Abstract
Psychophysical evidence suggests that sensations arising from our own movements are diminished when predicted by motor forward models and that these models may also encode the timing and intensity of movement. Here we report a functional magnetic resonance imaging study in which the effects on sensation of varying the occurrence, timing and force of movements were measured. We observed that tactile-related activity in a region of secondary somatosensory cortex is reduced when sensation is associated with movement and further that this reduction is maximal when movement and sensation occur synchronously. Motor force is not represented in the degree of attenuation but rather in the magnitude of this region’s response. These findings provide neurophysiological correlates of previously-observed behavioural forward-model phenomena, and advocate the adopted approach for the study of clinical conditions in which forward-model deficits have been posited to play a crucial role.
Introduction
Once organisms begin to move, sensory systems need to evolve to allow the differentiation of the sensory consequences of their actions from sensations attributable to the external environment. It is well established that this is achieved by a corollary discharge mechanism occurring across a range of species (Crapse and Sommer, 2008), where a corollary signal of the motor command is sent in parallel to the command to modify the sensory input related to that action (Sperry, 1950 and von Holst and Mittelstaedt, 1950). In more complex organisms, this mechanism has become more sophisticated to take account of a greater range of actions; in humans, a forward model of goal-directed action has been proposed (Wolpert, 1997 and Wolpert and Miall, 1996). Thus, provided with a spatiotemporal prediction of the sensory consequences of our actions, state and context estimation is enhanced. The secondary effect of this mechanism is that, by comparing actual and expected sensory feedback, it is possible to distinguish between alternative determinants of a sensory percept, thereby informing decisions of whether it is caused by oneself or another person’s action (Wolpert and Flanagan, 2001).
As it is likely that externally-attributable sensations will carry more novel information than our own actions, it is suggested that processing of environmental, exafferent stimuli should be facilitated by attenuation of the tactile signal associated with planned-motor, reafferent performance. Accordingly, we struggle to tickle ourselves and perceive identical stimuli as more intense when externally-imposed rather than self-generated (Blakemore et al., 1998b, Shergill et al., 2003, Shergill et al., 2005 and Weiskrantz et al., 1971). To exemplify this, when a force is applied (via a small lever attached to a torque motor) to one index finger and healthy individuals are required to match that force, they consistently overestimate the necessary force when they push directly on the lever with their other index finger. By contrast, they are able to accurately match that force when the task requires them to use a joystick to control the torque motor and replicate the force on the first finger (Shergill et al., 2005). These results suggest that in direct action of one body part on another, subjects anticipate the sensory consequences of their actions and attenuate the associated percept, thus requiring a larger force to match that experienced passively. The potential consequences of a failure in this system have been demonstrated in psychotic illness (Ford et al., 2001 and Shergill et al., 2005).
There are several key aspects of effective prediction which remain unclear: the role of timing and intensity of the motor action, and the neural level at which it operates. Our earlier work has suggested that attenuation of sensory processing related to movement is highly specific for the timing of events (Bays et al., 2006), but not so for the intensity of the action (Bays and Wolpert, 2007). Experimentally shifting the timing of the action relative to the sensory input revealed that perceptual attenuation is maximal when action and sensory stimulation are synchronous (Bays et al., 2005). However, the temporal range of the attenuation is approximately symmetrical over a period that is considerably broader than the specific duration of the action, suggestive of some uncertainty of the internal model in predicting the time of the contact event, or a ‘safety margin’ built into this systems to allow for the possibility of a prediction error (Bays and Wolpert, 2007).
Studies of the functional anatomy of sensorimotor prediction posit a role for both the cerebellum and parietal cortex (Blakemore and Sirigu, 2003); the cerebellum as an interface comparing signals between the motor prediction and sensory consequences, and functionally important for motor learning (Blakemore et al., 2001). The parietal operculum bilaterally is associated with sensory perception arising as a consequence of self-produced movement, relative to that due to externally produced movement (Blakemore et al., 1998a). Recent functional magnetic resonance imaging (fMRI) studies of somatosensory processing during motion and rest suggest a role for the secondary somatosensory cortex (SII) and insula during movement preparation (Jackson et al., 2011 and Parkinson et al., 2011). SII has been ascribed several functions which are predicated on manual attention, in tasks requiring manual exploration and tactile object recognition (Burton, 2001 and Valenza et al., 2001). Insular cortex responds more generally to cognitively, emotionally and homeostatically salient stimuli (Menon, 2011). Diminished activity in these regions during volitional movement suggests that attention towards behaviourally irrelevant objects is minimised to facilitate proprioceptive motor performance (Jackson et al., 2011; Nelson, 1996). This is mediated by a network of premotor regions including supplementary motor area, cingulate motor area and bilateral premotor cortex, which have been shown to significantly influence SII and insular activation during sensorimotor integration (Parkinson et al., 2011).
