Abstract
A gene homologous to rpoS was cloned from a fatal human pathogen, Vibrio vulnificus. The functional role of rpoS in V. vulnificus was accessed by using an rpoS knockout mutant strain. This mutant was impaired in terms of the ability to survive under oxidative stress, nutrient starvation, UV irradiation, or acidic conditions. The increased susceptibility of the V. vulnificus mutant in the exponential phase to H2O2 was attributed to the reduced activity of hydroperoxidase I (HPI). Although σS synthesis was induced and HPI activity reached the maximal level in the stationary phase, the mutant in the stationary phase showed the same susceptibility to H2O2 as the wild-type strain in the stationary phase. In addition, HPII activity, which is known to be controlled by σS in Escherichia coli, was not detectable in V. vulnificus strains under the conditions tested. The mutant in the exponential phase complemented with multiple copies of either the rpoS or katG gene of V. vulnificus recovered both resistance to H2O2 and HPI activity compared with the control strain. Expression of the katG gene encoding HPI in V. vulnificus was monitored by using a katG::luxAB transcriptional fusion. The expression of this gene was significantly reduced by deletion of σS in both the early exponential and late stationary phases. Thus, σS is necessary for increased synthesis and activity of HPI, and σS is required for exponentially growing V. vulnificus to develop the ability to survive in the presence of H2O2.
The life cycles of pathogenic bacteria involve periods in which they exist in a nongrowing state in stressed environments. Only if they survive such conditions are they able to proliferate with high metabolic activity in the proper host environments (7, 36). Thus, these organism have evolved several mechanisms that allow them to survive under stressful conditions, such as starvation, temperature fluctuation, oxidative stress, and osmotic shock, and that enable them to resume growth once the stress is removed (27). The cellular responses to environmental stimuli have been extensively studied in many bacterial species, most notably Escherichia coli.
To respond properly to diverse stresses, E. coli requires the rpoS gene product, which is a second principal sigma factor (σS); this product induces expression of many genes and allows the organism to mediate changes in cellular physiology and structure and to adapt, resist, and survive under stress conditions (9, 16, 19). σS is also required for eliciting phenotypes related to virulence in many pathogenic bacteria belonging to the γ subdivision of Proteobacteria (21, 32, 39, 45, 50).
It is generally believed that most microorganisms that communicate with, associate with, or colonize host animals are relatively well equipped with defense mechanisms to deal with oxidative stress (6, 15, 43). E. coli produces at least two enzymes to overcome the presence of hydrogen peroxide and to maintain a relatively constant concentration of intracellular H2O2 (8); these enzymes are KatG (hydroperoxidase I [HPI]), which has both catalase and peroxidase activities, and monofunctional KatE (HPII), which has catalase activity (25). KatG, one of the members of the OxyRS regulon, is induced by direct exposure to H2O2 (37). In contrast, KatE is known to be regulated by σS, and consequently cellular expression of this enzyme increases at the onset of the stationary phase (25, 30). Open reading frames homologous to both katG and katE are present in the genomes of Vibrio cholerae and Vibrio parahaemolyticus. The presence and role of monofunctional catalases have been studied in Vibrio fischeri and Vibrio rumoiensis, but the regulation of these enzymes has not been described (11, 44, 51).
The causative agent of septicemia, Vibrio vulnificus, has been considered an important pathogen in humans due to its rapid pathogenic progress and its high mortality rate (10, 38). A number of studies have been performed on virulence factors of this organism, including metalloprotease (13), hemolysin (48), and siderophores (35). Several regulators, including cyclic AMP receptor protein (CRP)/LuxR (12, 33), ToxRS/CRP (1, 17), and Fur (18), have been reported to control expression of these virulence factors. While survival of this bacterial species has been studied under diverse conditions (26), the molecular mechanisms underlying its survival strategies have not been studied well.
In an effort to isolate global regulators involved in survival of V. vulnificus, we cloned the rpoS gene and defined its physiological role in survival of V. vulnificus in the presence of various stresses. These analyses showed that V. vulnificus in the exponential phase requires σS for survival in the presence of low concentrations of hydrogen peroxide. In the present study we also observed regulation of the expression and activity of a catalase involved in this response, and the results were quite different from those obtained with E. coli.
MATERIALS AND METHODS
Isolation of the rpoS gene from V. vulnificus.
The genomic DNA of V. vulnificus ATCC 29307 was prepared by a standard technique (29) and then partially digested with Sau3AI and size fractionated by agarose gel electrophoresis. The DNA fragments, which ranged from 2 to 6 kb long, were pooled and ligated with the pUC19 vector which had been digested with BamHI and subsequently treated with bacterial alkaline phosphatase. The V. vulnificus library obtained was introduced into E. coli ZK918 having a deletion in its rpoS gene and a σS-dependent bolA::lacZ fusion in its chromosome (2). After transformation with the library, colonies were screened on Luria-Bertani (LB) medium supplemented with ampicillin (100 μg/ml) and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (10 μg/ml), which was blue as a result of induced expression of bolA::lacZ after addition of the RpoS homolog of V. vulnificus. Sequencing of the double-stranded DNA of the plasmid selected, pINE32, containing a 2,693-bp insert, was performed with an Applied Biosystems 373A automated DNA sequencer. The remaining gaps in the sequence were filled in by sequence analysis by using specifically designed internal primers that annealed to the insert region. Sequence analysis and database searches were performed by using the National Center for Biotechnology Information BLAST server.
Construction of rpoS knockout mutant KPR101.
