA-VB system (13) to ensure the precise timing of the virginiamycin production. To determine whether a similar regulation might operate for orf5, a plausible receptor-binding sequence was surveyed in the 5′-upstream region of orf5 using the consensus-binding sequence of Thompson et al. (TNANAWACNNACYNNNCGGTTTKTTT) (5). A probable 26-bp sequence (TAACATAGCGAACGCTCTCTACTTTT) was found at 63 to 88 bp upstream of the orf5 initiation codon together with a probable −10 element at 83 to 88 bp upstream and a −35 element at 106 to 111 bp upstream of orf5, suggesting that orf5 is likely to be under the transcriptional control of an autoregulator receptor. Because the intergenic region between orf5 and orf6 is 23 bp, because no plausible terminator sequence or attenuator sequence is present in the intergenic region, and because no promoter motif is present in the 5′-upstream region of orf6, orf5 and orf6 would seem to form a bicistronic operon, the transcription of which might be controlled by KsbA and the corresponding autoregulator (although direct evidence such as that from a gel-shift assay using recombinant KsbA [rKsbA] and the corresponding DNA fragment will be necessary to draw a definite conclusion).
Autoregulator-binding activity of KsbA.
To examine whether ksbA encodes a real autoregulator receptor which has autoregulator-binding ability, we expressed ksbA in E. coli DH5α. The coding region of ksbA was amplified by PCR and placed under the control of the lac promoter as described in Materials and Methods. After isopropyl-β-d-thiogalactopyranoside (IPTG) induction of transformants harboring pUC19-ksbA, crude cell extract containing rKsbA was prepared and assayed for autoregulator-binding activity. As shown in Table 1 and Fig. 3, it was confirmed that crude cell extract exhibited high binding activity toward [3H]SCB1, while that from the control cells harboring pUC19 alone showed no activity. When nonlabeled A-factor was used as a competitive ligand at a concentration of 125 μM (nonlabeled A-factor:labeled SCB1 = 1,450:1), the level of binding activity was similar to that using nonlabeled SCB1 (125 μM) as a competitive ligand, suggesting that either an SCB1-type or A-factor-type compound is the autoregulator for KsbA. However, when a 10-fold-lower concentration of A-factor (12.5 μM) was used, the binding activity dropped significantly to a level comparable to that observed with a 100-fold lower concentration of nonlabeled SCB1 (0.125 μM), indicating that the competition between the [3H]SCB1 and A-factor is 100-fold less than that between [3H]SCB1 and SCB1. Therefore, we can conclude that the autoregulator of rKsbA is an IM-2-type compound, rather than an A-factor-type compound, and that it has a long side chain with terminal branching, although further studies will be needed to isolate the native autoregulator and definitively identify the chemical structure. In addition to the binding assay with rKsbA, the binding activity in the cell-free lysates from K. setae was measured to evaluate the expression level of native KsbA. Similar to the tendency with rKsbA, cell-free lysates of K. setae showed clear binding activity toward [3H]SCB1 (Table 1), suggesting that KsbA may be the major γ-butyrolactone autoregulator receptor in K. setae under our cultivation condition. Typically, S. virginiae (VB producer), S. lavendulae FRI-5 (IM-2 producer), and S. griseus (A-factor producer) show autoregulator-binding activities of 0.347, 3.04, and 0.811 pmol/mg of protein in the crude cell-free lysates, respectively (23, 25, 29). The binding activity of the native receptor in the crude cell-free lysates of K. setae is 5- to 44-fold lower than those of typical Streptomyces autoregulator receptors. There are at least two possible explanations for this finding. First, the expression level of ksbA may be lower than that of typical receptor genes of Streptomyces species. Second, the binding of [3H]SCB1 to KsbA may be insufficient, because [3H]SCB1 may not be the true autoregulator of KsbA.
TABLE 1.
