Abstract
Background
The Damus-Kaye-Stansel (DKS) procedure is a method for mitigating the risk of systemic ventricular outflow tract obstruction (SVOTO). However, there have been few reports on which surgical technique shows a better outcome. The objective of this study was to compare the outcome of the DKS procedure according to the surgical technique used.
Methods
We retrospectively reviewed 12 consecutive patients who underwent the DKS procedure from March 2004 to April 2013. When the relationship of the great arteries was anterior-posterior, the double-barrel technique (group A) was performed. If the relationship was side-by-side, the ascending aortic flap technique (group B) was performed.
Results
There was no early mortality and 1 late mortality in group B. There was no statistically significant difference in the median peak pressure gradient of preoperative subaortic stenosis in both groups: 14 mmHg (range, 4 to 53 mmHg) in group A and 15 mmHg (range, 0 to 30 mmHg) in group B (p=0.526). Further, a significant postoperative pressure gradient was not observed in either group A or group B. More than moderate postoperative neoaortic regurgitation was observed in 1 patient of group B; this patient underwent neoaortic valve replacement 66 months after the DKS procedure. No one had a recurrent SVOTO during follow-up.
Conclusion
The DKS procedure is an effective way to minimize the risk of SVOTO, and there is little difference in the outcomes of the DKS procedure according to the surgical technique used.
Keywords: Great vessels, Pediatric, Congenital heart disease (CHD), Fontan operation
INTRODUCTION
Patients with a functional single ventricle undergo multiple-stage operations, including postnatal palliations, which restrict or increase pulmonary blood flow according to the amount of the existing pulmonary blood flow, bidirectional cavopulmonary shunt, and finally, the Fontan operation. However, any functional single ventricle anomalies (for example, tricuspid atresia and double inlet left ventricle with rudimentary bulboventricular foramen) have the problem of systemic ventricular outflow tract obstruction (SVOTO) after volume reduction surgery. SVOTO adversely affects the clinical outcome of the Fontan operation [1]. SVOTO could result in ventricular hypertrophy, impaired diastolic function, and subendocardial ischemia with subsequent deleterious effects on the single ventricle.
Typical approaches to the management of SVOTO include the enlargement of the bulboventricular foramen or the ventricular septal defect, and the resection of the subaortic conus. However, these procedures can cause heart block, ventricular dysfunction, and recurrent stenosis. Another method to relieve SVOTO is the Damus-Kaye-Stansel (DKS) procedure [2–5]. This operation was first introduced for biventricular repair in patients with dextro-transposition of the great arteries [6–8]. The original DKS procedure was an end-to-side anastomosis between the main pulmonary artery and the ascending aorta. Since then, there have been numerous articles proposing modified DKS procedures. Waldman et al. [9] demonstrated a double-barrel technique, which achieves a new bivalve single aorta. Laks et al. [10] reported the aortic flap technique as a modification of the DKS procedure [11].
We decided upon the DKS operation technique according to the relationship of the aorta and the main pulmonary artery. There have been a few reports about which surgical technique shows better outcomes. The objective of this study is to compare the outcomes of the DKS procedure according to the surgical technique used from the perspective of neo-aortic regurgitation and recurrent SVOTO, which are considered to be two major complications caused by the DKS procedure [12–14].
METHODS
1) Patient profiles
The clinical records of 12 consecutive patients with congenital heart disease who underwent a DKS operation in Seoul National University Children’s Hospital between March 2004 and April 2013 were retrospectively reviewed. Any patients who underwent the Norwood operation were excluded.
