Abstract
Single-port video-assisted thoracic surgery (VATS) has slowly established itself as an alternate surgical approach for the treatment of an increasingly wide range of thoracic conditions. The potential benefits of fewer surgical incisions, better cosmesis, and less postoperative pain and paraesthesia have led to the technique’s popularity worldwide. The limited single small incision through which the surgeon has to operate poses challenges that are slowly being addressed by improvements in instrument design. Of note, instruments and video-camera systems that are narrower and angulated have made single-port VATS major lung resection easier to perform and learn. In the future, we may see the development of subcostal or embryonic natural orifice translumenal endoscopic surgery access, evolution in anaesthesia strategies, and cross-discipline imaging-assisted lesion localization for single-port VATS procedures.
Keywords: DynaCT, Hookwire, Hybrid, Magnetic anchoring and guidance systems, Non-intubated lung resection, Natural orifice translumenal endoscopic surgery
INTRODUCTION
Single-port, also known as uniportal or single-incision, video-assisted thoracic surgery (VATS) has become increasingly popular [1,2]. The development of single-port VATS has come a long way, from the beginning for performing simple procedures such as sympathectomy and pleurodesis over a decade ago, to the rapid progression in the past 2 years of complex major lung resections, including segmentectomy, pneumonectomy, lung resection with chest wall resection, sleeve resection, and double-sleeve resection [3–9]. Furthermore, there is growing evidence that single-port VATS can be performed safely with good early outcomes that are at least comparable to those of conventional VATS major lung resection [10,11]. Although publications relating to single-port VATS account for less than 1% of all publications on VATS in general, there is a steady rise in literature, particularly from the Asian region, on this technique, accounting for over 50% of all publications on single-port VATS in the past 2 years.
The idea of performing thoracic surgery through a single small incision has led to a radical change in the thought process of VATS surgeons in terms of surgical access, approach to the procedure, and the need for specialized equipment. An added benefit of this new direction is the unprecedented growth in collaboration between thoracic surgeons and industry to achieve these goals. The current review will describe the developments in these areas, which should be of particular interest to surgeons who perform single-port VATS.
SURGICAL ACCESS & APPROACH
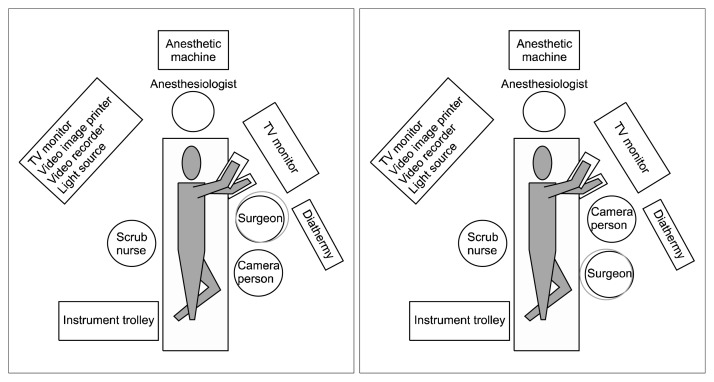
Single-port VATS represents a radical change in the approach to lung resection compared with the conventional three-port VATS. By requiring the placement of all the operating instruments and the camera within the same incision, single-port VATS can pose a challenge for both the surgeon and the assistant. Furthermore, the position of the single-port incision becomes even more important than in two- or three-port VATS. In general, our approach to the dissection of hilar structures is from the anterior; hence, the incision is centered around the anterior axillary line, at the 5th intercostal space [12]. The surgeon and the first assistant, who holds the camera, usually stand on the side that is anterior to the patient, while a second assistant may stand on the opposite side (Fig. 1). It should be highlighted that the relative positions of the surgeon and the first assistant can be exchanged during single-port VATS when surgery is performed for the upper and the lower chest cavities in order to facilitate instrumentation and improve ergonomics (Fig. 1). Often, the single-port surgeon may find the visualization and dissection of structures, as well as the identification of the correct angles for instrumentation and endostapler deployment, difficult. Changing the order of the instruments along the single-port incision can sometimes help facilitate these manoeuvres, without the need to enlarge the incision or exert force onto the wound. During most of the dissection, the scope is placed at the posterior aspect of the incision. It is noteworthy that the placement of the scope in the middle position is less often used because it is ‘floating’ in the incision wound and makes it difficult to keep the camera steady [13].