Autoregulator-binding activity of rKsbA and native KsbA
| Nonlabeled autoregulator | 3H-labeled autoregulator | Specific binding activity (pmol/mg)a with nonlabeled autoregulator at the indicated concn (μM)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| pUC19, 125 | pUC19 + ksbA
|
K. setae
|
|||||||
| 125 | 12.5 | 1.25 | 0.125 | 0.0125 | 125 | 12.5 | |||
| SCB1 | [3H]SCB1 | 0 | 0.168 | 0.131 | 0.058 | 0.042 | 0.018 | 0.069 | 0.049 |
| IM-2-C5 | [3H]IM-2-C5 | 0 | 0.021 | 0 | —b | — | — | 0.008 | 0 |
| VB-C6 | [3H]VB-C7 | 0 | 0.032 | 0 | — | — | — | 0.015 | 0 |
| A-factor | [3H]SCB1 | 0 | 0.159 | 0.035 | — | — | — | 0.058 | 0 |
Values are averages from three independent experiments.
—, not determined.
Disruption of the ksbA gene and phenotypic analyses.
To assess the in vivo role of KsbA in K. setae, the chromosomal ksbA gene was replaced with a mutated ksbA by using pSUC58, as described in Materials and Methods, resulting in a ksbA-disrupted strain (ΔksbA, strain RD1) (Fig. 4A). The deleted portion in ΔksbA consisted of 77 amino acid residues corresponding to the 44th to 120th amino acid residues of KsbA, which includes parts of those amino acid residues constituting the second helix of the HTH DNA binding motif (30th to 51st amino acid residues) in the N terminus (Fig. 5); thus, the resulting truncated protein should be devoid of DNA-binding ability, and the function of KsbA as a regulator should be lost. Conjugal transfer from E. coli ET12567(pUZ8002) harboring pSUC58 to K. setae gave apramycin-resistant exconjugants, in which pSUC58 integration by a single crossover was confirmed by Southern blot analysis (Fig. 4B). After three rounds of sporulation of the pSUC58-integrated strain, as shown in Fig. 4B, two types of apramycin-sensitive strains, namely, ksbA-disrupted mutants and a regenerated wild-type strain (wild-type segregant), were obtained. To complement the ksbA disruptants by intact ksbA, pKSBAC, a pSET152 derivative containing intact ksbA, was introduced into strain RD1 via attB-site mediated chromosomal integration. The integration of the intact ksbA gene in apramycin-resistant exconjugants was confirmed by Southern hybridization (data not shown).
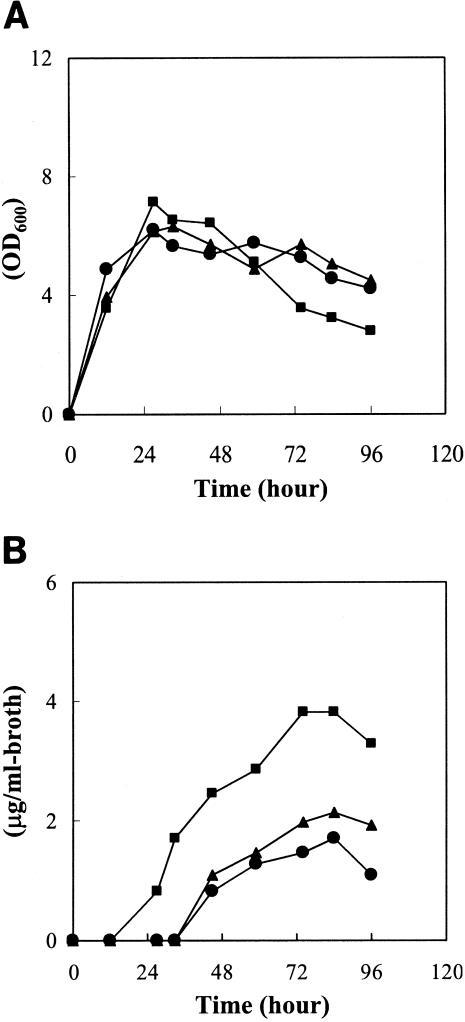
The growth characteristics of strain RD1 in liquid culture were indistinguishable from those of the wild-type strain (Fig. 6A). Furthermore, no morphological difference was observed on 10 kinds of agar media (Materials and Methods), indicating that ksbA does not participate in essential primary metabolism nor in the control of morphological differentiation in K. setae. However, when bafilomycin B1 production by the ksbA disruptant (strain RD1) was examined by bioassay using Saccharomyces cerevisiae, the ksbA disruptant started bafilomycin production 18 h earlier than that by the wild-type strain, and the