Abstract
Background
To determine the predictors of clinical outcomes following surgical descending thoracic aortic (DTA) repair.
Methods
We identified 103 patients (23 females; mean age, 64.1±12.3 years) who underwent DTA replacement from 1999 to 2011 using either deep hypothermic circulatory arrest (44%) or partial cardiopulmonary bypass (CPB, 56%).
Results
The early mortality rate was 4.9% (n=5). Early major complications occurred in 21 patients (20.3%), which included newly required hemodialysis (9.7%), low cardiac output syndrome (6.8%), pneumonia (7.8%), stroke (6.8%), and multi-organ failure (3.9%). None experienced paraplegia. During a median follow-up of 56.3 months (inter-quartile range, 23.1 to 85.1 months), there were 17 late deaths and one aortic reoperation. Overall survival at 5 and 10 years was 80.9%±4.3% and 71.7%±5.9%, respectively. Reoperation-free survival at 5 and 10 years was 77.3%±4.8% and 70.2%±5.8%. Multivariable analysis revealed that age (hazard ratio [HR], 1.10; 95% confidence interval [CI], 1.05 to 1.15; p<0.001) and left ventricle (LV) function (HR, 0.88; 95% CI, 0.82 to 0.96; p<0.003) were significant and independent predictors of long-term mortality. CPB strategy, however, was not significantly related to mortality (p=0.49).
Conclusion
Surgical DTA repair was practicable in terms of acceptable perioperative mortality/morbidity as well as favorable long-term survival. Age and LV function were risk factors for long-term mortality, irrespective of the CPB strategy.
Keywords: 1. Aorta, 2. Descending thoracic aorta, 3. Cardiopulmonary bypass
INTRODUCTION
Open surgical repair remains a gold standard therapeutic option in the management of descending thoracic aorta (DTA) aneurysm. Because of the progress in cardiopulmonary bypass (CPB) strategies, spinal cord protective adjuncts, as well as improvements in postoperative care, outcomes of open repair of DTA have improved [1–3]. Despite these improvements, morbidity and mortality rates in DTA replacement still remain high [2–4], with the recent data showing operative mortality rate of 4.7%, lower extremity paralysis rate of 3.4%, and stroke rate of 2.7% even in the high-volume aortic centers [5]. These figures might be challenging in low-volume aortic centers. In this context, concerns regarding the challenging risks of open surgical repair of DTA have led to the global atmosphere favoring thoracic endovascular aortic repair (TEVAR) more frequently even without hard evidence in support of the long-term benefits of TEVAR [6].
Reviewing the literature published in Korea, only a few studies have analyzed clinical outcomes of open surgical repair of DTA, of which the largest-scale study is one that evaluated 22 patients decades ago [7]. We therefore sought to evaluate the outcomes following open surgical repair of DTA in a reasonably sized cohort in the current era and to determine independent predictors of long-term outcomes.
METHODS
1). Patients and operative techniques
Out of 212 patients who underwent DTA repair between June 1999 and August 2011 at Asan Medical Center, we identified 103 patients (mean age, 53.8±13.1 years; 23 women) who underwent aortic surgery only for thoracic aortic segments. One hundred and nine patients who underwent aortic surgery combined with thoracoabdominal aorta repair and arch replacement were excluded in this study.
The operative procedure was conducted as follows: Patients were intubated with a double lumen endotracheal tube. Cerebrospinal fluid (CSF) drainage was in 56 patients (54%) who were supposed to receive lower thoracic aorta replacement. Left posterolateral thoracotomy was performed for all patients, and the level of entrance (4–6th intercostal spaces) was determined on the basis of the aortic segment replaced. The left femoral artery was the preferred site for arterial cannulation in the absence of atherosclerotic changes in the distal arteries. The left femoral vein was the most common site for venous cannulation. In general, arterial and venous cannulation was established with a wire-directed approach. Three mg/kg of heparin was given for systemic heparinization.
Deep hypothermic circulatory arrest (DHCA) was used in 44 patients (42.7%), and partial CBP was used in 58 patients (56.3%). The decision between DHCA versus partial CPB strategies depended on anatomic factors including involvement of the distal arch or feasibility of aortic cross-clamping, but was finally left to the attending surgeon’s discretion. The patients who underwent DHCA (n=44, 45%) were cooled to the core body temperature of 18°C, and when circulatory arrest was initiated, the aorta was opened. Proximal aortic anastomosis was made with a branched vascular graft in an open fashion, and then, an additional arterial cannula was inserted through the side branch of the aortic graft so that both the upper and the lower body could be perfused. For partial CPB strategies, proximal DTA clamping or distal arch clamping (between left common carotid and left subcalvian artery [LSCA]) with separate LSCA clamping was performed under mild hypothermia (>30°C). Distal anastomosis was performed in an open fashion if distal clamping was not feasible in both DHCA and partial CPB techniques. After CPB weaning, protamine was administered to return the activated clotting times to baseline.