Fig. 1.
Illustration of the positions of the surgeon and assistants during single-port video-assisted thoracic surgery lung resection. During surgery, the surgeon and the assistant may exchange positions for more ergonomic access. (A) Surgeon cranial. (B) Surgeon caudal.
Despite the minimally invasive nature of single-port VATS, placing an incision through an intercostal space can result in chronic pain or paraesthesia, even when strategies to reduce the incision size and decrease the risk of intercostal nerve injury are followed [14]. In 2013, Liu [15] successfully performed the first single-port VATS lobectomy via a subxiphoid approach for left upper lobectomy. The small incision placed inferior to the costal margin may reduce the risk of intercostal nerve injury and potentially avoid the limitations imposed by narrow rib spaces [15]. The subxiphoid approach, when implemented close to the midline, also allows for access to the bilateral thoracic cavities for bilateral lung resections through one incision [16]. However, the transmitted cardiac pulsation to the VATS instruments, particularly during left chest surgery, makes this approach challenging. Furthermore, in the event of a significant vascular injury, due to the distance of the subxiphoid incision from the hilar, bleeding control may be much more difficult. If a conversion to open surgery becomes necessary, an extension of the subxiphoid incision is unlikely to be useful and an additional thoracotomy incision may be necessary. As the VATS equipment continues to become smaller with more angles of articulation, another potential single-incision VATS approach would be through the axilla. Although such an approach would not address the problem of intercostal neuralgia, placing the incision in the axilla may be superior at least from a purely aesthetic point of view.
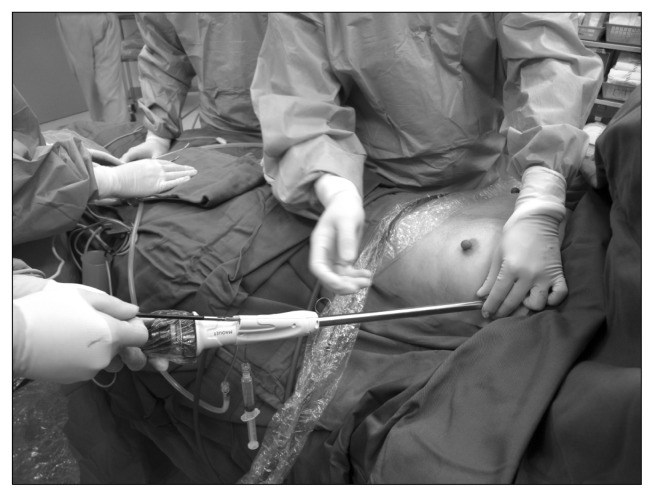
To improve ergonomics and particularly to avoid the fencing from individual instruments placed through the small incision of single-port VATS, rapid progress in instrument design has taken place. The narrower shafted double-hinged single-port instruments and thoracoscopes that have greater degrees of vision may all contribute to a reduction of some of the challenges associated with single-port VATS [13,17]. The greater lens flexibility improves visibility in single-port VATS when all the instruments and the scope pass through a single small incision in a parallel manner, particularly for surgeons on the learning curve and in difficult cases [13,17]. Further innovations that have led to a reduction in thoracoscope–instrument interference include the replacement of the conventional thoracoscope with remote wireless camera systems, such as the magnetic anchoring guidance system (MAGS) [17]. The MAGS uses magnets to hold and control a remote wireless camera placed within the operating cavity, eliminating the need for light and transmission cables. To further reduce the wound size and to avoid instrument fencing, the idea of having a combined instrument unit incorporating the various components of an endoscope, a retraction device, and an energy source has been developed. In fact, the first report was the utilization of a modified pediatric cysto-resectoscope in single-port bilateral VATS thoracic sympathectomy for hyperhidrosis [18]. Subsequently, along the same lines of thought, a more modern version of the single-instrument unit for single-port VATS sympathectomy uses the VasoView device. Through a 1-cm incision, the instrument originally designed for endoscopic vein harvesting can be placed into the thoracic cavity, and via the in-built instrument channel, camera, and carbon dioxide insufflation channel, the sympathectomy can be completed with good results [19,20] (Fig. 2).