Guided by this neuroimaging literature and previous psychophysical reports (Bays et al., 2005, Blakemore et al., 1998a, Blakemore et al., 1998b, Blakemore et al., 2000 and Jackson et al., 2011) we conducted an fMRI study in which we manipulated the link between action and its consequences as well as the timing of this link. We hypothesised that somatosensory blood-oxygenation level dependent (BOLD) response associated with sensory stimulation resulting from a synchronous motor act would be attenuated when compared to that produced by a qualitatively-identical tactile stimulus experienced in the absence of an action. We also predicted that this putative BOLD response attenuation would be significantly reduced when a temporal delay was introduced between performance the action and its sensory consequence. In light of previous neuroimaging findings (Blakemore et al., 1998b and Hesse et al., 2010), analysis was focused on response in SII, primary somatosensory cortex (SI) and cerebellum. In addition, on the basis that incorporating physical characteristics of movement within forward models is likely to improve their effectiveness, we investigated the relationship between the force of the movement and the associated modulation of sensorimotor BOLD responses.
Material and methods
Participants
Fifteen individuals with no reported personal history of neurological or psychiatric illness or drug dependence (age: 32.9 ± 7.8 years) were recruited to take part in this fMRI study. Ethical approval was provided by South London and Maudsley Research and Ethics Committee. All participants provided informed written consent and were given a monetary inconvenience allowance for participation in the study.
Experimental procedure
Participants performed a sensorimotor task comprising two 14-minute sessions, containing a total of 200 randomly-ordered experimental trials split equally between the experimental conditions and 60 randomly-interpolated null trials. To facilitate the required sustained attention, sessions were split by a short relaxation period, during which the participants remained in the scanner. Participants viewed a screen onto which visual stimuli were projected through appropriately aligned mirrors mounted on the scanner headcoil. The experimental apparatus is depicted in Fig. 1 and force measured through the use of two pressure sensors mounted one above the other (Bays et al., 2005). The upper sensor was fixed in space while the lower was mounted on the end of a lever that was attached to a small torque motor. This permitted a tap (by the right index finger) on the upper sensor to be transmitted synchronously, asynchronously with a 500 ms delay, or not at all to the left index finger. The tactile stimulus could also be presented with or without the right finger tap. The experiment was arranged as eight experimental conditions in a 2 × 2 × 2 factorial design. The factors were 1) the presence or absence of self-generated movement, that is the right finger tap on the upper sensor (M – 0/1); 2) the presence or absence of a tactile stimulus was delivered to the left finger (S – 0/1); and 3) the presence or absence of a 500-millisecond delay between the application of the right finger tap and its transmission to the left finger (D – 0/1). Thus the eight experimental conditions were self-produced tactile stimuli (M1S1), externally produced tactile stimuli (M0S1), self-produced movement without tactile stimuli (M1S0) and rest (M0S0) – each with and without a 500 ms delay (M1S1D0, M1S1D1, M1S0D0, M1S0D1, M0S1D0, M0S1D1, M0S0D0, M0S0D1). The use of a factorial design necessitated the inclusion of delay trials for each of the four primary conditions; although, there was no real difference between the trials where the delay coincided with an absence of tactile stimuli. Each trial lasted 6.5 s and consisted of a visual cue indicating ‘TAP’ or ‘DON’T TAP’ (1 s), a countdown (1.5 s), a response period (1 s) and a rest period (3 s).
Fig. 1.
The experimental set-up, showing right-index finger movement and effected left-index finger sensation. (RH — right hand; LH — left hand).
Blood oxygenation level-dependent (BOLD) functional images were acquired on a GE 3 Tesla system (Signa Excite, General Electric, Milwaukee, WI) with an 8-channel head coil using an echo planar imaging sequence with the following parameters: repetition time (TR): 2600 ms, echo time (TE): 30 ms, flip angle: 90°. In each of two 14-minute sessions, 166 volumes comprising forty descending, sequentially-ordered 2 mm axial slices (with 1.6 mm gap between slices) and an in-plane resolution of 3 mm × 3 mm were acquired.
fMRI data preprocessing and analysis
fMRI data were preprocessed using SPM5 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, University of London, UK). Data were realigned to the first image, normalised to a standard template of the MNI brain and smoothed using an 8-mm full-width at half-maximum Gaussian kernel.
First-level event-related general linear models (GLMs) were constructed for each participant. These included a regressor predicting the BOLD response to each condition by convolving a vector of delta functions for the onset of the response instruction for that condition with the canonical haemodynamic response function. The first and second derivatives of these timecourses were also calculated and included as further regressors for each condition. Effects of head motion were minimised by the inclusion of six realignment parameter vectors as regressors of no interest.
First-level contrast images were calculated for the canonical responses to each of the eight experimental conditions. The approach of modelling temporal and dispersion derivatives but not including these regressors in contrast images has been shown to optimally reflect canonical responses having accounted for non-standard responses (Steffener et al., 2010). First-level contrast images for the eight experimental conditions were entered into a full-factorial analysis of variance (ANOVA) model with a 2 × 2 × 2 design to include three within-subject binary factors: motion; sensation; and delay. Grand-mean scaling was omitted from this procedure. Main effects of these factors and their interaction were assessed by constructing F-contrast images. Clusters were judged to be significantly activated on the basis of spatial extent and number of contiguous supra-threshold voxels using an uncorrected voxel-level inclusion criterion of P < .001 and a cluster-level significance threshold of P < .05, corrected.