2). Data collection and statistical analysis
Data were collected from chart reviews, and the analysis was retrospective. Early mortality was defined as death within 30 days of surgery. Stroke was defined as any new clinically and radiographically evident brain injury including focal and global deficits. Paraplegia was defined as a deficit of the lower extremities including weakness or complete loss of motor function [8]. For study purposes, renal failure was defined as the new requirement for hemodialysis, and low cardiac output syndrome (LCOS) was defined as heart failure requiring mechanical ventricular support such as intra-aortic balloon pulsation or veno-arterial extracorporeal membrane oxygenation.
Categorical variables were presented as frequencies and percentages, and continuous variables were expressed as mean±standard deviation or medians with inter-quartile ranges. For multivariable analyses, Cox proportional hazards models were used to determine the risk factors of death. Variables listed in Table 1 were evaluated in the models, and those with a p-value of 0.20 or less in the univariable analyses were candidates for the multivariable Cox models. Multivariable analyses involved a backward elimination technique, and only variables with a p-value of less than 0.10 were used in the final model. Final models were validated in 1,000 bootstrap samples. All statistical analyses were performed with PASW SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Table 1.
Baseline characteristics and intraoperative profiles of patients
| Variable | Total (n=103) | Deep hypothermic circulatory arrest (n=45) | Partial CPB (n=58) | p-value |
|---|---|---|---|---|
| Age (yr) | 53.8±13.1 | 54.2±12.6 | 53.6±13.6 | 0.81 |
| Female gender | 23 (22.3) | 11 (24.4) | 12 (20.7) | 0.81 |
| Diabetes mellitus | 6 (5.8) | 0 | 6 (100.0) | 0.034 |
| Hypertension | 52 (50.4) | 22 (48.9) | 30 (51.7) | 0.84 |
| Aorta pathology | 0.092 | |||
| Acute dissection | 10 (9.7) | 3 (6.7) | 7 (12.1) | |
| Chronic dissection | 36 (34.9) | 21 (46.7) | 15 (25.9) | |
| Aneurysm without dissection | 57 (55.3) | 21 (46.7) | 36 (62.1) | |
| Emergency or urgent surgery | 11a) (10.7) | 5 (11.1) | 6 (10.3) | 0.90 |
| Rupture or impending rupture | 8 | 4 (8.9) | 4 (6.9) | 0.73 |
| Malperfusion | 1 | 0 | 1 (100.0) | >0.99 |
| Shock | 1 | 0 | 1 (100.0) | >0.99 |
| Hemothorax | 5 | 2 (4.4) | 3 (5.2) | 0.87 |
| Involvement of distal arch | 86 (83.5) | 42 (93.3) | 44 (75.9) | 0.030 |
| Left ventricular ejection fraction (%) | 61.8±5.3 | 61.5±5.0 | 62.0±5.5 | 0.61 |
| Blood hemoglobin level (g/dL) | 13.3±1.7 | 13.3±1.7 | 13.2±1.8 | 0.86 |
| Glomerular filtration rate (mL/min/1.73 m2) | 84.5±33.9 | 82.5±26.7 | 86.0±38.8 | 0.61 |
| Cerebrospinal fluid drainage | 56 (54.4) | 30 (66.7) | 26 (44.8) | 0.030 |
| Concomitant coronary artery bypass grafting | 5 (4.9) | 1 (2.2) | 4 (6.9) | 0.38 |
| Perfusion times (min) | ||||
| CPB time | 153.8±83.5 | 196.4±59.7 | 116.9±84.0 | <0.001 |
| Total circulatory arrest time | 23.3±13.5 | – | – |
Values are presented as mean±standard deviation or number (%). CPB, cardiopulmonary bypass.
Some patients had more than one indication for emergency or urgent surgery.
RESULTS
Baseline characteristics and intraoperative profiles of the patients are shown in Table 1. Eleven patients (10.7%) underwent emergency or urgent surgeries as indicated in Table 1. CPB time was longer in the DHCA group (196.4±59.7 minutes versus 116.9±84.0 minutes, p<0.001), and mean circulatory arrest time was 23.3±13.5 minutes in the DHCA group.