A 762-bp NruI fragment containing two-thirds of the rpoS coding sequence was deleted from pINE32. The resultant plasmid, pKP11, was digested with SmaI and XbaI, which resulted in a DNA fragment containing a region adjacent to the rpoS gene but not the rpoS gene. This DNA was cloned into suicide vector pDM4 (23), which was digested with ApaI and XbaI, yielding pKP13. pKP13 in E. coli SM10 λpir was mobilized into strain AR, a rifampin-resistant derivative of the wild-type strain V. vulnificus ATCC 29307. Conjugal transfer was performed by mixing aliquots of the strains that contained about 108 donor cells and about 108 recipient cells and then incubating the preparation overnight at 37°C in close contact on a membrane filter. The cell mixture was then resuspended in LBS (LB medium containing NaCl at a final concentration of 2.5%) broth and plated onto selective plates (LBS agar plates supplemented with 4 μg of chloramphenicol per ml and 50 μg of rifampin per ml). A colony showing indications of a double homologous recombination event (resistance to 5% sucrose and sensitivity to chloramphenicol) was isolated, and deletion of its rpoS region was confirmed by PCR by using primers F2 and R2 (Table 1).
TABLE 1.
Strains, plasmids, and oligonucleotide primers used in this study
| Strain, plasmid, or primer | Relevant characteristics | Reference or source |
|---|---|---|
| V. vulnificus strains | ||
| ATCC 29307 | Type strain | 12 |
| AR | ATCC 29307 but spontaneous rifampin resistance | This study |
| MO6-24/0 | Clinical isolate | 48 |
| CN7 | Clinical isolate | This study |
| CNUH94-4 | Clinical isolate | This study |
| SC9649 | Environmental isolate (seawater) | This study |
| SC9720 | Environmental isolate (sediment) | This study |
| SC97126 | Environmental isolate (seafood) | This study |
| KPR101 | AR but ΔrpoS (allelic exchange with pKP13) | This study |
| E. coli strains | ||
| DH5α | φ80dlacZΔM15 recA1 endA1 gyrA96 relA1 thi-1 hsdR17(rK− mK−) supE44 deoR Δ(lacZYA-argF)U169 | Laboratory collection |
| JM109 | endA1 recA1 gyrA96 thi-1 hsdR17(rK− mK−) relA1 supE44 Δ(lac-proAB) [F′ traD36 proAB lacIqZΔM15] | Promega |
| SM10λpir | thi-1 thr leu tonA lacY supE recA::Rp4-2-Tc::Mu λpir; Kmr | 34 |
| AMS6 | K-12 (λ− F− Δlac) | 31 |
| ZK918 | W3110 ΔlacU169 tna-2 rpos::kan bolA:lacZ | 2 |
| Plasmids | ||
| pUC19 | Cloning vector; lacZα Apr | 49 |
| pINE32 | pUC19 with 2.75-kb Sau3AI fragment of V. vulnificus DNA containing the complete coding sequence of V. vulnificus of rpoS | This study |
| pKP11 | pINE32 with deletion of 762-bp NruI fragment (V. vulnificus ΔrpoS) | This study |
| pDM4 | Suicide vector; oriR6K Cmr | 23 |
| pKP13 | pDM4 containing SmaI and XbaI fragment of pKP11 | This study |
| pLAFR5 | IncP Tcr; derivative of pLAFR3 containing a double cos cassette | 14 |
| pKP14 | pLAFR5 containing EcoRI and HindIII fragment of pINE32 | This study |
| pQE30 | Expression vector | Qiagen |
| pQErpoS | pET containg 1,072-bp PCR-amplified V. vulnificus rpoS coding region | This study |
| pHK0011 | pRK415 with promoterless luxAB; Tcr | 12 |
| pHL-03 | katGvv::luxAB transcriptional fusion in pHK0011 | This study |
| pRK415 | IncP ori; broad-host-range vector; oriT of RP4; Tcr | 14 |
| pKP51 | pRK415 containing KpnI and HindIII fragment of V. vulnificus katG | This study |
| Primers | ||
| rpoSvv-F | 5′-CGGATCCAGTATCAGCAACACAGTCACCAAAG-3′ | |
| rpoSvv-R | 5′-GGCCAAGCTTCTATGATTCCATAGCCTTTTTTC-3′ | |
| F2 | 5′-AACGCGCTAAGAGGTTATGGC-3′ | |
| R2 | 5′-AGCAAGAACGGTGGATATCGC-3′ | |
| katG-F-KpnI | 5′-GTTGGTACCTCAACTACCGC-3′ | |
| katG-R-XbaI | 5′-GGATGTCTAGATTGAGGGCC-3′ | |
| katG-R-HindIII | 5′-GCAAGTCTGATAAAAGCTTCG-3′ |
Determination of survival of V. vulnificus under various stress conditions.
Exponential-phase cultures (optical density at 600 nm [OD600] in LBS broth, 0.15 to 0.3) or stationary-phase cultures (OD600 in LBS broth, about 3 to 4; usually 8 to 10 h after an overnight culture was added to fresh LBS broth) were collected by centrifugation, washed with artificial seawater (ASW) (0.6 M NaCl, 0.1 M MgSO4, 0.02 M CaCl2, 0.02 M KCl, 50 mM Tris-HCl [pH 8.3] [28]), and resuspended in the appropriate medium at a density of approximately 106 to 107 cells/ml. During incubation under stress conditions at 30°C, aliquots were removed and spread on LBS agar plates to monitor the viability by determining the number of CFU. For survival studies under stress conditions, we used hyperosmosis (LBS medium containing 5 M NaCl), starvation (nutrient-free ASW), acidity (LBS medium titrated to pH 4.0 with HCl), UV irradiation (wavelength, 254 nm; energy, 120,000 μJ/cm2), and hydrogen peroxide (0.088 to 10 mM H2O2 in ASW).
Determination of catalase activities.
Cellular extracts of E. coli and various V. vulnificus strains (Table 1) were prepared in cold 50 mM potassium phosphate buffer (pH 7.0) by sonication (Vibracell; Sonics & Materials, Inc.) in ice. The amount of protein in a cell lysate was determined by the Bradford assay by using bovine serum albumin as the standard. After separation on an 8% nondenaturing polyacrylamide gel, the locations of HPI and HPII were visualized by staining the gel with a solution containing 1% K3Fe(CN)6 and 1% FeCl3 (47) and compared with the locations of the corresponding enzymes of E. coli.