Region of interest analyses of movement effects and their modulation by delay
In addition to the whole-head analysis, a region of interest (ROI) approach was adopted to investigate task effects in SI, SII and cerebellum. For these regions, mean data for a sphere of 6-mm radius were extracted and activity in these spheres assessed using the same ANOVA models as in whole-head mass univariate analysis. ROIs were centred on the foci of previously published forward-model effects for SII (x = 42, y = − 24, z = 18) and culmen of the anterior lobe of the cerebellum (x = 22, y = − 58, z = − 22) according to Blakemore and colleagues (Blakemore et al., 1998b). For SI, the ROI was centred on the index finger locus identified during somatotopic mapping of SI using fMRI (x = 49, y = − 19; z = 45) (Francis et al., 2000). SI and SII analyses were limited to grey-matter voxels within these, using a binarized template mask with the aim of enhancing sensitivity for neuronally-derived signals. The ROI mean betas were exported to SPSS (version 20, IBM Corp., New York) for statistical examination. Repeated-measures ANOVA tests were performed for each ROI independently, including movement, sensation and delay as within-subject factors and investigating the main effects of these factors and their interaction.
To more explicitly investigate effects of movement and delay on somatosensory activation, a further ROI analysis was conducted looking at the three most pertinent experimental conditions: M1S1D0, M1S1D1 and M0S1D0. To ascertain whether movement significantly reduced concomitant somatosensory responses, mean contrast estimates within these regions for the M1S1D0 and M0S1D0 conditions were compared using a paired-samples T-test for each region. To ascertain whether the introduction of delay modulated the predicted somatosensory attenuation, comparisons between the contrast estimates for the M1S1D0 and M1S1D1 conditions were judged using further paired-samples T-tests.
Force modulation of sensorimotor BOLD response
Condition-specific average forces following right index-finger movement were calculated for each individual (for conditions involving right finger movement), and their variation on the basis of experimental manipulation of sensation and delay on these forces assessed using a 2 × 2 repeated-measures ANOVA test.
To investigate the relationship between exerted force and BOLD activity on a single-trial basis, the recorded force measurements were included as first-order parametric modulators of BOLD activity for events in the four conditions including right index-finger movement (M1S1D0, M1S1D1, M1S0D0 and M1S0D1) in first-level GLMs otherwise identical to those described above (fMRI data preprocessing and analysis section). Contrast images were calculated for each individual for the overall effect of force modulation across all four conditions. Mean time-series contrast estimates of grey-matter voxels within three 6-mm radius sphere regions of interest centred on the peak locations found in primary motor cortex (MI; second-level main effect of movement), SI and SII (as described above) were calculated for each individual. One-sample T-tests were used for each region independently to test whether these contrast estimates significantly differed from zero.
Results
Brain activation
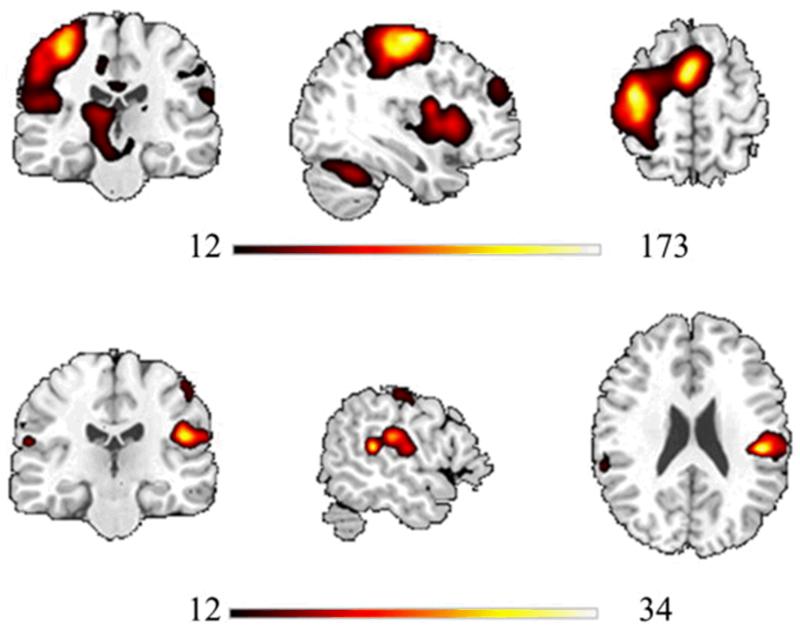
Significant self-generated-movement related activation (Table 1(A); Fig. 2) was found in regions including left precentral gyrus (MI), superior frontal gyrus, cerebellum, thalamus and basal ganglia. Significant tactile-sensation related effects (Table 1(B); Fig. 2) were observable in right postcentral gyrus (Brodmann Area 3; SI) and bilateral postcentral gyrus (Brodmann Areas 40 and 43; SII). No significant clusters of activation were observed for the main effect of delay or any interaction effects after correcting for multiple comparisons at whole-brain level.
