Abstract
orf7 (oppA1) and orf15 (oppA2) are located 8 kb apart in the clavulanic acid gene cluster of Streptomyces clavuligerus and encode proteins which are 48.0% identical. These proteins show sequence similarity to periplasmic oligopeptide-binding proteins. Mutant S. clavuligerus oppA1::acc, disrupted in oppA1, lacks clavulanic acid production. Clavulanic acid production is restored by transformation with plasmid pIJ699-oppA1, which carries oppA1, but not with the multicopy plasmid pIJ699-oppA2, which carries oppA2. The mutant S. clavuligerus oppA2::aph also lacks clavulanic acid production, shows a bald phenotype, and overproduces holomycin (5). Clavulanic acid production at low levels is restored in the oppA2-disrupted mutants by transformation with plasmid pIJ699-oppA2, but it is not complemented by the multicopy plasmid pIJ699-oppA1. Both genes encode oligopeptide permeases with different substrate specificities. The disrupted S. clavuligerus oppA2::aph is not able to grow on RPPGFSPFR (Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg; bradykinin), but both mutants grow on VAPG (Val-Ala-Pro-Gly) as the only nitrogen source, indicating differences in the peptide bound by the proteins encoded by both genes. The null S. clavuligerus oppA1::acc and S. clavuligerus oppA2::aph mutants are more resistant to the toxic tripeptide phosphinothricyl-alanyl-alanine (also named bialaphos) than the wild-type strain, suggesting that this peptide might be transported by these peptide-binding proteins.
Streptomyces clavuligerus produces the β-lactam antibiotic cephamycin C (27). Additionally it produces compounds with clavam structure, such as the β-lactamase inhibitor clavulanic acid or the antibacterial alanylclavam (26), and compounds with pyrrothin structure, such as holomycin (5). Each of these secondary metabolites has different precursors (α-aminoadipic acid, cysteine, and valine for cephamycin C; arginine for clavulanic acid; and probably cysteine for holomycin), although it is believed that clavulanic acid and clavams share the initial steps of their pathways (8). Genes for clavulanic acid biosynthesis are located adjacent to those of the cephamycin C cluster in S. clavuligerus (7, 8, 33), forming the so-called β-lactam supercluster. Several enzymes encoded by these genes have been purified to homogeneity, yielding information on different steps of the pathway (2, 9, 29, 34), and the β-lactam synthetase and clavaminate synthetase have been crystallized (17, 35). However, the studies on clavulanic acid biosynthesis are confused by the presence of two sets of paralogous genes that encode the early enzymes of the pathway (8). The cas1 and cas2 genes, which encode the multifunctional clavaminate synthases 1 and 2, are located, respectively, in the clavam gene cluster (18) and in the clavulanic acid gene cluster (15, 22). The gene ceaS (pyc) plays an essential role in the formation of the C3 unit of clavulanic acid in certain media (9); however, mutants disrupted in ceaS are still able to produce clavulanic acid in glycerol-sucrose-proline-glutamate (GSPG) medium when glycerol is added (26). This suggests that a gene alternative to ceaS encodes an enzyme able to form the C3 unit from glycerol. Knockout mutants disrupted in bls, pah, or orf6 as well as the above-mentioned ceaS and cas2 were found to be blocked in clavulanic acid and clavam production when grown in the starch-asparagine (SA) medium but partially regained the ability to produce clavulanic acid and clavams when grown in peptone-based soy medium (8). The degree of restoration on soy medium was higher for clavam production, which reached up to 100% of wild-type production for the pah-disrupted strain. However, restoration of clavulanic acid production in soy medium was 40 to 60% for the cas2-, orf6-, and pah-disrupted mutants and lower for the ceaS- and bls-disrupted strains. The restoration of clavulanic acid production may be related to the induction of alternative biosynthetic pathways by peptides released from soy meal.
Nodwell and Losick (19) proposed that aerial mycelium formation in Streptomyces coelicolor is induced by an uncharacterized peptide. Similarly, Sánchez and Braña (31) proposed that peptides accumulated in the spent culture broth of S. clavuligerus at nanomolar concentrations stimulated antibiotic biosynthesis in cells grown in fresh medium. Peptides are known to act as inducers of the competence state in Bacillus subtilis (13) and may also be important signals for triggering antibiotic production and differentiation in Streptomyces.
The known clavulanic acid gene cluster has been extended recently by sequencing an additional region upstream of the car gene, which is essential for clavulanic acid biosynthesis (14, 16). While studying this region we found an open reading frame (ORF), orf15, which is similar to orf7, a gene previously described (7). In this work we present the characteristics of both genes and the effects exerted by their disruption on the formation of clavulanic acid or holomycin and on peptide transport.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in this work are listed in Table 1. Escherichia coli strains were grown at 37°C in TB medium (containing, per liter, 12 g of tryptone, 24 g of yeast extract, and 5 mg of glycerol; pH 7.5) supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), or apramycin (25 μg/ml) when required. S. clavuligerus ATCC 27064 and the strains derived from it were grown in TSB medium (containing, per liter, 30 g of Trypticaseine soy broth) for 36 h at 220 rpm and 28°C. Five milliliters of this culture was used to inoculate 100 ml of TSB, and the culture was grown under the same conditions. Alternatively the culture was made in SA medium (22) or in GSPG medium (27). Cultures of S. clavuligerus transformants were supplemented with thiostrepton (5 μg/ml) to select pIJ699-derived plasmids, and the disrupted mutants were supplemented with apramycin (50 μg/ml) or kanamycin (50 μg/ml) when required. All the fermentations were repeated twice using triplicate 500-ml tripled-baffled flasks. DNA was measured as reported previously in adequately diluted samples (26). The relation y = 37.70x + 1.41, where y is the cell dry weight and x is DNA (both in milligrams per milliliter) was used for values of DNA between 0.05 and 0.20 μg/ml. Colony morphology and sporulation were tested in ME medium (31).
