Abstract
Biological (ie, sex) differences as well as cultural (ie, gender) norms influence the acceptance and efficacy of vaccines for males and females. These differences are often overlooked in the design and implementation of vaccination strategies. Using seasonal and pandemic influenza vaccines, we document profound differences between the sexes in the acceptance, correlates of protection, and adverse reactions following vaccination in both young and older adults. Females develop higher antibody responses, experience more adverse reactions to influenza vaccines, and show greater vaccine efficacy than males. Despite greater vaccine efficacy in females, both young and older females are often less likely to accept influenza vaccines than their male counterparts. Identification of the biological mechanisms, including the hormones and genes, that underlie differential responses to vaccination is necessary. We propose that vaccines should be matched to an individual's biological sex, which could involve systematically tailoring diverse types of FDA-approved influenza vaccines separately for males and females. One goal for vaccines designed to protect against influenza and even other infectious diseases should be to increase the correlates of protection in males and reduce adverse reactions in females in an effort to increase acceptance and vaccine-induced protection in both sexes.
Keywords: aging, gender, immunogenicity, influenza, reactogenicity, sex difference, sex hormone, vaccine
Sex (ie, the biological differences between males and females) and gender (ie, cultural norms associated with being male or female) impact acceptance of, responses to, and the outcome of vaccination [1]. Females are often less likely to accept vaccines [2] and develop higher antibody responses to vaccines than males. After vaccination against influenza, yellow fever, rubella, measles, mumps, hepatitis A and B, herpes simplex 2, rabies, smallpox, and dengue viruses, protective antibody responses can be twice as high in females as males [1]. Measures of cell-mediated immunity following vaccination are also higher in females than males for some vaccines [3–5]. Females develop more frequent and severe adverse reactions, including fever, pain, and inflammation, to vaccines [1, 6, 7]. Because information about adverse events is often acquired through passive reporting, it is assumed that this reflects a gender difference, in which females might be more likely to report adverse side effects than males. Alternatively, sex-based biological differences might also be involved, in which inflammatory responses to vaccines might be higher in females and result in increased adverse biological reactions to vaccines in females compared with males. The goal of this review is to translate clinical and epidemiological observations of male-female differences into recommendations for rational design and use of influenza vaccines. Sex-based differences in humoral immune responses and adverse reactions to diverse viral and bacterial vaccines have been reviewed extensively [1, 8, 9]. Because influenza vaccines are administered annually worldwide, and multiple vaccine formulations are approved to be used in humans, there is a plethora of data from which to systematically evaluate the roles of sex and gender in the outcome of vaccination and make recommendations about priorities for improving vaccine design. Using influenza vaccines, we propose that vaccine design should be explicitly matched to an individual's biological sex.
INFLUENZA VACCINE DESIGN AND DOSAGE IN ADULTS DOES NOT ACCOUNT FOR AN INDIVIDUAL'S SEX
Influenza vaccines are updated annually without extensive clinical trials and must be produced in large quantities in a short period of time—especially during pandemics [10, 11]. These 2 factors have led to the development of a number of different influenza vaccines, vaccine formulations, and vaccine delivery methods. Whether these diverse formulations and delivery methods affect influenza vaccine efficacy differently for males and females has not been adequately addressed despite a growing body of literature documenting that the responses to and outcome of some influenza vaccines differ between the sexes. Before systematically reviewing male-female differences, we will briefly highlight the diverse influenza vaccine designs, dosages, and measures of protection.
INFLUENZA VACCINE FORMULATIONS
Inactivated and Subunit Based Vaccines
The vast majority of influenza vaccines available are inactivated or subunit vaccines and are considered safe and efficacious for most individuals, including children 6 months to 2 years of age, pregnant women, and individuals 65 years and older [10]. These vaccines are made from virus that has been inactivated, then partially purified, and are usually administered via intramuscular inoculation. The virus used to generate inactivated influenza vaccine can be grown either in the allantoic cavity of embryonated hen's eggs or in mammalian cells.