Table 1.
Significant grey-matter activations associated with movement and sensation.
| Brain structure (Brodmann area) | Talairach coordinates | Peak voxel F-value |
|---|---|---|
| (A) Local maxima for main effect of movement | ||
| Precentral gyrus (4) | − 39 – 12 56 | 169.80 |
| Superior frontal gyrus (6) | 0 5 49 | 171.94 |
| Inferior frontal gyrus (9) | − 59 7 30 | 70.28 |
| Precentral gyrus (44) | − 56 9 11 | 44.80 |
| Cingulate gyrus (32) | − 6 19 32 | 73.34 |
| Cingulate gyrus (23) | − 3 – 16 28 | 20.05 |
| Insula (13) | − 36 1 14 | 73.34 |
| Superior temporal gyrus (42) | 62 – 31 21 | 37.38 |
| Cuneus (17) | 15 – 90 5 | 37.48 |
| Cerebellum | 15 – 53 – 12 | 160.92 |
| Thalamus, ventral lateral nucleus | − 12 – 17 4 | 186.90 |
| Thalamus, ventral anterior nucleus | 12 – 6 9 | 42.44 |
| Putamen | − 21 9 0 | 73.77 |
| Putamen | 18 12 – 1 | 51.63 |
| (B) Local maxima for main effect of sensation | ||
| Postcentral gyrus (43) | 50 – 14 17 | 27.44 |
| Postcentral gyrus (40) | − 56 – 20 18 | 15.93 |
| Postcentral gyrus (3) | 56 – 10 42 | 14.19 |
| Middle temporal gyrus (22) | − 48 – 58 11 | 18.51 |
Fig. 2.
Significant main effects of movement (top) and tactile sensation (bottom) overlaid on a standardised T1-weighted image. These maps were produced using the whole-brain ANOVA carried out to investigate whole-brain effects. Voxels significant at P < .001 uncorrected threshold. Condition-specific colour bars below each effect display F-value scales.
As Table 2 illustrates, SII exhibited significant main effects of sensation and delay, and movement by sensation, movement by delay and movement by sensation by delay interactions. SI displayed significant main effects of movement and sensation, while only a highly-significant main effect of movement was observed in cerebellum. However, noteworthy trends towards interaction effects were observed between sensation and delay in cerebellum, and between movement and sensation in SI.
Table 2.
Region-specific repeated-measures analysis of variance results. Bold type denotes significant main effect of task or associated interaction.
| Region of interest | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SI | SII | Cerebellum | |||||||
| Effect/interaction | Mean sum of squares | F-value | P-value | Mean sum of squares | F-value | P-value | Mean sum of squares | F-value | P-value |
| M | 3.81 | 5.07 | .041 | 0.71 | 0.38 | .548 | 127.65 | 69.67 | 8 × 10− 7 |
| S | 7.34 | 10.24 | .006 | 13.44 | 11.20 | .005 | 2.18 | 1.58 | .270 |
| D | 0.44 | .59 | .455 | 5.60 | 9.57 | .008 | 0.97 | 1.92 | .187 |
| M × S | 1.66 | 3.88 | .069 | 1.98 | 5.78 | .033 | 2.15 | 2.60 | .129 |
| M × D | 0.37 | 0.37 | .554 | 4.05 | 7.12 | .018 | 0.38 | 0.22 | .650 |
| S × D | 0.78 | 1.34 | .267 | 1.62 | 2.13 | .166 | 4.06 | 4.22 | .059 |
| M × S × D | 0.02 | 0.02 | .902 | 3.41 | 7.20 | .018 | 0.34 | 0.42 | .527 |
M, motion; S, sensation; D, delay.
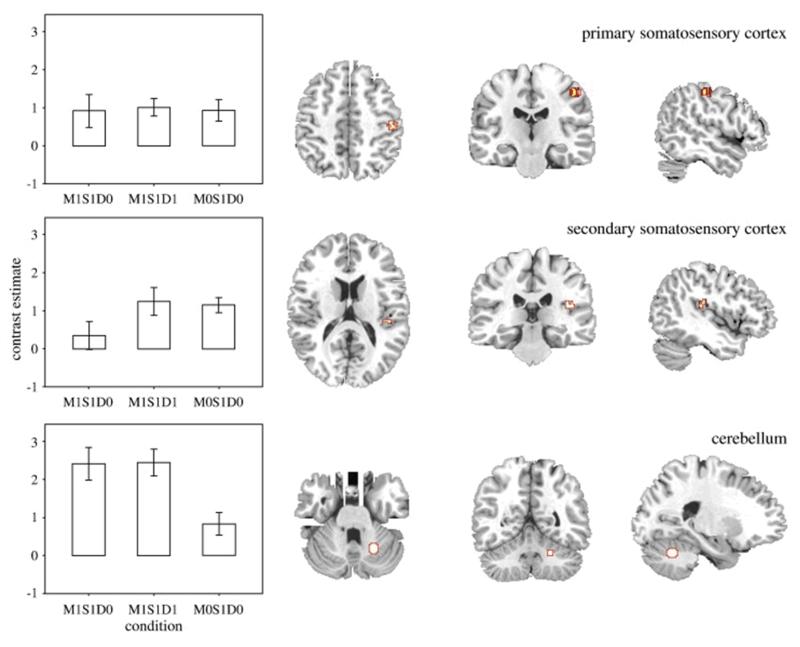
Investigation of condition-specific effects revealed that the response during movement and synchronous sensation (M1S1D0) was reduced in SII when compared to sensation alone (M0S1D0; T(14) = 2.20, P = .045) and also when compared to movement accompanied by a delayed sensation (M1S1D1; T(14) = 3.25, P = .006; Fig. 3). This pattern of response was specific to SII. By contrast, in SI no significant difference in activation was observed between the three conditions (Fig. 3). In cerebellum, significantly increased response was observed during M1S1D0 when compared to the M0S1D0 condition (T(14) = 6.23, P = 2 × 10− 5; Fig. 3).
