Abstract
Human immunodeficiency virus (HIV) remains a global infectious diseases threat that disproportionally affects women. Beyond social and political factors, biological and genetic differences have been identified that lead to differential disease courses and outcomes in men and women. Following HIV type 1 (HIV-1) seroconversion, women have up to 40% lower HIV loads and higher CD4+ T-cell counts than men. However, at the same level of viremia, progression to AIDS is faster in women. After adjustment for viral load, HIV-positive women also display increased levels of generalized immune activation and experience the consequences of elevated inflammatory activity more frequently than men. Part of these observations are linked to sex-based differences in innate immunity, in which the differential ability of plasmacytoid dendritic cells to produce interferon α following stimulation of Toll-like receptor 7 and upregulation of interferon-stimulated genes play a central role. Here, we review the current knowledge and remaining gaps therein regarding sex-based differences in HIV-1 pathogenesis.
Keywords: HIV-1, sex, Immune activation, interferon-alpha, ISG
Differential susceptibility to and outcomes of infections between women and men have been well documented in the literature for a variety of pathogens [1]. Sexually transmitted infections caused by viruses, including herpes simplex virus type 2, human papillomavirus, and human immunodeficiency virus type 1 (HIV-1) are among the most prevalent infections worldwide and are known to disproportionally affect women. Despite major advances in treatment and prevention of HIV-1 infection over the past 3 decades, the AIDS pandemic and the >35 million people living with HIV-1 remain among the major health challenges of our time [2]. In sub-Saharan Africa, the epicenter of the global epidemic, women still account for approximately 60% of all people living with HIV-1, and young women aged 15–24 years represent a particularly vulnerable population for HIV-1 acquisition, accounting for more than a quarter of new infections. Gender inequalities and harmful gender norms that promote unsafe sex and limit access to health services for HIV-1 infection and reproduction continue to drive the HIV-1 epidemic in many countries.
Gender inequalities include vulnerability to rape, sex with older men, and unequal access to education and economic opportunities. In comparison to men, women are more likely to acquire HIV-1 at an early age, resulting in a global HIV-1 prevalence among girls and young women that is double or greater than that among males of the same age [2]. While multiple social, political, economic, and legal issues require attention and urgently need to be addressed to ensure decreased HIV-1 acquisition rates among females at risk, increased access to care, and improved health outcomes for women living with HIV-1 [2], we will herein focus on reviewing the biological factors influencing the differential outcomes of HIV-1 infection in women and men, with particular focus on genetic and immunological factors.
INFLUENCE OF SEX ON THE NATURAL HISTORY OF HIV-1 INFECTION
Sex-based differences in disease comprise genetic differences that can be attributable to differences in the expression of steroid hormones, to differences in anatomy, and to X and Y chromosome–linked factors, such as X chromosome inactivation or genes encoded on the Y chromosome. It has been well established through cross-sectional and longitudinal studies in HIV-1 infection that in acute [3–7] and chronic HIV-1 infection [8, 9], women have lower plasma viral loads and higher CD4+ T-cell counts than men. It has been shown that HIV-1 loads in untreated women are up to 40% lower than those in males; however, despite harboring lower viral loads for a given level of viremia, women had a 1.6-fold higher risk of progressing to AIDS [10], ultimately resulting in similar times to AIDS between infected women and men. Despite these sex-specific differences observed in HIV loads, their impact on the establishment and maintenance of HIV latency and the nature of the viral reservoir remains unknown, warranting further investigation (Table 1).
Table 1.