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Characteristic(s) | Origin or reference |
|---|---|---|
| Strains | ||
| S. clavuligerus 27064 | Wild type, clavulanic acid producer | ATCCa |
| S. clavuligerus oppA1::acc | Disrupted in oppA1; resistant to apramycin | This work |
| S. clavuligerus oppA2::aph | Disrupted in oppA2; resistant to kanamycin | This work |
| S. clavuligerus oppA1::acc oppA2::aph | Double disruptant in oppA1 and oppA2; resistant to apramycin and kanamycin | This work |
| E. coli ET12567(pUZ8002) | Strain used in E. coli-Streptomyces conjugation | 11 |
| Plasmids | ||
| pIJ699 | Streptomyces positive selection vector; Thior | 10 |
| pIJ699-oppA2 | pIJ699 containing an NdeI-SpeI DNA fragment with oppA2, downstream of the PtipA promoter | This work |
| pIJ699-oppA1 | pIJ699 containing an NdeI-SpeI DNA fragment with oppA1, downstream of the PtipA promoter | This work |
| p30Krel | pBSKS+ containing a 6.5-kb KpnI-EcoRI fragment with the 3′ end of cs2, ORF6, oppA1, claR, and car | 24 |
| pALCL26 | pBSKS+ containing a 4.5-kb fragment containing ORF14, oppA2, and ORF16 of the clavulanic acid cluster | 16 |
| pMS17-oppA2 | Contains oppA2 downstream of the anhydrotetracycline-induced promoter tcp830 in plasmid pMS17; an E. coli-Streptomyces integrative-conjugative plasmid that integrates in attB, the insertion site for the wide-host-range phage φC31 | This workb |
ATCC, American Type Culture Collection.
pMS17 was provided by M. Smith and A. Rodríguez-García.
Peptide incorporation.
To test the use or uptake of peptides as nitrogen source, microtiter plates with 24 wells (diameter, 15 mm) were used. The wells were filled with 1 ml of solid (2% agarose) SA medium or SA medium lacking asparagine and supplemented with phosphinothricyl-alanyl-alanine (Pt-AA) or the peptides used as nitrogen source. Cells (5 ml) from a 24-h culture grown in TSB medium were thoroughly washed twice with saline solution in order to remove all the carryover of the medium and suspended in the same volume of saline solution, and a 20-μl aliquot was used to inoculate the surface of each well. The plates were incubated at 28°C, and the growth was monitored by direct observation for up to 4 days.
Antibiotic determination.
Cephamycin C was measured by bioassay against E. coli Ess22-31 (27). Clavulanic acid was measured both by the inhibition of Klebsiella pneumoniae growth in the presence of penicillin G (27) and by derivatization with imidazole and analysis by high-performance liquid chromatography (HPLC) using the conditions described by Foulstone and Reading (4). Alanylclavam was measured by the inhibition of Bacillus sp. strain ATCC 27860 (25). Holomycin was measured by HPLC as described by Fuente et al. (5).
DNA methods.
Restriction endonuclease digestions of DNA were carried out according to the manufacturer's recommendations. DNA ligation, plasmid isolation, and E. coli or Streptomyces transformations were performed by standard procedures (11, 30).
PCR amplification.
Plasmids pIJ699-oppA1 and pIJ699-oppA2 were obtained by digesting the PCR-amplified genes with appropriate restriction enzymes and subcloning them in pIJ699. The genes oppA1 and oppA2 were obtained by PCR amplification using the following oligonucleotide pairs: (i) oppA1-1 (5′-GGCAGGAGGCATATGGAGACCACT-3′) and oppA1-2 (5′-CATACGGTACTAGTGCCGGACCCCGTGCTCA-3′) to amplify oppA1, (ii) oppA2-1 (5′-CGATCAGAAGAGGACCGCCATATGACCA-3′) and oppA2-2 (5′-GTGATGGAACTAGTCTTCTTCGGCGA-3′) to amplify oppA2, (iii) orf14-1 (5′-ACAAGGGACCCATATGAACGACA-3′) and orf14-2 (5′-AAACTAGTCGCCGTTCTCGATGCTGGTT-3′) to amplify orf14, and (iv) oppA2-1 (5′-CGATCAGAAGAGGACCGCCATATGACCA-3′) and orf14-2 to amplify the oppA2 to orf14 segment. The initiation and stop codons of the ORFs are shown in boldface type, and restriction sites for NdeI (oppA1-1, oppA2-1, and orf14-1) and SpeI (oppA1-2, oppA2-2, and orf14-2) are underlined.
For PCR amplification the nucleotide concentrations were adapted to the 70% G+C content of Streptomyces. To amplify oppA1 the PCR conditions were as follows: Tris-HCl, 70 mM (pH 8.8); ammonium sulfate, 20 mM; Tween 20, 0.01%; MgCl2, 1 mM; dGTP and dCTP, 28 μM each; dATP and dTTP, 12 μM each; oligonucleotides, 0.2 μM each; dimethyl sulfoxide, 5%; Taq polymerase, 2.5 U/100 μl; linearized plasmid p30Krel, 20 ng/100 μl. The amplification steps were as follows: 96°C for 3 min, 53°C for 1 min, and 74°C for 50 s; 25 cycles of 96°C for 30 s, 53°C for 15 s, 50°C for 15 s, and 74°C for 50 s; and 74°C for 10 min. To amplify oppA2, linearized plasmid pALCL26 (20 ng/100 μl) was used, and the amplification reaction was as follows: 96°C for 3 min; 45°C for 30 s; 30 cycles of 74°C for 1 min, 96°C for 1 min, and 45°C for 30 s; and 74°C for 10 min. The fragments obtained by PCR were confirmed by sequencing.