Fig. 3.
Condition-specific task estimates and region of interest location, for primary somatosensory cortex (top), secondary somatosensory cortex (middle) and cerebellum (bottom). Error bars denote standard error of the mean.
Force
No significant difference was observed in force exerted between the four conditions involving right index-finger movement (M1S1D0: 2.11 ± 0.51 N (mean ± standard deviation); M1S1D1: 2.16 ± 0.51 N; M1S0D0: 2.14 ± 0.48 N; M1S0D1: 2.16 ± 0.48 N).
Across movement-containing conditions force significantly and linearly modulated BOLD response in MI (contrast estimate: 2.55 ± 1.09; T(14) = 2.34, P = .035), and notably, also in SII (contrast estimate: 2.30 ± 0.97; T(14) = 2.38, P = .032). Force did not significantly modulate BOLD activity in SI.
Discussion
By systematically controlling the occurrence and timing of a left index-finger sensation associated with a right index-finger movement, it was possible to investigate the neural basis of the modification of sensory processing by putative forward models of motor planning in this fMRI study. A localised region of SII exhibited activity consistent with aspects of these models observed in previous psychophysical experiments.
In this region of SII, activation was significantly reduced when left-finger sensation was a direct consequence of self-generated movement as compared with when it was generated mechanically, demonstrating it to be a focus of movement-induced tactile attenuation. Importantly, SII activation was also significantly attenuated in the synchronous movement and sensation condition as compared with the same condition with a delay of 500 ms between movement and sensation. The data demonstrate that the attenuation of activation within SII is maximal when sensation occurs at a predicted time point, in accord with previous behavioural force-matching studies (Bays et al., 2005 and Johansson and Westling, 1988), and thereby providing a neural correlate of these reported psychophysical effects. While it has been previously shown that this region’s activity is attenuated by self-movement (Blakemore et al., 1998b and Blakemore et al., 2000), this study shows for the first time that the attenuation evident here is lessened if there is onset delay between the associated motor and tactile events.
While the data provide strong evidence of forward-model outputs being expressed in SII, the pattern of activation exhibited by SI and cerebellum also implicates these regions. Targeted evaluation of the activity in these latter regions in three pertinent conditions suggested that their activity was not attenuated in the same manner as SII. However, there was a trend for an interaction effect between movement and sensation in SI when considering all eight experimental conditions. This latter finding supports previous magnetoencephalography reports of movement-induced tactile attenuation in SI (Hesse et al., 2010). Moreover, electroencephalographic reports of attenuated somatosensory evoked potential (SEP) amplitude in a time window representative of SI function in response to peripheral nerve stimulation during movement (Jones et al., 1989 and Rushton et al., 1981), and the finding that the relative timing of motion and sensation determines degree of attenuation (Rushton et al., 1981), suggest that sensory suppression is coded in SI. However, since SI SEP attenuation is equivalent during both passive and active movement (Rushton et al., 1981), SI gating may not be specifically a forward-model phenomenon.
Despite the characteristics of the investigated region of anterior cerebellar lobe suggesting it to directly influence motion, there was nevertheless a trend for this region to exhibit a sensation by delay interaction. The results on the whole do not therefore preclude SI or cerebellar involvement in predictive motor models but rather suggest primacy of SII as the downstream locus of the sensory attenuation that they encode. That several cortical regions encode forward-model correlates has also been found in relation to vocalisation, where both middle and superior temporal structures are seen to be involved (Doehrmann et al., 2010 and Wild et al., 2012). It is feasible that the characterisation of regional-specific aspects of motor prediction is complicated by non-linearity and the influence of local inhibitory processes. However, several notable findings implicating the cerebellum have been recently made. In an elegant study using transcranial magnetic stimulation to dampen motor cortex reactivity, the source of forward models was demonstrated to be upstream of primary motor cortex (Voss et al., 2006), indirectly implicating cerebellum. Furthermore, while cerebellum is essential for visually-guided movement perhaps the most direct evidence of cerebellar involvement in internal predictive models was provided by recent single-unit observations from cat cerebellar Purkinje cells. Tonic activity initiated and correlated with visual target movement, but dissociated from eye or limb movement, was found to be sustained during transient disappearance of the target suggesting it to relate not directly to motor performance but rather to the spatiotemporal prediction of movement (Cerminara et al., 2009).