Current Gaps in Knowledge About Sex-Based Differences in Human Immunodeficiency Virus Type 1 (HIV-1) Pathogenesis and Warranted Future Studies
| Identification of molecular mechanisms and pathways leading to sex-based differences in HIV pathogenesis |
| Analysis of sex-based differences in the establishment and maintenance of HIV latency |
| Evaluation of the size and nature of HIV reservoirs in women and men |
| Investigation of sex-based differences in aging and its impact on HIV-1 infection |
| Impact of menopause on HIV-1 pathogenesis |
| Analysis of sex-based differences in T-cell regulation |
| Evaluation of sex-specific outcomes in immunity to HIV vaccines |
| Understanding the interplay between sex hormones and the microbiome in women and men |
It has been suggested that women experience or report fewer symptoms during primary infection, which may delay diagnosis in this population [7]. Meditz et al also demonstrated in a large cohort of North American HIV-1 seroconverters that women experienced >2-fold more combined HIV- and AIDS-related illnesses than men and that the morbidity was particularly increased among nonwhite women. However, in this study the authors suggest that part of the observed sex-based differences in morbidity were not biologically based but resulted from socioeconomic factors and possibly race [7]. Dissecting the reason for the differences in markers of disease progression and the differential risk for progression to AIDS between individuals of opposite sexes has been an intense area of research in recent years. Differences in immunological responses to HIV-1, possibly mediated through the effects of gonadal steroids or as a result of genetic variation, have been implicated as probable mechanisms and are discussed below.
Female Sex Hormones in HIV-1 Infection
Studies in animal models have examined the direct role of sex hormones on acquisition of sexually transmitted infections. While rhesus macaques were found to be more susceptible to intravaginal simian immunodeficiency virus (SIV) infection during the luteal phase (when the progesterone level is high), compared with the follicular phase (when the estrogen level is high), of their reproductive cycle [11] and had enhanced SIV acquisition following administration of depot-medroxyprogesterone acetate (DMPA) [12], the systemic administration of estrogens protected female rhesus macaques against intravaginal challenge with highly pathogenic SIV [13]. In humans, sex hormones have been implicated in modulating HIV-1 acquisition, both during the menstrual cycle and in pregnancy. In a prospective cohort study of discordant couples, pregnancy was associated with increased risks of acquisition among females and of transmission from females to males [14], but recent data from population-based studies did not find these associations and in fact suggested a possibly decreased risk of HIV-1 acquisition during pregnancy [15]. The suggested impact of hormonal contraception on HIV-1 acquisition represents an important public health issue and remains a matter of ongoing controversy. A detailed recent meta-analysis concluded that all reviewed trials studying norethisterone enanthate and most of the studies on oral contraceptives did not demonstrate an increased acquisition risk [16]. The authors suggested that more evidence was needed to draw definitive conclusions on the risk of HIV-1 acquisition associated with the use of injectable DMPA, an inexpensive, effective, female-controlled method of contraception used by millions of women worldwide. This conclusion was based on the heterogeneity of investigative methods and some studies reporting an increased risk of HIV acquisition, with other studies demonstrating no association [16].
Following HIV-1 infection, women with self-reported normal menses were found to have normal levels of progesterone and estradiol during the menstrual cycle, similar to HIV-seronegative controls. HIV-1 RNA levels were shown to vary with the menstrual cycle in the female genital tract but not in the peripheral blood compartment [17] and were more elevated in endocervical canal fluid than in blood plasma [18]. It has also recently been suggested that the female genital tract may serve as a reservoir of persistent HIV-1 replication during highly active antiretroviral therapy (HAART) [19]. Meditz et al recently described increased levels of CCR5 expression on endocervical CD4+ T cells in postmenopausal women, which may increase the risk for HIV-1 acquisition in older women [20]. However, overall the exact interplay between sex hormones, mucosal immunity in the female reproductive tract, and HIV acquisition remains inadequately understood and warrants further investigation to optimally design preventive and therapeutic interventions.