Early operative outcomes are summarized in Table 2. Early mortality occurred in five patients (4.9%). The causes of early deaths were LCOS in all patients, among which four patients showed multi-organ failure. Regarding the early mortality cases, one patient had low left ventricle (LV) ejection fraction (35%) preoperatively, in whom partial CPB strategy was applied. The other four patients underwent DHCA strategies. Early major complications occurred in 21 patients (20.3%) including 7 patients (6.8%) of re-exploration for postoperative bleeding. Ten patients (9.7%) required new dialysis for acute renal failure and 7 patients (6.8%) developed stroke, but no cases of paraplegia were reported. Between the DHCA group and the partial CPB group, the DHCA group had a higher rate of LCOS (13.3% versus 1.7%, p=0.041) and the duration of mechanical ventilation was longer than in the partial CPB group.
Table 2.
Early operative outcomes
| Variable | Total (n=103) | Deep hypothermic circulatory arrest (n=45) | Partial cardiopulmonary bypass (n=58) | p-value |
|---|---|---|---|---|
| Early mortality | 5 (4.9) | 4 (8.9) | 1 (1.7) | 0.17 |
| No. of patients with major complications | 21 (20.3) | 14 (31.1) | 7 (12.1) | 0.026 |
| Re-exploration due to surgical bleeding | 7 (6.8) | 5 (11.1) | 2 (3.4) | 0.24 |
| Requirement for dialysis | 10 (9.7) | 6 (13.3) | 4 (6.9) | 0.33 |
| Requirement for hemodynamic mechanical support | 7 (6.8) | 6 (13.3) | 1 (1.7) | 0.041 |
| Permanent neurologic injury | 7 (6.8) | 5 (11.1) | 2 (3.4) | 0.24 |
| Stroke | 7 (6.8) | 5 (11.1) | 2 (3.4) | 0.24 |
| Paraplegia | 0 | 0 | 0 | – |
| Multi-organ failure | 4 (3.9) | 3 (6.7) | 1 (1.7) | 0.32 |
| Surgical wound revision | 4 (3.9) | 1 (2.2) | 3 (5.2) | 0.63 |
| Pneumonia | 8 (7.8) | 5 (11.1) | 3 (5.2) | 0.29 |
| Ventilator support duration (hr) | 25 (13–55) | |||
| Ventilator support>12 hr | 79 (77.7) | 41 (91.1) | 38 (65.5) | 0.004 |
| Ventilator support>24 hr | 52 (50.5) | 34 (75.6) | 18 (31.0) | <0.001 |
| Intensive care unit stay (day) | 5 (3–7) | 6 (4–9) | 4 (3–5) | 0.28 |
Values are presented as number (%) or median (inter-quartile range).
During the median follow-up duration of 56.3 months (inter-quartile range, 23.1 to 85.1 months), there were 17 (16.5%) late mortalities including 11 cardiovascular-related deaths. Non-cardiovascular causes of death were malignancy in one, respiratory failure in four, and alcoholic liver disease in one patient. The overall survival rates at 5 and 10 years were 80.9%±4.3% and 71.7%±5.9%, respectively (Fig. 1). The 5- and 10-year reoperation-free survival rates were 77.3%±4.8% and 70.2%±5.8%, respectively (Fig. 2). There was one aortic reoperation case during follow-up, in whom asymptomatic pseudoaneurysm at the anastomoses site was seen at regular follow-up computed tomography performed 3 months after surgery. Reoperative aortic replacement was performed without leaving significant morbidity.
Fig. 1.
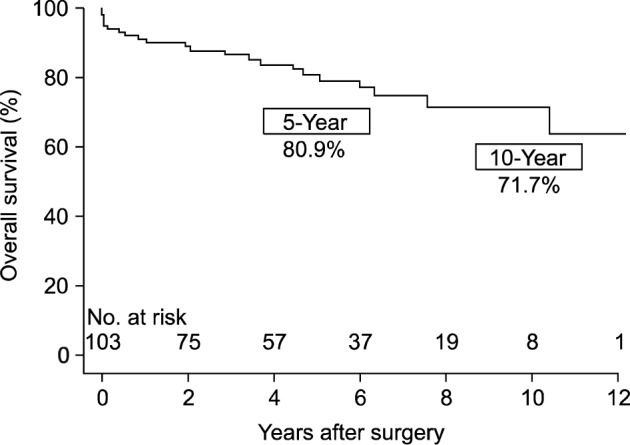
A Kaplan-Meier plot for overall survival.