This work does not provide strong evidence for thalamic involvement in forward-model estimation, in line with previous work demonstrating SI somatosensory gating persists in thalamotomised Parkinson’s patients (Rushton et al., 1981). However, activation was observed in two first-order motor relay nuclei, the ventral anterior and ventral lateral nuclei, as a main effect of motion. It is feasible that these structures contribute to sensorimotor prediction in light of their respective driver input from cerebellum and basal ganglia and direct connections with primary and premotor cortical structures (for review, see Sherman, 2005). Targeted investigation of their role is a worthwhile future objective but must make use of optimised high-resolution scanning protocols given the scale of these structures.
It is has been suggested that motor forward models may code physical attributes of movement, such as intensity, in addition to their timing; however, psychophysical data suggest that tactile attenuation is not modulated by movement force (Bays and Wolpert, 2007). This study found a significant positive linear relationship between movement-related force and sensorimotor BOLD activity in MI in line with monkey cellular activity findings (Georgopoulos et al., 1992). Another principal finding of this study is the observation of a significant positive linear relationship between motor force and activation in SII but not SI. In other words, while synchronous self-action attenuates the magnitude of SII tactile response, the force of the action is positively related with the magnitude of the activation rather than the magnitude of its attenuation. This makes ecological sense – a sensorimotor system in which tactile attenuation was positively related to self-action magnitude would be insensitive to large and potentially meaningful environmental events occurring simultaneously with self-movements. The specificity of this effect to SII (but not SI) further implicates this region as a fundamental correlate of previously reported psychophysical forward-model effects.
Several fMRI studies have previously investigated force modulation of BOLD activity. Dai et al. (2001) reported widespread BOLD signal intensity increases with increased force suggesting that force is encoded in a distributed network including MI, SI, SII, prefrontal cortex, premotor cortex and cerebellum. Ehrsson et al. (2000) reported greater activity in a combined MI/SI region and SII associated with large as compared to a small precision-grip force. More recently, Kuhtz-Buschbeck et al. (2008) showed a positive linear relationship with grip force in MI/SI and cerebellum but not SII. As such, while our current trend towards a positive modulation of SII but not SI activity by force suggests SII to be a directed focus of downstream physical characteristics of action, the literature is not unequivocal. An intriguing recent report of mouse whisker control suggests a more active role of sensory cortex in the initiation of motor action (Matyas et al., 2010). While this phenomenon has not been reported in humans, there are therefore some grounds to suggest that our current understanding of the regional specialisation of sensorimotor control warrants re-evaluation. It also remains to be seen whether the regions previously implicated all code force directly or whether the force signal is transmitted from one hub around this sensorimotor network. Movement-related force magnitudes in the current study were small; given this and the findings presented in this work, systematic study of SII attenuation of neural response associated with actions more wide-ranging force magnitudes is suggested.
There are two task-related caveats of our results. First, it is noteworthy that the movements, although self-generated were visually cued. A recent fMRI meta-analysis of finger-tapping experiments demonstrated that the neural systems employed during finger tapping differed on the basis of the presence and sensory modality of cue (Witt et al., 2008). Therefore, while it is considered unlikely that the current results are entirely dependent on the movements being visually prompted, it is plausible that the systems contributing to tactile suppression differ according to the contextual foundations of the movement involved. Second, the experimental design makes a categorical distinction between conditions that evoke tactile sensation and those that do not. However, those conditions in which the right finger moved induced sensation on the moving hand. While it appears that SII responds bilaterally to unilateral peripheral stimulation, it has been shown previously that SII response is lateralised to the hemisphere contralateral to response (White et al., 2009), and as such our investigation of right-hemispheric SII effects can be reasonably asserted to reflect tactile responses of the left index finger. Nevertheless, these considerations highlight the complexity of studying sensorimotor prediction using bimanual interactions.
The work presented in this paper has potential clinical relevance. Forward-model deficits have been hypothesised to play an important generative role in hallucinatory perception (Feinberg, 1978, Ford et al., 2001, Frith and Done, 1988, Shergill et al., 2005 and Simons et al., 2010). Accordingly, prediction failures result in diminished attenuation of tactile sensation associated with inner speech. We therefore advocate investigation of motor-induced tactile attenuation in SII in individuals with psychosis as a means of directly testing this hypothesis.
Highlights.
▶ We report perceptual effects of varying timing and intensity of willed movement.
▶ Secondary somatosensory cortex activation is reduced in association with action.
▶ The introduction of delay between movement and sensation reduces this attenuation.
Acknowledgments
This work was supported by a Medical Research Council New Investigator award to S.S.S, and developed by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, and a joint infrastructure grant from Guy’s and St Thomas’ Charity and the Maudsley Charity. We would like to thank all the volunteers for their participation in this study.