INNATE IMMUNITY AND IMMUNE ACTIVATION IN HIV-1 INFECTION
Sex Differences in the Toll-like Receptor (TLR) Response to HIV-1
It has now been well established that generalized immune activation represents a strong predictor for HIV-1 disease progression, independent of viral load. Plasmacytoid dendritic cells (pDCs) can contribute importantly to HIV-1–associated immune activation through their ability to sense HIV-1 single-stranded RNA via TLR7 [21, 22]. Building on data by Berghoefer et al, who reported higher production of interferon α (IFN-α) by pDCs from women following stimulation with TLR7 ligands [23], Meier et al demonstrated substantial sex-based differences in the response of pDCs to HIV-1 and showed that pDCs derived from women produced significantly higher amounts of IFN-α in response to HIV-1–derived TLR7/8 ligands or whole inactivated HIV-1 (AT-2 virus), compared with men. These sex-based differences in TLR7-mediated IFN-α production by pDCs furthermore resulted in stronger activation of CD8+ T cells in vitro. The percentage of IFN-α–expressing pDCs following stimulation with HIV-1–derived TLR7/8 ligands was significantly correlated to plasma progesterone levels in study participants, suggesting that sex hormone levels can modulate the ability of pDCs to produce IFN-α [24]. In line with this observation in humans, recent studies by Seillet et al that used humanized mice demonstrated that 17β-estradiol can indeed enhance the ability of pDCs to respond to TLR7 stimulation [25]. The sex-based differences in HIV triggered in vitro IFN-α production by pDCs observed in humans were reflected in vivo by higher levels of HIV-1–associated T-cell activation in women, compared with men, after adjustment for viral load. Women had an average of 5% more activated HLA-DR+CD38+CD8+ T cells for a given viral load and reached the same levels of CD8+ T-cell activation as men at approximately 1 log10 lower HIV-1 load. Taken together, these data suggested a critical role of the TLR7/IFN-α pathway in inducing HIV-1–associated immune activation and thereby possibly in the described sex-based differences in the manifestations of HIV-1 disease.
Higher Expression of IFN-Stimulated Genes (ISGs) in HIV-1–Infected Women
To investigate the sex-dependent differences in HIV-1–induced IFN-α production, Chang et al performed expression analysis of 98 genes involved in the TLR and type I IFN signaling pathways on populations of flow-sorted T cells and DCs derived from men and women with untreated chronic HIV-1 infection [26]. In this study, expression of a number of ISGs, including those encoding ISG15, MX1, IFIT3 and IFIT1, IRF7, and OAS2, correlated with markers of disease progression, and after adjustment for HIV-1 load, higher expression of several ISGs was observed in females. These data suggest that HIV-1 infection leads to stronger induction of ISGs in women, compared with men, for the same amount of virus replication and are in line with a number of recent studies suggesting sex-based differences in the IFN-α pathway. This has been specifically illustrated in the context of autoimmunity, such as systemic lupus erythematosus, an autoimmune disease with a female to male predominance of 7:1 and for which it was recently reported that the TLR7 and IFN-α pathways play a critical role [27]. Similarly, hepatitis C virus infection, in which the IFN-α pathways exhibit a central role and IFN-α is part of the currently used treatment regimens, demonstrates a strong sex-based difference in outcome and treatment response, as reviewed by Baden and Rockstroh elsewhere in this supplement.
In recent studies using the LCMV model, IFN-α has been shown to have a differential impact on the outcome of viral infections, depending on whether this pathway is active in the acute or chronic phase of infection [28]. Wilson et al and Tejiaro et al demonstrated that blocking of IFN-α during acute lymphocytic choriomeningitis virus (LCMV) infection has detrimental consequences for disease outcome, reflecting the important antiviral activity of IFN-α and IFN-α–induced genes during acute infection [29, 30]. In contrast, during persistent chronic LCMV infection, continuous production of IFN-α contributed to immune pathology, including impairment of T-cell function, and blocking of IFN-α allowed for enhanced immune control and viral clearance. IFN-α may have a similar dichotomous effect during HIV-1 infection, and sex-based differences in IFN-α and ISG induction might explain some of the differences in the clinical manifestations of HIV-1 infection between women and men, including the better control of HIV-1 viremia by women during primary infection (as a consequence of the anti–HIV-1 effect of IFN-α) and the higher level of immune activation and accelerated disease progression in women during chronic infection, after adjustment for the level of viral replication (as a consequence of the proinflammatory effect of persistent IFN-α production; Figure 1).
Figure 1.