Fig. 2.
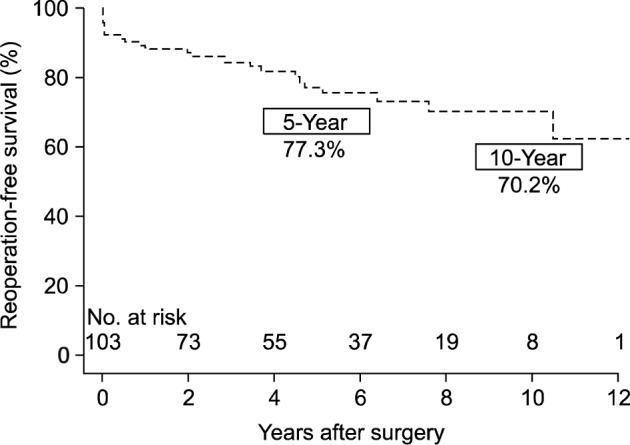
A Kaplan-Meier plot for aortic reoperation-free survival.
The patient variables associated with mortality in the univariate analysis were age (p<0.001), LV ejection fraction (p=0.001), and glomerular filtration rate (p=0.002). Multivariable analyses revealed that age (hazard ratio [HR], 1.10; 95% confidence interval [CI], 1.05 to 1.15; p<0.001) and LV function (HR, 0.88; 95% CI, 0.82 to 0.96; p<0.003) were significantly associated with mortality (Table 3). Ruptured aorta and urgent/emergency cases, however, were not significantly associated with an increased risk of mortality in this study. Furthermore, different CPB strategies (p=0.49) and distal arch involvement (p=0.92) did not significantly affect mortality.
Table 3.
Risk factor analysis for long-term mortality
| Variable | Univariate
|
Multivariable
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age | 1.10 | 1.05–1.15 | <0.001 | 1.10 | 1.05–1.15 | <0.001 |
| Sex | 0.75 | 0.25–2.21 | 0.60 | |||
| Diabetes mellitus | 1.31 | 0.31–5.63 | 0.72 | |||
| Hypertension | 2.33 | 1.18–6.66 | 0.24 | |||
| Aorta pathology | 0.89 | 0.46–1.70 | 0.72 | |||
| Rupture | 2.15 | 0.63–7.33 | 0.22 | |||
| Hemothorax | 1.76 | 0.41–7.60 | 0.45 | |||
| Emergency | 2.00 | 0.67–5.92 | 0.23 | |||
| Left ventricular ejection fraction (by 1% increment) | 0.87 | 0.80–0.94 | 0.001 | 0.88 | 0.82–0.96 | 0.003 |
| Hemoglobin | 1.04 | 0.81–1.35 | 0.74 | |||
| Glomerular filtration rate (by 1 mL/min/1.73 m2 increment) | 0.98 | 0.96–0.99 | 0.002 | – | – | – |
| Distal arch involvement | 0.95 | 0.32–2.80 | 0.92 | |||
| Cerebrospinal fluid drain | 0.61 | 0.26–1.45 | 0.26 | |||
| Deep hypothermic circulatory arrest | 1.35 | 0.58–3.17 | 0.49 | |||
HR, hazard ratio; CI, confidence interval.
DISCUSSION
Open surgical repair remains a gold standard approach in the management of DTA lesions requiring invasive intervention [9]. Progress in pre-operative management and CPB strategies as well as adjunctive maneuvers to protect the kidneys, visceral organs, and central nervous system has significantly improved surgical outcomes [3,4,8,10,11]. However, open surgical repair of DTA is still regarded as a surgical challenge having a reported early mortality rate of 4.8% to 22.4% [8,12]. In addition, in the era of endovascular alternatives, patients who undergo open repair of DTA generally have highly complex aortic pathology where TEVAR approaches are regarded inappropriate, and these circumstances make it more challenging for practicing surgeons to undertake open surgery for DTA, particularly for surgeons in low-volume aortic centers [3,8].