A variation of this vaccine approach is to generate one component of the virus—usually the hemagglutinin (HA) protein, which is the primary target of the host antibody response to infection—using recombinant DNA technology. Subunit vaccines are often faster to generate and are more pure than inactivated vaccines. Currently, the only subunit vaccine available for influenza is an HA-based vaccine.
Live, Attenuated Influenza Vaccines
The live, attenuated influenza vaccine (LAIV) is approved for use in individuals ages 2–50. LAIV is administered intranasally and has reduced ability to replicate and cause disease due to multiple mutations in the viral genome. LAIV induces both antibody and cellular immune responses in the respiratory tract. LAIV is grown in embryonated hen's eggs and partially purified before formulation into a vaccine.
Vaccine Components
Three distinct types of influenza cause seasonal influenza epidemics: influenza A virus H1N1, influenza A virus H3N2, and influenza B virus. Until 2013, all influenza vaccine formulations contained components from all 3 virus strains. Influenza B virus exists as 2 related but distinct types and in 2013, a quadrivalent influenza vaccine formulation containing both influenza B virus types, along with H1N1 and H3N2 components, was made available and has been shown to induce strong immune responses to all 4 vaccine components [10].
In the event of a pandemic, monovalent vaccines consisting of only the newly emerged influenza virus strain can be administered to limit disease from that specific virus strain. The 2009 influenza pandemic vaccine (pH1N1) was a monovalent formulation that was used in tandem with the trivalent seasonal influenza vaccine.
Routes of Delivery and Formulations
Influenza vaccines can be administered in a number of ways [10]. LAIV is delivered to the respiratory tract via a nasal spray, which deposits the virus on respiratory epithelial cells in the upper respiratory tract. Inactivated or subunit vaccines are most frequently delivered via intramuscular inoculation. Recently, an intradermal inoculation via microneedles has been used as an alternative to intramuscular delivery.
Adjuvants are compounds that help stimulate immune responses to an antigen. Their use in vaccines allows for a strong immune response, which correlates with increased vaccine efficacy. The amount of antigen used in combination with an adjuvant is typically less than when the antigen is administered alone, thereby allowing more vaccine doses per amount of antigen produced. In the United States, adjuvants are not present in influenza vaccine formulations. Internationally, adjuvants have been used in some seasonal influenza vaccine formulations and most recently in the monovalent 2009 H1N1 vaccine.
Dosage
The LAIV dosage is set so a specific number of infectious virus particles is administered for each virus. Inactivated or subunit vaccines delivered via intramuscular inoculation are standardized to a dose of 15 micrograms of HA protein per virus component in a volume of 0.5 mL. The intradermal vaccine delivers a smaller volume (0.1 mL) and amount (9 micrograms of HA per vaccine component) when compared to intramuscular inoculation. The intradermal influenza vaccine induces a strong immune response using a smaller dose of antigen presumably because more antigen presenting cells are exposed to antigen. By stimulating a more robust immune response to a given amount of antigen, adjuvants play a role analogous to intradermal inoculation, though the increased immune responses are mediated by different immunological mechanisms.
Because influenza vaccination in the >65 year-old population often results in a suboptimal immune response, a high-dose (60 micrograms of HA) inactivated influenza vaccine is available for that population. The vaccine strategy is based on the concept that providing more antigen to an individual will induce a more robust immune response. The indications for influenza vaccine formulations, doses, and delivery routes are currently modified depending on the age, but not the sex, of an individual.
DETERMINANTS OF INFLUENZA VACCINE EFFICACY
Correlates of Protection
Historically, 2 in vitro parameters have been used as surrogates for assessing influenza vaccine efficacy, both of which are based on the generation of antibodies that prevent influenza virus-mediated hemagglutination (HAI) of red blood cells. Individuals possessing a 1:40 serum dilution of HAI antibodies against a specific influenza virus strain or a 4-fold rise in HAI titers have both been associated with effective vaccination and protection from infection and are considered relative correlates of protection. It is important to note that LAIV vaccination induces minimal serum HAI antibodies, and there is no known correlate of protection for this vaccine. As detailed below, the correlates of protection for diverse influenza vaccines show higher responsiveness in females than males.