References
- Bays and Wolpert, 2007.Bays PM, Wolpert DH. Predictive attenuation in the perception of touch. In: Haggard P, Rossetti Y, Kawato M, editors. Sensorimotor Foundations of Higher Cognition: Attention and Performance XXII. Oxford University Press; Oxford: 2007. [Google Scholar]
- Bays et al., 2005.Bays PM, Wolpert DM, Flanagan JR. Perception of the consequences of self-action is temporally tuned and event driven Curr. Biol. 2005;15:1125–1128. doi: 10.1016/j.cub.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Bays et al., 2006.Bays PM, Flanagan JR, Wolpert DM. Attenuation of self-generated tactile sensations is predictive, not postdictive. PLoS Biol. 2006;4:e28. doi: 10.1371/journal.pbio.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore and Sirigu, 2003.Blakemore SJ, Sirigu A. Action prediction in the cerebellum and in the parietal lobe. Exp. Brain Res. 2003;153:239–245. doi: 10.1007/s00221-003-1597-z. [DOI] [PubMed] [Google Scholar]
- Blakemore et al., 1998a.Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: the role of sensorimotor context estimation. J. Neurosci. 1998;18:7511–7518. doi: 10.1523/JNEUROSCI.18-18-07511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore et al., 1998b.Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat. Neurosci. 1998;1:635–640. doi: 10.1038/2870. [DOI] [PubMed] [Google Scholar]
- Blakemore et al., 2000.Blakemore SJ, Wolpert D, Frith C. Why can’t you tickle yourself? Neuroreport. 2000;11:R11–R16. doi: 10.1097/00001756-200008030-00002. [DOI] [PubMed] [Google Scholar]
- Blakemore et al., 2001.Blakemore SJ, Frith CD, Wolpert DM. The cerebellum is involved in predicting the sensory consequences of action. Neuroreport. 2001;12:1879–1884. doi: 10.1097/00001756-200107030-00023. [DOI] [PubMed] [Google Scholar]
- Burton, 2001.Burton H. Cerebral cortex regions devoted to the somatosensory system: results from brain imaging studies in humans. In: Nelson RJ, editor. The Somatosensory System: Deciphering the Brain’s Own Body Image. CRC Press; New York: 2001. pp. 27–72. [Google Scholar]
- Cerminara et al., 2009.Cerminara NL, Apps R, Marple-Horvat DE. An internal model of a moving visual target in the lateral cerebellum. J. Physiol. 2009;587:429–442. doi: 10.1113/jphysiol.2008.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse and Sommer, 2008.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat. Rev. Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai et al., 2001.Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH. Relationship between muscle output and functional MRI-measured brain activation. Exp. Brain Res. 2001;140:290–300. doi: 10.1007/s002210100815. [DOI] [PubMed] [Google Scholar]
- Doehrmann et al., 2010.Doehrmann O, Weigelt S, Altmann CF, Kaiser J, Naumer MJ. Audiovisual functional magnetic resonance imaging adaptation reveals multisensory integration effects in object-related sensory cortices. J. Neurosci. 2010;30:3370–3379. doi: 10.1523/JNEUROSCI.5074-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson et al., 2000.Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision - versus power-grip tasks: an fMRI study. J. Neurophysiol. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Feinberg, 1978.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr. Bull. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- Ford et al., 2001.Ford JM, Mathalon DH, Heinks T, Kalba S, Faustman WO, Roth WT. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am. J. Psychiatry. 2001;158:2069–2071. doi: 10.1176/appi.ajp.158.12.2069. [DOI] [PubMed] [Google Scholar]
- Francis et al., 2000.Francis ST, Kelly EF, Bowtell R, Dunseath WJ, Folger SE, McGlone F. fMRI of the responses to vibratory stimulation of digit tips. Neuroimage. 2000;11:188–202. doi: 10.1006/nimg.2000.0541. [DOI] [PubMed] [Google Scholar]
- Frith and Done, 1988.Frith CD, Done DJ. Towards a neuropsychology of schizophrenia. Br. J. Psychiatry. 1988;153:437–443. doi: 10.1192/bjp.153.4.437. [DOI] [PubMed] [Google Scholar]
- Georgopoulos et al., 1992.Georgopoulos AP, Ashe J, Smyrnis N, Taira M. The motor cortex and the coding of force. Science. 1992;256:1692–1695. doi: 10.1126/science.256.5064.1692. [DOI] [PubMed] [Google Scholar]
- Hesse et al., 2010.Hesse MD, Nishitani N, Fink GR, Jousmaki V, Hari R. Attenuation of somatosensory responses to self-produced tactile stimulation. Cereb. Cortex. 2010;20:425–432. doi: 10.1093/cercor/bhp110. [DOI] [PubMed] [Google Scholar]
- Jackson et al., 2011.Jackson SR, Parkinson A, Pears SL, Nam SH. Effects of motor intention on the perception of somatosensory events: a behavioural and functional magnetic resonance imaging study. Q. J. Exp. Psychol. (Hove) 2011;64:839–854. doi: 10.1080/17470218.2010.529580. [DOI] [PubMed] [Google Scholar]