2) Surgical methods
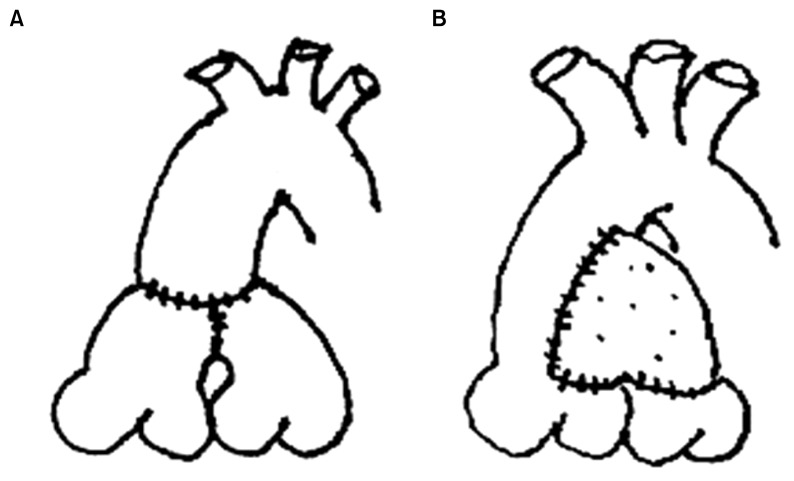
We decided upon the surgical technique to use for the DKS procedure according to the relationship between the aorta and the main pulmonary artery. If the great arteries had the relationship of anterior-posterior, we chose the double-barrel technique (group A), and if they lay side-by-side, we performed the ascending aorta flap technique (group B), which is a type of end-to-side DKS procedure with patch augmentation [12]. We resected the ascending aorta in an ‘L’ shape and anastomosed with the posterior wall of the pulmonary artery stump. Thereafter, a bovine pericardium was used for repairing the anterior defect (Fig. 1). All DKS operations were performed by a single surgeon.
Fig. 1.
Illustration of surgical technique. (A) Double barrel technique (group A). (B) Ascending aorta flap technique (group B).
3) Evaluation and follow-up
We evaluated the preoperative echocardiographic results: aortic regurgitation (AR), pulmonary regurgitation (PR), and pressure gradient of subaortic stenosis. Further, we followed-up with the postoperative echocardiographic results: AR, PR (that is, ‘neoAR’), and recurrent SVOTO.
4) Statistical analysis
Data are expressed as mean ± standard deviation or median (range) as appropriate. A comparison between the two groups was performed using the Wilcoxon signed-rank test. A p-value of less than 0.05 was considered statistically significant. IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA) was used for the data analysis.
RESULTS
1) Patient characteristics
The preoperative cardiac diagnosis is summarized in Table 1. The median age at the time of the DKS operation was 5.9 months (range, 2.9 to 16.8 months), and the median body weight was 6.8 kg (range, 3.5 to 9.7 kg). Ten patients underwent pulmonary artery banding (PAB) previously, and the median duration from PAB to the DKS operation was 6.1 months (range, 3.1 to 14.8 months). The median peak pressure gradient of subaortic stenosis was 15 mmHg (range, 0 to 53 mmHg). Only 1 patient had mild preoperative AR, and the rest had a degree of trivial. Further, 1 patient had mild preoperative PR, and the rest had a degree of trivial. There were no statistically significant differences between group A and group B in age, body weight, body surface area, duration, and subaortic stenosis at the time of both PAB and the DKS procedure (Table 2).
Table 1.