For the catalase assay, cell samples were obtained at various growth phases (OD600, 0.1 to 4.2) and were resuspended in chilled catalase buffer (5 mM potassium phosphate buffer [pH 7.0], 5 mM EDTA, 10% glycerol, 25 μM phenylmethylsulfonyl fluoride) (42) and sonicated in ice. Each cellular extract was then mixed with 25 mM potassium phosphate buffer (pH 7.0) containing 5.9 mM H2O2, and the amount of remaining H2O2 in the reaction mixture was estimated by monitoring the absorbance at 240 nm at 30-s intervals for 10 min. One unit of specific activity was defined as 1 μmol of H2O2 degraded per min per mg of protein (42).
Construction of katG::luxAB transcriptional fusion and measurement of expression of the katG gene.
The 504-bp DNA fragment which included nucleotide positions −318 to 115 relative to the initiation codon of katG (gene VV12755 in GenBank accession no. NC_004459) was amplified by PCR by using two primers, katG-F-KpnI and katG-R-XbaI (Table 1). The PCR product was digested with appropriate restriction enzymes (KpnI and XbaI) and inserted into luxAB-based plasmid pHK0011 (12) digested with the same enzymes. The resultant transcriptional fusion, pHL-03, was transferred into V. vulnificus cells via conjugative transfer. Overnight (16- to 18-h) cultures of the cells containing pHL-03, which exhibited the basal level of bioluminescence, were inoculated into fresh LBS medium containing 3 μg of tetracycline per ml, and the expression from the katG promoter was measured by monitoring light production in the presence of 0.006% n-decyl aldehyde by using luminometers (TD-20/20; Turners Designs). The specific bioluminescence was calculated by normalizing the relative light units with cell mass (OD600).
Complementation of KPR101 with a broad-host-range vector containing V. vulnificus rpoS (pKP14) or V. vulnificus katG (pKP51).
An EcoRI-HindIII DNA fragment that included the intact rpoS gene was obtained from pINE32 and cloned into the broad-host-range vector pLAFR5 (14) to obtain plasmid pKP14. The 2,569-bp DNA fragment that included the intact katG gene was amplified by PCR by using two primers, katG-F-KpnI and katG-R-HindIII. The PCR product was digested with appropriate restriction enzymes (KpnI and HindIII) and cloned into the broad-host-range vector pRK415 (14) to obtain plasmid pKP51. Each plasmid was introduced into the rpoS mutant by conjugative transfer, as described above.
Western analysis of σS.
Two oligonucleotides, rpoSvv-F and rpoSvv-R (Table 1), were used to amplify a 1,032-bp DNA fragment containing the complete sequence of the rpoS gene from the genomic DNA of V. vulnificus. BamHI and HindIII sites located at both ends of the resultant rpoS DNA were used to clone this DNA into the pQE30 expression plasmid (Qiagen) to generate plasmid pQErpoS. Recombinant σS was overexpressed in E. coli JM109 by adding isopropylthio-β-d-galactoside (Sigma) at a concentration of 1.0 mM and was purified by using an Ni+-nitrilotriacetic acid affinity column as directed by the manufacturer (Qiagen). Polyclonal antibodies against V. vulnificus σS were produced in a rabbit by intravenous immunization with 200 μg of the recombinant V. vulnificus σS, and this initial immunization was followed by additional immunizations at 1 and 4 weeks. Ten days after the last injection, the blood of the immunized rabbit was collected, and its serum was used for Western blot analysis. Cell extracts of the V. vulnificus wild type and the rpoS mutant containing either pLAFR5 or pKP14 were prepared by sonication in TNT buffer (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.05% Tween 20) (29), and 40-μg aliquots of the extracts were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After transfer to a Hybond P membrane (Amersham), Western blot analysis was performed by serially incubating the filter with V. vulnificus σS polyclonal antibodies (1:1,000 dilution) and alkaline phosphatase-conjugated rabbit anti-rat immunoglobulin G (1:1,000 dilution; Sigma). The V. vulnificus σS band was visualized by using the nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) system (Promega).
Nucleotide sequence accession number.
The V. vulnificus rpoS nucleotide sequence has been deposited in the GenBank database under accession number AY187681.
RESULTS AND DISCUSSION
Isolation of V. vulnificus rpoS by functional complementation.
In E. coli, σS is known to regulate the expression of several genes involved in cellular adaptation to diverse stresses. Since the σS homologue of V. vulnificus, if there is one, may also be able to play an equivalent role in regulation of these genes, E. coli strain ZK918 containing the σS-dependent bolA promoter::lacZ fusion (2) was utilized. Upon transformation of ZK918 with the genomic library of V. vulnificus, the bluish transformants on LB medium supplemented with X-Gal were chosen as candidates for complementing plasmids for the rpoS function. One of the plasmids, plasmid pINE32 carrying a 2,693-bp insert (Table 1), was used for further investigation.
Analysis of the pINE32 insert DNA.
Sequence analysis of the insert in pINE32 revealed that it coded for the proteins of V. vulnificus homologous to NlpD, RpoS, and MutS. Although the genetic organization of the open reading frames flanking rpoS is the same as that found in other bacteria, the lengths of the intergenic spaces between nlpD and rpoS and between pcm (the upstream gene of nlpD) and nlpD were quite different from the lengths of the intergenic spaces in E. coli or Pseudomonas. This finding suggests that the regulation of rpoS expression at the transcriptional level in V. vulnificus may be different from the regulation in other organisms which have been extensively studied (20, 30, 41). The amino acid sequence deduced from the gene homologous to rpoS, which codes for 343 amino acid residues corresponding to ca. 39.6 kDa with a pI of 4.92, was aligned with other known σS sequences and was found to exhibit overall levels of identity of 83, 79, and 70% with the sequences of V. parahaemolyticus, V. cholerae, and E. coli, respectively. There is complete homology in subregions 2.3 and 2.4 of σS, which are involved in promoter recognition (e.g., RpoD box and 14-mer) (4), and there is significant conservation in subregion 2.1, which is involved in core binding. The helix-turn-helix motif in subregion 3.1 and the −35 recognition region in subregion 4.2 showed relatively weak similarity.
Generation of an rpoS-deficient strain, KPR101.