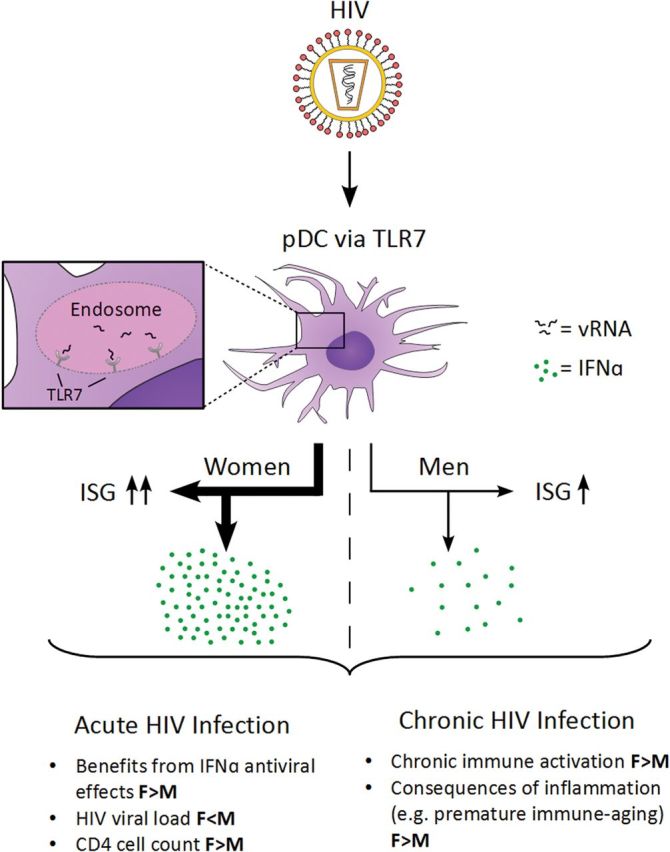
Differential interferon α (IFN-α; green circles) response and IFN-stimulated gene (ISG) upregulation following plasmacytoid dendritic cell (pDC) stimulation with human immunodeficiency virus (HIV) through Toll-like receptor 7 (TLR7) triggering. Beneficial antiviral effects of IFN-α may lead to lower HIV loads and higher CD4+ T-cell counts in females (F) during primary HIV-1 infection. However, in chronic infection, immunomodulatory effects of IFN-α may contribute to the enhanced immune activation and lead to increased sequelae of a chronic inflammatory state in females, compared with males (M).
Clinical Effects of Immune Activation and Premature Aging
Increasing evidence links immune activation in HIV-1 infection to non–AIDS-related comorbidities, cardiovascular events, and premature aging. In the context of the sex-based differences in immune activation described above, it is not surprising that sex plays a role in the sequelae of increased inflammatory activity with differential clinical outcomes. Data from large registry studies recently indicated that relative increases in myocardial infarction rates are higher among HIV-1–infected women relative to noninfected women, compared with HIV-1–infected men relative to noninfected men (relative risk, 2.98 vs 1.40, respectively) [31, 32]. Fitch et al demonstrated that HIV-1–infected women had significantly higher percentages of coronary segments with noncalcified plaque than HIV-1–negative women but also higher percentages than HIV-1–infected and uninfected men [33]. Similarly, immune activation parameters such as soluble CD163 (sCD163), soluble CD14 (sCD14), and the percentage of CD4+HLA-DR+ T cells were significantly higher in HIV-1–infected women than in infected men. In addition, the authors revealed a 2-way interaction between age and sex, demonstrating that sCD163 increased more with age among women than among men [33]. These results are in line with recent data from Martin et al, who investigated markers of innate immune activation in HIV-1–infected women, compared with uninfected women [34]. Their findings indicated that the plasma levels of the innate immune activation markers CXCL10, sCD163, sCD14, and neopterin and an increased proportion of CD16+ monocytes were elevated in HIV-1–infected women, as opposed to HIV-1–negative controls. The authors concluded from their data that HIV-1 infection confers the equivalent of a 10–14-year increase in the levels of innate immune aging markers, which in turn may contribute to the elevated risk of inflammation-related diseases in HIV-1–positive women [34].