Fig. 2.
Endoscopic vein harvesting device with an instruments channel, a carbon dioxide insufflator channel, an endoscope, and a lens cleaning system, as an example of an integrated instrument design for single-port video-assisted thoracic surgery surgery.
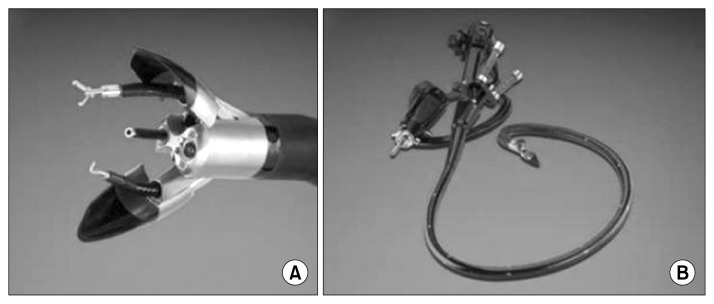
To avoid an unsightly incision scar, embryonic natural orifice translumenal endoscopic surgery (e-NOTES) has been successfully performed for certain simple thoracic procedures. E-NOTES involves the passage of an endoscope through a single incision at the umbilicus in order to reach the thoracic cavity transdiaphragmatically. Zhu et al. [21] reported the use of a 5-mm ultrathin flexible gastroscope to perform e-NOTES thoracic sympathectomy in patients suffering from palmar hyperhidrosis. The 35 patients who underwent this procedure suffered no early or late complications, and experienced less pain and higher satisfaction with the aesthetic results when compared with conventional needlescopic sympathectomy [22]. Most recently, a group in Taiwan has ventured into e-NOTES anatomic pulmonary lobectomy in a canine model, and developments in this area are eagerly awaited [23]. Although the e-NOTES approach using the currently available endoscopes may be adequate for performing simple thoracic procedures, a more sophisticated endoscopic platform is needed to accomplish more complex surgical tasks. Flexible endoscopic platforms such as Anubiscope (Karl Storz, Tuttlingen, Germany) are under development for NOTES [24] (Fig. 3). A steerable endoscope has three channels that allow specialized instruments mimicking standard surgical cautery, graspers, and clips to perform the necessary surgery. Therefore, these advanced endoscopic platforms provide the operator with better control and ability to have traction and counter-traction as compared to standard endoscopes. However, the use of these endoscopes for NOTES of the chest by breeching the integrity of the trachea or the oesophagus to enter the pleural cavity requires further refinement.
Fig. 3.
(A, B) Advanced single-incision endoscopic platforms for natural orifice translumenal endoscopic surgery, such as the Anubiscope, are being developed to allow more complex surgical procedures to be performed.
CROSSING DISCIPLINES
The single-port VATS approach may further reduce access trauma and has brought about a new line of thought on the role of an ‘awake’ non-intubated technique in fast-tracking patients postoperatively. Rocco et al. [25] first described the use of mild sedation, single-shot epidural regional anaesthesia, and bronchoscopically guided Fogarty balloon placement into the lobar bronchus to facilitate selective lung collapse as techniques for ‘awake,’ non-intubated single-port VATS wedge resection. Subsequently, the same group described their ‘awake’ technique for performing single-port VATS pleurodesis for spontaneous pneumothorax [26]. More recently, Gonzalez-Rivas reported the first successful non-intubated single-port VATS right middle lobectomy case without epidural or vagus blockade in a 46-year-old man with carcinoid tumour [27]. However, it should be emphasized that case selection is important for this approach and that the anaesthetists should be familiar with the technique and be skilled at intubation under difficult conditions. The gradual maturation of the ‘awake’ non-intubated lung surgery concept over the years may allow it to become a useful adjunct in single-port VATS for early postoperative ambulation and hospital discharge.