A major portion of the V. vulnificus rpoS gene in pINE32 was deleted by digestion with NruI and subsequent ligation. The nlpD-rpoS-mutS region with the rpoS gene deleted was transferred to a conjugative plasmid, pDM4, resulting in pKP13. Replacement of the wild-type rpoS gene located on the chromosome of V. vulnificus AR with this plasmid was accomplished through homologous recombination. Deletion of the rpoS gene in the V. vulnificus mutant was confirmed by PCR by using primers F2 and R2 (Table 1). A PCR analysis of a deletion of the internal region of the rpoS gene in the mutant revealed the expected size for the DNA fragment (754 bp); meanwhile, the intact rpoS gene in the wild-type produced a 1,516-bp DNA fragment (data not shown). The resultant mutant was designated KPR101.
Survival characteristics of KPR101.
The survival of KPR101 in the exponential phase was examined when it was exposed to various stresses, including hyperosmotic conditions, starvation, an acid environment, UV irradiation, and oxidative conditions, and was compared to the survival of the wild type. In contrast to enteric bacteria (9, 19), rpoS-deficient V. vulnificus exhibited the same survival pattern in the presence of a high salt concentration (5 M NaCl) that the wild type exhibited (data not shown). The survival of the mutant, however, was severely impaired in the presence of other stresses. For example, the abilities of the mutant to survive under starvation, acidic, and UV-irradiated conditions (6 days, 1 h, and 12 s, respectively) were estimated to be 25-, 1,300-, and 16-fold less than those of the corresponding controls, respectively (Fig. 1A ∼ C).
FIG. 1.
Survival of exponential-phase wild-type and ΔrpoS mutant KPR101 cultures under stress conditions. Wild-type and KPR101 cells in the exponential phase were challenged by starvation (ASW) (A), acidic conditions (LBS medium titrated to pH 4.0) (B), and irradiation with UV light (254 nm; 120,000 μJ/cm2) (C). At several times during exposure, aliquots of each culture were removed, and the numbers of CFU per milliliter were estimated as described in Materials and Methods. The resultant values were expressed as percentages of the initial cell density, which ranged from 106 to 107 CFU/ml.
The ΔrpoS mutant cells in the exponential phase also showed significantly increased susceptibility to 880 μM H2O2; there was up to a 1,000-fold difference after 30 min of exposure (Fig. 2A). The KPR101 cells in the stationary phase showed more resistance than the cells in the exponential phase, but the susceptibility was basically similar to the susceptibility of the wild type in the presence of various concentrations of H2O2 up to 10 mM (Fig. 2B). This σS-independent increase in H2O2 resistance in the stationary phase is an unusual observation, since synthesis of σS is induced in the stationary phase in V. vulnificus (data not shown), and one of catalases (HPII) is known to be induced by σS in E. coli (30, 37, 40). In addition, V. vulnificus cells were generally more sensitive to H2O2 than other enteric bacteria were, because exposure to the concentrations of H2O2 used for E. coli or V. cholerae (e.g., ca. 10 mM for 30 min) resulted in survival of only 0.1 to 0.01% of the cells present initially (Fig. 2B). Thus, it is possible that V. vulnificus may have mechanisms for oxidative stress response that are distinct from those found in E. coli, at least under the conditions which we used.
FIG. 2.
Survival of the wild-type and ΔrpoS mutant KPR101 cells in the presence of H2O2. (A) Wild-type and KPR101 cells in the exponential phase were challenged with an oxidative stress (ASW containing 880 μM H2O2). At several times during exposure, aliquots of each culture were removed, and the numbers of CFU per milliliter were estimated. The asterisks indicate values lower than the detection limit used in this analysis. (B) Wild-type and KPR101 cells in the stationary phase (OD600 in LBS broth, approximately 3 to 4) were harvested and incubated in ASW containing concentrations of H2O2 ranging from 0.6 to 10 mM. After 30 min of exposure, the numbers of remaining culturable cells were estimated. The resultant values were expressed as percentages of the initial cell density, which was about 107 CFU/ml.
Characterization of the catalase of V. vulnificus.
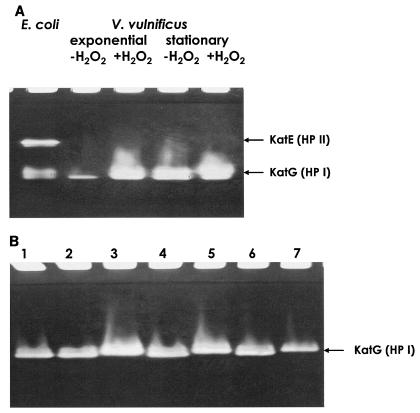
The distinct susceptibility of KPR101 to H2O2, especially during the exponential phase, led us to examine the innate properties of hydroperoxidase(s) in wild-type V. vulnificus. Protein extracts of the type strain of V. vulnificus (ATCC 29307) were separated on a nondenaturing polyacrylamide gel, and the bands representing hydroperoxidase activities were compared with those of a stationary-phase E. coli extract. In contrast to E. coli, which has both KatG (HPI; a multimer composed of two 80.0-kDa subunits) and KatE (HPII; a multimer composed of four 84.2-kDa subunits), only the band corresponding to HPI was visualized in both exponential- and stationary-phase V. vulnificus extracts (Fig. 3A). The calculated molecular mass of the deduced KatG polypeptide of V. vulnificus (VV12755 in GenBank accession no. NC_004459) is estimated to be 80.4 kDa. The absence of HPII activity seemed to be common in this bacterial species, since various V. vulnificus strains exhibited only HPI activity, at least under the conditions which we tested (Fig. 3B).
FIG. 3.