amount of accumulated bafilomycin became 2.4-fold higher than that by the wild-type strain (Fig. 6B). This change in the profile of bafilomycin production was restored to the original wild-type phenotype by the introduction of intact ksbA to the ksbA disruptant (Fig. 6B), confirming that the loss-of-function mutation in the ksbA gene is responsible for the change in bafilomycin production and suggesting that KsbA as an autoregulator receptor likely acts as a primary negative regulator on the biosynthesis of bafilomycin. The phenomena caused by the ksbA disruption are similar to those observed in the autoregulator receptor (farA) disruptant of S. lavendulae FRI-5 (17). In both cases, disruption of the autoregulator receptor gene resulted in overproduction of secondary metabolites (bafilomycin in K. setae, and nucleoside antibiotics in S. lavendulae FRI-5), while no apparent effect was observed on the growth or morphological differentiation, indicating that the corresponding autoregulator receptors only act as primary negative regulators on the biosynthesis of cognate secondary metabolite. Such noninvolvement of autoregulator receptors in morphological differentiation has also been reported for S. virginiae (19, 20) and S. coelicolor A3(2) (33), but not for S. griseus (21, 25), in which disruption of the A-factor receptor gene resulted in 10-fold higher production of streptomycin but also earlier sporulation.
FIG. 6.
Growth curves (A) and bafilomycin B1 production (B) in liquid culture of a wild-type strain (solid circles), a ksbA disruptant (strain RD1) (solid squares), and a ksbA-complemented strain (strain RDC1) (solid triangles). Each strain was grown at 28°C in ISP2 medium. The amounts of bafilomycin B1 at the indicated times were measured as described in Materials and Methods. Growth was monitored by measuring the optical density at 600 nm (OD600). Three independent ksbA disruptants and ksbA-complemented strains showed an identical pattern; only the representative data on strain RD1 and RDC1 are shown.
In summary, our results demonstrate that the γ-butyrolactone autoregulator receptor gene, ksbA, acts as a primary repressor for bafilomycin B1 production in K. setae. Therefore, much as in the well-known cases in Streptomyces species, γ-butyrolactone autoregulator receptors may exist widely in the genera of non-Streptomyces actinomycetes and play an important role(s) in controlling the production of secondary metabolites. To our knowledge, this is the first report on the cloning and characterization of a butyrolactone autoregulator receptor gene in the genera of non-Streptomyces actinomycetes, as well as in the genus of Kitasatospora.
Acknowledgments
This work was supported in part by grants from the Ministry of Agriculture, Forestry, and Fisheries of Japan and from the Brain Korea 21 Project in 2003.