- Johansson and Westling, 1988.Johansson RS, Westling G. Programmed and triggered actions to rapid load changes during precision grip. Exp. Brain Res. 1988;71:72–86. doi: 10.1007/BF00247523. [DOI] [PubMed] [Google Scholar]
- Jones et al., 1989.Jones SJ, Halonen JP, Shawkat F. Centrifugal and centripetal mechanisms involved in the ‘gating’ of cortical SEPs during movement Electroencephalogr. Clin. Neurophysiol. 1989;74:36–45. doi: 10.1016/0168-5597(89)90049-x. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck et al., 2008.Kuhtz-Buschbeck JP, Gilster R, Wolff S, Ulmer S, Siebner H, Jansen O. Brain activity is similar during precision and power gripping with light force: an fMRI study. Neuroimage. 2008;40:1469–1481. doi: 10.1016/j.neuroimage.2008.01.037. [DOI] [PubMed] [Google Scholar]
- Matyas et al., 2010.Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CC. Motor control by sensory cortex. Science. 2010;330:1240–1243. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- Menon, 2011.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Nelson, 1996.Nelson RJ. Interactions between motor commands and somatic perception in sensorimotor cortex. Curr. Opin. Neurobiol. 1996;6:801–810. doi: 10.1016/s0959-4388(96)80031-6. [DOI] [PubMed] [Google Scholar]
- Parkinson et al., 2011.Parkinson A, Plukaard S, Pears SL, Newport R, Dijkerman C, Jackson SR. Modulation of somatosensory perception by motor intention. Cogn. Neurosci. 2011;2:47–56. doi: 10.1080/17588928.2010.525627. [DOI] [PubMed] [Google Scholar]
- Rushton et al., 1981.Rushton DN, Rothwell JC, Craggs MD. Gating of somatosensory evoked potentials during different kinds of movement in man. Brain. 1981;104:465–491. doi: 10.1093/brain/104.3.465. [DOI] [PubMed] [Google Scholar]
- Shergill et al., 2003.Shergill SS, Bays PM, Frith CD, Wolpert DM. Two eyes for an eye: the neuroscience of force escalation. Science. 2003;301:187. doi: 10.1126/science.1085327. [DOI] [PubMed] [Google Scholar]
- Shergill et al., 2005.Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am. J. Psychiatry. 2005;162:2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- Sherman, 2005.Sherman SM. Thalamic relays and cortical functioning. Prog. Brain Res. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- Simons et al., 2010.Simons CJ, Tracy DK, Sanghera KK, O’Daly O, Gilleen J, Dominguez MD, Krabbendam L, Shergill SS. Functional magnetic resonance imaging of inner speech in schizophrenia. Biol. Psychiatry. 2010;67:232–237. doi: 10.1016/j.biopsych.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Sperry, 1950.Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J. Comp. Physiol. Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Steffener et al., 2010.Steffener J, Tabert M, Reuben A, Stern Y. Investigating hemodynamic response variability at the group level using basis functions. Neuroimage. 2010;49:2113–2122. doi: 10.1016/j.neuroimage.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza et al., 2001.Valenza N, Ptak R, Zimine I, Badan M, Lazeyras F, Schnider A. Dissociated active and passive tactile shape recognition: a case study of pure tactile apraxia. Brain. 2001;124:2287–2298. doi: 10.1093/brain/124.11.2287. [DOI] [PubMed] [Google Scholar]
- von Holst and Mittelstaedt, 1950.von Holst E, Mittelstaedt H. Das Reafferenzprincip. Naturwissenschaft. 1950;37(37):464–476. [Google Scholar]
- Voss et al., 2006.Voss M, Ingram JN, Haggard P, Wolpert DM. Sensorimotor attenuation by central motor command signals in the absence of movement. Nat. Neurosci. 2006;9:26–27. doi: 10.1038/nn1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz et al., 1971.Weiskrantz L, Elliott J, Darlington C. Preliminary observations on tickling oneself. Nature. 1971;230:598–599. doi: 10.1038/230598a0. [DOI] [PubMed] [Google Scholar]
- White et al., 2009.White TP, Francis ST, Joseph V, O’Regan E, Head KE, Liddle PF. Evidence for reduced somatosensory lateralisation and focalisation in schizophrenia. Psychiatry Res. 2009;174:24–31. doi: 10.1016/j.pscychresns.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Wild et al., 2012.Wild CJ, Davis MH, Johnsrude IS. Human auditory cortex is sensitive to the perceived clarity of speech. Neuroimage. 2012;60:1490–1502. doi: 10.1016/j.neuroimage.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Witt et al., 2008.Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage. 2008;42:343–356. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert, 1997.Wolpert DM. Computational approaches to motor control. Trends Cogn. Sci. 1997;1:209–216. doi: 10.1016/S1364-6613(97)01070-X. [DOI] [PubMed] [Google Scholar]
- Wolpert and Flanagan, 2001.Wolpert DM, Flanagan JR. Motor prediction. Curr. Biol. 2001;11:R729–R732. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Wolpert and Miall, 1996.Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]