The preoperative cardiac diagnosis and operation performed in stages
| Diagnosis | 1st | 2nd | 3rd | 4th/Cx | |
|---|---|---|---|---|---|
| 1 | DORV, VSD, hypoplastic LV | PAB, CoA repair, atrial septectomy | DKS, BCPS | ||
| 2 | DILV, rudimentary RV | PAB (another hospital) | DKS, BCPS, atrial septectomy | Fontan, neoAV repair | neoAVR |
| 3 | DORV with noncommitted VSD, hypoplastic MV, LV | PAB, CoA repair | DKS, BCPS, atrial septectomy | Fontan | Bleeding control |
| 4 | ccTGA, VSD, criss-cross heart, small AV annulus | PAB, CoA repair | DKS, BCPS, atrial septectomy | Fontan | pexy |
| 5 | DORV, VSD, LV type UVH, small RV | PAB, CoA repair | DKS, BCPS, PA angioplasty, neoaortopexy | ||
| 6 | cAVSD, DORV, heterotaxia | PAB, CoA & TAPVR repair | DKS, BCPS | ||
| 7 | cAVSD, heterotaxia | PAB | DKS, bilat. BCPS | Fontan | |
| 8 | DILV, VSD, rudimentary RV | PAB, CoA repair, atrial septetomy | DKS, BCPS, recurrent CoA repair, both PA patch angioplasty | Fontan | |
| 9 | TGA, VSD, mitral atresia, small LV | PAB, s/p balloon atrial septostomy | DKS, BCPS, atrial septectomy | Fontan | |
| 10 | DORV, VSD, criss-cross heart | PAB | DKS, BCPS, atrial septectomy, PA angioplasty | ||
| 11 | DILV, rudimentary RV, VSD | DKS, BCPS, atrial septectomy | |||
| 12 | Critical AS dysplastic MV | DKS, PAB, atrial septectomy, MV obliteration s/p balloon aortic valvotomy |
BCPS, PAPVR | ||
Cx, complication; DORV, double outlet right ventricle; VSD, ventricular septal defect; LV, left ventricle; PAB, pulmonary artery banding; CoA, coartation of aorta; DKS, Damus-Kaye-Stansel; BCPS, bidirectional cavopulmonary shunt; DILV, double inlet left ventricle; RV, right ventricle; AV, aortic valve; AVR, aortic valve replacement; MV, mitral valve; ccTGA, congenitally corrected transposition of the great arteries; UVH, univentricular heart; PA, pulmonary artery; cAVSD, complete atrioventricular septal defect; TAPVR, total anomalous pulmonary venous return; TGA, transposition of the great arteries; s/p, status post; AS, aortic stenosis.
Table 2.
Patient characteristics of the two groups. Group A underwent double barrel technique, and group B was performed ascending aorta flap technique
| Characteristic | Group A (n=7) | Group B (n=5) | p-value |
|---|---|---|---|
| Sex (male:female) | 5:2 | 3:2 | |
| Pulmonary artery banding | |||
| Age (mo) | 1.1±1.0 | 0.9±0.6 | 0.8 |
| Bwt (kg) | 3.1±0.7 | 3.0±1.1 | 0.9 |
| BSA (m2) | 0.2±0.0 | 0.2±0.0 | 1.0 |
| Duration (mo) | 7.0±4.2 | 6.9±4.5 | 1.0 |
| Damus-Kaye-Stensel | |||
| Age (mo) | 6.8±4.2 | 7.8±5.0 | 0.7 |
| Bwt (kg) | 6.6±2.0 | 6.7±2.0 | 0.9 |
| BSA (m2) | 0.3±0.1 | 0.4±0.1 | 0.8 |
| Duration (yr) | 2.0±2.3 | 3.2±2.1 | 0.4 |
| Preoperative aortic regurgitation | |||
| ≤Mild | 7 | 5 | |
| ≥Moderate | 0 | 0 | |
| Preoperative pulmonary regurgitation | |||
| ≤Mild | 7 | 5 | |
| ≥Moderate | 0 | 0 | |
| Subaortic stenosis (mmHg) | 14 (4–53) | 15 (0–30) | 0.526 |
Values are presented as mean±standard deviation except sub-aortic stenosis, which is presented as median (range).
Bwt, body weight; BSA, body surface area.
2) Surgical data
The DKS operation was performed with a bidirectional cavopulmonary shunt (BCPS) in 11 of the 12 patients. Only 1 patient underwent DKS with PAB, and he also underwent BCPS surgery 6 months later. We divided the 12 patients into two groups according to the surgical technique used. Seven of the 12 patients underwent the double-barrel technique (group A), and 5 patients underwent the ascending aorta flap technique (group B). In group A, 3 patients underwent the Gore-Tex or polytetrafluoroethylene graft interposition for preserving the shape of the pulmonary sinus, and 1 patient underwent aortopulmonary window creation. The other associated procedures were atrial septectomy in 7 patients, pulmonary angioplasty with bovine pericardium in 3 patients, and neoaortopexy in 1 patient. The mean aortic cross-clamping time was 82.7±7.9 minutes, and the mean cardiopulmonary bypass time was 190.4±11.4 minutes.