Gene disruption.
Gene disruption was performed by double recombination between the genome and pHZ1351-derived plasmids containing either the acc3(IV) gene for apramycin resistance or the aphII gene for kanamycin resistance, resulting in partial replacement of the genes to be disrupted by the resistance genes. Spores of the putative Streptomyces disruptants were analyzed for the correct antibiotic resistance as described by Pérez-Redondo et al. (26).
Streptomyces-E. coli conjugation.
Conjugation was performed using S. clavuligerus oppA2::aph and E. coli ET12567(pU28002) as described by Kieser et al. (11).
Southern hybridization analysis.
For Southern hybridization analysis, genomic DNA was isolated as described by Kieser et al. (11), digested with suitable restriction enzymes, and probed using standard methods.
Sequence analysis.
Sequence analysis was performed using the DNASTAR package (Madison, Wis.) and the Clustal V program (6). Searches of the European Bioinformatics Institute EMBL data banks of nucleotide sequences and Swiss-Prot protein sequences were made using the FASTA and BLAST programs.
RESULTS
Characteristics of the proteins encoded by orf7 and orf15.
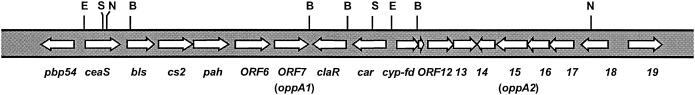
orf7 (8), located downstream of claR and in the opposite orientation, has 1,668 nucleotides (nt) with a 72% G+C content and encodes a protein of 555 amino acids. An intergenic region of 164 bp is present between orf6 and orf7 and contains two palindromic sequences (nt 9122 to 9136 and nt 9144 to 9158; nt 9222 to 9228 and nt 9232 to 9238 in U87786) that may form stem-and-loop structures in the RNA with free energies of −41.8 and −16.6 kcal/mol. These stem-and-loop structures might act as transcription terminators of readthrough transcripts from genes upstream of orf7.
orf15 is located in the recently reported DNA fragment upstream of car (16), 8 kb upstream of orf7 (Fig. 1). It encodes a protein of 562 amino acids 48% identical to the ORF7 protein. These proteins resemble BldK of S. coelicolor (24.2 and 24.4% identity with oppA1 and oppA2, respectively), an oligopeptide importer which is also involved in aerial mycelium formation (19, 20), and also resemble putative periplasmic oligopeptide transport proteins (OppA) from S. coelicolor (37.8% identity with OppA1 and 35.6% with OppA2, respectively) (Fig. 2) and Streptomyces avermitilis (36.6 and 41.9% identity, respectively, to protein SAV2768).
FIG. 1.
Cluster of genes for clavulanic acid biosynthesis. Restriction sites for BglII (B), SacI (S), EcoRI (E), and NotI (N) are indicated.
FIG. 2.
Comparison of amino acid sequences of proteins encoded by the oppA1 and oppA2 genes of S. clavuligerus and the BldK and OppA1 proteins of S. coelicolor. Isoleucine (I) and leucine (L) have been considered equivalent. Boxed is a motif characteristic of proteins binding soluble substrates. The underlined motif is characteristic for lipid binding in membrane-docking proteins.
A motif (PS01040 [PROSITE]) characteristic of extracellular proteins able to bind soluble compounds is present in both orf7- and orf15-encoded proteins (ORF15, 88GPGEVSDGGRTWTYRLRRGLRY109; ORF7, 84SLGESSEDGRVWTYRLREGLRY105), where identical amino acids have been underlined.
However, the amino acid sequences of ORF7 and ORF15 lack the characteristic motif for lipid binding which is found in membrane-docking proteins. Since those genes are similar to the oligopeptide carrier proteins of S. coelicolor and S. avermitilis, they have been tentatively designated as oppA1 and oppA2. In this work we obtained knockout mutants of oppA1 and oppA2 in order to study the possible cross-complementation of the proteins encoded by these genes.
Disruption of oppA1 and oppA2 and isolation of double-disrupted mutants.
In order to disrupt oppA1, plasmid pHZ-oppA1::acc, a pHZ1351-derived plasmid, was constructed by replacing most of the oppA1 gene by the apramycin resistance gene acc, leaving 189 nt of the 5′ end of oppA1 and 157 nt of the 3′ end of oppA1. In parallel plasmid pHZ-oppA2::aph was constructed for the inactivation of oppA2. This plasmid contains the aph gene, for kanamycin resistance, flanked by 758 bp of the 3′ end and 70 bp of the 5′ end of oppA2, lacking therefore 861 nt of the central part of this gene. S. clavuligerus transformants were selected for kanamycin resistance and thiostrepton sensitivity (S. clavuligerus oppA2::aph) or for apramycin resistance and thiostrepton sensitivity (S. clavuligerus oppA1::acc). Moreover, a double mutant, S. clavuligerus oppA1::acc oppA2::aph, was obtained from S. clavuligerus oppA2::aph using the same strategy.
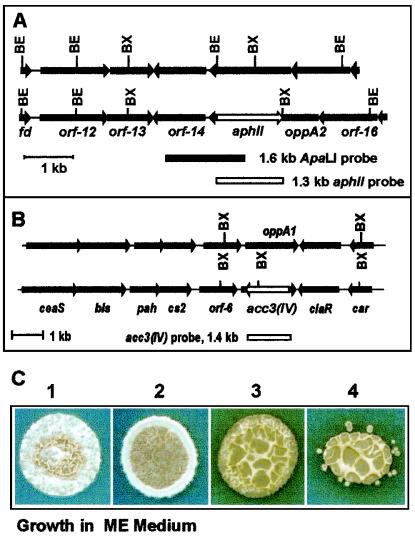
DNA from the putative disrupted strains was hybridized with (i) a 1.6-kb ApaLI DNA probe containing the 5′ end of orf14 and the 3′ end of oppA2 (Fig. 3A), (ii) a 1.3-kb DNA probe containing the aph gene which only hybridized with the disrupted mutants (not shown), and (iii) a 1.4-kb DNA probe containing the acc3(IV) gene (Fig. 3B). The pattern of hybridization shown for the three probes agreed with the restriction map expected for the disrupted strains.
