Abstract
A 44-year-old pregnant female patient gave stillbirth while being treated for pneumonia. She developed acute respiratory failure, which resulted in mechanical ventilator support. Diagnostic lung biopsy revealed a cryptogenic organizing pneumonia. The patient’s condition deteriorated and a venous-venous extracorporeal membrane oxygenation was placed. She was listed for lung transplantation. Because of her worsening condition lung transplantation was performed despite positive cross matching result. She was treated with rituximab, intravenous immunoglobulin, and plasmapheresis and recovered without event. There is no sign of rejection at the time of last follow-up.
Keywords: Histocompatibility testing, Lung transplantation, Plasmapheresis
CASE REPORT
A 44-year-old pregnant female patient at a gestational age of 23 weeks was admitted to the hospital with the symptoms of pneumonia. During the course of the antibiotic treatment, fetal death in utero was detected, and she had a stillbirth. Her pneumonia deteriorated and required mechanical ventilator support. A video-assisted thoracic surgery lung biopsy was performed, and the pathological report revealed a diagnosis of cryptogenic organizing pneumonia. Following a worsening of her respiratory symptoms, a venous-venous extracorporeal membrane oxygenation (ECMO) was inserted, and she was listed for lung transplantation (Fig. 1). The lung transplantation work-up revealed a high panel reactive antibody (PRA) score (which was tested by using the luminex technique; class I, 78%; class II, 53%), and a very weak level of donor-specific antibody (DSA) against DR9 was detected (mean fluorescent intensity [MFI]<1,000). Thirteen days later, a matched donor was registered. The donor was a 45-year-old male patient who had died of cerebral infarction. An arterial blood gas analysis (ABGA) revealed a pO2 to FiO2 ratio of 425, and the chest X-ray was normal. The T-cell cross-match was negative in both the complement-dependent cytotoxicity and the flow cytometric cross-match tests. However, the B-cell flow cytometric cross-match test was weakly positive (MFI ratio=2.5). Despite the fact that the cross-matching result was positive, lung transplantation was proceeded because of the worsening condition of the patient. Bilateral sequential lung transplantation was performed under cardiopulmonary bypass. To compensate for the size discrepancy, a right middle lobe wedge resection was performed. The cold ischemic time was 5 hours 29 minutes for the right lung and 7 hours for the left lung. The warm ischemic time of the right and left lung was 1 hour 18 minutes and 28 minutes, respectively.
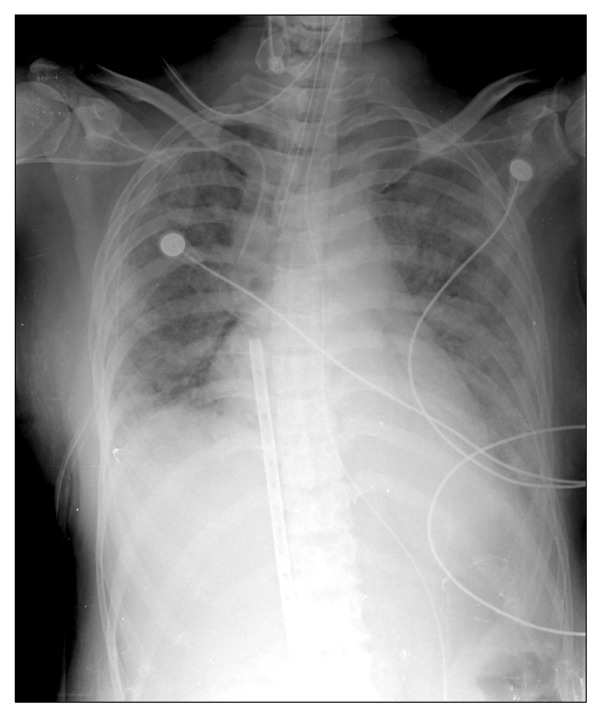
Fig. 1.
Preoperative chest X-ray. Note veno-veno extracorporeal membrane oxygenation cannula.
Because of the positive cross-match, the recipient was treated with rituximab (375 mg/m2) before the transplantation and intravenous immunoglobulin (IVIG, 2 g/kg) for 4 days during the perioperative period. Although the level was weak, anti-DR9 DSA persisted on the 4th postoperative day (1,000 <MFI<3,000). Therefore, the recipient was treated with plasmapheresis on the 5th and 7th postoperative days, and the anti-DR9 DSA completely disappeared on the 9th postoperative day. On the 12th postoperative day, bronchoscopy did not reveal any abnormal findings. On the 20th postoperative day, the mechanical ventilator was weaned to BiPAP, which was subsequently weaned on the 28th postoperative day with the ABGA results of pCO2 42.2 and pO2 72.7 without a supply of oxygen. On the 43rd postoperative day, the T-cannula was removed, and the patient was discharged from the hospital with no specific complications on the 49th postoperative day. During the 3-month follow-up, no graft rejection was observed (Fig. 2).
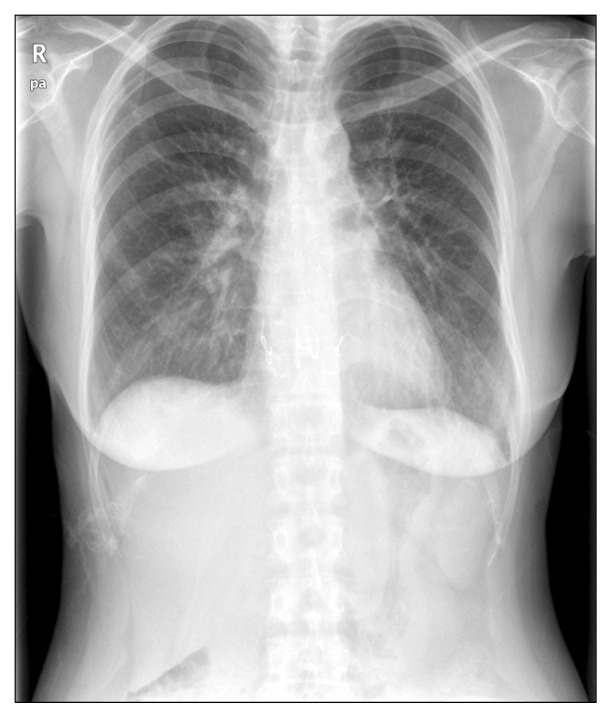
Fig. 2.