3) Postoperative outcomes
There were no statistically significant differences in the postoperative course, such as extubation, intensive care unit stay, or chest tube removal between group A and group B. Recurrent SVOTO was not observed in either group in the immediate postoperative period (Table 3).
Table 3.
The postoperative results in each group
| Variable | Group A (n=7) | Group B (n=5) | p-value |
|---|---|---|---|
| Early mortality | 0 | 0 | |
| Late mortality | 0 | 1 | 0.22 |
| Postoperative AR | |||
| ≤Mild | 7 | 5 | 0.44 |
| ≥Moderate | 0 | 0 | |
| Postoperative neoAR | |||
| ≤Mild | 7 | 4 | 0.87 |
| ≥Moderate | 0 | 1 | 0.22 |
| Recurrent systemic ventricular outflow tract obstruction | 0 | 0 | |
| Extubation (day) | 3.8±4.3 | 1.4±0.7 | 0.26 |
| Intensive care unit stay (day) | 13.0±19.0 | 3.9±2.0 | 0.32 |
| Chest tube removal (day) | 4.7±1.9 | 5.0±1.6 | 0.79 |
| Complication | |||
| Neo-aortic valve replacement | 0 | 1 | 0.22 |
| Bleeding control | 0 | 1 | 0.22 |
Values are presented as number or mean±standard deviation.
AR, aortic regurgitation.
There was no early death and 1 late death in group B. He was 5.6 months old with right isomerism and a complete atrioventricular septal defect. This patient underwent PAB, coarctoplasty, and infracardiac obstructive total anomalous pulmonary venous connection repair before the DKS procedure. He was discharged on postoperative day 10 without any problems after the DKS with BCPS operation. However, 7 months later, he was admitted via the emergency room owing to general weakness. In echocardiography, there was no evidence of AR or neoAR. However, mild-to-moderate atrioventricular valve regurgitation and an ejection fraction of <40% were found. The patient’s condition aggravated to a low cardiac output syndrome in spite of intensive care, and the patient died of heart failure.
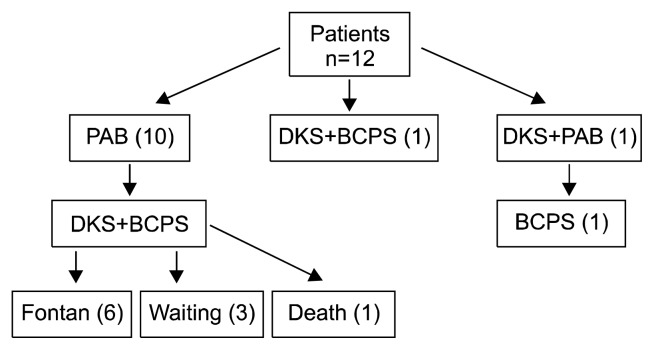
The mean follow-up time was 36.3±8.2 months. Six of the 12 patients underwent Fontan completion after the DKS operation, and 3 patients were waiting (Fig. 2). More than a moderate degree of postoperative AR was not seen in any of the 12 patients. However, more than moderate degree of postoperative neoAR was observed in 1 patient, and he underwent neo-aortic valve repair concomitant with the Fontan operation 21 months after the DKS procedure. However, neoAR was aggravated in the process, and the patient underwent neo-aortic valve replacement with ATS 16 mm (ATS Medical Inc., Minneapolis, MN, USA) 45 months after the neo-aortic valve repair. He has been followed-up for 10 months without any evidence of neoAR or recurrent SVOTO.
Fig. 2.
Flowchart of patients. PAB, pulmonary artery banding; DKS, Damus-Kaye-Stensel; BCPS, bidirectional cavopulmonary shunt.