FIG. 3.
(A) Schemes of the DNA for both strains show the restriction sites for BstXI (BX) and BstE (BE). (B) Growth for 4 days in ME sporulation medium of S. clavuligerus 27064 (plate 1), S. clavuligerus oppA1::acc (plate 2), S. clavuligerus oppA2::aph (plate 3), and S. clavuligerus oppA1::acc oppA2::aph (plate 4).
Effects of oppA1 and oppA2 disruption on colony morphology: oppA2-null mutants form bald colonies, whereas oppA1 mutants sporulate normally.
In solid SA or ME medium S. clavuligerus oppA1::acc-disrupted mutants produced aerial mycelium and showed spore morphology similar to that of the wild-type strain, but their growth rate was slightly lower (Fig. 4 and 5). However, the strains disrupted in oppA2 were unable to produce aerial mycelium or spores, showing therefore a Bld phenotype (Fig. 3C). In both media the colonies of S. clavuligerus oppA2::aph or the double mutant were yellow and formed a yellow-pigmented zone around the colonies due to holomycin overproduction (5). However, the oppA1-disrupted clones sporulated normally and did not produce enough pigment to be clearly detectable in the plates.
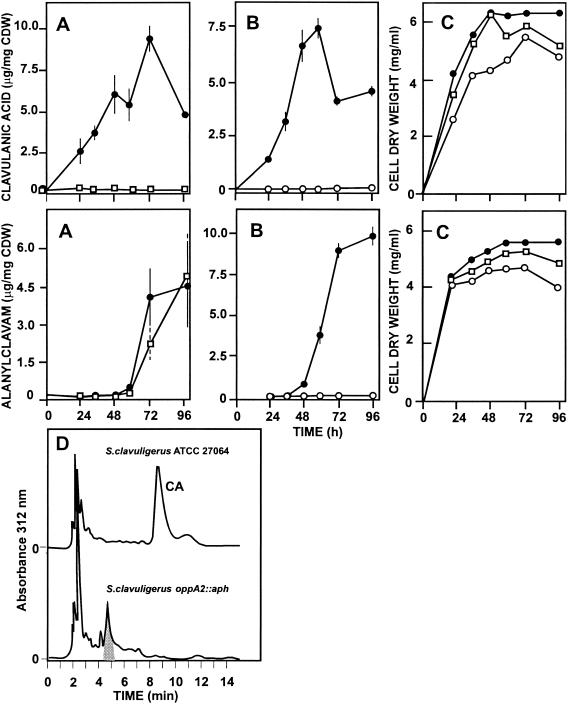
FIG. 4.
Production of clavulanic acid in SA medium and alanylclavam in GSPG medium by S. clavuligerus 27064 (•) and S. clavuligerus oppA1::acc (□) (A) or S. clavuligerus 27064 (•) and S. clavuligerus oppA2::aph (○) (B). (C) Growth of the different strains. (D) HPLC analysis of the fermentation broth of cultures of S. clavuligerus ATCC 27064 and S. clavuligerus oppA2::aph grown for 35 h in TSB medium. Vertical bars indicate standard deviations.
FIG. 5.
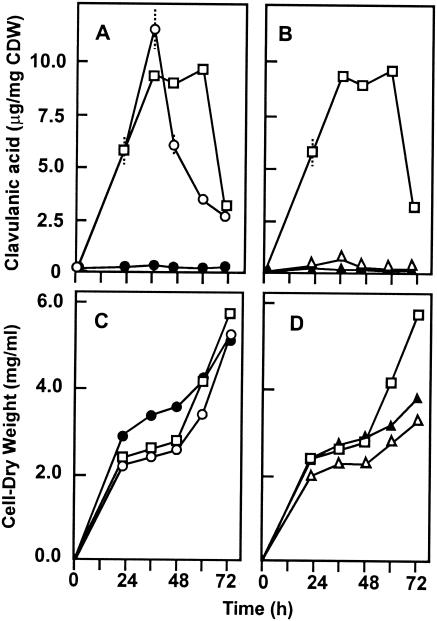
Clavulanic acid production in SA medium by S. clavuligerus (pIJ699) (□) and the following transformants: S. clavuligerus oppA1::acc(pIJ699-oppA2) (•) and S. clavuligerus oppA1::acc(pIJ699-oppA1) (○) (A) and S. clavuligerus oppA2::aph(pIJ699-oppA1) (▴) and S. clavuligerus oppA2::aph(pIJ699-oppA2) (▵) (B). (C and D) Growth of the corresponding strains.
Effects of oppA1 or oppA2 disruption on holomycin, cephamycin, clavulanic acid, and alanylclavam formation.
Production of holomycin in TSB medium by oppA1- and oppA2-disrupted mutants and that of clavulanic acid and cephamycin C in TSB, SA, and GSPG media were compared with production by the wild-type strain. Alanylclavam production was studied in GSPG medium.