Chest X-ray at the time of last follow-up.
DISCUSSION
The prevention of graft rejection affects both the short- and long-term survival of the recipient. Therefore, the selection of the most compatible donor is a critical part of a successful transplantation. However, the shortage of donors and the worsening condition of recipients often force surgeons to proceed lung transplantations from incompatible donors.
A high PRA score (>50% or >80%) is known to be a poor prognostic marker for transplantation [1]. Lee et al. [2] reported the graft rejection rate among renal allograft recipients. The graft rejection rate was 8.6% in recipients whose PRA score was zero. However, the graft rejection rate was 20% in recipients whose PRA score was less than 50%, and 25% when the PRA score was more than 50% (p<0.05). Data from the United Network for Organ Sharing Standard Transplant Analysis and Research files from 1987 to 2005 indicate that survival decreased with increasing PRA, and the difference was significant when the PRA score exceeded 25%. A multivariable analysis indicated that PRA was associated with an increase in the 30-day (hazard risk, 2.6) and the overall mortality (hazard risk, 1.3) [3].
A high PRA score indicates a high probability of the pre-existing donor-specific antibody. To remove the pre-existing donor-specific antibody, desensitization protocols were developed in the late 1990s to deal with the increasing number of sensitized patients. These approaches, which employ combinations of IVIG, plasma exchange, and rituximab, have gained acceptance [4].
Haririan et al. [5] compared the short-term and long-term results of patients who received kidney transplantations with positive cross-match patients who were treated with plasma-pheresis and IVIG. The 1-year graft survival rate was 89.9% and the 5-year graft survival rate was 69.4% for the positive cross-match recipients, whereas the 1-year graft survival rate was 97.6% and the 5-year graft survival rate was 80.6% for the control group. A positive cross-match was associated with an increased risk of graft loss (hazard risk, 2.6; p=0.04).
It is known that antibody-mediated rejection (AMR) is related to the pre-existing donor-specific antibody. Earlier studies of renal transplant examined the role of IVIG alone during the treatment of AMR. Later studies evaluated IVIG plus rituximab or IVIG plus plasmapheresis plus rituximab. More recently, bortezomib, a proteasome inhibitor, and eculizumab, a monoclonal antibody against terminal complement protein C5 that inhibits terminal complement activation, have been investigated [6]. Daoud et al. [6] reported a treatment for AMR. Two AMR patients after lung transplantation received plasmapheresis and IVIG, resulting in clinical improvement and the ultimate elimination of DSA [6].
Lung transplantation involving a positive cross-match result is not yet a common practice. Yu et al. [7] reported a lung transplantation between an O-type blood donor and an A-type blood recipient. The recipient was treated with OKT3 immediately and did not show graft rejection during a 4-month follow-up.
In our case, the preoperative PRA level was high, and the preoperative cross-matching result was positive. To prevent acute graft rejection, the recipient was treated with rituximab, IVIG, and plasmapheresis. Although the long-term result remains to be determined, graft rejection was not observed 3 months after the transplant.
In conclusion, positive cross-match lung transplantation is a feasible option when combined with plasmapheresis and appropriate medication. However, a more delicate protocol for a positive cross-match is required. Moreover, the long-term results of positive cross-match lung transplantation are needed.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Jang JY, Kim YJ, Kim Y, Park YJ, Han K, Oh EJ. Application of calculated panel reactive antibody using HLA frequencies in Koreans. Ann Lab Med. 2012;32:66–72. doi: 10.3343/alm.2012.32.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KW, Kim SJ, Lee DS, et al. Effect of panel-reactive antibody positivity on graft rejection before or after kidney transplantation. Transplant Proc. 2004;36:2009–10. doi: 10.1016/j.transproceed.2004.08.137. [DOI] [PubMed] [Google Scholar]
- 3.Glanville AR. Antibody-mediated rejection in lung transplantation: myth or reality? J Heart Lung Transplant. 2010;29:395–400. doi: 10.1016/j.healun.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Vo AA, Petrozzino J, Yeung K, et al. Efficacy, outcomes, and cost-effectiveness of desensitization using IVIG and rituximab. Transplantation. 2013;95:852–8. doi: 10.1097/TP.0b013e3182802f88. [DOI] [PubMed] [Google Scholar]
- 5.Haririan A, Nogueira J, Kukuruga D, et al. Positive cross- match living donor kidney transplantation: longer-term outcomes. Am J Transplant. 2009;9:536–42. doi: 10.1111/j.1600-6143.2008.02524.x. [DOI] [PubMed] [Google Scholar]
- 6.Daoud AH, Betensley AD. Diagnosis and treatment of antibody mediated rejection in lung transplantation: a retrospective case series. Transpl Immunol. 2013;28:1–5. doi: 10.1016/j.trim.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Yu SH, Kim HK, Lee DY, et al. Lung transplantation in ABO compatible but nonidentical patients. Korean J Thorac Cardiovasc Surg. 2001;34:94–6. [Google Scholar]