DISCUSSION
Our study shows that the DKS operation is a safe option for solving the problem of SVOTO and there are no significant differences in the clinical outcomes of the DKS operation according to the surgical technique used. In 2006, Masuda et al. [15] reported that the aortic flap technique is a safe, useful, and reproducible method for treating SVOTO. Further, in 2007, Fiore et al. [16] demonstrated that the double-barrel technique relieves a single ventricle to the aortic gradient effectively. However, these studies did not compare the clinical outcomes of the other surgical techniques. There are few papers in the English literature comparing the results of the DKS operation according to the surgical technique used. In 2011, Fujii et al. [17] reported that the double-barrel technique is better than the end-to-side technique for preserving the pulmonary valve function. They chose the DKS surgical technique according to the diameter of the ascending aorta. Further, they suggested that an almost equal diameter of the ascending aorta to that of the pulmonary trunk was a good indication for the double-barrel technique. We performed PAB prior to the DKS operation. This remains an issue under debate. From the perspective of systemic ventricular hypertrophy and the subsequently developed diastolic dysfunction, PAB prior to DKS could aggravate SVOTO. Some groups prefer the DKS procedure as an initial palliation for this reason. However, functional single ventricle anomalies are usually associated with excessive pulmonary blood flow. Therefore, we performed PAB prior to the DKS operation to control the pulmonary blood flow and prevent the development of pulmonary vascular resistance [18]. Low pulmonary vascular resistance improves the outcome of the Fontan operation. Moreover, DKS as an initial palliation in neonates is not technically easy.
We performed the DKS operation at the time of BCPS, except in 1 patient. There are numerous reports that demonstrate that concomitant BCPS and DKS are effective in eliminating the risk of SVOTO events [1,11]. Shimada et al. [3] even proposed a prophylactic DKS operation in a patient without subaortic stenosis in order to ensure that the degree of SVOTO does not progress during volume reduction surgery.
We decided upon the DKS operation technique according to the relationship of the aorta and the main pulmonary artery. It is possible to perform the double-barrel technique in the case of a side-by-side relationship, and it is also possible to choose the ascending aorta flap technique in the case of the anterior-posterior relationship. However, the DKS procedure is technically difficult, and it is not easy to preserve the shape of the pulmonary sinus.
In our study, one of the patients presented with a more than moderate degree of postoperative neoAR. He underwent neo-aortic valve replacement 66 months after the DKS procedure. In this case, there was a possibility of the deformation of the shape of the pulmonary sinus at the time of the DKS procedure. For such cases, Fujii et al. [17] recommend the double-barrel technique for preserving the pulmonary valve function.
This article is meaningful in that it compares the outcomes of two different surgical techniques, and to the best of our knowledge, in Korea, this is the first report to demonstrate the clinical outcomes of DKS according to the surgical technique used. However, our study has certain limitations. We reviewed a small group of 12 patients, and the operation was performed by one surgeon in one institution. The results of long-term follow-up remain to be seen.
In conclusion, the DKS procedure is an effective way to minimize the risk of SVOTO, and there are few differences in the outcomes of the DKS procedure according to the surgical technique used.
Footnotes
This article was originally presented with the title: ‘Clinical outcomes of Damus-Kaye-Stansel procedure’ at the 44th Korean Society for Thoracic & Cardiovascular Surgery, autumn symposium (2012. 11. 2).