Adverse Side Effects
Inactivated influenza vaccines have a consistent record of safety, with the most commonly reported side effects being redness or soreness at the site of inoculation. Severe adverse effects are infrequent, with allergic reactions or hypersensitivity most commonly reported. LAIV also has a strong safety record with a runny nose and or nasal congestion being the primary adverse effect reported after LAIV administration. An association of influenza vaccination with disorders such as Bell palsy and Guillain-Barre syndrome has not been clearly made, and the benefits of vaccination (ie, protection from severe influenza) far outweigh any risk of developing either of these syndromes [10]. Although the prevalence of autoimmune diseases is higher in females than males, there also is no documented link between the development of autoimmune diseases and receipt of influenza vaccines. Across diverse age groups, females are more likely to report adverse effects following influenza vaccination than males.
SEX AND GENDER DIFFERENCES RELATED TO INFLUENZA VACCINATION OF ADULTS
Acceptance and Receipt
Acceptance of (ie, the intention of receiving) influenza vaccines is passively measured through questionnaires. The intention of receiving either pandemic or avian influenza vaccines is reportedly 2–3 times lower for females than males, even among healthcare providers (eg, nurses and general practitioners) [2, 12–14]. Receipt of seasonal trivalent influenza vaccines (TIV) in the United States and in several European countries is consistently lower among both young and older adult females than their male counterparts [15–18]. A systematic review revealed that during the 2009 H1N1 pandemic (pH1N1), receipt of the monovalent vaccine was consistently higher in males than females worldwide [5]. Concerns about pH1N1 vaccine efficacy and safety were more likely to be reported among females, despite pregnant women being more likely to receive the pH1N1 than seasonal influenza vaccines [5]. Because male-biased acceptance and receipt of influenza vaccines is observed among older adults, as well as younger adults, pregnant women refusing vaccination cannot solely be responsible for this gender difference.
Antibody Responses
Among both younger (18–64 years) and older (>65 years) adults, females consistently have higher HAI antibody titers than males following TIV vaccination. Receipt of either a full or half-dose of seasonal TIV in adults 18–49 years of age results in HAI antibody titers that are at least twice as high in females as males [19]. Elevated HAI and neutralizing antibody titers among adult females are observed against H1N1, H3N2, and influenza B antigens [5, 19]. Among older adults, the route of administration determines the extent of sex differences in HAI titers. Receipt of TIV by intramuscular injection results in significantly higher HAI titers among older females than males, whereas vaccination via intradermal injection results in HAI titers that are equivalent between the sexes [7]. Among older adults that received the standard intramuscular seasonal TIV, higher HAI titers are associated with lower rates of hospitalization and mortality in females than males, suggesting that the efficacy of TIV in older adults might be higher for females [20, 21]. Among older adults, antibody responses to high-dose TIV are consistently higher than responses to standard-dose TIV for both males and females. Sex differences in HAI titers to high-dose TIV are still apparent, in which antibody responses are significantly higher in older females than males against each of the three influenza antigens [22]. Whether sex differences occur in response to seasonal LAIV has not been reported. Similar to seasonal TIV, older females were reported to have higher HAI antibody titers against the monovalent pH1N1 inactivated vaccine than males, resulting in a 2–3 times higher seroprotection and seroconversion rate in females than males [23]. Although older females produced higher antibody responses to the pH1N1 vaccine, the avidity of their antibodies after pH1N1 vaccination was significantly lower than that of older males [24]. If higher avidity is a measure of a superior antibody response in the elderly, then these data suggest that the quality of the antibody response might be better for males than females. Conversely, cross-reactivity of antibody might be higher for females than males.
Animal models often provide insights into differential efficacy of vaccines. When immunized with an H1N1 or an H3N2 influenza virus, adult female mice of reproductive ages mount higher neutralizing and total antibody responses than males [25]. Following vaccination, female mice are better protected against lethal challenge with a novel influenza strain than males [25]. Although elevated immunity afforded females greater cross-protection than males against lethal challenge with heterosubtypic viruses, both sexes are equally protected against lethal challenge with homologous virus (ie, the strain of virus in the vaccine) [25].