Cephamycin C biosynthesis was normal in the wild-type strain and the two disrupted mutants. However, no clavulanic acid production by the oppA1- or the oppA2-disrupted mutants was found at any time in the fermentation in the three media tested (Fig. 4). When HPLC analysis of clavulanic acid of S. clavuligerus oppA2::aph and S. clavuligerus oppA1::acc broths was performed, no peak corresponding to clavulanic acid was found, but in the former strain a new peak appeared with a retention time of 5.2 min. This substance, which gave a positive reaction with imidazole typical of clavam compounds, peaked at 24 h of fermentation in TSB medium in the oppA2-disrupted mutant, decreasing thereafter. In summary both oppA1 and oppA2 are required for clavulanic acid production (although clavam intermediates are still formed in the oppA2 mutant) but not for cephamycin biosynthesis.
Alanylclavam was not produced by S. clavuligerus oppA2::aph or the double mutant (not shown), but production of alanylclavam by S. clavuligerus oppA1::acc was practically normal (Fig. 4C). The yellow antibiotic holomycin was overproduced by S. clavuligerus oppA2::aph with a steady increase during 100 h of culture, reaching titers on the order of 1,200 μg/mg of DNA (5). Holomycin formation by S. clavuligerus oppA1::acc increased steadily in a similar manner to that by oppA2::aph during the first 48 h of culture, up to a titer of 700 μg/mg of DNA, and then decreased thereafter. Both null mutants accumulated holomycin to DNA levels well above those of the wild-type strain S. clavuligerus ATCC 24076 (10 μg/mg of DNA), although the kinetics of pigment accumulation by the two mutants were different. No substantial differences in penicillin G resistance (>100 μg/ml) or cephalosporin C resistance (>1,000 μg/ml) were found between the wild type and the mutants, indicating that the opp genes are not involved in cephamycin export or in β-lactam resistance.
Complementation of clavulanic acid production in oppA1- and oppA2-disrupted mutants by transformation with oppA1 and oppA2, respectively.
Transformation of S. clavuligerus oppA1::acc with plasmid pIJ699-oppA1, a multicopy plasmid containing oppA1, resulted in full restoration of the clavulanic acid titers (Fig. 5A). However, complementation of S. clavuligerus oppA2::aph with pIJ699-oppA2, a pIJ699-derived plasmid containing oppA2 downstream of the strong promoter of the tipA gene (PtipA), which is efficiently expressed in S. clavuligerus (5, 32), restored only 6 to 10% of clavulanic acid production compared with the control strain, S. clavuligerus (pIJ699), in SA (Fig. 5B) or TSB (not shown) medium. Complementation to aerial mycelium formation was not observed, probably due to the presence of thiostrepton in the plates, but the holomycin production by the transformant was restored to almost wild-type levels (5). These results indicate that the large increase of holomycin formation in S. clavuligerus oppA2::aph is due to lack of the OppA2 protein. Furthermore, the poor recovery of clavulanic acid production might indicate that more than one protein is affected in the disrupted mutant. Downstream of oppA2 in the clavulanic acid cluster is orf14, which encodes a protein of unknown function that affects clavulanic acid production. A possible explanation of the poor restoration of clavulanic acid formation by plasmid pIJ699-oppA1 could be the lack of expression of orf14 in oppA2-disrupted strains, if orf14 is expressed as part of a bicistronic oppA2-orf14 operon. To elucidate this hypothesis plasmids pIJ699-orf14 and pIJ699-orf14/oppA2 were constructed, in which orf14 or orf14/oppA2 were expressed from the PtipA promoter. However, transformants S. clavuligerus oppA2::aph(pIJ699-orf14) or S. clavuligerus oppA2::aph(pIJ699-orf14/oppA2) only recovered 5 to 10% of the clavulanic acid production in relation to the control strain. In order to study whether the lack of aerial mycelium complementation of S. clavuligerus oppA2::aph(pIJ699-oppA2) was due to the presence of thiostrepton in the plates, the thiostrepton-induced promoter was replaced by an anhydrotetracycline-induced one. oppA2 was inserted as a blunt DNA fragment in the XbaI site of the integrative plasmid pMS17 (A. Rodriguez and M. C. M. Smith, personal communication) to produce pMS17-oppA2, which carries oppA2 downstream of the synthetic tcp830 anhydrotetracycline-inducible promoter. pMS17-oppA2 was introduced in S. clavuligerus oppA2::aph by conjugation, and the nalidixic acid-apramycin-resistant exconjugants were plated in unsupplemented ME medium or ME medium supplemented with anhydrotetracycline (1 μg/ml). Anhydrotetracycline does not affect aerial mycelium formation in Streptomyces lividans but strongly induces the expression of genes located downstream of the tcp830 promoter (A. Rodríguez-García, and M. C. M. Smith, unpublished results). A clear formation of aerial mycelium was observed in S. clavuligerus oppA2::aph(pMS17-oppA2) growing in ME plates supplemented with anhydrotetracycline but not in the absence of this inducer. The aerial mycelium formation was on the order of 20% of that of the control strain S. clavuligerus (pMS17), reflecting a behavior similar to that of S. clavuligerus oppA2::aph(pIJ699-oppA2) in relation to clavulanic acid formation or bialaphos resistance.
No cross-complementation was found between oppA1 and oppA2. In order to know if the expression of oppA1 in high copy number complements the defect of S. clavuligerus oppA2::aph and, vice versa, whether expression of oppA2 in a high-copy-number plasmid would complement the defect of S. clavuligerus oppA1::acc, the transformants S. clavuligerus oppA2::aph(pIJ699-oppA1) and S. clavuligerus oppA1::acc(pIJ699-oppA2) were compared with the corresponding control strains transformed with plasmid pIJ699. No production of clavulanic acid either in SA (Fig. 5B) or TSB (not shown) medium was observed in any of the cases. These results clearly indicate that oppA1 and oppA2 exert different roles in clavulanic acid biosynthesis.
Peptide transport by oppA1- and oppA2-disrupted mutants.
Since the proteins encoded by oppA1 and oppA2 resemble oligopeptide permeases, we decided to test the transport of several peptides by the wild type and the disrupted mutants.