In the past decades, a number of researches have been conducted to improve the preservation of end-organ functions from ischemic damages. Several perfusion techniques were developed such as DHCA and partial CPB including femoral-femoral bypass and left heart bypass [4,9]. Adjunctive strategies such as intercostal reimplantation, CSF drainage, motor/somatosensory-evoked potential monitoring, and rapid renal cooling have also been developed. Despite these improvements in surgical techniques and adjuncts for end-organ protection, the optimal CPB strategy between DHCA and partial CPB still remains controversial [9]. Recent studies reviewed that DHCA for open repair of DTA has several advantages over non-DHCA [9,11,13–15]. First, a bloodless field without clamping the aorta can be obtained. In particular, since clamping of the aorta is not safe if the aortic disease involves the distal aortic arch, DHCA may offer a safe environment for surgery in this situation. Patel et al. [11] demonstrated that half of the patients suffered from postoperative stroke when cross-clamping was conducted for the atheromatous aortic arch, but the stroke rate decreased to 11% when DHCA was used without aortic clamping. Secondly, hypothermia has a protective effect on the spinal cord and visceral organs by decreasing the oxygen demand [13]. In support of this hypothesis, Rokkas et al. [14] reported that the spinal cord is protected by DHCA not only by decreased metabolism but also by the inhibition of the release of excitatory amino acids in the extracellular neuronal space [14]. In DHCA strategies, however, prolonged CPB time may require more transfusion, and hypothermia induces impairment of coagulation cascade [15].
Partial CPB strategies have certain advantages such as requirement for shorter CPB time than DHCA, which consequently reduce the risks of coagulopathy and lethal intrapulmonary hemorrhage that may hamper postoperative recovery [15]. Moreover, several recent studies reported excellent spinal cord protection using a mild-hypothermic distal perfusion technique in combination with CSF drainage [4,16].
Acher [16] suggested that the quality of spinal cord protection relies mainly on perfusion, metabolism, and oxygen delivery to the spinal cord during mild hypothermia rather than the CPB strategies themselves. Spinal cord perfusion can be further augmented by CSF drainage and maintaining the mean arterial pressure above 80 mmHg. Along with the optimization of the hemoglobin level, oxygen saturation and cardiac index are regarded important to prevent postoperative delayed paraplegia.
In support of these previous reports, the present study also showed that the CPB strategy was not significantly related to early mortality, multi-organ failure, neurologic injury, and ARF. Although the rate of immediate postoperative LCOS requiring mechanical support was higher in the DHCA group (Table 2), the CPB strategy did not affect long-term survival even in a univariate analysis (Table 3). The independent predictors were age (HR, 1.10; 95% CI, 1.05 to 1.15; p<0.001) and LV ejection fraction (HR, 0.88; 95% CI, 0.82 to 0.96; p <0.003), which are not factors adjustable by surgery. Because of the retrospective nature of this study, we could not determine why LV venting was not routinely used. However, considering the higher rates of LCOS (p=0.041) in the DHCA group, we recently changed our strategy to assertively insert an LV vent (via LV apex) when conducting DHCA.
Despite the improvements in the operative outcomes of open surgical repair of DTA, it still regarded too aggressive, particularly in the current era of TEVAR. Early mortality rates following TEVAR are reported to be up to 7.4% [17] and 4-year mortality after TEVAR for ruptured thoracic aneurysm has been estimated to be 25.4% [18]. For elective cases, TEVAR was associated with lower risks of early death and complications compared with open repair of DTA [6,18,19]. However, there are only a few studies that have compared long-term outcomes between TEVAR and open repair in DTA. Of note, Makaroun et al. [19] reported that TEVAR showed a comparable 5-year survival rate (68% versus 67%, p-value=0.433) with that of open surgery. Nevertheless, the risk of vascular complications following TEVAR is reported to be substantial, and the 5-year re-intervention rate was reported to be 3.6% to 17% [19,20]. In this regard, overall clinical outcomes including intervention-related complications should be compared between TEVAR and open surgery in further large-scale studies in order to determine optimal candidates for each treatment option.
1). Limitation
This study is limited by a retrospective analysis that includes an inherent bias. Non-randomized design may have affected the results because of unmeasured confounders, procedure bias, or detection bias, even with the use of rigorous statistical adjustment. Further, this study represents the experience of a single large tertiary referral center and might not be generalized to other centers.