One of the concerns with single-port VATS lung resection is the limited access for accurately palpating and assessing the thoracic tumour. Despite the overall satisfactory accessibility to most of the thoracic cavity through a single incision usually positioned around the mid-thoracic region, localization of small tumours with a low solid component and ground-glass opacities may be difficult. Classically, the use of pre-operative computed tomography (CT)-guided marking by dye or even hookwire localization can be very useful to aid the intraoperative identification of the lung lesion. In selected cases, hookwire localization may also allow the single-port VATS incision to be minimized [28]. However, dye marking from a percutaneous image-guided approach can be associated with significant dye diffusion and artifacts, thereby reducing its accuracy and applicability. The disadvantages of hookwire localization are pneumothorax, hemothorax, and hookwire dislodgement. Furthermore, when multiple lung lesions require pre-operative marking, these percutaneous techniques become even less desirable. Electromagnetic navigational bronchoscopy (ENB) may become a very useful tool in the future, either preoperatively or intraoperatively, to localize pulmonary lesions by dye injection or by the placement of metal fiducials [29,30]. The accuracy of the ENB systems has improved over the past decade, and these systems can now competently localize peripheral lung lesions that are several millimetres in size. The risks of complications following ENB are few as compared to those of percutaneous image-guided methods, and multiple lesions can be conveniently marked in the same session.
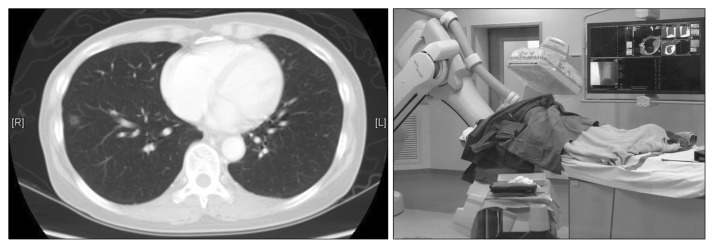
The recent development of hybrid operating rooms and their application in surgery have opened new opportunities in the image-guided localization of lung lesions. The integration of real-time on-table image guidance technology into clinical practice is well-established in other specialties, including cardiology and vascular surgery. However, its use in minimally invasive thoracic surgery, also known as image-guided VATS, was only first reported in 2013 by Prof. Raphael Bueno at Harvard University, Brigham and Women Hospital, Boston. In the subsequent year, the group at The Chinese University of Hong Kong, Hong Kong, became the first to pioneer hybrid operating room image guidance for single-port VATS (iSPVATS) for small ground-glass pulmonary lesions [31] (Fig. 4). The real-time DynaCT scan in the hybrid theatre can be used to guide hookwire insertion, then proceeding immediately to single-port VATS lung resection of the lesion in the same setting, thereby reducing risks associated with transfers and delays when the patient may have pneumothorax, hook-wire displacement, and discomfort. Should the hookwire migrate or dislodge, for example during lung deflation, a CT scan must be performed immediately to re-hookwire or re-localize the lesion. For lung lesions that are not feasible for hookwire insertion, a real-time on-table scan can aid in localizing the lung lesion for resection and may provide additional information on resection margins [31]. In the future, a combination of ENB-guided metal fiducial placement and hybrid operating theatre image-guided localization may further improve the resection accuracy and safety of iSPVATS.
Fig. 4.
Hybrid operating room with capabilities for performing image-guided video-assisted thoracic surgery. Ground-glass opacity in the right lower lobe located by real-time on-table DynaCT, with a frozen section showing adenocarcinoma, followed by image-guided single-port video-assisted thoracic lobectomy.