Adverse Reactions
Passive reporting of local reactions (eg, muscle pain, redness, and inflammation) to inactivated split or whole influenza vaccines is consistently more frequent for females than males among both younger and older adults [9]. Measurements of local erythema and induration, both of which are associated with inflammation, reveal that both younger and older adult females have larger (≥6 mm) injection site reactions to TIV than their male counterparts [26]. Systemic reactions (eg, fever, chills, nausea, headaches, and body aches) to TIV also are more commonly reported by females than males, with fatigue and headache being the most notable systemic reactions that occur more frequently in adult females than males [27]. Reports of local and systemic adverse reactions also are more frequent among adult females than males following receipt of the inactivated monovalent pH1N1 vaccine [28, 29]. The types of adverse reactions to the pH1N1 vaccine that were reported, however, were similar between the sexes [28]. To date, whether altering the dose or route for administration of the vaccine could reduce adverse reactions in females has not been analyzed.
Reports of higher frequency of adverse reactions in females than males were observed following receipt of the MF59-adjuvanted monovalent H5N1 vaccine [30]. When administered alone either intramuscularly or intradermally, the adjuvant aluminum hydroxide causes greater injection site reactions in adult females than males [31]. A recent analysis of reports to the Vaccine Adverse Event Reporting System following receipt of either inactivated or live attenuated monovalent pH1N1 vaccines revealed that immediate hypersensitivity reactions were higher in females than males (ages 10–69) [32].
BIOLOGICAL BASIS FOR SEX DIFFERENCES IN RESPONSE TO VACCINES
Hormones Affect Immune Responses
The prevailing hypothesis for immunological differences between the sexes is that sex steroids, particularly testosterone, estradiol, and progesterone, influence the functioning of immune cells. Sex steroids alter the functioning of immune cells by binding to specific receptors, expressed in many immune cells, including lymphocytes, macrophages, and dendritic cells [33]. The binding of sex steroids to their respective steroid receptors directly influences cell signaling pathways, resulting in differential production of cytokines and chemokines [33]. Sex steroids also affect migration, proliferation, and activity of CD4+ and CD8+ T cells as well as antibody production [34].
Estradiol, at physiological concentrations, can stimulate antibody production by B cells [35], including antibody responses to an inactivated influenza vaccine administered in mice [36] suggesting one possible mechanism mediating higher antibody production in females, at least prior to reproductive senescence. In humans, reduced neutralizing antibody responses to influenza vaccination are correlated with higher serum testosterone concentrations [5]. Elevated testosterone concentrations in males is associated with greater lipid metabolism, suggesting a novel pathway mediating reduced antibody responses in males [5].
Genetic Differences Between the Sexes
Sex-based differences in humoral immune responses are observed prior to puberty, during the reproductive years, and after reproductive senescence [1, 19], suggesting that sex hormones are not the only mediators of sex differences in humoral immune responses to vaccines [1]. Alternatively, genetic differences might underlie sex-based differences in adaptive immune responses to viral vaccines. Some sex differences might be caused by the inherent imbalance in the expression of genes encoded on the X and Y chromosomes. Several immune-related genes and regulatory microRNAs are encoded on the X chromosome, and there is some evidence of greater activation of X-linked genes in immune cells from females than males [37, 38]. Polymorphisms in sex chromosomal and autosomal genes that encode for immunological proteins also can contribute to sex differences in immune responses and antibody responses to vaccination [39].
FUTURE DIRECTIONS
Males and females are biologically different. The mechanisms mediating these differences, both hormonal and genetic factors, can alter immune responses to infection or vaccination and require further systematic evaluation. Application of sex-based biology to the rational design of influenza vaccines should not be delayed while the specific underlying mechanisms are investigated. Instead, we should match vaccine design to our biological sex. Now is the time to consider how vaccine design could be used to overcome the reduced immunogenicity of influenza vaccines in males and the heightened adverse reactions to influenza vaccines in females (Figure 1).