The strains were grown in SA medium containing increasing amounts of the tripeptide Pt-AA (0.05 to 1 μg/ml). This tripeptide (also named bialaphos), once transported intracellularly, releases phosphinothricin, which is known to inhibit the glutamine synthetase, therefore preventing cell growth (12).
Pt-AA at 0.3 μg/ml inhibited the growth of the parental strain, indicating that it is transported in S. clavuligerus. Both strains disrupted in either oppA1 or oppA2 showed partial Pt-AA resistance, being able to grow in the presence of Pt-AA at concentrations up to 1 μg/ml, although their growth was not homogeneous (Fig. 6). The patterns of growth were similar for both of them, although due to the lack of aerial mycelium, the oppA2-disrupted strain shows a bald aspect in Fig. 6. This suggests that both oppA1- and oppA2-encoded proteins are able to transport the tripeptide Pt-AA to some extent.
FIG. 6.
Growth in SA medium or SA medium supplemented with Pt-AA (0.6 μg/ml). Panels: 1, S. clavuligerus 27064; 2, S. clavuligerus oppA1::acc; 3, S. clavuligerus oppA2::aph; 4, S. clavuligerus oppA1::acc oppA2::aph.
Complementation with plasmid pIJ699-oppA1 restored the bialaphos sensitivity phenotype fully to S. clavuligerus mutants oppA1::acc and partially to S. clavuligerus mutant oppA2::aph. The oppA2 gene restored the sensitivity to bialaphos to an almost-wild-type level in S. clavuligerus oppA2::aph and affected slightly the bialaphos sensitivity in S. clavuligerus oppA1::acc.
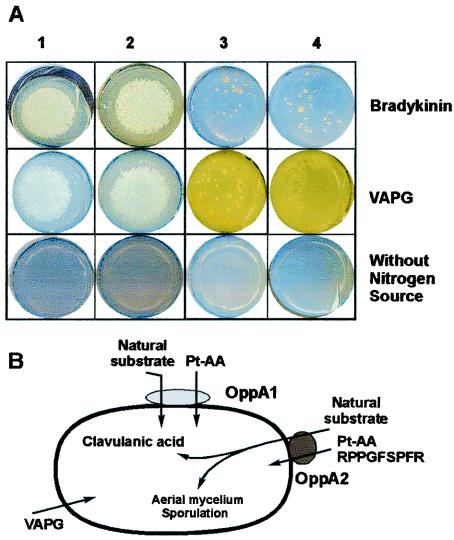
Since the natural substrates transported by oppA1 and oppA2 are not known, two other peptides were tested as nutrients providing the only nitrogen source for growth. The tetrapeptide Val-Ala-Pro-Gly (VAPG) and Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg (RPPGFSPFR; bradykinin) at 1.5 and 1 mM concentrations, respectively, were added to a solid, nitrogen-free SA medium (lacking asparagine). Nitrogen-free SA medium in which total lack of growth was observed in all the strains was used as a negative control (Fig. 7A). All the media were inoculated with 20 μl of a twice-washed 48-h-grown cell suspension. The disrupted strain S. clavuligerus oppA1::acc grew as well as the wild-type strain both on VAPG and on RPPGFSPFR as nitrogen sources, indicating that these two peptides are transported by other systems different from oppA1 (Fig. 7A). However, the growth of S. clavuligerus oppA2::aph on these two peptides was clearly different. VAPG was used by this strain, giving a film of strongly yellow (holomycin-forming) bald mycelium. However, no growth was observed on RPPGFSPFR (only small pellets due to inoculum accumulation are observed) (Fig. 7A); i.e., oppA2 seems to be involved in transport of peptides similar to RPPGFSPFR.
FIG. 7.
(A) Growth in nitrogen-free SA medium (lacking asparagine) supplemented with RPPGFSPFR (1.0 mM) or VAPG (1.5 mM) or without supplementation. Panels: 1, S. clavuligerus 27064; 2, S. clavuligerus oppA1::acc; 3, S. clavuligerus oppA2::aph; 4, S. clavuligerus oppA1::acc oppA2::aph. (B) Scheme for the putative function of OppA1 and OppA2.
DISCUSSION
OppA1 and OppA2 resemble oligopeptide-binding lipoproteins of S. coelicolor and S. avermitilis (3, 21) as well as transport lipoproteins of the ABC type such as BldK (19, 20). The oppA1 and oppA2 genes of S. clavuligerus, located in the clavulanic acid gene cluster, encode oligopeptide permeases that are involved in the transport of tripeptides such as Pt-AA. Neither oligopeptide permease is involved in the transport of the tetrapeptide VAPG. The oppA1 mutant is able to grow on the nine-amino-acid peptide RPPGFSPFR, while the oppA2 mutant is unable to grow on this nonapeptide as sole nitrogen source, indicating that this nonapeptide, and probably other related peptides, are transported by OppA2 (Fig. 7B).
Both oppA1 and oppA2 are strictly required for clavulanic acid production, and they cannot replace each other even at low levels when expressed in multicopy plasmids (Fig. 4), confirming that they are functionally distinct in spite of a 48% amino acid identity of the proteins OppA1 and OppA2. Antibiotic production of oppA1-null mutants can be complemented with the oppA1 gene in multicopy plasmids, and the mutant is restored to Pt-AA sensitivity when complemented, showing that the oppA1 gene is functional in the transport of this peptide. However, the clavulanic acid production in oppA2 is very poorly complemented by the oppA2 gene (6 to 10%) using the oppA2 gene itself or in the construction containing oppA2-orf14, indicating that the poor complementation of oppA2 is not due to a polar effect on the expression of orf14, the only gene located downstream of oppA2, which may be expressed from the same promoter. Similar results were found in relation to complementation of Pt-AA resistance.