Nondenaturing gels showing activity staining of catalases in bacterial crude extracts. (A) Crude extracts (120 μg) prepared from V. vulnificus ATCC 29307 in the exponential or stationary phase which had been treated with either 0 or 10 μM H2O2 for 10 min were loaded into an 8% polyacrylamide gel, and the HPI and HPII activities were examined. The typical HPI (dimer consisting of 80.0-kDa subunits) and HPII (tetramer consisting of 84.2-kDa subunits) bands found in E. coli AMS6 (60 μg) were visualized by staining the gel with 1% K3Fe(CN)6 and 1% FeCl3, and the results were compared to the results obtained for V. vulnificus. (B) Crude extracts of various V. vulnificus strains (60 μg) were loaded into an 8% polyacrylamide gel. The strains used were ATCC 29307 (lane 1), MO6-24/0 (lane 2), clinical isolate CN7 (lane 3), clinical isolate CNUH94-4 (lane 4), environmental (seawater) isolate SC9649 (lane 5), environmental (sediment) isolate SC9720 (lane 6), and environmental (seafood) isolate SC97126 (lane 7).
When the katG gene of V. vulnificus was disrupted, no hydroperoxidase band appeared (data not shown), and the missing HPI band was restored when the V. vulnificus katG gene was supplied in a multicopy plasmid (J. H. Rhee, personal communication). The katE gene is present on chromosomal DNA of V. vulnificus (VV21473 in GenBank accession no. NC_004460), and the molecular mass of the deduced polypeptide is predicted to be 55.4 kDa. However, no apparent KatE (HPII) activity was observed under any of the conditions tested, even when we used large amounts of cellular extract (up to 200 μg of crude protein extract [data not shown]) and cellular extracts were exposed to H2O2 (Fig. 3A). In addition, no transcript for katE was detected in the total RNA (30 μg) by Northern analysis with a 1,523-bp katE gene probe (data not shown). Exposure of the exponential-phase cells to H2O2 resulted in the induced level of HPI found in the stationary-phase cells, which suggests that a redox-operated regulator, possibly OxyR, is involved in activating katG (46; I. Kong, A. Huelsmann, and J. D. Oliver, 102nd Gen. Meet. Am. Soc. Microbiol., poster no. K-94, 2002).
Determination of HPI activity in the wild-type strain and KPR101.
To identify the possible role of σS in regulation of the activity of KatG, HPI activities were monitored along the growth curve (Table 2). The HPI catalase specific activity of the wild-type strain was about 2 U during the early exponential phase (for the first 2 h of growth) and gradually decreased during the mid-exponential phase. Then it reached a maximal level, approximately 6 to 8 U, after the cells entered the late stationary phase. The activity of HPI in V. vulnificus cells during the whole growth period, except for an initiation period during the stationary phase (OD600, 2.2 and 3.2 [Table 2]), was regulated by the presence of σS, since the rpoS mutant contained about twofold less HPI than the wild-type strain contained. Thus, the induction of HPI at the onset of the stationary phase may depend on other regulators.
TABLE 2.
Catalase activities of the wild type and ΔrpoS mutant KPR101 in various growth phases
| OD600 | Catalase sp acta
|
Ratio (wild type/KPR101) | |
|---|---|---|---|
| Wild type | KPR101 | ||
| 0.1 | 2.25 ± 0.19 | 1.33 ± 0.12 | 1.7 |
| 0.3 | 0.86 ± 0.10 | 0.51 ± 0.10 | 1.7 |
| 0.6 | 0.71 ± 0.07 | 0.41 ± 0.05 | 1.7 |
| 1.3 | 0.60 ± 0.14 | 0.32 ± 0.05 | 1.9 |
| 2.2 | 0.98 ± 0.06 | 0.68 ± 0.19 | 1.4 |
| 3.2 | 2.85 ± 0.07 | 2.43 ± 0.05 | 1.2 |
| 3.9 | 7.60 ± 0.07 | 3.56 ± 0.70 | 2.1 |
| 4.2 | 6.39 ± 0.55 | 2.60 ± 0.70 | 2.4 |
Wild-type and KPR101 cells grown in LBS broth were harvested at different cell densities, and the crude extracts were processed in order to estimate the rates of H2O2 degradation by a spectrophotometric assay, as described in Materials and Methods. The concentrations of H2O2 were determined by using an extinction coefficient of 43.6 mM (3). The catalase specific activities were obtained from two independent experiments and were calculated by using the following equation (42): (1,000 × average ΔOD240/min)/[(43.6 × milligrams of enzyme)/milliliters of reaction mixture].
Similarly, the HPI catalase specific activity in KPR101 was 2 to 3 U at the onset of the stationary phase, but it did not reach the maximal level observed in the wild type (Table 2). However, the twofold reduction in HPI activity in late-stationary-phase KPR101 compared to the activity in the wild type did not result in a difference in survival in the presence of H2O2 (Fig. 2B), because this amount of HPI activity (i.e., more than 2 to 3 U) might provide as much resistance to the concentrations of H2O2 used (up to 10 mM) as the amount in the wild type provides.
The medium used to grow KPR101 contained less HPI activity than the medium used to grow the wild type contained. No activity was detected in a cell-free medium (glucose-based ASW) which was used to grow KPR101 at the exponential phase, whereas significant HPI activity (0.38 ± 0.05 U) was found in the spent medium used for the wild type at the exponential phase. In the medium used to culture KPR101 at the stationary phase, the extracellular HPI activity (0.97 ± 0.06 U) was about 70% of the activity of the wild type (1.32 ± 0.08 U). This suggests that the smaller amount of KatG activity in KPR101 than in the wild type was not due to increased excretion of this enzyme.
Complementation of KPR101 with a broad-host-range vector containing the V. vulnificus rpoS gene, pKP14.