X CHROMOSOMAL FACTORS ASSOCIATED WITH DIFFERENTIAL HIV-1 DISEASE OUTCOME
While direct effects of sex hormones may account for many of the described differences in immune function between women and men, some sex-based differences may also be caused by an inherent imbalance in the expression of genes encoded by the X and Y chromosomes. Multiple genes on the X chromosome, such as those encoding TLR7 and TLR8, CD132 (IL-2Rγ), FOXP3, and CD40L, regulate immune function and might play an important role in modulating sex-based differences in immune-related diseases [35]. Through a recent microsatellite genome-wide association study of SIV-infected macaques, Siddiqui et al identified an X chromosomal locus as being associated with the rate at which SIV infection leads to the onset of AIDS-related disease in rhesus macaques [36]. On the basis of this finding, the X chromosomal single-nucleotide polymorphism rs5968255, located at human Xq.21.1, was identified as a significant genetic determinant of HIV disease progression in females but not in males. Heterozygous female carriers of the C allele demonstrated significantly lower decreases in CD4+ T-cell counts and lower HIV-1 set points than TT homozygous female carriers and males. These data suggested that some differences in sex-based disease outcome in HIV-1 infection may be the result of sex-linked genetic variation and that elucidating the mechanisms and pathways underlying these sex-specific effects may assist in the rational design of biomedical interventions with high efficacy in both sexes [36].
FOXP3, which is encoded by another gene on the X chromosome, is best known for its role as a transcription factor in regulatory T cells, a CD4+ lymphocyte subset involved in the maintenance of peripheral tolerance. More recently, FOXP3 has also been detected in epithelial cells of breast and ovaries and may play a role in carcinogenesis in these organs in females. Some FOXP3 (also known as Scurfin) gene polymorphisms have been associated with autoimmune disease, especially in women [37], and modulation of regulatory T cells (Tregs) through synergistic action of 17b-estradiol and interleukin 6 is thought to be responsible for the sex bias in immune-mediated drug-induced liver injury [38]. In the context of HIV-1 infection, FOXP3 was shown to enhance HIV-1 gene expression by modulating NFκB-p65 binding to the HIV-1 long terminal repeat [39] and may contribute to the promotion of Tregs as part of the HIV-1 latent reservoir [40]. FOXP3-expressing regulatory T cells expand during the late follicular phase of the menstrual cycle, tightly linked to serum levels of estradiol and followed by a sharp decrease in Treg numbers at the luteal phase [41]. Tregs also contribute to establishment of fetal-maternal tolerance during pregnancy. In HIV-1–infected pregnant women immunoregulatory profiles differ from HIV-1–uninfected women [42, 43], an immune unbalance that is thought to interfere with the prevention of fetal rejection and may in part explain the increased risk of abortion in HIV-1–infected women [42].
We and others have recently shown increased frequencies (ie, increased percentages of Tregs among CD4+ cells) in HIV-1–infected individuals [43–45], which may in part be explained by increased thymic output of naive Tregs [43], peripheral conversion from conventional T cells, or preferential survival of Tregs during HIV-1 infection. This increase in frequencies coincides with a decrease in absolute CD4 Treg counts during chronic HIV-1 infection and is associated with elevated T-cell activation. While some studies suggest decreased levels of Tregs in women, compared with men [46], detailed comparative studies investigating the effect of sex in the context of T-cell regulation of HIV-1 are not available to date. In addition, given the significant fluctuation of Treg levels in fertile women, the menstrual cycle should be taken into consideration when Treg numbers are investigated clinically. Further studies are therefore needed to understand the impact of sex and sex hormones on T-cell regulation in HIV-1 infection and other viral infections, with careful consideration of sex, phase of menstrual cycle, tissue compartment, and age.