Figure 1.
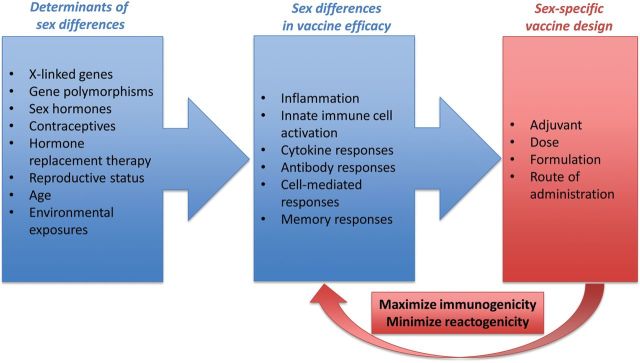
There are a number of determinants of sex differences, including age, reproductive status, sex hormone activity, and genetic factors. The biological differences between the sexes affect innate, adaptive, and memory immune responses as well as adverse reactions (eg, inflammatory responses) to vaccines, resulting in sex differences in vaccine efficacy. Manipulating the biological differences between the sexes is not currently feasible, so we propose sex-specific rational design of vaccines, which involves tailoring vaccines separately to males and females in an effort to increase immunogenicity in males and reduce adverse reactions (reactogenicity) in females. The long-term goal of sex-specific rational vaccine design is to increase acceptance, uptake, and protection following vaccination in both sexes. Sex-specific rational design of vaccines might involve the use of adjuvanted vaccines in males, but not females; reduced doses of vaccines in females; or modified routes of administration, such as use of intradermal routes, or modified delivery methods to reduce local adverse reactions in females.
Mechanisms to Potentiate Immunogenicity in Males
Broadening the immune response in males might be a productive mechanism for increasing vaccine efficacy in males. Increasing the induction of innate immune responses to influenza vaccines might be one strategy that could potentiate adaptive immune responses and, presumably, vaccine efficacy in males. Adjuvants could be used to increase innate immune responses to vaccine in males. Live viral vaccines also induce a broader adaptive immune response that includes activation of T cells and production of cytokines. Limited data reveal that cell-mediated immune responses following vaccination are higher in females than males for some vaccines [3–5], and finding ways to potentiate the cell-mediated immune response following vaccination of males could be advantageous. Finally, increased antigen doses in males might be another mechanism for increasing antibody responses to inactivated or subunit influenza vaccines.
Mechanisms to Reduce Adverse Reactions in Females
The defined strategy to improve influenza vaccines in females might be very different from those indicated for males. In females, the goal would be to reduce inflammation and adverse reactions while retaining elevated neutralizing antibody responses and presumably vaccine efficacy. We propose that both the dose and route of administration must be systematically evaluated for sex-specific effects to determine if lower amounts of antigen combined with intradermal delivery might reduce adverse reactions in females while maintaining sufficient antibody responses to confer adequate protection.
CONCLUDING REMARKS
This concept of sex-specific dosage recommendations has been more readily applied to drugs than to biologics, such as vaccines. The diverse array of Food and Drug Administration (FDA)-approved influenza vaccines available and the evidence of sex-specific responses to influenza infection and vaccination present a unique opportunity to investigate the use of sex-specific vaccine design as a possible means of increasing vaccine efficacy and reducing adverse side effects in the population (Figure 1). The proposed modifications for the design and administration of influenza vaccines could be applied to other vaccines for which sex differences in antibody responses and adverse reactions are reported [1, 8, 9].
Notes
Financial support. NIAID R01 AI097417 from a Supplement for Research on Sex/Gender Differences from the Office of the NIH Director.