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Alsoufi B, Al-Wadai A, Khan M, et al. Outcomes of Damus-Kaye-Stansel anastomosis at time of cavopulmonary connection in single ventricle patients at risk of developing systemic ventricular outflow tract obstruction. Eur J Cardiothorac Surg. 2014;45:77–82. doi: 10.1093/ejcts/ezt251. [DOI] [PubMed] [Google Scholar]
- 2.Lim HG, Lee CH, Kim SJ, et al. The clinical application and results of palliative Damus-Kaye-Stansel procedure. Korean J Thorac Cardiovasc Surg. 2008;41:1–11. [Google Scholar]
- 3.Shimada M, Hoashi T, Kagisaki K, Shiraishi I, Yagihara T, Ichikawa H. Clinical outcomes of prophylactic Damus-Kaye- Stansel anastomosis concomitant with bidirectional Glenn procedure. J Thorac Cardiovasc Surg. 2012;143:137–43. 143.e1. doi: 10.1016/j.jtcvs.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Miura T, Kishimoto H, Kawata H, Hata M, Hoashi T, Nakajima T. Management of univentricular heart with systemic ventricular outflow obstruction by pulmonary artery banding and Damus-Kaye-Stansel operation. Ann Thorac Surg. 2004;77:23–8. doi: 10.1016/s0003-4975(03)01248-7. [DOI] [PubMed] [Google Scholar]
- 5.Hiramatsu T, Imai Y, Kurosawa H, et al. Midterm results of surgical treatment of systemic ventricular outflow obstruction in Fontan patients. Ann Thorac Surg. 2002;73:855–60. doi: 10.1016/s0003-4975(01)03440-3. [DOI] [PubMed] [Google Scholar]
- 6.Damus PS. Correspondence. Ann Thorac Surg. 1975;20:724–5. [Google Scholar]
- 7.Kaye MP. Anatomic correction of transposition of great arteries. Mayo Clin Proc. 1975;50:638–40. [PubMed] [Google Scholar]
- 8.Stansel HC., Jr A new operation for d-loop transposition of the great vessels. Ann Thorac Surg. 1975;19:565–7. doi: 10.1016/s0003-4975(10)64433-5. [DOI] [PubMed] [Google Scholar]
- 9.Waldman JD, Lamberti JJ, George L, et al. Experience with Damus procedure. Circulation. 1988;78(5 Pt 2):III32–9. [PubMed] [Google Scholar]
- 10.Laks H, Gates RN, Elami A, Pearl JM. Damus-Stansel-Kaye procedure: technical modifications. Ann Thorac Surg. 1992;54:169–72. doi: 10.1016/0003-4975(92)91174-8. [DOI] [PubMed] [Google Scholar]
- 11.Gates RN, Laks H, Elami A, et al. Damus-Stansel-Kaye procedure: current indications and results. Ann Thorac Surg. 1993;56:111–9. doi: 10.1016/0003-4975(93)90413-c. [DOI] [PubMed] [Google Scholar]
- 12.Freedom RM, Williams WG, Fowler RS, Trusler GA, Rowe RD. Tricuspid atresia, transposition of the great arteries, and banded pulmonary artery: repair by arterial switch, coronary artery reimplantation, and right atrioventricular valved conduit. J Thorac Cardiovasc Surg. 1980;80:621–8. [PubMed] [Google Scholar]
- 13.Huddleston CB, Canter CE, Spray TL. Damus-Kaye-Stansel with cavopulmonary connection for single ventricle and sub-aortic obstruction. Ann Thorac Surg. 1993;55:339–45. doi: 10.1016/0003-4975(93)90994-s. [DOI] [PubMed] [Google Scholar]
- 14.Clarke AJ, Kasahara S, Andrews DR, et al. Mid-term results for double inlet left ventricle and similar morphologies: timing of Damus-Kaye-Stansel. Ann Thorac Surg. 2004;78:650–7. doi: 10.1016/j.athoracsur.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Masuda M, Tanoue Y, Ohno T, Tominaga R. Modified Damus-Kaye-Stansel procedure using aortic flap technique for systemic ventricular outflow tract obstruction in functionally univentricular heart. Eur J Cardiothorac Surg. 2006;29:1056–8. doi: 10.1016/j.ejcts.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Fiore AC, Rodefeld M, Vijay P, et al. Subaortic obstruction in univentricular heart: results using the double barrel Damus-Kaye Stansel operation. Eur J Cardiothorac Surg. 2009;35:141–6. doi: 10.1016/j.ejcts.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Fujii Y, Kasahara S, Kotani Y, et al. Double-barrel Damus-Kaye-Stansel operation is better than end-to-side Damus-Kaye-Stansel operation for preserving the pulmonary valve function: the importance of preserving the shape of the pulmonary sinus. J Thorac Cardiovasc Surg. 2011;141:193–9. doi: 10.1016/j.jtcvs.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Chang YH, Kim WH, Lee JY, et al. Pulmonary artery banding before the Damus-Kaye-Stansel procedure. Pediatr Cardiol. 2006;27:594–9. doi: 10.1007/s00246-006-1038-4. [DOI] [PubMed] [Google Scholar]