REFERENCES
- 1.Bibb, M. J., P. R. Findlay, and M. W. Johnson. 1984. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene 30:157-166. [DOI] [PubMed] [Google Scholar]
- 2.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 3.Choi, S. U., C. K. Lee, Y. I. Hwang, H. Kinosita, and T. Nihira. 2003. γ-Butyrolactone autoregulators and receptor proteins in non-Streptomyces actinomycetes producing commercially important secondary metabolites. Arch. Microbiol. 180:303-307. [DOI] [PubMed] [Google Scholar]
- 4.Choi, S. U., C. K. Lee, Y. I. Hwang, H. Kinosita, and T. Nihira. 2004. Intergeneric conjugal transfer of plasmid DNA from Escherichia coli to Kitasatospora setae, a bafilomycin B1 producer. Arch. Microbiol. -298.181:244. [DOI] [PubMed] [Google Scholar]
- 5.Folcher, M., H. Gaillard, L. T. Nguyen, K. T. Nguyen, P. Lacroix, N. Bamas-Jacques, M. Rinkel, and C. J. Thompson. 2001. Pleiotropic functions of a Streptomyces pristinaespiralis autoregulator receptor in development, antibiotic biosynthesis, and expression of a superoxide dismutase. J. Biol. Chem. 276:44297-44306. [DOI] [PubMed] [Google Scholar]
- 6.Grant, S. G. N., J. Jessee, F. R. Bloom, and D. Hanahan. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 87:4645-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto, K., T. Nihira, and Y. Yamada. 1992. Distribution of virginiae butanolides and IM-2 in the genus Streptomyces. J. Ferment. Bioeng. 73:61-65. [Google Scholar]
- 8.Hobbs, G., C. M. Frazer, D. C. J. Gardner, J. A. Cullum, and S. G. Oliver. 1989. Dispersed growth of Streptomyces in liquid culture. Appl. Microbiol. Biotechnol. 31:272-277. [Google Scholar]
- 9.Kawabuchi, M., Y. Hara, T. Nihira, and Y. Yamada. 1997. Production of butyrolactone autoregulators by Streptomyces coelicolor A3(2). FEMS Microbiol. Lett. 157:81-85. [Google Scholar]
- 10.Kieser, T., M. J. Bibb., M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 11.Kim, H. S., H. Tada, T. Nihira, and Y. Yamada. 1990. Purification and characterization of virginiae butanolide C-binding protein, a possible pleiotropic signal-transducer in Streptomyces virginiae. J. Antibiot. 43:692-706. [DOI] [PubMed] [Google Scholar]
- 12.Kim, H. S., T. Nihira, H. Tada, M. Yanagimoto, and Y. Yamada. 1989. Identification of binding protein of virginiae butanolide C, an autoregulator in virginiamycin production, from Streptomyces virginiae. J. Antibiot. 42:769-778. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita, H., H. Ipposhi, S. Okamoto, H. Nakano, T. Nihira, and Y. Yamada. 1997. Butyrolactone autoregulator receptor protein (BarA) as a transcriptional regulator in Streptomyces virginiae. J. Bacteriol. 179:6986-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinoshita, H., T. Tsuji, H. Ipposhi, T. Nihira, and Y. Yamada. 1999. Characterization of binding sequences for butyrolactone autoregulator receptors in streptomycetes. J. Bacteriol. 181:5075-5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitani, S., B. Mervyn, T. Nihira, and Y. Yamada. 2000. Conjugal transfer of plasmid DNA from Escherichia coli to Streptomyces lavendulae FRI-5. J. Microbiol. Biotechnol. 10:535-538. [Google Scholar]
- 16.Kitani, S., H. Kinoshita, T. Nihira, and Y. Yamada. 1999. In vitro analysis of the butyrolactone autoregulator receptor protein (FarA) of Streptomyces lavendulae FRI-5 reveals that FarA acts as a DNA-binding transcriptional regulator that controls its own synthesis. J. Bacteriol. 181:5081-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitani, S., Y. Yamada, and T. Nihira. 2001. Gene replacement analysis of the butyrolactone autoregulator receptor (FarA) reveals that FarA acts as a novel regulator in secondary metabolism of Streptomyces lavendulae FRI-5. J. Bacteriol. 183:4357-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuno, K., Y. Yamada, C. K. Lee, and T. Nihira. 2004. Identification by gene deletion analysis of barB as a negative regulator controlling an early process of virginiamycin biosynthesis in Streptomyces virginiae. Arch. Microbiol. 181:52-59. [DOI] [PubMed] [Google Scholar]