SEX, VACCINES, AND ANTIRETROVIRAL THERAPY (ART)
Biological differences between the sexes are associated with variations in the immune response to vaccination, and for many vaccines women elicit much stronger humoral immune responses than men, as reviewed by Klein and Pekosz in this supplement issue. However, among the 6 completed phase 2b and 3 HIV vaccine trials [47], study populations in 3 were exclusively or >94% male. The other 3 trials of candidate vaccines recruited both women and men but largely lacked specific analysis for sex-based differences in immunogenicity, adverse events, or protection in the published data. While only the RV144 trial showed marginal overall vaccine efficacy [48], it has been illustrated in other vaccine models that failure to perform analysis with stratification by sex can lead to underestimation of vaccine protection, skewing of immunogenicity data, and an incomplete side effect profile [49].
In HIV-1 drug therapy, it has now been demonstrated that, with equal access to care and correct administration of and compliance with ART, there is no significant sex-based difference in treatment response [50]. Following seroconversion, similar percentages of women and men achieve undetectable viral loads after 6 months of ART [7]. However, multiple studies suggest that side effects of ART and adverse events can differ dramatically between women and men. This was most notable for first and second generation antiretrovirals, when women experienced more frequent occurrences of skin rashes, mitochondrial toxicity, lactic acidosis, gastrointestinal intolerance, and lipodystrophy [51], which led to increased rates of treatment nonadherence among women [52]. However, even clinical studies with newer antiretroviral drugs such as rilpivirine (RPV) and efavirenz (EFV) report sex-based differences in adverse effects. In recent randomized, controlled trials, the incidence of nausea was greater among women than men with regimens containing either EFV or RPV. In contrast, the incidence of diarrhea was greater among men in the EFV group, compared with women, and the incidence of abnormal dreams or nightmares was more frequent among men in both the EFV and RPV groups [53]. A recently published randomized, controlled trial investigated treatment responses to atazanavir plus ritonavir (ATV/r) or EFV in initial antiretroviral regimens among females and males, to determine whether treatment outcomes differed by sex. In this study, Smith et al demonstrated that women assigned to receive ATV/r displayed a higher risk of virologic failure than men assigned to receive the same regimen. Self-reported adherence did not differ significantly by sex, but interestingly, females had slower ATV clearance and higher predose levels of ATV, compared with men [54]. Addressing the underrepresentation of women in randomized, controlled trials and dissecting the detailed mechanisms of drug metabolism and toxicities may lead to more and much needed individually tailored and sex-specific approaches to ART and represents an important area for future individualized therapy and personalized medicine.
CONCLUSION
Beyond gender and cultural norms, there are distinct biological differences in the natural history of and immunity to HIV-1 infection between women and men. The persistent higher expression of IFN-α and ISGs in females may in part explain the higher immune activation with subsequent faster disease progression and premature aging observed in chronically infected women, compared with men, for the same level of viral replication. However, X-linked and other genetic associations related to sex-based differences in HIV-1 infection remain incompletely understood and warrant further investigation. A few key gaps in knowledge, including questions relevant to ongoing HIV cure research efforts, aging, and the microbiome, that are in need of further study are summarized in Table 1. It is conceivable that reversal of latency and HIV eradication efforts may well need distinct approaches in women, compared with men. Careful experimental design and dedicated sex-based analysis of HIV clinical and vaccine trial data will be required to gain further insights into sex-based differences in the immune pathogenesis of HIV-1 infection, which will serve as the basis for strategic design of individualized treatment and prevention efforts.
Notes
Financial support. This work was supported by the National Institutes of Health (NIH; grant RO1 AI074405 to M. A.); the German Center for Infectious Disease Research (to M. M. A.); the Harvard University Center for AIDS Research, an NIH-funded program (P30 AI060354), which is supported by the following NIH cofunding and participating institutes and centers: the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, the Eunice Kennedy Shriver National Institute of Child Health and Development, the National Heart, Lung, and Blood Institute, the National Institute of Drug Abuse, the National Institute of Mental Health, the National Institute on Aging, the National Center for Contemporary and Alternative Medicine, the Fogarty International Center, and the Office of AIDS Research.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Acknowledgements: We thank Hans Christian Stubbe, MD for support with the revision of figure 1.