Potential conflicts of interest. Both authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–49. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulcini C, Massin S, Launay O, Verger P. Factors associated with vaccination for hepatitis B, pertussis, seasonal and pandemic influenza among French general practitioners: a 2010 survey. Vaccine. 2013;31:3943–9. doi: 10.1016/j.vaccine.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 3.Umlauf BJ, Haralambieva IH, Ovsyannikova IG, et al. Associations between demographic variables and multiple measles-specific innate and cell-mediated immune responses after measles vaccination. Viral Immunol. 2012;25:29–36. doi: 10.1089/vim.2011.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Castelli FA, Zhu X, Wu M, Maillere B, BenMohamed L. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol. 2008;15:1436–49. doi: 10.1128/CVI.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furman D, Hejblum BP, Simon N, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2013;111:869–74. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poland GA, Ovsyannikova IG, Jacobson RM. Adversomics: the emerging field of vaccine adverse event immunogenetics. Pediatr Infect Dis J. 2009;28:431–2. doi: 10.1097/INF.0b013e3181a6a511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook IF, Barr I, Hartel G, Pond D, Hampson AW. Reactogenicity and immunogenicity of an inactivated influenza vaccine administered by intramuscular or subcutaneous injection in elderly adults. Vaccine. 2006;24:2395–402. doi: 10.1016/j.vaccine.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 8.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–5. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 9.Cook IF. Sex differences in injection site reactions with human vaccines. Hum Vaccin. 2009;5:441–9. doi: 10.4161/hv.8476. [DOI] [PubMed] [Google Scholar]
- 10.CDC. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices--United States, 2013–2014. MMWR Recomm Rep. 2013;62:1–43. [PubMed] [Google Scholar]
- 11.WHO. Influenza vaccines. Wkly Epidemiol Rec. 2005;80:279–87. [PubMed] [Google Scholar]
- 12.Opstelten W, van Essen GA, Ballieux MJ, Goudswaard AN. Influenza immunization of Dutch general practitioners: vaccination rate and attitudes towards vaccination. Vaccine. 2008;26:5918–21. doi: 10.1016/j.vaccine.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 13.Chor JS, Ngai KL, Goggins WB, et al. Willingness of Hong Kong healthcare workers to accept pre-pandemic influenza vaccination at different WHO alert levels: two questionnaire surveys. BMJ. 2009;339:b3391. doi: 10.1136/bmj.b3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.To KW, Lee S, Chan TO, Lee SS. Exploring determinants of acceptance of the pandemic influenza A (H1N1) 2009 vaccination in nurses. Am J Infect Control. 2010;38:623–30. doi: 10.1016/j.ajic.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endrich MM, Blank PR, Szucs TD. Influenza vaccination uptake and socioeconomic determinants in 11 European countries. Vaccine. 2009;27:4018–24. doi: 10.1016/j.vaccine.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Merrill RM, Beard JD. Influenza vaccination in the United States, 2005–2007. Med Sci Monit. 2009;15:PH92–100. [PubMed] [Google Scholar]
- 17.Bean-Mayberry B, Yano EM, Mor MK, Bayliss NK, Xu X, Fine MJ. Does sex influence immunization status for influenza and pneumonia in older veterans? J Am Geriatr Soc. 2009;57:1427–32. doi: 10.1111/j.1532-5415.2009.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez-Garcia R, Hernandez-Barrera V, de Andres AL, Jimenez-Trujillo I, Esteban-Hernandez J, Carrasco-Garrido P. Gender influence in influenza vaccine uptake in Spain: time trends analysis (1995–2006) Vaccine. 2010;28:6169–75. doi: 10.1016/j.vaccine.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Engler RJ, Nelson MR, Klote MM, et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch Intern Med. 2008;168:2405–14. doi: 10.1001/archinternmed.2008.513. [DOI] [PubMed] [Google Scholar]