2). Conclusions
The outcomes of open surgical repair of DTA were feasible in terms of excellent perioperative mortality/morbidity as well as favorable long-term outcomes. Age and LV function were independent predictors of long-term mortality, whereas the surgical strategies did not significantly affect the clinical outcomes.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 2.Patel HJ, Williams DM, Upchurch GR, Jr, et al. A comparison of open and endovascular descending thoracic aortic repair in patients older than 75 years of age. Ann Thorac Surg. 2008;85:1597–603. doi: 10.1016/j.athoracsur.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 3.Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Variables predictive of outcome in 832 patients undergoing repairs of the descending thoracic aorta. Chest. 1993;104:1248–53. doi: 10.1378/chest.104.4.1248. [DOI] [PubMed] [Google Scholar]
- 4.Estrera AL, Miller CC, 3rd, Chen EP, et al. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann Thorac Surg. 2005;80:1290–6. doi: 10.1016/j.athoracsur.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85(1 Suppl):S1–41. doi: 10.1016/j.athoracsur.2007.10.099. [DOI] [PubMed] [Google Scholar]
- 6.Chaikof EL, Mutrie C, Kasirajan K, et al. Endovascular repair for diverse pathologies of the thoracic aorta: an initial decade of experience. J Am Coll Surg. 2009;208:802–16. doi: 10.1016/j.jamcollsurg.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Lee HS, Lee SH, Yoon YC, Kuh BI, Kim CH. Surgical treatment of the descending thoracic aorta: an analysis of 22 cases. Korean J Thorac Cardiovasc Surg. 1999;32:532–5. [Google Scholar]
- 8.Bozinovski J, Coselli JS. Outcomes and survival in surgical treatment of descending thoracic aorta with acute dissection. Ann Thorac Surg. 2008;85:965–70. doi: 10.1016/j.athoracsur.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Weiss AJ, Lin HM, Bischoff MS, et al. A propensity score-matched comparison of deep versus mild hypothermia during thoracoabdominal aortic surgery. J Thorac Cardiovasc Surg. 2012;143:186–93. doi: 10.1016/j.jtcvs.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Coselli JS, LeMaire SA, Koksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002;35:631–9. doi: 10.1067/mva.2002.122024. [DOI] [PubMed] [Google Scholar]
- 11.Patel HJ, Shillingford MS, Mihalik S, Proctor MC, Deeb GM. Resection of the descending thoracic aorta: outcomes after use of hypothermic circulatory arrest. Ann Thorac Surg. 2006;82:90–5. doi: 10.1016/j.athoracsur.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 12.Coselli JS, Plestis KA, La Francesca S, Cohen S. Results of contemporary surgical treatment of descending thoracic aortic aneurysms: experience in 198 patients. Ann Vasc Surg. 1996;10:131–7. doi: 10.1007/BF02000756. [DOI] [PubMed] [Google Scholar]
- 13.Kouchoukos NT, Masetti P, Rokkas CK, Murphy SF, Blackstone EH. Safety and efficacy of hypothermic cardiopulmonary bypass and circulatory arrest for operations on the descending thoracic and thoracoabdominal aorta. Ann Thorac Surg. 2001;72:699–707. doi: 10.1016/s0003-4975(01)02800-4. [DOI] [PubMed] [Google Scholar]
- 14.Rokkas CK, Sundaresan S, Shuman TA, et al. Profound systemic hypothermia protects the spinal cord in a primate model of spinal cord ischemia. J Thorac Cardiovasc Surg. 1993;106:1024–35. [PubMed] [Google Scholar]
- 15.Kestin AS, Valeri CR, Khuri SF, et al. The platelet function defect of cardiopulmonary bypass. Blood. 1993;82:107–17. [PubMed] [Google Scholar]
- 16.Acher C. It is not just assisted circulation, hypothermic arrest, or clamp and sew. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S136–41. doi: 10.1016/j.jtcvs.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 17.Shah AA, Craig DM, Andersen ND, et al. Risk factors for 1-year mortality after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg. 2013;145:1242–7. doi: 10.1016/j.jtcvs.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Coselli JS, Gopaldas RR. Ruptured thoracic aneurysms: to stent or not to stent? Circulation. 2010;121:2705–7. doi: 10.1161/CIRCULATIONAHA.110.961631. [DOI] [PubMed] [Google Scholar]
- 19.Makaroun MS, Dillavou ED, Wheatley GH, Cambria RP Gore TAG Investigators. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J Vasc Surg. 2008;47:912–8. doi: 10.1016/j.jvs.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Lee CJ, Rodriguez HE, Kibbe MR, Malaisrie SC, Eskandari MK. Secondary interventions after elective thoracic endovascular aortic repair for degenerative aneurysms. J Vasc Surg. 2013;57:1269–74. doi: 10.1016/j.jvs.2012.10.124. [DOI] [PubMed] [Google Scholar]