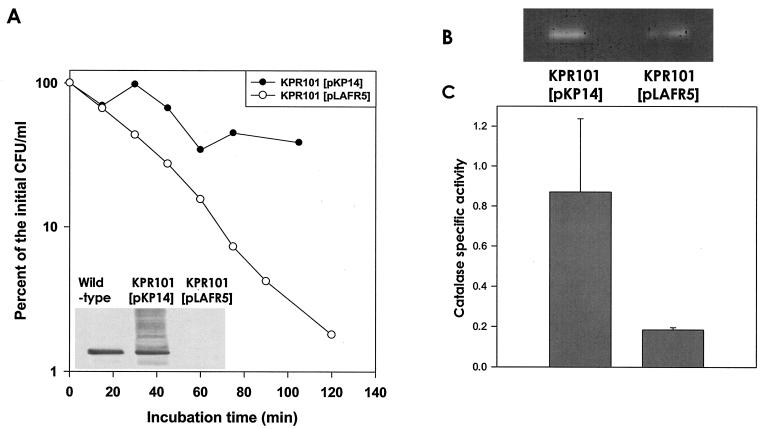
After pKP14, a pLAFR5-based plasmid containing the V. vulnificus rpoS gene, was added to KPR101, the expression of V. vulnificus σS in this strain was confirmed by Western blotting by using V. vulnificus σS-specific polyclonal antibody, which clearly showed the presence of an ∼39-kDa V. vulnificus σS (Fig. 4A, inset). The exponential-phase KPR101 culture, containing pKP14 grown in glucose-based ASW supplemented with tetracycline, was exposed to 880 μM H2O2, and changes in the number of CFU were monitored for 2 h. This strain was found to be resistant to H2O2, whereas KPR101 containing pLAFR5 was found to be sensitive to the same concentration of H2O2 (Fig. 4A).
FIG. 4.
Complementation of ΔrpoS mutant KPR101 with intact V. vulnificus rpoS. (A) Complementation of KPR101 with the rpoS gene (in the broad-host-range pLAFR5 vector containing the V. vulnificus rpoS gene or pKP14) resulted in synthesis of σS, as determined by Western blotting (inset). Exponential-phase KPR101 containing pKP14 or pLAFR5 was grown in ASW supplemented with tetracycline and exposed to 880 μM H2O2. At several times during exposure, aliquots of the culture were removed, and the numbers of CFU per milliliter were estimated as described in Materials and Methods. The resultant values were expressed as percentages of the initial cell density, which was approximately 106 CFU/ml. (B and C) The HPI activity in a crude extract from an exponential-phase KPR101 culture containing pKP14 was compared to the HPI activity of KPR101 containing pLAFR5 by either the catalase staining method with a nondenaturing gel (B) or the H2O2 degradation assay with a spectrophotometer (C).
When the V. vulnificus rpoS gene was present in trans, apparently increased HPI activity was observed by the gel staining assay (Fig. 4B), and the increase was estimated to be about fourfold as determined by an H2O2 degradation kinetic study (Fig. 4C). These results show that in the exponential phase the rpoS mutant complemented with the V. vulnificus rpoS gene exhibited both resistance to H2O2 and HPI activity.
Complementation of KPR101 with a broad-host-range vector containing the V. vulnificus katG gene, pKP51.
If the effect of an rpoS mutation on increased sensitivity to H2O2 was mainly due to a lower level of HPI, KPR101 should become resistant to H2O2 if multiple copies of the katG gene are provided. Thus, KPR101 was complemented with pKP51, a pRK415-based plasmid containing the V. vulnificus katG gene. In the exponential phase KPR101 containing pKP51 grown in LBS medium supplemented with tetracycline exhibited much greater HPI activity (Fig. 5A). This strain also exhibited greater resistance to 880 μM H2O2 than KPR101 containing pRK415 exhibited (Fig. 5B). However, it is possible that the other gene products regulated by σS play an important role in survival in the presence of higher concentrations of H2O2 than we tested. Currently, the proteins which are induced in the wild type in the exponential phase by exposure to H2O2 but are not induced in KPR101 in the exponential phase under the same conditions are being investigated by proteome analyses by using two-dimensional matrix-assisted laser desorption ionization—time of flight mass spectrometry.
FIG. 5.
Complementation of the ΔrpoS mutant KPR101 with V. vulnificus katG. (A) Complementation of KPR101 with the katG gene (in the broad-host-range vector pRK415 containing the V. vulnificus katG gene or pKP51) resulted in increased activity of HPI, as determined by the catalase staining method with a nondenaturing gel. The HPI activity in the crude extract of an exponential-phase KPR101 culture containing pKP51 was compared to the HPI activities of KPR101 and the wild type containing pRK415. (B) An exponential-phase KPR101 culture containing pKP51 or pRK415 in LBS medium was grown in LBS broth supplemented with tetracycline and exposed to 880 μM H2O2. At several times during exposure, aliquots of the culture were removed, and the numbers of CFU per milliliter were estimated as described in Materials and Methods. The resultant values were expressed as percentages of the initial cell density, which was approximately 107 CFU/ml.
Determination of katG expression in the wild type and KPR101.
Since the presence of σS apparently increases HPI activity, its role in controlling HPI was analyzed further by monitoring expression of the katG gene in the wild type and KPR101. To do this, a luxAB-based transcriptional fusion, pHL-03 containing the promoter region of the katG gene, was constructed and transferred to both strains. In the wild-type cells, induction of the katG fusion was apparently initiated during the early exponential phase (during the first 1 to 2 h of incubation), and the level decreased to the basal level during the mid-exponential phase. A high level of expression during the initial incubation period was also observed in E. coli (8), and the HPI activity was also high at this stage of growth (Table 2). Then induction occurred again at the onset of the stationary phase, and the level of expression reached the maximal level (about 10-fold greater than the basal level) when the cells entered the late stationary phase (Fig. 6). This expression profile essentially parallels the pattern of catalase activity measured directly (Table 2).
FIG. 6.
Expression of the katG gene determined by using a katG::luxAB transcriptional fusion (pHL-03). Wild-type (•) and KPR101 (○) cells containing the katG::luxAB fusion were freshly grown in LBS broth supplemented with 3 μg tetracycline per ml by inoculating overnight cultures, whose levels of bioluminescence were the basal levels. Aliquots were removed, and the cell masses (OD600) (A) and bioluminescence (relative light units [RLU]) (B) were estimated. The luciferase activities were expressed as normalized values obtained by dividing the number of RLU by the OD600 of each sample. The activities of three independent experiments were averaged, and the error bars indicate the standard deviations.