References
- 1.Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays. 2012;34:1050–9. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS Global summary of the HIV/AIDS epidemic UD. 2013. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf . Accessed 14 April 2014.
- 3.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344:720–5. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 4.Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180:666–72. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- 5.Delmas MC, Jadand C, De Vincenzi I, et al. Gender difference in CD4+ cell counts persist after HIV-1 infection. SEROCO Study Group. AIDS. 1997;11:1071–3. [PubMed] [Google Scholar]
- 6.Touloumi G, Pantazis N, Babiker AG, et al. Differences in HIV RNA levels before the initiation of antiretroviral therapy among 1864 individuals with known HIV-1 seroconversion dates. AIDS. 2004;18:1697–705. doi: 10.1097/01.aids.0000131395.14339.f5. [DOI] [PubMed] [Google Scholar]
- 7.Meditz AL, MaWhinney S, Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis. 2011;203:442–51. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35:313–22. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- 9.Prins M, Robertson JR, Brettle RP, et al. Do gender differences in CD4 cell counts matter? AIDS. 1999;13:2361–4. doi: 10.1097/00002030-199912030-00007. [DOI] [PubMed] [Google Scholar]
- 10.Farzadegan H, Hoover DR, Astemborski J, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–4. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 11.Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S119–23. [PubMed] [Google Scholar]
- 12.Marx PA, Spira AI, Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–9. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 13.Smith SM, Mefford M, Sodora D, et al. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. AIDS. 2004;18:1637–43. doi: 10.1097/01.aids.0000131393.76221.cc. [DOI] [PubMed] [Google Scholar]
- 14.Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25:1887–95. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marston M, Newell ML, Crampin A, et al. Is the risk of HIV acquisition increased during and immediately after pregnancy? A secondary analysis of pooled HIV community-based studies from the ALPHA network. PLoS One. 2013;8:e82219. doi: 10.1371/journal.pone.0082219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13:797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 17.Cu-Uvin S, Wright DJ, Anderson D, et al. Hormonal levels among HIV-1-seropositive women compared with high-risk HIV-seronegative women during the menstrual cycle. Women's Health Study (WHS) 001 and WHS 001a Study Team. J Womens Health Gend Based Med. 2000;9:857–63. doi: 10.1089/152460900750020883. [DOI] [PubMed] [Google Scholar]
- 18.Reichelderfer PS, Coombs RW, Wright DJ, et al. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. AIDS. 2000;14:2101–7. doi: 10.1097/00002030-200009290-00005. [DOI] [PubMed] [Google Scholar]
- 19.Fiscus SA, Cu-Uvin S, Eshete AT, et al. Changes in HIV-1 subtypes B and C genital tract RNA in women and men after initiation of antiretroviral therapy. Clin Infect Dis. 2013;57:290–7. doi: 10.1093/cid/cit195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meditz AL, Moreau KL, MaWhinney S, et al. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr. 2012;59:221–8. doi: 10.1097/QAI.0b013e31823fd215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 22.Beignon AS, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–75. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177:2088–96. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- 24.Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seillet C, Laffont S, Tremollieres F, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119:454–64. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 26.Chang JJ, Woods M, Lindsay RJ, et al. Higher expression of several interferon-stimulated genes in HIV-1-infected females after adjusting for the level of viral replication. J Infect Dis. 2013;208:830–8. doi: 10.1093/infdis/jit262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guiducci C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931–42. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odorizzi PM, Wherry EJ. Immunology. An interferon paradox. Science. 2013;340:155–6. doi: 10.1126/science.1237568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson EB, Yamada DH, Elsaesser H, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–7. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teijaro JR, Ng C, Lee AM, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–11. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–30. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 33.Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208:1737–46. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin GE, Gouillou M, Hearps AC, et al. Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One. 2013;8:e55279. doi: 10.1371/journal.pone.0055279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–44. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqui RA, Sauermann U, Altmuller J, et al. X chromosomal variation is associated with slow progression to AIDS in HIV-1-infected women. Am J Hum Genet. 2009;85:228–39. doi: 10.1016/j.ajhg.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwase K, Shimada A, Kawai T, et al. FOXP3/Scurfin gene polymorphism is associated with adult onset type 1 diabetes in Japanese, especially in women and slowly progressive-type patients. Autoimmunity. 2009;42:159–67. doi: 10.1080/08916930802488258. [DOI] [PubMed] [Google Scholar]
- 38.Cho J, Kim L, Li Z, Rose NR, Talor MV, Njoku DB. Sex bias in experimental immune-mediated, drug-induced liver injury in BALB/c mice: suggested roles for Tregs, estrogen, and IL-6. PLoS One. 2013;8:e61186. doi: 10.1371/journal.pone.0061186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes D, Knudsen G, Mackey-Cushman S, Su L. FoxP3 enhances HIV-1 gene expression by modulating NFkappaB occupancy at the long terminal repeat in human T cells. J Biol Chem. 2007;282:15973–80. doi: 10.1074/jbc.M702051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran TA, de Goer de Herve MG, Hendel-Chavez H, et al. Resting regulatory CD4 T cells: a site of HIV persistence in patients on long-term effective antiretroviral therapy. PLoS One. 2008;3:e3305. doi: 10.1371/journal.pone.0003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–8. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 42.Richardson K, Weinberg A. Dynamics of regulatory T-cells during pregnancy: effect of HIV infection and correlations with other immune parameters. PLoS One. 2011;6:e28172. doi: 10.1371/journal.pone.0028172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolte L. Thymic function in HIV-infection. Dan Med J. 2013;60:B4622. [PubMed] [Google Scholar]
- 44.Schulze Zur Wiesch J, Thomssen A, Hartjen P, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol. 2010;85:1287–97. doi: 10.1128/JVI.01758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angin M, Kwon DS, Streeck H, et al. Preserved function of regulatory T cells in chronic HIV-1 infection despite decreased numbers in blood and tissue. J Infect Dis. 2012;205:1495–500. doi: 10.1093/infdis/jis236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afshan G, Afzal N, Qureshi S. CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clin Lab. 2012;58:567–71. [PubMed] [Google Scholar]
- 47.Schiffner T, Sattentau QJ, Dorrell L. Development of prophylactic vaccines against HIV-1. Retrovirology. 2013;10:72. doi: 10.1186/1742-4690-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 49.Stanberry LR, Spruance SL, Cunningham AL, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 50.Moore AL, Kirk O, Johnson AM, et al. Virologic, immunologic, and clinical response to highly active antiretroviral therapy: the gender issue revisited. J Acquir Immune Defic Syndr. 2003;32:452–61. doi: 10.1097/00126334-200304010-00017. [DOI] [PubMed] [Google Scholar]
- 51.Ofotokun I, Pomeroy C. Sex differences in adverse reactions to antiretroviral drugs. Top HIV Med. 2003;11:55–9. [PubMed] [Google Scholar]
- 52.Currier JS, Spino C, Grimes J, et al. Differences between women and men in adverse events and CD4+ responses to nucleoside analogue therapy for HIV infection. The Aids Clinical Trials Group 175 Team. J Acquir Immune Defic Syndr. 2000;24:316–24. doi: 10.1097/00126334-200008010-00003. [DOI] [PubMed] [Google Scholar]
- 53.Hodder S, Arasteh K, De Wet J, et al. Effect of gender and race on the week 48 findings in treatment-naive, HIV-1-infected patients enrolled in the randomized, phase III trials ECHO and THRIVE. HIV Med. 2012;13:406–15. doi: 10.1111/j.1468-1293.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- 54.Smith KY, Tierney C, Mollan K, et al. Outcomes by sex following treatment initiation with atazanavir plus ritonavir or efavirenz with abacavir/lamivudine or tenofovir/emtricitabine. Clin Infect Dis. 2014;58:555–63. doi: 10.1093/cid/cit747. [DOI] [PMC free article] [PubMed] [Google Scholar]