CONCLUSION
Although the increasing application of single-port VATS has brought about new challenges both for the surgeon and for the industry that provides us the technology to push the boundaries of surgery, it has also created unique opportunities for collaboration and to explore novel techniques. In the future, the difficulty will be to decide upon the most appropriate approach or technology to apply for each unique patient.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Ng CS, Lau KK, Gonzalez-Rivas D, Rocco G. Evolution in surgical approach and techniques for lung cancer. Thorax. 2013;68:681. doi: 10.1136/thoraxjnl-2012-203157. [DOI] [PubMed] [Google Scholar]
- 2.Ng CS. Uniportal VATS in Asia. J Thorac Dis. 2013;5(S3):S221–5. doi: 10.3978/j.issn.2072-1439.2013.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng CS, Rocco G, Yim AP. Video-assisted thoracoscopic surgery (VATS) pleurodesis for pneumothorax. Multimed Man Cardiothorac Surg. 2005;2005 doi: 10.1510/mmcts.2004.000349. doi: mmcts.2004.000349. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez D, Paradela M, Garcia J, Dela Torre M. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg. 2011;12:514–5. doi: 10.1510/icvts.2010.256222. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Rivas D, Fieira E, Mendez L, Garcia J. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg. 2012;42:e169–71. doi: 10.1093/ejcts/ezs482. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Rivas D, de la Torre M, Fernandez R, Garcia J. Video: Single-incision video-assisted thoracoscopic right pneumonectomy. Surg Endosc. 2012;26:2078–9. doi: 10.1007/s00464-011-2127-x. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Rivas D, Fernandez R, Fieira E, Rellan L. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg. 2013;145:1676–7. doi: 10.1016/j.jtcvs.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Rivas D, Fernandez R, Fieira E, Mendez L. Single-incision thoracoscopic right upper lobectomy with chest wall resection by posterior approach. Innovations (Phila) 2013;8:70–2. doi: 10.1097/IMI.0b013e3182852005. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Rivas D, Delgado M, Fieira E, Mendez L. Single- port video-assisted thoracoscopic lobectomy with pulmonary artery reconstruction. Interact Cardiovasc Thorac Surg. 2013;17:889–91. doi: 10.1093/icvts/ivt340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau RW, Ng CS, Kwok MW, et al. Early outcomes following uniportal video-assisted thoracic surgery lung resection. Chest. 2014;145(3 Suppl):50A. [Google Scholar]
- 11.Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg. 2013;95:426–32. doi: 10.1016/j.athoracsur.2012.10.070. [DOI] [PubMed] [Google Scholar]
- 12.Ng CS, Lau KK, Wong RH, et al. Single port video-assisted thoracoscopic lobectomy for early stage non-small cell lung carcinoma. Surg Pract. 2013;17:35–6. [Google Scholar]
- 13.Ng CS, Wong RH, Lau RW, Yim AP. Minimizing chest wall trauma in single-port video-assisted thoracic surgery. J Thorac Cardiovasc Surg. 2014;147:1095–6. doi: 10.1016/j.jtcvs.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 14.Yim AP. Minimizing chest wall trauma in video-assisted thoracic surgery. J Thorac Cardiovasc Surg. 1995;109:1255–6. doi: 10.1016/S0022-5223(95)70217-2. [DOI] [PubMed] [Google Scholar]
- 15.Liu CC. First report: 20131209 subxiphoid single port VAT. [Internet]. [place unknown]: [publisher unknown]. 2013. [cited 2014 May 10]. Available from: http://www.youtube.com/watch?v=Hi_gn8s0VzU.
- 16.Liu CC. First report: 20131211 subxiphoid single port VATS for bilateral NPC lung mets. 2013. [Internet]. [place unknown]: [publisher unknown] [cited 2014 May 10]. Available from: http://www.youtube.com/watch?v=xNBTuFJV3bQ.