The katG expression in KPR101 followed the same pattern as the katG expression in the wild type, but the degrees of induction in both the early exponential and late stationary phases were significantly reduced (Fig. 6). This pattern of V. vulnificus katG expression is quite different from the katG expression in E. coli. In E. coli, the maximal katG expression was only slightly reduced in an rpoS mutant (8), the maximal induction of katG occurred during the late exponential phase (22), and the duration of induced katG expression was not prolonged during the late stationary phase (8). Interestingly, an HPII-deficient E. coli mutant has been reported to exhibit higher HPI activity than the parental strain exhibits (42).
While no difference in katG expression was observed during the mid-exponential phase (Fig. 6), the estimated HPI activities in KPR101 were about 50% of those in the wild type at the same growth stage (Table 2). This difference in activity might result from different expression during the early exponential phase. Otherwise, σS might not directly regulate KatG at the transcriptional level, at least during the mid-exponential phase. Instead, some factors regulated by σS are involved in increased (or sustained) activity of HPI. It is necessary to identify these factors in order to elucidate the regulatory pathways for catalase via σS in exponential-phase V. vulnificus. The experiments in which we examined the effect of H2O2 on synthesis of σS revealed a slightly increased amount of σS in the exponential-phase cells exposed to nonlethal concentrations of H2O2 (data not shown). However, additional studies are necessary to clarify whether the slightly increased amount of σS upregulates the synthesis and/or activity of KatG during the exponential phase.
Several research groups have emphasized the importance of σS in bacterial survival in the stationary phase and in resistance to H2O2 via regulation of HPII. Although it has been reported that in the stationary phase HPI is partially induced by σS in E. coli (8, 24) and in the exponential phase some virulence genes are regulated by σS in Salmonella dublin (5), exponential-phase induction of HPI by σS has not been examined previously. In the present study, however, we found that in V. vulnificus this global regulator plays a role in the response to oxidative stress during the exponential phase by increasing the amount of HPI with no involvement of HPII. Investigation of the other roles of σS in hierarchical regulatory cascades and the expression of rpoS in the presence of specific stresses is in progress.
Acknowledgments
This research was supported by the 21C Frontier Microbial Genomics and Applications Center Program, Ministry of Science and Technology (grant MG02-0201-004-2-1-0 to K.-H.L.), Republic of Korea.
We thank E.-K. Jeon and J. H. Lee for technical assistance, J. H. Rhee for providing a V. vulnificus ΔkatG mutant strain, H.-J. Myung for overexpression of V. vulnificus σS, and J. K. Lee for helpful discussions.
REFERENCES
- 1.Bang, Y. B., S. E. Lee, J. H. Rhee, and S. H. Choi. 1999. Evidence that expression of the Vibrio vulnificus hemolysin gene is dependent on cyclic AMP receptor protein. J. Bacteriol. 181:7639-7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohannon, D. E., N. Connell, J. Keener, A. Tormo, M. Espinosa-Urgel, M. M. Zambrano, and R. Kolter. 1991. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and role of σ70. J. Bacteriol. 173:4482-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, S. M., M. L. Howell, M. L. Vasil, A. J. Anderson, and D. J. Hassett. 1995. Cloning and characterization of the katB gene of Pseudomonas aeruginosa encoding a hydrogen peroxide-inducible catalase: purification of KatB, cellular localization, and demonstration that it is essential for optimal resistance to hydrogen peroxide. J. Bacteriol. 177:6536-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess, R. R., and L. Anthony. 2001. How sigma docks to RNA polymerase and what sigma does. Curr. Opin. Microbiol. 4:126-131. [DOI] [PubMed] [Google Scholar]
- 5.El-Gedaily, A., G. Paesold, C.-Y. Chen, D. G. Guiney, and M. Krause. 1997. Plasmid virulence gene expression induced by short-chain fatty acids in Salmonella dublin: identification of rpoS-dependent and rpoS-independent mechanisms. J. Bacteriol. 179:1409-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faruque, S. M., M. J. Albert, and J. J. Mekalanos. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62:1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Flecha, B., and B. Demple. 1997. Homeostatic regulation of intracellular hydrogen peroxide concentration in aerobically growing Escherichia coli. J. Bacteriol. 179:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the σs (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollis, D. G., R. E. Weaver, C. N. Baker, and C. Thornsberry. 1976. Halophilic Vibrio species isolated from blood cultures. J. Clin. Microbiol. 3:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichise, N., N. Morita, T. Hoshino, K. Kawasaki, I. Yumoto, and H. Okuyama. 1999. A mechanism of resistance to hydrogen peroxide in Vibrio rumoiensis S-1. Appl. Environ. Microbiol. 65:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong, H. S., K. C. Jeong, H. K. Choi, K.-J. Park, K.-H. Lee, J. H. Rhee, and S. H. Choi. 2001. Differential expression of Vibrio vulnificus elastase gene in a growth phase-dependent manner by two different types of promoters. J. Biol. Chem. 276:13875-13880. [DOI] [PubMed] [Google Scholar]
- 13.Jeong, K. C., H. S. Jeong, J. H. Rhee, S. E. Lee, S. S. Chung, A. M. Starks, G. M. Escudero, P. A. Gulig, and S. H. Choi. 2000. Construction and phenotypic evaluation of a Vibrio vulnificus vvpE mutant for elastolytic protease. Infect. Immun. 68:5096-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmid for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 15.Klotz, M. G., G. R. Klassen, and P. C. Loewen. 1997. Phylogenetic relationships among prokaryotic and eukaryotic catalases. Mol. Biol. Evol. 14:951-958. [DOI] [PubMed] [Google Scholar]
- 16.Kolter, R., D. A. Siegele, and A. Tormo. 1993. The stationary phase of the bacterial life cycle. Annu. Rev. Microbiol. 47:855-874. [DOI] [PubMed] [Google Scholar]