- 19.Nakano, H., C. K. Lee, T. Nihira, and Y. Yamada. 2000. A null mutant of the Streptomyces virginiae barA gene encoding a butyrolactone autoregulator receptor and its phenotypic and transcriptional analysis. J. Biosci. Bioeng. 90:204-207. [DOI] [PubMed] [Google Scholar]
- 20.Nakano, H., E. Takehara, T. Nihira, and Y. Yamada. 1998. Gene replacement analysis of the Streptomyces virginiae barA gene encoding the butyrolactone autoregulator receptor reveals that BarA acts as a repressor in virginiamycin biosynthesis. J. Bacteriol. 180:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohnishi, Y., S. Kameyama, H. Onaka, and S. Horinouchi. 1999. The A-factor regulatory cascade leading to streptomycin biosynthesis in Streptomyces griseus: identification of a target gene of the A-factor receptor. Mol. Microbiol. 34:102-111. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto, S., K. Nakamura, T. Nihira, and Y. Yamada. 1995. Virginiae butanolide binding protein from Streptomyces virginiae. J. Biol. Chem. 270:12319-12326. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto, S., T. Nihira, H. Kataoka, A. Suzuki, and Y. Yamada. 1992. Purification and molecular cloning of a butyrolactone autoregulator receptor from Streptomyces virginiae. J. Biol. Chem. 267:1093-1098. [PubMed] [Google Scholar]
- 24.Omura, S., K. Otoguro, T. Nishikiori, R. Oiwa, and Y. Iwai. 1981. Setamycin, a new antibiotic. J. Antibiot. 34:1253-1256. [DOI] [PubMed] [Google Scholar]
- 25.Onaka, H., N. Ando, T. Nihira, Y. Yamada, T. Beppu, and S. Horinouchi. 1995. Cloning and characterization of the A-factor receptor gene from Streptomyces griseus. J. Bacteriol. 177:6083-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otoguro, K., A. Nakagawa, and S. Omura. 1988. Setamycin, a 16-membered macrolide antibiotic identification and nematocidal activity. J. Antibiot. 41:250-252. [DOI] [PubMed] [Google Scholar]
- 27.Paranthaman, S., and K. Dharmalingam. 2003. Intergeneric conjugation in Streptomyces peucetius and Streptomyces sp. strain C5: chromosomal integration and expression of recombinant plasmids carrying the chiC gene. Appl. Environ. Microbiol. 69:84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao, R. N., M. A. Richardson, and S. Kuhstoss. 1987. Cosmid shuttle vectors for cloning and analysis of Streptomyces DNA. Methods Enzymol. 153:166-198. [DOI] [PubMed] [Google Scholar]
- 29.Ruengjitchatchawalya, M., T. Nihira, and Y. Yamada. 1995. Purification and characterization of the IM-2-binding protein from Streptomyces sp. strain FRI-5. J. Bacteriol. 177:551-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stegmann, E., S. Pelzer, K. Wilken, and W. Wohlleben. 2001. Development of three different gene cloning systems for genetic investigation of the new species Amycolatopsis japonicum MG417-CF17, the ethylenediaminedisuccinic acid producer. J. Biotechnol. 92:195-204. [DOI] [PubMed] [Google Scholar]
- 33.Takano, E., R. Chakraburtty, T. Nihira, Y. Yamada, and M. Bibb. 2001. A complex role for the γ-butyrolactone SCB1 in regulating antibiotic production in Streptomyces coelicolor A3(2). Mol. Microbiol. 41:1015-1028. [DOI] [PubMed] [Google Scholar]
- 34.Takano, E., T. Ninira, Y. Hara, J. J. Jones, C. J. L. Gershater, Y. Yamada, and M. Bibb. 2000. Purification and structural determination of SCB1, a γ-butyrolactone that elicits antibiotic production in Streptomyces coelicolor A3(2). J. Biol. Chem. 275:11010-11016. [DOI] [PubMed] [Google Scholar]
- 35.Waki, M., T. Nihira, and Y. Yamada. 1997. Cloning and characterization of the gene (farA) encoding the receptor for an extracellular regulatory factor (IM-2) from Streptomyces sp. strain FRI-5. J. Bacteriol. 179:5131-5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright, F., and M. J. Bibb. 1992. Codon usage in the G+C-rich Streptomyces genome. Gene 113:55-65. [DOI] [PubMed] [Google Scholar]