An important finding is the bald phenotype of oppA-null mutants. The oppA1-null mutant produces aerial mycelium and sporulates normally. However, the oppA2-encoded permease transports a signal peptide molecule that is involved in triggering both aerial mycelium formation and clavulanic acid biosynthesis, as occurs with BldK of S. coelicolor. This aerial bald phenotype is partially complemented only in integrative monocopy transformants in which the oppA2 gene is expressed from the anhydrotetracycline-induced promoter tcp380 but not in noninduced transformants or in multicopy transformants selected by thiostrepton, suggesting that thiostrepton exerts an additional negative effect on aerial mycelium formation. Since only partial complementation of the bald phenotype in oppA2-null mutants has been achieved with an integrative plasmid, which does not require the presence of antibiotics, and only partial complementation of antibiotic production and Pt-AA resistance has been achieved either with a multicopy or with an integrative plasmid, it is possible that the optimal expression of oppA2 in these transformants requires the presence of sequences upstream of oppA2 which modulate the expression of this gene, suggesting that optimal expression is only possible when expressed in cis.
So far oligopeptide transporters have not been reported in antibiotic biosynthesis clusters. However, the protein CalT6 (Q8KNE6), of unknown function, encoded by a gene present in the calicheamicin cluster of Micromonospora echinospora (1), has a 29.2% identity to oppA1.
Oligopeptide ABC transporters are involved in morphological differentiation in S. coelicolor (19), sporulation and the competence state in Bacillus (23, 28), and the mating pheromones in Enterococcus faecalis. Most Opp proteins are thought to transport peptides nonspecifically, mainly as carbon or nitrogen sources (13), but this evidence also suggests that quorum sensing mediated by peptides may play an important role in the physiology of the bacteria, converging with starvation sensing to lead to stationary phase or to secondary metabolism. The presence of two nonexchangeable opp genes in the clavulanic acid cluster suggests that the sensing of signaling peptides that trigger clavulanic acid biosynthesis may be an important mechanism as reported by Sánchez and Braña (31). The findings reported in this work open the way for the full characterization of the peptide mediators.
ADDENDUM IN PROOF
The unknown compound with a retention time of 5.2 min has been characterized recently as N-acetylglycilclavaminic acid (S. E. Jensen, A. S. Paradkar, R. H. Mosher, C. Anders, P. H. Beatty, M. J. Brumlik, A. Griffin, and B. Barton, Antimicrob. Agents Chemother. 48:192-202, 2004).
Acknowledgments
This work was supported by grants BIO2000-0272 from the Spanish Ministry of Education, Culture, and Sports and by the Areces Foundation. Luis M. Lorenzana was the recipient of a fellowship from the University of León (León, Spain).
The technical assistance of Angeles Vidales and Maria Mediavilla is appreciated. We thank Maggy Smith and A. Rodríguez-García for providing the unpublished plasmid pMS17.
REFERENCES
- 1.Ahlert, J., E. Shepard, N. Lomovskaya, E. Zazopoulos, A. Staffa, B. O. Bachmann, K. Huang, L. Fonstein, A. Czisny, R. E. Whitwam, C. M. Farnet, and J. S. Thorson. 2002. The calicheamicin gene cluster and its iterative type I enediyne PKS. Science 16:1173-1176. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, B. O., R. Li, and C. A. Townsend. 1998. β-Lactam synthetase: a new biosynthetic enzyme. Proc. Natl. Acad. Sci. USA 95:9082-9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley, S. D., K. F. Chater, A. M. Cerdeño-Tárraga, G. L. Challis, et al. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 4.Foulstone, M., and C. Reading. 1982. Assay of amoxicillin and clavulanic acid, the components of augmentin, in biological fluids with high-performance liquid chromatography. Antimicrob. Agents Chemother. 178:6310-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuente, A., L. M. Lorenzana, J. F. Martín, and P. Liras. 2002. Mutants of Streptomyces clavuligerus disrupted in different genes for clavulanic acid biosynthesis produce large amounts of holomycin: possible cross-regulation of two unrelated secondary metabolite pathways. J. Bacteriol. 184:6559-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins, D. G., A. J. Bleasby, and R. Fuchs. 1989. Clustal V: improved software for multiple sequence alignments. Comput. Appl. Biosci. 8:189-191. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson, J. E., A. P. Fosberry, N. S. Rawlinson, H. N. Ross, R. J. Neal, J. C. Arnell, A. J. Earl, and E. J. Lawlor. 1995. Clavulanic acid biosynthesis in Streptomyces clavuligerus: gene cloning and characterization. Gene 166:49-55. [DOI] [PubMed] [Google Scholar]
- 8.Jensen, S. E., K. J. Elder, K. A. Aidoo, and A. S. Paradkar. 2000. Enzymes catalyzing the early steps of clavulanic acid biosynthesis are encoded by two sets of paralogous genes in Streptomyces clavuligerus. Antimicrob. Agents Chemother. 44:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaleeli, N., R. Li, and C. A. Townsend. 1999. Origin of the β-lactam carbons in clavulanic acid from an unusual thiamine pyrophosphate-mediated reaction. J. Am. Chem. Soc. 121:9223-9224. [Google Scholar]
- 10.Kieser, T., and R. E. Melton. 1988. Plasmid pIJ699, a multi-copy positive-selection vector for Streptomyces. Gene 65:83-91. [DOI] [PubMed] [Google Scholar]
- 11.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, United Kingdom.