- 17.Lee, S. E., S. H. Shin, S. Y. Kim, Y. R. Kim, D. H. Shin, S. S Chung, Z. H. Lee, J. Y. Lee, K. C. Jeong, S. H. Choi, and J. H. Rhee. 2000. Vibrio vulnificus has the transmembrane transcription activator ToxRS stimulating the expression of the hemolysin gene vvhA. J. Bacteriol. 182:3405-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litwin, C. M., and S. B. Calderwood. 1993. Cloning and genetic analysis of the Vibrio vulnificus fur gene and construction of a fur mutant by in vivo marker exchange. J. Bacteriol. 175:706-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matin, A., E. A. Auger, P. H. Blum, and J. E. Schultz. 1989. Genetic basis of starvation survival in nondifferentiating bacteria. Annu. Rev. Microbiol. 43:293-316. [DOI] [PubMed] [Google Scholar]
- 20.McCann, M. P., C. D. Fraley, and A. Matin. 1993. The putative σ factor KatF is regulated posttranscriptionally during carbon starvation. J. Bacteriol. 175:2143-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrell, D. S., A. D. Tischler, S. H. Lee, and A. Camilli. 2000. Vibrio cholerae requires rpoS for efficient intestinal colonization. Infect. Immun. 68:6691-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michan, C., M. Manchado, G. Dorado, and C. Pueyo. 1999. In vivo transcription of the Escherichia coli oxyR regulon as a function of growth phase and in response to oxidative tress. J. Bacteriol. 181:2759-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milton, D. L., R. O'Toole, O. Horstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhopadhyay, S., and H. E. Schellhorn. 1994. Induction of Escherichia coli hydroperoxidase I by acetate and other weak acids. J. Bacteriol. 176:2300-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulvey, M. R., J. Switala, A. Borys, and P. C. Loewen. 1990. Regulation of transcription of katE and katF in Escherichia coli. J. Bacteriol. 172:6713-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver, J. D. 1993. Formation of viable but nonculturable cells, p. 239-272. In S. Kjelleberg (ed.), Starvation in bacteria. American Society for Microbiology, Washington, D.C.
- 27.Oliver, J. D., F. Hite, D. McDougald, N. L. Andon, and L. M. Simpson. 1995. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl. Environ. Microbiol. 61:2624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruby, E. G., and K. H. Nealson. 1977. Pyruvate production and excretion by the luminous marine bacteria. Appl. Environ. Microbiol. 34:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Schellhorn, H. E., and V. L. Stones. 1992. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J. Bacteriol. 174:4769-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz, J. E., G. I. Latter, and A. Matin. 1988. Differential regulation by cyclic AMP of starvation protein synthesis in Escherichia coli. J. Bacteriol. 170:3903-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seshadri, R., and J. E. Samuel. 2001. Characterization of a stress-induced alternate sigma factor, RpoS, of Coxiella burnetii and its expression during the development cycle. Infect. Immun. 69:4874-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao, C.-P., and L.-I. Hor. 2001. Regulation of metalloprotease gene expression in Vibrio vulnificus by a Vibrio harveyi LuxR homologue. J. Bacteriol. 183:1369-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 35.Simpson, L. M., and J. D. Oliver. 1983. Siderophore production by Vibrio vulnificus. Infect. Immun. 42:644-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slauch, J., R. Taylor, and S. Maloy. 1997. Survival in a cruel world: how Vibrio cholerae and Salmonella respond to an unwilling host. Genes Dev. 11:1761-1774. [DOI] [PubMed] [Google Scholar]
- 37.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 38.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 39.Suh, S.-J., L. Silo-Suh, D. W. Woods, D. J. Hassett, S. E. H. West, and D. E. Ohman. 1999. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J. Bacteriol. 181:3890-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Switala, J., J. O. O'Neil, and P. C. Loewen. 1999. Catalase HPII from Escherichia coli exhibits enhanced resistance to denaturation. Biochemistry 38:3895-3901. [DOI] [PubMed] [Google Scholar]
- 41.Venturi, V. 2003. Control of rpoS transcription in Escherichia coli and Pseudomonas: why so different? Mol. Microbiol. 49:1-9. [DOI] [PubMed] [Google Scholar]
- 42.Visick, J. E., and S. Clarke. 1997. RpoS- and OxyR-independent induction of HPI catalase at stationary phase in Escherichia coli and identification of rpoS mutations in common laboratory strains. J. Bacteriol. 179:4158-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Visick, K. L., and M. J. McFall-Ngai. 2000. An exclusive contract: specificity in the Vibrio fischeri-Euprymna scolopes partnership. J. Bacteriol. 182:1779-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visick, K. L., and E. G. Ruby. 1998. The periplasmic, group III catalase of Vibrio fischeri is required for normal symbiotic competence and is induced both by oxidative stress and by approach to stationary phase. J. Bacteriol. 180:2087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vivas, E. I., and H. Goodrich-Blair. 2001. Xenorhabdus nematophilus as a model for host-bacterium interactions: rpoS is necessary for mutualism with nematodes. J. Bacteriol. 183:4687-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldron, D. E., P. Owen, and C. J. Dorman. 2002. Competitive interaction of the OxyR DNA-binding protein and the Dam methylase at the antigen 43 gene regulatory region in Escherichia coli. Mol. Microbiol. 44:509-520. [DOI] [PubMed] [Google Scholar]
- 47.Wayne, L. G., and G. A. Diaz. 1986. A double staining method for differentiation between two classes of mycobacterial catalase in polyacrylamide electrophoresis gels. Anal. Biochem. 157:89-92. [DOI] [PubMed] [Google Scholar]
- 48.Wright, A. C., J. G. Morris, Jr., D. R. Maneval, Jr., K. Richardson, and J. B. Baker. 1985. Cloning of the cytotoxin-hemolysin gene of Vibrio vulnificus. Infect. Immun. 50:922-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 50.Yildiz, F. H., and G. K. Schoolnik. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yumoto, I., D. Ichihashi, H. Iwata, A. Istokovics, N. Ichise, H. Matsuyama, H. Okuyama, and K. Kawasaki. 2000. Purification and characterization of a catalase from the facultatively psychrophilic bacterium Vibrio rumoiensis S-1T exhibiting high catalase activity. J. Bacteriol. 182:1903-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]