- 17.Ng CS, Wong RH, Lau RW, Yim AP. Single port video-assisted thoracic surgery: advancing scope technology. Eur J Cardiothorac Surg. 2014 doi: 10.1093/ejcts/ezu236. In press. [DOI] [PubMed] [Google Scholar]
- 18.Lardinois D, Ris HB. Minimally invasive video-endoscopic sympathectomy by use of a transaxillary single port approach. Eur J Cardiothorac Surg. 2002;21:67–70. doi: 10.1016/s1010-7940(01)01042-9. [DOI] [PubMed] [Google Scholar]
- 19.Ng CS, Yeung EC, Wong RH, Kwok MW. Single-port sympathectomy for palmar hyperhidrosis with Vasoview Hemopro 2 endoscopic vein harvesting device. J Thorac Cardiovasc Surg. 2012;144:1256–7. doi: 10.1016/j.jtcvs.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Ng CS, Lau RW, Wong RH, Ho AM, Wan S. Single-port vasoview sympathectomy for palmar hyperhidrosis: a clinical update. J Laparoendosc Adv Surg Tech A. 2014;24:32–4. doi: 10.1089/lap.2013.0346. [DOI] [PubMed] [Google Scholar]
- 21.Zhu LH, Chen L, Yang S, et al. Embryonic NOTES thoracic sympathectomy for palmar hyperhidrosis: results of a novel technique and comparison with the conventional VATS procedure. Surg Endosc. 2013;27:4124–9. doi: 10.1007/s00464-013-3079-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhu LH, Du Q, Chen L, et al. One-year follow-up period after transumbilical thoracic sympathectomy for hyperhidrosis: outcomes and consequences. J Thorac Cardiovasc Surg. 2014;147:25–8. doi: 10.1016/j.jtcvs.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 23.Yin SY, Chu Y, Wu YC, et al. Feasibility of transumbilical anatomic pulmonary lobectomy in a canine model. Surg Endosc. 2014 May 23; doi: 10.1007/s00464-014-3561-3. [Epub] [DOI] [PubMed] [Google Scholar]
- 24.Perretta S, Dallemagne B, Barry B, Marescaux J. The ANUBISCOPE® flexible platform ready for prime time: description of the first clinical case. Surg Endosc. 2013;27:2630. doi: 10.1007/s00464-013-2818-6. [DOI] [PubMed] [Google Scholar]
- 25.Rocco G, Romano V, Accardo R, et al. Awake single-access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting. Ann Thorac Surg. 2010;89:1625–7. doi: 10.1016/j.athoracsur.2010.01.087. [DOI] [PubMed] [Google Scholar]
- 26.Rocco G, La Rocca A, Martucci N, Accardo R. Awake single-access (uniportal) video-assisted thoracoscopic surgery for spontaneous pneumothorax. J Thorac Cardiovasc Surg. 2011;142:944–5. doi: 10.1016/j.jtcvs.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Rivas D, Fernandez R, de la Torre M, Rodriguez JL, Fontan L, Molina F. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection? Interact Cardiovasc Thorac Surg. 2014 Jul 7; doi: 10.1093/icvts/ivu209. [Epub] [DOI] [PubMed] [Google Scholar]
- 28.Ng CS, Hui JW, Wong RH. Minimizing single-port access in video-assisted wedge resection, with a hookwire. Asian Cardiovasc Thorac Ann. 2013;21:114–5. doi: 10.1177/0218492312467448. [DOI] [PubMed] [Google Scholar]
- 29.Gildea TR, Mazzone PJ, Karnak D, Meziane M, Mehta AC. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med. 2006;174:982–9. doi: 10.1164/rccm.200603-344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kupelian PA, Forbes A, Willoughby TR, et al. Implantation and stability of metallic fiducials within pulmonary lesions. Int J Radiat Oncol Biol Phys. 2007;69:777–85. doi: 10.1016/j.ijrobp.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 31.Ng CS, Chu CM, Kwok MW, Wong RH. Hybrid DynaCT guided localization single port lobectomy. Chest. 2015 doi: 10.1378/chest.14-1503. In press. [DOI] [PubMed] [Google Scholar]