- 12.Kumada, Y., E. Takano, K. Nagaoka, and C. J. Thompson. 1990. Streptomyces hygroscopicus has two glutamine synthetase genes. J. Bacteriol. 172:5343-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazzazera, B. A. 2001. The intracellular function of extracellular signaling peptides. Peptides 22:1519-1527. [DOI] [PubMed] [Google Scholar]
- 14.Li, R., N. Khaleeli, and C. A. Townsend. 2000. Expansion of the clavulanic acid gene cluster: identification and in vivo functional analysis of three new genes required for biosynthesis of clavulanic acid by Streptomyces clavuligerus. J. Bacteriol. 182:4087-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh, E. N., M. D. Chang, and C. A. Townsend. 1992. Two isozymes of clavaminate synthase central to clavulanic acid formation: cloning and sequencing of both genes from Streptomyces clavuligerus. Biochemistry 22:12648-12657. [DOI] [PubMed] [Google Scholar]
- 16.Mellado, E., L. M. Lorenzana, M. Rodríguez-Sáiz, B. Díez, P. Liras, and J. L. Barredo. 2002. The clavulanic acid biosynthetic cluster of Streptomyces clavuligerus: genetic organization of the region upstream of the car gene. Microbiology 148:1427-1438. [DOI] [PubMed] [Google Scholar]
- 17.Miller, M. T., B. O. Bachmann, C. A. Townsend, and A. C. Rosenzweig. 2002. The catalytic cycle of beta-lactam synthetase observed by x-ray crystallographic snapshots. Proc. Natl. Acad. Sci. USA 99:14752-14757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosher, R. H., A. S. Paradkar, C. Anders, B. Barton, and S. E. Jensen. 1999. Genes specific for the biosynthesis of clavam metabolites antipodal to clavulanic acid are clustered with the gene for clavaminate synthase 1 in Streptomyces clavuligerus. Antimicrob. Agents Chemother. 43:1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nodwell, R., and R. Losick. 1998. Purification of an extracellular signaling molecule involved in production of aerial mycelium by Streptomyces coelicolor. J. Bacteriol. 180:1334-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nodwell, R., K. McGovern, and R. Losick. 1996. An oligopeptide permease responsible for the import of an extracellular signal governing aerial mycelium formation in Streptomyces coelicolor. Mol. Microbiol. 22:881-893. [DOI] [PubMed] [Google Scholar]
- 21.Omura, S., H. Ikeda, J. Ishikawa, A. Hanamoto, C. Takahashi, M. Shinose, Y. Takahashi, H. Horikawa, H. Nakazawa, T. Osonoe, H. Kikuchi, T. Shiba, Y. Sakaki, and M. Hattori. 2001. Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. USA 98:12215-12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paradkar, A. S., and S. E. Jensen. 1995. Functional analysis of the gene encoding the clavaminate synthase 2 isoenzyme involved in clavulanic acid biosynthesis in Streptomyces clavuligerus. J. Bacteriol. 177:1307-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perego, M., C. F. Higgins, S. R. Pearce, M. P. Gallagher, and J. A. Hoch. 1991. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol. Microbiol. 5:173-185. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Redondo, R. 2000. Genética de la producción de ácido clavulánico en Streptomyces clavuligerus. Ph.D. thesis. University of León, León, Spain.
- 25.Pérez-Redondo, R., A. Rodríguez-García, J. F. Martín, and P. Liras. 1998. The claR gene of Streptomyces clavuligerus, encoding a LysR-type regulatory protein controlling clavulanic acid biosynthesis, is linked to the clavulanate-9-aldehyde reductase (car) gene. Gene 211:311-321. [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Redondo, R., A. Rodríguez-García, J. F. Martín, and P. Liras. 1999. Deletion of the pyc gene blocks clavulanic acid biosynthesis except in glycerol-containing medium: evidence for two different genes in formation of the C3 unit. J. Bacteriol. 181:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero, J., P. Liras, and J. F. Martín. 1984. Dissociation of cephamycin and clavulanic acid biosynthesis in Streptomyces clavuligerus. Appl. Microbiol. Biotechnol. 20:318-325. [Google Scholar]
- 28.Rudner, D. Z., J. R. LeDeaux, K. Ireton, and A. D. Grossman. 1991. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J. Bacteriol. 173:1388-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salowe, S. P., E. N. Marsh, and C. A. Townsend. 1990. Purification and characterization of clavaminate synthase from Streptomyces clavuligerus: an unusual oxidative enzyme in natural product biosynthesis. Biochemistry 29:6499-6508. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and J. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Sánchez, L., and A. F. Braña. 1996. Cell density influences antibiotic biosynthesis in Streptomyces clavuligerus. Microbiology 142:1209-1220. [DOI] [PubMed] [Google Scholar]
- 32.Santamarta, I., A. Rodríguez-García, R. Pérez-Redondo, J. F. Martín, and P. Liras. 2002. CcaR is an autoregulatory protein that binds to the ccaR and the cefD-cmcI promoters of the cephamycin C-clavulanic acid cluster in Streptomyces clavuligerus. J. Bacteriol. 184:3106-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward, J. M., and J. E. Hodgson. 1993. The biosynthetic genes for clavulanic acid and cephamycin production occur as a “super-cluster” in three Streptomyces. FEMS Microbiol. Lett. 110:239-242. [DOI] [PubMed] [Google Scholar]
- 34.Wu, T. K., R. W. Busby, T. A. Houston, D. B. McIlwaine, L. A. Egan, and C. A. Townsend. 1995. Identification, cloning, sequencing, and overexpression of the gene encoding proclavaminate amidino hydrolase and characterization of protein function in clavulanic acid biosynthesis. J. Bacteriol. 177:3714-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, Z., J. Ren, K. Harlos, C. H. McKinnon, I. J. Clifton, and C. J. Schofield. 2002. Crystal structure of a clavaminate synthase-Fe(II)-2-oxoglutarate-substrate-NO complex: evidence for metal centered rearrangements. FEBS Lett. 517:7-12. [DOI] [PubMed] [Google Scholar]