- 20.Wang CS, Wang ST, Chou P. Efficacy and cost-effectiveness of influenza vaccination of the elderly in a densely populated and unvaccinated community. Vaccine. 2002;20:2494–9. doi: 10.1016/s0264-410x(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 21.Fleming DM, Watson JM, Nicholas S, Smith GE, Swan AV. Study of the effectiveness of influenza vaccination in the elderly in the epidemic of 1989–90 using a general practice database. Epidemiol Infect. 1995;115:581–9. doi: 10.1017/s095026880005874x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 23.Kao TM, Hsieh SM, Kung HC, et al. Immune response of single dose vaccination against 2009 pandemic influenza A (H1N1) in the Taiwanese elderly. Vaccine. 2010;28:6159–63. doi: 10.1016/j.vaccine.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Khurana S, Verma N, Talaat KR, Karron RA, Golding H. Immune response following H1N1pdm09 vaccination: differences in antibody repertoire and avidity in young adults and elderly populations stratified by age and gender. J Infect Dis. 2012;205:610–20. doi: 10.1093/infdis/jir791. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo ME, Hodgson A, Robinson DP, Kaplan JB, Pekosz A, Klein SL. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine. 2011;29:9246–55. doi: 10.1016/j.vaccine.2011.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cate TR, Couch RB, Parker D, Baxter B. Reactogenicity, immunogenicity, and antibody persistence in adults given inactivated influenza virus vaccines - 1978. Rev Infect Dis. 1983;5:737–47. doi: 10.1093/clinids/5.4.737. [DOI] [PubMed] [Google Scholar]
- 27.Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med. 1996;156:1546–50. [PubMed] [Google Scholar]
- 28.Kim JH, Cho HY, Hennessey KA, Lee HJ, Bae GR, Kim HC. Adverse events following immunization (AEFI) with the novel influenza a (H1N1) 2009 vaccine: findings from the national registry of all vaccine recipients and AEFI and the passive surveillance system in South Korea. Jpn J Infect Dis. 2012;65:99–104. [PubMed] [Google Scholar]
- 29.Marques JI, Ribeiro Vaz I, Santos C, Polonia J. Adverse events with the influenza A(H1N1) vaccine Pandemrix(R) at healthcare professionals in Portugal. Acta Med Port. 2013;26:107–12. [PubMed] [Google Scholar]
- 30.Huang WT, Chang CH, Peng MC. Telephone monitoring of adverse events during an MF59-adjuvanted H5N1 influenza vaccination campaign in Taiwan. Hum Vaccin Immunotherapeutics. 2013;10 doi: 10.4161/hv.26737. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pittman PR. Aluminum-containing vaccine associated adverse events: role of route of administration and gender. Vaccine. 2002;20(Suppl 3):S48–50. doi: 10.1016/s0264-410x(02)00172-x. [DOI] [PubMed] [Google Scholar]
- 32.Halsey NA, Griffioen M, Dreskin SC, et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: Reports to VAERS. Vaccine. 2013;31:6107–12. doi: 10.1016/j.vaccine.2013.09.066. [DOI] [PubMed] [Google Scholar]
- 33.Kovats S, Carreras E, Agrawal H. Sex steroid receptors in immune cells. In: Klein SL, Roberts CW, editors. Sex hormones and immunity to infection. Berlin: Springer-Verlag; 2010. pp. 53–92. [Google Scholar]
- 34.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–71. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu FX, Abel K, Ma Z, et al. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. 2002;128:10–20. doi: 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen DC, Masseoud F, Lu X, Scinicariello F, Sambhara S, Attanasio R. 17beta-Estradiol restores antibody responses to an influenza vaccine in a postmenopausal mouse model. Vaccine. 2011;29:2515–8. doi: 10.1016/j.vaccine.2011.01.080. [DOI] [PubMed] [Google Scholar]
- 37.Stamova B, Tian Y, Jickling G, et al. The X-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke. 2012;43:326–34. doi: 10.1161/STROKEAHA.111.629337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinheiro I, Dejager L, Libert C. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays. 2011;33:791–802. doi: 10.1002/bies.201100047. [DOI] [PubMed] [Google Scholar]
- 39.Gordeeva LA, Shabaldin AV, Semenova EM, Glushkov AN. Influence of genetic and phenotypical factors on the efficiency of the vaccination of young children against diphtheria and measles. Zh Mikrobiol Epidemiol Immunobiol. 2006;2:42–6. [PubMed] [Google Scholar]


